Abstract
Submerged macrophytes can profoundly influence interactions between aquatic predators and their prey due to changes in foraging efficiencies, pursuit time and swimming behaviors of predator–prey participants. Water hyacinth, Eichhornia crassipes (Mart.) Solms-Laub. (Pontederiaceae), is the most widely distributed of the aquatic invasive weeds in South Africa. This invasive weed contributes to changes in physicochemical (turbidity, temperature and water column stratification) and biological (total chlorophyll-a (Chl-a) concentrations and species composition and distribution of vertebrates and invertebrates) variables within freshwater systems of the region. The current study assessed the influence of varying levels of water hyacinth cover (0, 25, 50 and 100% treatments) on the total Chl-a concentration, size structure of the phytoplankton community and the strength of the interaction between a predatory notonectid, Enithares sobria, and zooplankton using a short-term 10-day long mesocosm study. There were no significant differences in selected physicochemical (temperature, dissolved oxygen, total nitrogen and total phosphate) variables in these different treatments over the duration of this study (ANOVA; p > 0.05 in all cases). Results of this study indicate that treatment had a significant effect on total Chl-a concentrations and total zooplankton abundances. The increased surface cover of water hyacinth contributed to a significant reduction in total Chl-a concentrations and a significant increase in total zooplankton abundances (ANCOVA; p < 0.05 in both cases). The increased habitat complexity conferred by the water hyacinth root system provided refugia for zooplankton. The decline in total Chl-a concentration and the size structure of the phytoplankton community under elevated levels of water hyacinth cover can therefore probably be related to both the unfavorable light environment conferred by the plant cover and the increased grazing activity of zooplankton. The presence of the water hyacinth thus suppressed a predator–prey cascade at the base of the food web. Water hyacinth may, therefore, have important implications for the plankton food web dynamics of freshwater systems by reducing food availability (Chl-a), changing energy flow and alternating the strength of interactions between predators and their prey.
1. Introduction
Invasive aquatic weeds are a key ecological threat to freshwater ecosystem structures and functions worldwide [1,2]. Invasive weeds contribute to decreased water flow, increased thermal stratification, reduced transportation of nutrients, loss of habitat and alterations in hydrological parameters such as freshwater discharge and flow [1]. From a biological perspective, the presence of freshwater invasive species is associated with the loss of biodiversity, reductions in primary production and changes in nutrient and energy flow driven by shifts in the community structure, biotic interactions and the establishment of trophic cascades [1,3,4].
Predation has been identified as a key ecological process in structuring aquatic ecosystems [5,6,7,8]. Predators play a key role in top-down structuring of prey populations in aquatic ecosystems [7,8]. The impact of predators can, however, extend beyond their prey, as they can influence the lower trophic levels by establishing trophic cascades [6,7]. Predation-induced trophic cascades have been linked to changes in energy flows and food web structures that may enable regime shifts within aquatic systems [5,6]. The strength and nature of the interaction between predators and their prey are, however, complex and affected by, among other things, physicochemical (temperature, turbidity and oxygen concentrations) and biological variables such as prey type, prey size and availability, predator diversity and habitat complexity conferred by submerged macrophytes [6,9,10].
Water hyacinth, Eichhornia crassipes (Mart.) Solms-Laub. (Pontederiaceae), is the most problematic of the invasive aquatic weeds in South Africa and, indeed, around the world [11,12,13]. This weed is widespread across South Africa’s aquatic ecosystems due to favorable environmental conditions (elevated levels of eutrophication), a lack of natural competitors and the absence of biological control agents [12]. The persistence of mats of this invasive weed in southern African freshwater lotic systems has been linked to changes in nutrient supply, water column stability, sediment dynamics and flow rates [10,11]. Mats of water hyacinth also contribute to decreased phytoplankton productivity, shifts in the phytoplankton community structure and changes in invertebrate and vertebrate communities [11,12,13]. At the ecosystem level, extensive mats of water hyacinth can have far-reaching consequences, including interfering with the interface between the air and the water, increasing evapotranspiration, altering gaseous exchange and generating both chemical and thermal stratification [10]. At present, there is a paucity of information on the effect of water hyacinth in mediating biological interactions in freshwater systems within the region.
Submerged macrophytes play an important role in determining the outcome of the interaction between predators and prey in shallow-water ecosystems [8,9,10]. The persistence of extensive beds of invasive water hyacinth in open freshwater systems in southern Africa [11,12,13] suggests that their presence may be important in mediating interactions between aquatic predators and their prey, with possible knock-on effects for the food web structure and energy flow. The aim of the current study was to assess the influence of different levels of water hyacinth cover on the total Chl-a concentration, the size structure of the phytoplankton community and the strength of the interaction between a predatory notonectid Enithares sobria and zooplankton, comprising mainly daphnia, using a short-term 10-day long mesocosm study.
2. Materials and Methods
This study was conducted in the Department of Zoology and Entomology, Rhodes University, in the Eastern Cape province of South Africa. Water hyacinth plants (stem length 6–8 cm) were sourced from stock cultures maintained by the Centre of Biological Control (CBC) at Rhodes University. Water, Enithares sobria and zooplankton for this study were collected from a small pond (33°15′ S, 26°26′ E) in the southeastern temperate region of South Africa in June 2017. Enithares sobria, a common local notonectid that preys extensively on zooplankton, were collected via active searching using a kick net (1000 μm mesh size), while the zooplankton, comprising mainly Daphnia, were collected using a 57 cm diameter, 200 μm mesh size zooplankton net towed horizontally, perpendicular to the shoreline. Zooplankton and notonectids collected were transported to the laboratory within 1 h of collection and stored separately in aerated source water for 48 h prior to the commencement of the experiment in a constant environment (CE) room. Experiments were conducted in aerated 500 L carboys (dimensions: 0.9 m × 0.6 m × 0.9 m) maintained in a CE room at 18 °C with a light/dark phase of 14:10. Due to the volume of the carboys, the mesocosm experiment was conducted over 10 days to minimize possible bottling effects. To simulate natural conditions, the sides of the carboys were painted black to ensure light exposure only occurred from directly above.
2.1. Experimental Setup
The experimental setup comprised five treatments with three replicates prepared for each treatment. The control consisted of source water gravimetrically filtered through an 80 μm mesh to isolate large metazoans. Preliminary analysis indicated that the filtration of the water through the mesh removed < 1% of the total chlorophyll-a (Chl-a) and > 95% of metazoans. Treatment 1 comprised source water sieved through an 80 μm mesh to which zooplankton were added. Treatments 2, 3 and 4 were prepared as above, except that the carboys were stocked with 25%, 50% and 100% surface covers of water hyacinth (stem length 6–8 cm) with the addition of three active adult notonectids (SL 9–11 mm). The abundance of zooplankton and notonectids in the carboys were in the range recorded in their natural environment (zooplankton abundances ranged from 5.50 to 1.880 × 103 ind m−3; notonectid densities ranged from 2 to 16 ind m−3). Treatments were set up in a random design within the CE room.
Water temperature, dissolved oxygen (DO), total nitrate and total phosphate concentrations were measured at the start and thereafter on every second day for the duration of this study. Surface temperature (°C) and dissolved oxygen (mg L−1) measurements were made using an Aquaread probe at a depth of 10 cm. Nutrient concentrations were determined from a 100 mL water sample collected from a depth of 10 cm and analyzed using a multiparameter bench photometer (HI 83203, Hanna Instruments Inc., Woonsocket, RI, USA) within 2 h of water sample collection. Total nitrogen (photometer range of 0–10 mg L−1 ± 0.05 mg L−1 accuracy) and total phosphorus concentration (photometer range of 0–30 mg L−1, ±1 mg L−1 accuracy) were expressed in mg L−1.
To assess the effect of treatment on the size-fractionated chlorophyll-a (Chl-a) concentration, a 250 mL water sample was collected from each carboy using a 1 L Schott bottle at the start of this study and thereafter on every second day for the duration of the experiment. Water samples collected at a depth of 10 cm were serially filtered through a 5.0 μm (Nucleopore) and GF/F filter (47 mm diameter) (vacuum; <5 cm Hg) and extracted in 90% acetone in the dark at −20 °C for 24 h [8]. Chl-a concentrations within the two size fractions were then determined fluorometrically using a Turner Designs 10 AU fluorometer and expressed as µg Chl- a L−1 [8,14]. The total Chl-a concentration was assumed to correspond to the sum of the two size fractions.
Each mesocosm was monitored daily to ensure that no mortality of notonectids occurred during the experiment. The abundances of zooplankton within different treatments were determined from water samples collected at night (19:00) to account for zooplankton vertical migrations from vertical tows by hand using a 25 cm modified WP-2 (mesh size of 100 μm) fitted with a codend. Zooplankton collected were immediately preserved with 70% alcohol. Counts were made using a plankton-counting tray with a Zeiss inverted microscope operated at 100× magnification [8]. At the end of the incubation period, the roots of the water hyacinth were gently shaken to ensure that no zooplankton were entwined in the root system. The water in Treatments 1 to 4 was then gravimetrically fed through an 80 μm mesh to retain all zooplankton and notonectids. The total numbers of zooplankton and notonectids within each treatment at the end of the experiment were then counted as above. Zooplankton abundance data were expressed as ind L−1.
2.2. Statistical Analysis
One-way ANOVA with treatment as a fixed factor was employed to determine if there were any significant differences in the physicochemical characteristics (temperature, dissolved oxygen concentrations, total nitrogen and total phosphorous concentrations), total Chl-a concentrations, zooplankton abundances and size ratios of the Chl-a (ratio of <5.0 and >5.0 μm fractions) within different treatments at the start of the experiment. Data were checked for homogeneity of variance using Levine’s test. Normality of data was assessed using the Shapiro test. Thereafter, an analysis of covariance (ANCOVA) test was run to determine the effect of treatment on the mean total Chl-a concentration, size structure of the Chl-a and mean total zooplankton abundances. Post hoc Tukey tests were then employed to determine which group means differed significantly. All statistical analyses were performed in R v3.5.1 [15].
3. Results
3.1. Physicochemical Variables
Water temperatures and dissolved oxygen (DO) concentrations during the incubation period ranged from 18.1 to 18.5 °C and from 6.8 to 7.4 mg L−1, respectively (Figure 1). There were no significant differences in either temperature or DO concentrations in the different treatments at the start of the experiment (temperature: F = 0.46; p = 0.76; DO: F = 1.07; p = 0.56). Total nitrogen concentrations in the various treatments ranged from 28.2 to 36.1 mg L−1, while total phosphorous concentrations varied between 6.4 and 7.3 mg L−1 (Figure 1). Again, there were no significant differences in the total nitrogen or phosphorous concentrations at the start of the experiment (nitrogen: F = 2.994; p = 0.075; phosphorus: F = 1.396; p = 0.304). ANCOVA indicated that treatment had no significant effect on selected physiochemical variables during the experiment (p > 0.05 in all cases).
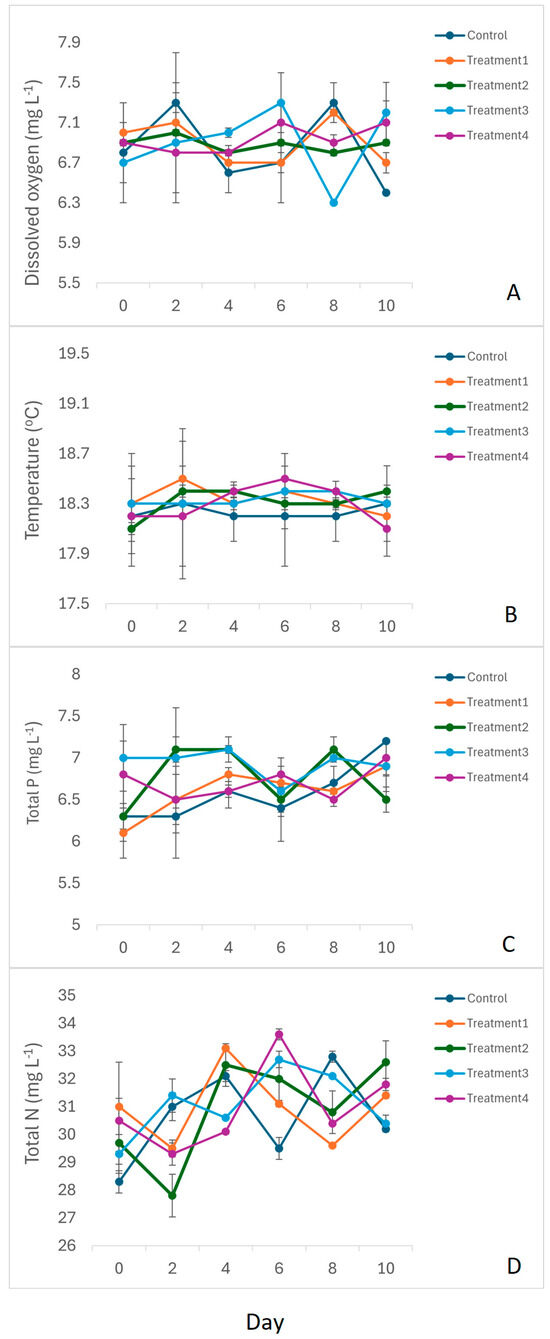
Figure 1.
Selected physicochemical variables: (A) dissolved oxygen; (B) temperature; (C) total phosphorous; and (D) total nitrogen measured over the 10-day long mesocosm study. Control = source water; Treatment 1 = source water + zooplankton; Treatment 2 = source water + zooplankton + notonectids + 25% water hyacinth cover; Treatment 3 = source water + zooplankton + notonectids + 50% water hyacinth cover; Treatment 4 = source water + zooplankton + notonectids + 100% water hyacinth cover. n = 3 for each treatment.
3.2. Biological Variables
There were no significant differences in the mean total Chl-a concentrations (range 0.981 to 1. 513 μg L−1) in the control and different treatments at the start of the experiment (F = 0.204; p = 0.094). ANCOVA indicated that treatment had a significant effect on the total Chl-a concentration (F = 37.56; p < 0.001). Post hoc Tukey tests revealed that the mean total Chl-a concentration in the control was significantly higher than those recorded in Treatments 1 and 4. There were no significant differences in total Chl-a concentrations between the control and Treatments 2 and 3. Similarly, there were no significant differences in mean total Chl-a concentrations between Treatments 1 and 4 (Figure 2).
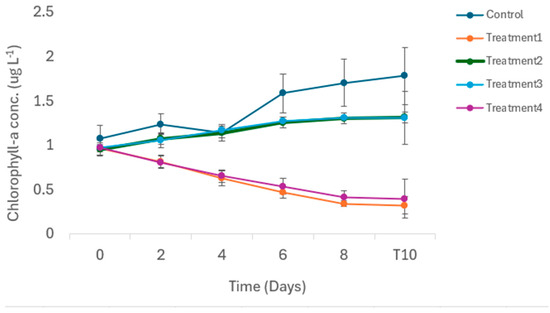
Figure 2.
Temporal changes in total Chl-a concentrations in different treatments during the 10-day long mesocosm study. Treatment 1 = zooplankton + predator; Treatment 2 = zooplankton + predator + 25% water hyacinth cover; Treatment 3 = zooplankton + predator + 50% water hyacinth cover; Treatment 4 = zooplankton + predator + 100% water hyacinth cover. Error bars are standard error (n = 3 for each treatment).
No notonectid mortality was recorded during the experiment. Total zooplankton abundances in the control ranged from 0 to 13 ind L−1. In contrast, the mean total zooplankton abundances in the treatments ranged from 89 to 138 ind L−1. There were no significant differences in the total zooplankton abundances in Treatments 1 to 4 at the start of the study (F = 5.23; p = 0.027). ANCOVA indicated that treatment again had a significant effect on total zooplankton abundances (Figure 3; F = 115. 37; p < 0.001). Post hoc Tukey tests conducted after ANCOVA indicated that the mean total zooplankton abundance in the control was significantly lower than it was in Treatments 1 to 4. Similarly, total zooplankton abundances in Treatment 1 were significantly lower than those recorded in Treatments 2 to 4 (p < 0.05). There were no significant differences in total zooplankton abundances between Treatments 2, 3 and 4 (p > 0.05).
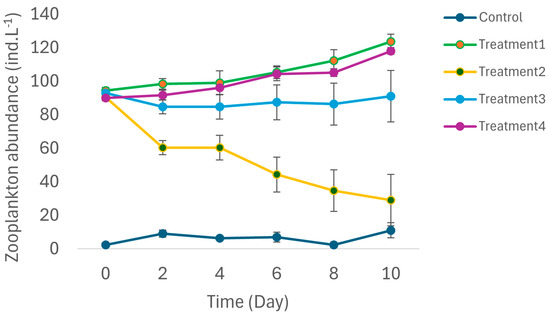
Figure 3.
Temporal changes in total zooplankton abundances in different treatments during the 10-day long mesocosm study. Treatment 1 = zooplankton + predator; Treatment 2 = zooplankton + predator + 25% water hyacinth cover; Treatment 3 = zooplankton + predator + 50% water hyacinth cover; Treatment 4 = zooplankton + predator +100% water hyacinth cover. Error bars are standard error (n = 3 for each treatment).
The ratio of small (<5.0 m) to large (>5 μm) phytoplankton size classes to the total Chl-a concentration was highly variable and ranged from 0.57: 1 to 2.38:1 during this mesocosm study (Figure 4). There were no significant differences in the size ratio of the phytoplankton community in different treatments at the start of this study (F = 1.56; p > 0.05). Overall, the contribution of the small-size class to the total Chl-a concentration increased over the duration of this study. The shift in the size structure of the phytoplankton community was most evident in the predation treatments, particularly in Treatment 4. ANCOVA, however, indicated that treatment had no significant effect on the size ratio of the Chl-a (p > 0.05).
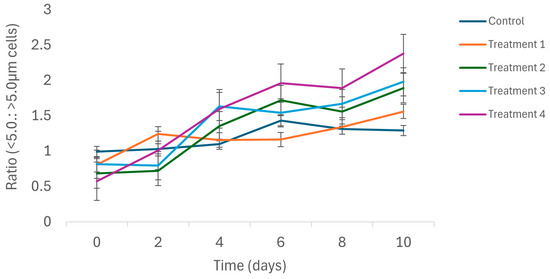
Figure 4.
Temporal shifts in size compositions of phytoplankton assemblages in different treatments during the 10-day long mesocosm study. Treatment 1 = zooplankton + predator; Treatment 2 = zooplankton + predator + 25% water hyacinth cover; Treatment 3 = zooplankton + predator + 50% water hyacinth cover; Treatment 4 = zooplankton + predator + 100% water hyacinth cover. Error bars are standard error (n = 3 for each treatment).
4. Discussion
Freshwater aquatic ecosystems worldwide are facing unparalleled threats attributed to development, globalization, eutrophication, global climate change and biological invasions [16,17,18,19]. Invasive aquatic weeds have been identified as a key ecological threat to freshwater ecosystems, as their presence is associated with changes in biogeochemical and nutrient cycles, productivity, species distributions and diversity within freshwater aquatic ecosystems [11,20,21,22,23,24]. In southern Africa, the invasive water hyacinth, Eichhornia crassipes, is widespread throughout the region due to the eutrophication of inland water bodies and the absence of natural control [12,13]. The presence of this weed is associated with changes in productivity and species composition within lotic systems within the region [10,11,12,13]. The current study investigated the effect of the invasive water weed, E. crassipes, on the total chlorophyll-a (Chl-a) concentration and phytoplankton community structure and in mediating the strength of the interaction between a predator, the notonectid Enithares sobria, and zooplankton using a short-term mesocosm study.
Mesocosm experiments attempt to provide a realistic representation of an ecosystem while offering the statistical power of replicated experiments [25,26,27,28,29]. However, the mesocosms employed during this study did not adequately represent the food web complexity of lotic systems. The absence of a benthic community and other components of the pelagic community, such as fish, within these mesocosms suggests that the main findings of this study should be viewed with caution. Indeed, animals may exert strong impacts on the species composition of primary producers via effects on nutrient supply rates and ratios [30,31]. Nonetheless, the main findings of this study provide insights into the direct and indirect effects of the presence of the invasive water hyacinth in structuring the phytoplankton community and mediating trophic interactions between components of plankton within freshwater ecosystems.
Treatment had a significant effect on total Chl-a concentrations, with estimates for water hyacinth treatments significantly lower than those of the control (p < 0.05). The decrease in total Chl-a concentration with increases in water hyacinth cover is in agreement with the published literature [11,13]. The absence of any significant difference in the selected physicochemical variables in the various treatments suggests that the top-down (resource availability) control of primary production can be discounted (Figure 1; p > 0.05 in all cases). Rather, differences observed in the various treatments indicate that the observed trend in total Chl-a concentration was underpinned by biological processes. Water hyacinths caused a reduction in phytoplankton productivity because the weed mats shaded out any photoautotrophs (both phytoplankton and submersed macrophytes) beneath them [11,12,13]. In addition, water hyacinth can exhibit allelopathic effects on algae by biosynthesizing the algaecidal compound N-phenyl-2-naphthylamine (PNA) in its roots and secreting them into the water to inhibit algal growth [32,33]. The unfavorable light environment conferred by the floating weeds also likely accounted for the decrease in the size structure of the phytoplankton community observed during the experiment (Figure 4), as small cells are physiologically better adapted to grow under low light conditions than large cells.
Results of this study indicate that treatment had a significant effect on total zooplankton abundances, with estimates for water hyacinth treatments significantly higher than those for the control (Figure 3). Although a laboratory-based study previously showed that structural complexity increases the predation impact of Enithares sobria on zooplankton, the complexity employed in that study was less that that generated by the complex root system of the floating water hyacinth [34]. In that study, high complexity corresponded to four plant stalks [34]. The presence of submerged macrophytes often contributes to reduced predation rates within plankton due to several factors, including the restricted movement of predators, increased prey handling times and the provision of refugia for prey [8,9,10]. The significant increase in total zooplankton abundances with an increase in cover of water hyacinth (p < 0.05) suggests that habitat complexity conferred by the water hyacinth root system afforded zooplankton with an effective refuge against predation by notonectids. The unfavorable light environment conferred by the mats of tis invasive weed would have further contributed to the reduced predation impact because notonectids are active predators that detect their prey using visual cues. The absence of any significant difference in zooplankton abundances between Treatments 1 (absence of predator) and 4 (100% cover) attests to the effectiveness of the water hyacinth root system as a refuge (Figure 3).
Zooplankton are important grazers of phytoplankton in aquatic ecosystems and can exert a strong top-down control of phytoplankton production [14,25]. The significant decrease in the total Chl-a concentration in the absence of notonectids (Treatment 1) was, therefore, expected (Figure 3). The rise in total zooplankton abundances associated with increased water hyacinth cover suggests that in addition to unfavorable light conferred by the water hyacinth cover, the decrease in total Chl-a concentrations recorded in Treatments 2 to 4 can also be attributed to an increased grazing impact. Zooplankton, including Daphnia sp., preferentially consume particles in the nano-size (2–20 μm) class [35]. The general decrease in the size ratio of the phytoplankton observed in treatments with increased zooplankton abundance can thus likely also be attributed to their selective grazing activities.
5. Conclusions
The current study demonstrated that an increased cover of water hyacinth contributed to decreases in total Chl-a concentrations and a shift in the size structure of phytoplankton communities. The observed pattern was likely mediated by the unfavorable light environment under the floating mats. In addition, through the provision of a refuge, the predation of notonectids on zooplankton was reduced, contributing to increased zooplankton abundances that exerted a higher grazing impact. The water hyacinth, therefore, suppressed a predator–prey cascade at the base of the food web [36]. Importantly, the effect of the water hyacinth was evident even at the lowest percentage of cover, highlighting the strong adverse ecological effects of invasive weeds on freshwater ecosystems. The shift in the size structure of the phytoplankton community mediated by the water hyacinth is likely to have far-reaching consequences on the food web dynamics of lotic systems, including changes in nutrient cycles, energy flow, trophic transfer energy and biogeochemical cycles. Such changes are likely to drive regime shifts within these systems. This suggests that control of the water hyacinth is important in maintaining the ecological functioning of lotic systems within the region, especially as it is predicted that the water hyacinth distribution range in South Africa will increase in response to global warming [37].
Funding
This work was funded by a Joint Research Council (JRC) Grant awarded to the corresponding author by Rhodes University, grant number 6621.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to its focus on plankton and insects.
Data Availability Statement
Data are available on request from the corresponding author.
Acknowledgments
Funds and facilities for this investigation were provided by Rhodes University. The author would like to thank the Centre for Biological Control (CBC), Department of Zoology and Entomology, Rhodes University, for providing water hyacinth plants for this study.
Conflicts of Interest
The author declares no competing financial interests or personal relationships that could have influenced the work reported in this paper.
References
- Hassan, A.; Nawchoo, I.A. Impact of Invasive Plants in Aquatic Ecosystems. In Bioremediation and Biotechnology; Hakeem, K., Bhat, R., Qadri, H., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Strayer, D.L. Alien species in fresh waters: Ecological effects, interactions with other stressors and prospects for future. Fresh. Biol. 2010, 55, 152–174. [Google Scholar] [CrossRef]
- Stiers, I.; Crohain, N.; Josens, G.; Triest, L. Impact of three aquatic invasive species on native plants and macroinvertebrates in temperate ponds. Biol. Invasions 2011, 13, 2715–2726. [Google Scholar] [CrossRef]
- Crooks, J.A. Characterising ecosystem-level consequences of biological invasions: The role of ecosystem engineers. Oikos 2002, 97, 153–166. [Google Scholar] [CrossRef]
- Pace, M.L.; Cole, J.; Carpenter, S.R.; Kitchell, R.F. Trophic cascades revealed in diverse ecosystems. TREE Trends Ecol. Evol. 1999, 14, 483–488. [Google Scholar] [CrossRef]
- Bruno, J.F.; Connor, M.O. Cascading effects of predator diversity and omnivory in a marine food web. Ecol. Letters 2005, 8, 1048–1056. [Google Scholar] [CrossRef]
- Wasserman, R.J.; Vink, T.J.F.; Kramer, R.; Froneman, P.W. Hyperbenthic and pelagic predators regulate alternate key planktonic predators in shallow temperate estuaries. Mar. Freshw. Res. 2014, 65, 791–801. [Google Scholar] [CrossRef]
- Froneman, P.W.; Cuthbert, R.N. Habitat complexity alters predator-prey interactions in a shallow water ecosystem. Diversity 2022, 14, 431. [Google Scholar] [CrossRef]
- Klecka, J.; Boukal, D.S. The effect of habitat structure on prey mortality depends on predator and prey microhabitat use. Oecologia 2014, 176, 183–191. [Google Scholar] [CrossRef]
- Alexander, M.E.; Kaiser, H.; Weyl, O.L.F.; Dick, J.T.A. Habitat simplification increases the impact of a freshwater invasive fish. Environ. Biol. Fishes 2015, 98, 477–486. [Google Scholar] [CrossRef]
- Coetzee, J.A.; Hill, M.P.; Byrne, M.J.; Bownes, A. A Review of the Biological Control Programmes on Eichhornia crassipes (C.Mart.) Solms (Pontederiaceae), Salvinia molesta D.S.Mitch. (Salviniaceae), Pistia stratiotes L. (Araceae), Myriophyllum aquaticum (Vell.) Verdc. (Haloragaceae) and Azolla filiculoide. Afr.Ento. 2011, 19, 451–468. [Google Scholar] [CrossRef]
- King, A.M. The Effect of Temperature on Biological Control of Water Hyacinth, Eichhornia crassipes (Pontederiaceae) in South Africa. Master’s Thesis, University of Witwatersrand, South Africa, 2011; p. 152. [Google Scholar]
- Coetzee, J.A.; Jones, R.W.; Hill, M.P. Water hyacinth, Eichhornia crassipes (Pontederiaceae), reduces benthic macroinvertebrate diversity in a protected subtropical lake in South Africa. Biodivers. Conserv. 2014, 23, 1319–1330. [Google Scholar] [CrossRef]
- Holm-Hansen, O.; Riemann, B. Chlorophyll a Determination: Improvements in Methodology. Oikos 1978, 30, 438–447. [Google Scholar] [CrossRef]
- Venables, W.N.; Smith, D.M. The R development core team. In An Introduction to R, Version 1.0; R Core Team: Vienna, Austria, 2003. [Google Scholar]
- Dudgeon, D. Threats to Freshwater Biodiversity in a Changing World. In Global Environmental Change: Handbook of Global Environmental Pollution; Freedman, B., Ed.; Springer: Dordrecht, The Netherlands, 2014; Volume 1, pp. 243–253. [Google Scholar] [CrossRef]
- Vilà, M.; Espinar, J.L.; Hejda, M.; Hulme, P.E.; Jarošík, V.; Maron, J.L.; Pergl, J.; Schaffner, U.; Sun, Y.; Pyšek, P. Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 2011, 14, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Chaurisia, S. Role of macrophytes: A review. Adv. Zoo. Bot. 2022, 10, 75–81. [Google Scholar] [CrossRef]
- Schultz, R.; Dibble, E. Effects of invasive macrophytes on freshwater fish and macroinvertebrate communities: The role of invasive plant traits. Hydrobiologia 2012, 684, 1–14. [Google Scholar] [CrossRef]
- Toft, J.D.; Simenstadt, C.; Cordell, J.; Grimaldo, M. The effects of introduced water hyacinth on habitat structure, invertebrate assemblages and fish diet. Est. Coasts 2003, 26, 746–758. [Google Scholar] [CrossRef]
- Midgley, J.M.; Hill, M.P.; Villet, M.H. The effect of water hyacinth, Eichhornia crassipes (Martius) Solms-Laubach (Pontederiaceae), on benthic biodiversity in two impoundments on the New Year’s River, South Africa. Afr. J. Aquat. Sci. 2006, 31, 25–30. [Google Scholar] [CrossRef]
- Quirino, B.A.; Thomaz, S.M.; Jeppesen, E.; Søndergaard, M.; Dainez-Filho, M.S.; Fugi, R. Aquatic Macrophytes Shape the Foraging Efficiency, Trophic Niche Breadth, and Overlap among Small Fish in a Neotropical River. Water 2022, 14, 3543. [Google Scholar] [CrossRef]
- Cambray, J.A. Impact on indigenous species biodiversity caused by the globalisation of alien recreation freshwater fisheries. Hydrobiologia 2003, 500, 217–230. [Google Scholar] [CrossRef]
- Keller, R.P.; Masoodi, A.; Shackleton, R.T. The impact of invasive aquatic plants on ecosystem services and human well-being in Wular Lake, India. Reg. Environ. Change 2018, 18, 847–857. [Google Scholar] [CrossRef]
- Bartholomew, A. Space size relative to prey width and total cover in an area both influence the habitat choices of freshwater angelfish Pterophyllum scalare in mesocosms. Mar. Freshw. Behav. Physiol. 2012, 45, 29–43. [Google Scholar] [CrossRef]
- Auffan, M.; Tella, M.; Santaella, C.; Brousset, L.; Paillès, C.; Barakat, M.; Espinasse, B.; Artells, E.; Issartel, J.; Masion, A.; et al. An adaptable mesocosm platform for performing integrated assessments of nanomaterial risk in complex environmental systems. Sci. Rep. 2014, 4, 5608. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Caughlin, T.T.; Civitello, D.J.; Flory, S.L. Combining mesocosm and field experiments to predict invasive plant performance: A hierarchical Bayesian approach. Ecology 2015, 96, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.; Hodgkins, G.A.; Stoner, A.W. A mesocosm system for ecological research with marine invertebrate larvae. Mar. Ecol. Prog. Ser. 1996, 130, 97–104. [Google Scholar] [CrossRef][Green Version]
- Graney, R.L. Aquatic Mesocosms in Ecological Risk Assessment; CRC Press: Boca Raton, FL, USA, 2020; 736p. [Google Scholar] [CrossRef]
- Withers, P.J.A.; Jarvie, H.P. Delivery and cycling of phosphorus in rivers: A review. Sci. Total Environ. 2008, 400, 379–395. [Google Scholar] [CrossRef]
- Atkinson, C.L.; Capps, K.A.; Rugenski, A.T.; Vanni, M.J. Consumer driven nutrient dynamics in freshwater ecosystems: From Individuals to ecosystems. Bio. Rev. Camb. Philos. Soc. 2017, 94, 2003–2023. [Google Scholar] [CrossRef]
- Pei, Y.; Liu, L.; Hilt, S.; Xu, R.; Wang, B.; Li, C.; Chang, X. Root exudated algicide of Eichhornia crassipes enhances allelopathic effects of cyanobacteria Microcystis aeruginosa on green algae. Hydrobiologia 2018, 823, 67–77. [Google Scholar] [CrossRef]
- Lao, X.F.K. Isolation and identification of antialgal compounds in root system of water hyacinth. Acta. Phytophysiol. Sin. 1992, 18, 399–402. [Google Scholar]
- Wasserman, R.J.; Alexander, M.E.; Weyl, O.L.F.; Froneman, P.W.; Dalu, T. Emergent effects of structural complexity and temperrture on predator-prey interactions. Ecosphere 2016, 7, e01239. [Google Scholar] [CrossRef]
- Sarnelle, O. Daphnia as keystone predators: Effects on phytoplankton diversity and grazing resistance. J. Plankton Res. 2005, 27, 1229–1238. [Google Scholar] [CrossRef]
- Sommer, U. Trophic cascades in marine and freshwater plankton. Int. Rev. Hydrobiol. 2008, 93, 506–516. [Google Scholar] [CrossRef]
- Hoveka, L.N.; Bezeng, B.S.; Yessoufou, K.; Baotwright, J.S.; Van der Bank, M. Effects of global climate change on the future distributions of the top five freshwater invasive plants in South Africa. S. Afr. J. Bot. 2016, 102, 33–38. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).