Abstract
Interspecific interactions among sympatric carnivores are critical for understanding patterns of coexistence, competition, and community structure. Among mesocarnivores, dominance hierarchies are typically shaped by differences in body size, social organization, and competitive ability. The golden jackal (Canis aureus) is generally assumed to dominate the red fox (Vulpes vulpes) across shared landscapes, particularly at high-value resources such as carcasses. However, here, we present rare behavioral evidence that challenges this prevailing assumption. Using motion-triggered camera traps deployed at a carcass in Lake Kerkini National Park, Greece, we recorded a sequence of interactions in which a golden jackal displayed clear submissive behavior toward a red fox, including lowered body posture, tail tucking, and conflict avoidance. Subsequent footage revealed two additional agonistic encounters, during which the same red fox successfully displaced two separate jackals, one of which emitted a distress vocalization while retreating. These findings represent the first documented case of interspecific submission by golden jackals toward a red fox and suggest that context-specific factors—such as immediate carcass possession, individual experience, or body condition—may modulate expected dominance outcomes. Our observations underscore the importance of fine-scale behavioral studies in revealing plasticity in interspecific relationships and contribute to a more nuanced understanding of carnivore competition under semi-natural conditions.
1. Introduction
Carnivores are key regulators of ecological communities, influencing prey populations [1,2], structuring food webs [3], and biodiversity patterns [4]. These regulatory effects emerge not only through predator–prey relationships but also through interactions among carnivore species themselves. Interspecific interactions among carnivores, especially sympatric ones, represent a fundamental component of ecosystem functioning [5,6], as they govern the structure and stability of carnivore guilds across diverse ecosystems [7,8]. In such assemblages where ecological niches overlap, species are frequently brought into competition [9] over limited and spatially aggregated resources such as carcasses, den sites, and high-value foraging areas [8,10]. These interactions are mediated through a range of behavioral mechanisms—spatial avoidance [11,12], temporal segregation [12,13], and agonistic encounters [14]—which collectively reduce conflict and promote the functional coexistence of species.
Among these behavioral mechanisms, dominance and submission play a pivotal role in shaping the outcomes of competitive interactions and are fundamental in organizing principles within carnivore social systems [15,16,17,18]. Dominance behaviors—such as resource monopolization [19,20,21], threat posturing [14,22,23,24], and spatial displacement [25,26]—function to establish hierarchical relationships and secure preferential access to critical resources [9]. Conversely, submissive behaviors—such as lowered body posture, tail tucking, and gaze aversion—serve as conflict mitigation signals [27,28,29,30,31], that reduce the risk of injury and maintain social stability. These interactions are well documented within species [32], particularly in group-living carnivores. However, their expression between species—especially true interspecific submission in the wild—remains largely undocumented, especially between ecologically similar mesocarnivores (body mass of <15 kg [2]), where behavioral plasticity and competitive asymmetries typically produce subtle outcomes that evade detection in broader-scale ecological studies.
One of the most illustrative models for exploring these dynamics is the interaction between the golden jackal (Canis aureus) and the red fox (Vulpes vulpes), two generalist mesocarnivores with extensive, overlapping distributions and broadly similar ecological roles. The golden jackal ranges across northern and eastern Africa, the Middle East, the Caucasus, South and Central Asia, and southeastern Europe [33,34], occupying habitats from arid lowlands to temperate forests. The red fox, the most widespread mesocarnivore in the Northern Hemisphere, exhibits comparable ecological flexibility and occupies nearly all major habitat types used by golden jackals [35,36,37,38].
Although golden jackals and red foxes occupy broadly overlapping ecological niches, accumulating evidence suggests that their coexistence is often shaped by pronounced competitive asymmetries. In southern Europe and Mediterranean ecosystems, multiple studies have documented consistent patterns of food-based interference competition between the two species, typically favoring the jackal and resulting in spatial displacement or reduced resource access for red foxes [39,40,41]. These asymmetries are reinforced by differences in body mass (jackals: 9.6–10.8 kg; foxes: 5.4–6.3 kg; [42,43], morphology (jackals possess longer limbs and stronger dentition), activity patterns (jackals are more arrhythmic; foxes are primarily nocturnal), and foraging strategies (jackals may hunt in pairs or cooperatively, while foxes forage solitarily; [44,45]). Both species are generalist predators and scavengers, and in landscapes where apex predators have been extirpated, they often function as top carnivores [46]. In such contexts, predictable carrion sources—particularly the remains of domestic livestock—represent high-value, spatially restricted resources that commonly elicit interspecific encounters and potential conflict [47,48,49,50]. These settings provide a natural arena for observing the mechanisms by which behavioral dominance is asserted or contested.
Consistent with these ecological dynamics, an increasing number of studies have documented that golden jackals actively suppress red foxes through interference competition. Across multiple spatial scales, red foxes have been shown to avoid the core activity zones of jackals, exhibiting spatial displacement and reduced foraging efficiency in overlapping territories [40,51]. This spatial segregation likely reflects an adaptive avoidance strategy by the subordinate species (e.g., the red fox), intended to minimize costly confrontations with the dominant competitor. In some cases, red foxes have been observed abandoning energetically profitable food patches in the presence of jackals, even when alternative resources are scarce [40]. In more extreme instances, golden jackals have been recorded engaging in intraguild predation on red foxes [51,52]. Although rare cases of tolerance and affiliative behavior have been reported—such as co-feeding and non-aggressive co-occurrence between a solitary male jackal and a red fox family in Germany [53]—these events remain exceptional and do not contradict the prevailing pattern of dominance asymmetry across sympatric carnivores.
Here, we present a novel series of behavioral observations recorded at a water buffalo (Bubalus bubalis) carcass in a semi-natural landscape. Using motion-triggered camera traps, we documented one instance of clear submissive behavior by a golden jackal toward a red fox, followed by two agonistic encounters involving separate jackals and the same fox.
2. Materials and Methods
2.1. Study Area
Lake Kerkini National Park, located in northern Greece along the border with Bulgaria, represents a complex socio-ecological landscape where natural and anthropogenic elements coexist. The lake encompasses a wide range of habitat types, including riparian forests, marshes, reed beds, grasslands, agricultural fields, and extensive open-water areas. This heterogeneity supports a high degree of ecological richness and has earned the site formal protection as both a Ramsar Wetland of International Importance and a designated Natura 2000 area within the European Union’s conservation framework.
Ecological processes in the area are shaped not only by habitat diversity but also by sustained human presence and land use [54]. Livestock grazing, particularly by water buffaloes (Bubalus bubalis), is a dominant feature of the local landscape. More than 6000 individuals are raised under extensive pastoralist systems, with herds allowed to roam freely across large distances [55]. These herds are typically accompanied by livestock guardian dogs, which operate without continuous human oversight (pers. obs.).
Crucially, the absence of direct supervision, combined with the common practice of leaving deceased or moribund animals in the field, often results in the abandonment of sick, injured, or aging animals in remote parts of the grazing territory. When mortality occurs under such conditions, carcasses are frequently left uncollected in open pastures, leading to spatially predictable carrion availability. While no precise data exist on carcass density or turnover rates in Lake Kerkini, this consistent nutrient input creates conditions that may intensify interspecific competition among scavengers. These remain accessible to scavengers and may persist long enough to serve as stable food resources. Consequently, the area sustains a functional scavenger guild, including red foxes, golden jackals, wolves (Canis lupus), and a variety of facultative avian scavengers. High numbers of free-ranging and/or semi-feral domestic dogs are also present in the area (pers. obs.). While no quantitative data are available regarding the population densities of carnivores, mesocarnivores, or the frequency of carcass availability, the presence of these species is well-documented through repeated field observations. The predictable but unquantified carrion availability provides opportunities for studying interspecific interactions, behavioral plasticity, and carcass-based trophic dynamics under semi-natural conditions.
The climate is transitional between Mediterranean and continental types. It is characterized by hot, arid summers and relatively mild, humid winters.
2.2. Camera Trap Deployment and Behavioral Observation of Carnivore Interactions
The carcass site was situated in the northern sector of Lake Kerkini National Park (Figure 1), within a mixed grassland–marsh mosaic (41.228177° N, 23.199577° E). The carcass originated from a herd of approximately 2000 domesticated water buffaloes that graze year-round in the area under semi-extensive management. The surrounding area consisted of a matrix of temporarily inundated grasslands, and open pastures, creating a structurally diverse foraging environment for scavengers. To provide a consistent ecological context, habitat types depicted in Figure 1 were mapped using the Level 2 typology of the European Nature Information System (EUNIS), a standardized habitat classification framework developed by the European Environment Agency [56] and widely adopted for biodiversity monitoring and conservation planning across the European Union.
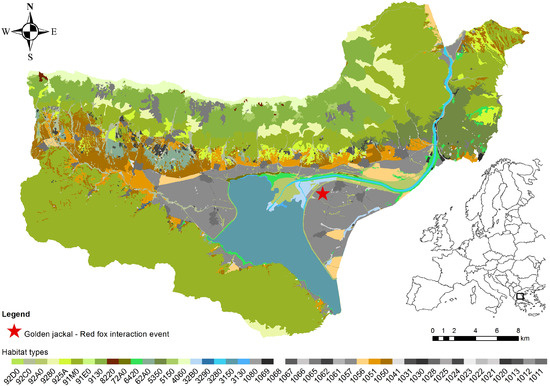
Figure 1.
Location of the observed golden jackal (Canis aureus)–red fox (Vulpes vulpes) interaction events at a water buffalo carcass in Lake Kerkini National Park, northern Greece. Habitat types are classified according to the EUNIS Level 2 habitat typology. The red star indicates the carcass site where the interactions were recorded.
To monitor carnivore behavior at the carcass site, we deployed three passive infrared (PIR) motion-triggered camera traps: two SunTek HC-800M units and one SunTek HC-300A unit (Hong Kong Suntek International Co., Ltd., Hong Kong, China). The carcass, identified as an adult water buffalo, was already partially consumed upon discovery and was left undisturbed to preserve natural conditions. No modifications were made to the carcass itself or to its immediate surroundings. To avoid influencing scavenger behavior, no physical measurements of biomass were taken. However, based on visual assessment a substantial portion of the carcass remained intact, constituting a high-value food resource capable of attracting multiple scavenger species over several days.
In order to minimize visual disturbance and reduce potential neophobic responses among scavenging species [57,58,59], each camera was mounted on a 1.2-m-high metal pole carefully concealed within dense shrub vegetation. This precaution aimed to prevent behavioral alterations typically associated with the sudden presence of novel objects in the environment. The cameras were positioned approximately 4–6 m from the carcass and arranged at roughly 120° intervals, allowing for comprehensive and overlapping coverage of the site. Inter-camera distances varied between 5 and 7 m, depending on vegetation density and terrain complexity.
All cameras were programmed to record high-definition video clips of 1-min duration upon motion detection, with a 5 s PIR retrigger interval (i.e., the minimum delay between consecutive recordings after motion is detected). Infrared illumination enabled the capture of nocturnal activity without disturbing animal behavior. The passive infrared (PIR) sensors had a detection range of 120°, with a trigger speed of 0.3 s and a night vision range of up to 20 m. The camera traps remained active for five consecutive days. Field visits were conducted daily at midday to replace batteries and retrieve data from memory cards, ensuring continuous and uninterrupted monitoring throughout the observation period.
Following data collection, all video recordings were reviewed and coded using the Behavioural Observation Research Interactive Software (BORIS, v. 9.4.1, Turin, Italy) [60], a freely available open-source application designed for logging and analyzing behavioral data from video recordings. A predefined ethogram was used to classify behaviors based on posture, movement, and interaction outcomes. Specifically, observed behaviors included the following: uninterrupted feeding at the carcass; vigilant monitoring; slow or direct approach with assertive or submissive posture; halting or hesitation during approach; stationary submission (e.g., crouching, tail tucked); defensive crouching with piloerection; pursuit or escape behaviors; aggressive lunges (without contact); high-pitched distress vocalizations; withdrawal or full retreat; co-feeding tolerance; and complete displacement.
The ethogram was informed by established literature on interspecific interactions and carnivore behavior [14,16]. Submission was defined as any posture indicating reduced threat and appeasement, including body crouching, tail tucking, lateral ear flattening, or motionless lying while oriented away from the conspecific [27,28,29,30,31]. Aggression was defined as overtly threatening or displacing behavior (e.g., chasing, growling, lunging) [22,23,24], while displacement was assigned when one individual actively caused the other to abandon the carcass [19,20]. Tolerance referred to co-feeding or lack of reaction despite proximity, and retreat was defined as voluntary withdrawal without direct provocation.
All annotations were performed by a single trained observer (Yiannis G. Zevgolis) to ensure consistency and minimize inter-observer bias. Species identification was confirmed visually based on established morphological characteristics. All observed behavioral interactions were recorded, encompassing initial responses to the carcass, reactions to the presence of other scavengers, escape strategies, and any atypical behaviors displayed. Special attention was given to documenting displays of tolerance, aggression, submission, or displacement among scavenger species.
All fieldwork was conducted non-invasively and in accordance with Greek national legislation (Presidential Decree 67/81: “On the protection of native flora and wild fauna and the determination of the coordination and control procedure of related research”) on the humane use of animals. No animals were captured, handled, or disturbed during the course of this study.
3. Results
During the five-day monitoring period at the carcass site in Lake Kerkini National Park, a series of noteworthy interspecific interactions between a red fox and golden jackals were recorded. Among these, three consecutive events captured on 16 and 19 March 2025 were of particular significance, revealing distinct behavioral strategies employed by golden jackals toward a red fox in a competitive scavenging context. These events, encompassing both submissive and aggressive interactions, offer valuable insights into the flexibility of interspecific behavioral responses under semi-natural scavenging conditions.
3.1. Submissive Interaction of a Golden Jackal Toward a Red Fox
At 21:52 EEST on 16 March 2025, a solitary red fox was recorded feeding undisturbed at a water buffalo carcass within the monitoring site. At approximately 10 s into the recording, a group of five golden jackals became visible in the background, maintaining a distance of approximately 30 m and exhibiting no direct engagement with the carcass. One jackal detached from the group and cautiously approached the feeding fox.
Throughout its approach, the jackal displayed conspicuous submissive body language. It maintained a lowered posture close to the ground, with the tail tightly tucked against the hindlimbs and the ears flattened laterally. Its gait was slow and indirect, punctuated by intermittent pauses, consistent with heightened hesitancy and an apparent intent to avoid provoking aggression (Figure 2). No vocalizations, piloerection, lunging, or overt aggressive displays were observed.

Figure 2.
Sequential camera trap images recorded on 16 March 2025 at 21:52 EEST, illustrating the submissive approach of a golden jackal (Canis aureus) toward a red fox (Vulpes vulpes) at a water buffalo carcass site in Lake Kerkini National Park. Panel (A): Golden jackal cautiously approaching the feeding red fox; Panel (B): Jackal adopts a lowered posture while maintaining distance; Panel (C): Red fox remains near the carcass without exhibiting aggressive behavior.
The fox exhibited intermittent vigilance toward the approaching jackal, briefly lifting its head and directing its gaze toward the intruder, but remained primarily engaged in feeding and did not initiate any agonistic behavior. Upon reaching an estimated distance of approximately 2–3 m from the carcass, the jackal adopted a stationary submissive posture. It remained motionless in a lowered position, displaying continued conflict-avoidaning behavior while the fox resumed uninterrupted feeding. A detailed sequence illustrating the progression from approach to stationary submission and eventual withdrawal is presented in Figure 3.
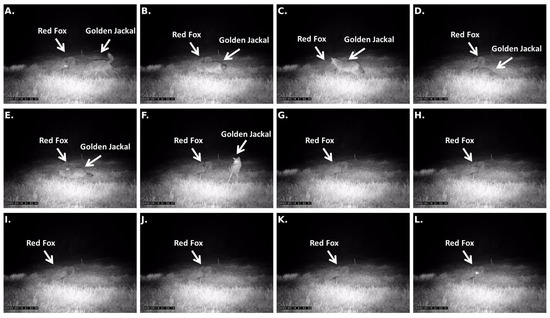
Figure 3.
Detailed sequential frames (Panels (A–L)) documenting the golden jackal’s cautious approach, active submission posture, stationary submission, and final withdrawal during the interaction with the feeding red fox.
After maintaining this submissive posture for approximately 10 s, the jackal gradually retreated from the carcass area without attempting to access the resource or provoke further interaction. Throughout the encounter, the red fox maintained exclusive control of the carcass without displaying overt aggression or displacement behavior.
The complete behavioral sequence observed during this submissive interaction, including the red fox’s vigilance responses and the golden jackal’s progression from cautious approach to withdrawal, is summarized in Table 1.

Table 1.
Observed behaviors and responses during the submissive interaction between a golden jackal (Canis aureus) and a red fox (Vulpes vulpes) at a water buffalo carcass on 16 March 2025. Behavioral events are listed sequentially with corresponding time intervals. The first two entries refer to initial fox activity recorded prior to jackal approach. Letter codes correspond to photographic panels in Figure 3.
3.2. Aggressive Interactions Between a Golden Jackal and a Red Fox
On 19 March 2025, a markedly more dynamic series of agonistic interactions between two golden jackals and the same red fox was recorded at the water buffalo carcass site (Figure 4). In contrast to the previously observed submissive interaction, these events were characterized by direct physical chases, repeated confrontations, and a distress vocalization emitted by the jackals.
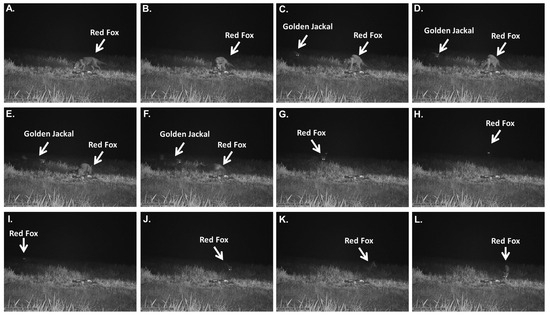
Figure 4.
Detailed sequential frames (Panels (A–L)) documenting the aggressive interaction between the red fox (Vulpes vulpes) and a golden jackal (Canis aureus) at a water buffalo (Bubalus bubalis) carcass on 19 March 2025 (22:47 local time).
At 22:47 EEST, the red fox was initially observed feeding at the carcass. A solitary golden jackal entered the frame and adopted a more assertive approach than previously documented, advancing directly toward the carcass. Upon reaching approximately 3–4 m from the fox, the jackal initiated an abrupt forward movement, attempting to challenge the fox’s control of the resource.
The fox immediately responded with an active chase, pursuing the jackal over a short distance and temporarily displacing it from the immediate vicinity. After this first displacement, the fox returned to the carcass and resumed feeding. However, the jackal continued to hover nearby, prompting a second aggressive chase by the fox at approximately 23 s into the recording.
Despite multiple retreats, the jackal persisted in trailing the fox at a cautious distance. A third, more intense chase occurred around the 30 s mark, culminating in a brief close-range confrontation. During this encounter, the jackal emitted an audible distress vocalization at approximately 34–35 s, signaling social subordination and perceived threat. Following this vocalization, the jackal fully withdrew from the immediate area.
At approximately 51 s, the jackal reappeared at the periphery of the camera’s field of view. It cautiously approached the earlier conflict site, displaying a low body posture and engaging in ground-sniffing behavior, suggestive of submissive investigation. The jackal did not attempt to re-engage and ultimately retreated, while the fox maintained undisturbed access to the carcass throughout the recording period.
Approximately one minute after the previous encounter, a third aggressive interaction between the red fox and a second golden jackal was recorded at the same carcass site. The fox was initially observed sniffing and feeding at the carcass when, at 6 s into the recording, another jackal entered the frame, approaching cautiously from behind (Figure 5).
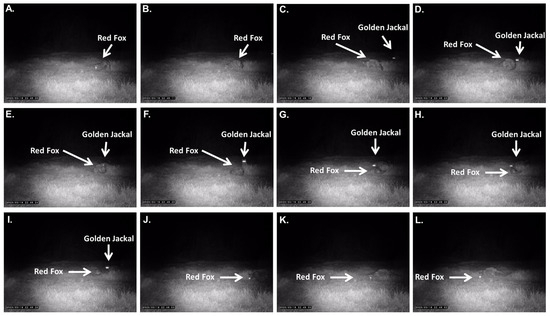
Figure 5.
Detailed sequential frames (Panels (A–L)) documenting the aggressive interaction between the red fox (Vulpes vulpes) and a golden jackal (Canis aureus) at a water buffalo (Bubalus bubalis) carcass on 19 March 2025 (22:48 local time).
Between 6 and 12 s, the jackal advanced slowly toward the fox. Upon establishing visual contact, the fox turned to face the jackal directly. In response, the jackal rapidly executed a series of backward and forward stepping movements, alternating between advancing and retreating without fully committing to an approach. During this period (12–16 s), the fox displayed clear piloerection, raising the fur along its back and tail, a classic defensive threat display commonly observed in carnivores.
At approximately 17 s, the jackal made a brief forward lunge, interpreted as a non-contact bite attempt, while the fox simultaneously adopted a defensive crouching posture. Immediately thereafter (18 s), the fox launched an aggressive pursuit, actively chasing the jackal for approximately 24 s. During the chase, at 42 s into the recording, the jackal emitted a high-pitched distress vocalization, typical of subordinate individuals under social pressure or perceived threat. Following this prolonged pursuit, the fox ceased chasing and returned to the carcass, resuming feeding behavior without further disturbance. The jackal withdrew from the vicinity and did not reappear during the remainder of the recording.
A detailed ethogram summarizing the observed behaviors, species involved, and corresponding timing during the two aggressive interactions is presented in Table 2.

Table 2.
Observed behaviors and responses during agonistic interactions between golden jackals (Canis aureus) and a red fox (Vulpes vulpes) at a water buffalo carcass on 19 March 2025, combining events from two consecutive camera trap recordings. Behaviors are listed chronologically by event timing. The first entry (4A) refers to initial fox activity recorded prior to jackal approach. Letter codes correspond to photographic panels in Figure 4 and Figure 5.
4. Discussion
The interspecific submission displayed by a golden jackal toward a red fox at the carcass site represents a rare and behaviorally novel observation within mesocarnivore guilds. While dominance hierarchies among carnivores are well recognized, they are typically expressed through aggressive displacement or spatial avoidance [17,61], particularly in resource-rich contexts such as carcasses [62,63,64]. The overtly submissive behavior documented here—including lowered body posture, tail tucking, and approach inhibition—stands in stark contrast to the widely accepted assumption that golden jackals are consistently behaviorally dominant over red foxes in overlapping ranges [40,41,51,65]. To the best of our knowledge, this is the first empirical evidence of active interspecific submission directed from a jackal to a red fox under competitive conditions.
In the days that followed this unusual interaction, the dynamics at the carcass site temporarily shifted. During the nights of 17 and 18 March, both species continued to visit the site; however, their activity patterns did not overlap. The red fox and golden jackals appeared at different hours, effectively avoiding direct encounters. This short-lived temporal segregation may represent a form of interference avoidance [13,17,66,67], a tactic often employed by sympatric carnivores to mitigate conflict when shared resources are contested and the cost of escalation is high.
The subsequent aggressive interactions observed on 19 March involving two different jackals and the same red fox indicate a dynamic shift in the competitive relationship. In both cases, the fox not only defended exclusive access to the carcass but initiated repeated chases, eliciting high-pitched vocalizations interpreted as distress calls from the jackals. These responses, particularly vocal distress signals and retreat postures, further support the notion of momentary subordination [51,68]. The repeated failure of the jackals to displace the fox suggests a temporary inversion of the expected dominance structure. Importantly, the sequence of interactions—ranging from passive submission to escalated agonism—occurred without any reinforcement or group support, underscoring the importance of individual-level variation in shaping behavioral outcomes [63,69,70,71].
This apparent reversal in dominance hierarchy invites closer examination of individual traits influencing these encounters. The jackals involved in the submissive and initial agonistic interactions were clearly different individuals, distinguishable by their body size, coat pattern, and tail morphology. In contrast, although the jackals in the second and third interactions appeared distinct, we cannot entirely exclude the possibility of repeated appearances due to limitations in image resolution. The red fox, however, was consistently identifiable and exhibited unusually assertive behaviors across all events. This behavioral consistency points toward a potentially atypical individual, whose assertiveness may reflect traits such as boldness, prior experience, or familiarity with the carcass site. After all, interspecific dominance is not solely determined by body size [72,73] but is shaped by a combination of behavioral traits such as territoriality, social structure, and spatial familiarity [74], particularly in flexible or transitional competitive settings.
These findings contribute to a growing body of literature highlighting the context dependence of interspecific interactions among mesocarnivores. While golden jackals are generally larger, more social, and behaviorally dominant in most ecological contexts [40,42,51], our observations suggest that dominance is not fixed but rather subject to modulation by local conditions. Factors such as immediate carcass possession [75,76,77], individual boldness [78], body condition [63,79], or previous experience [26] may have enabled the red fox to assert dominance despite the usual asymmetry. This interpretation is consistent with experimental and field-based evidence from both Europe and the Americas, where carnivore dominance hierarchies have been shown to shift in response to habitat structure, prey distribution, and recent interspecific encounters [80,81]. Moreover, the behavioral plasticity exhibited by both species aligns with theoretical expectations that carnivore interactions are governed not solely by morphological or social traits, but by immediate situational factors and cost–benefit calculations.
From an ecological perspective, such plasticity in competitive behavior may have broader implications for species coexistence and trophic structuring. As golden jackals continue to expand their range in Europe [82,83] and in Greece [84], encounters with red foxes and other mesocarnivores are expected to become more frequent. The assumption of a rigid dominance hierarchy may oversimplify the true complexity of these relationships. Behavioral observations such as ours, though rare, reveal that subordinate species may assert control over key resources under certain ecological configurations. For example, apex predator suppression has been shown to influence mesocarnivore abundance and behavior, indirectly altering interspecific interactions at the carcass level [85]. Such reversals, even if temporary, can influence scavenging dynamics, feeding efficiency, and local space use, ultimately shaping carnivore assemblages.
This behavioral nuance acquires particular ecological significance in human modified landscapes such as Lake Kerkini, where extensive pastoralism creates a semi-predictable abundance of carrion. The routine abandonment of deceased or dying water buffaloes by local herders (pers. obs.) results in spatially clumped, high-energy food subsidies that can intensify competitive interactions among facultative scavengers. In such resource-rich yet socially dynamic contexts, behavioral flexibility—such as transient assertions of dominance by typically subordinate species—may be more likely to manifest [26,86]. These context-dependent deviations from established dominance hierarchies suggest that competitive interactions among mesocarnivores are not fixed but may shift according to ecological opportunity and individual condition.
Nevertheless, the interpretative power of our findings is constrained by methodological limitations. The observations derive from a short-term monitoring effort and represent interactions among a small number of individuals. Without identification of specific jackals or foxes, it is not possible to assess sex, age class, prior experience, or social affiliation—all of which may influence behavior. Additionally, the lack of repeated trials or multiple carcass sites constrains the generalizability of the findings. The known presence of other carnivores in the area (pers. obs.), such as wolves (Canis lupus) and livestock guardian dogs, further highlights the need for research examining how broader carnivore communities influence mesocarnivore interactions at shared resources. Incorporating multi-species monitoring at carcass sites, along with individual identification (e.g., via GPS collars or genetic sampling), replicated carcass placement, and longer-term monitoring could help clarify the conditions under which interspecific submission and dominance reversals occur.
In conclusion, the behavioral interactions documented here challenge conventional assumptions about fixed dominance hierarchies among sympatric mesocarnivores. Emerging evidence, such as ours, suggests that rare or anomalous behavioral events may signal latent plasticity in interspecific relationships, offering a more nuanced understanding of the ecological roles species can occupy under varying environmental and social conditions [87]. The observation of submissive and subordinate behaviors by golden jackals toward a red fox—across both passive and agonistic encounters—illustrates the importance of individual variation and situational context in shaping interspecific relationships. By capturing a rare but ecologically informative sequence of events, our findings contribute to a greater awareness of the complexity of carnivore competition and coexistence, with implications for both behavioral ecology and conservation management.
Author Contributions
Conceptualization, Y.G.Z. and C.K.; methodology, Y.G.Z., C.K. and B.G.; validation, Y.G.Z.; investigation, C.K. and B.G.; resources, Y.G.Z., C.K., B.G., A.L. and A.C.; data curation, Y.G.Z., C.K., B.G. and A.C.; writing—original draft preparation, Y.G.Z.; writing—review and editing, Y.G.Z., C.K., B.G., A.L. and A.C.; supervision, Y.G.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
We would like to thank the anonymous reviewers for their constructive suggestions and comments. All aspects of this study were conducted in full compliance with Hellenic national law (Presidential Decree 67/81: “On the protection of native flora and wild fauna and the determination of the coordination and control procedure of related research”) on the humane use of animals.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Terborgh, J.; Estes, J.A.; Paquet, P.; Ralls, K.; Boyd-Heger, D.; Miller, B.J.; Noss, R.F. The Role of Top Carnivores in Regulating Terrestrial Ecosystems; Island Press: Washington, DC, USA, 1999. [Google Scholar]
- Roemer, G.W.; Gompper, M.E.; Van Valkenburgh, B. The Ecological Role of the Mammalian Mesocarnivore. Bioscience 2009, 59, 165–173. [Google Scholar] [CrossRef]
- Winnie, J.; Creel, S. The many effects of carnivores on their prey and their implications for trophic cascades, and ecosystem structure and function. Food Webs 2017, 12, 88–94. [Google Scholar] [CrossRef]
- Ripple, W.J.; Estes, J.A.; Beschta, R.L.; Wilmers, C.C.; Ritchie, E.G.; Hebblewhite, M.; Berger, J.; Elmhagen, B.; Letnic, M.; Nelson, M.P.; et al. Status and Ecological Effects of the World’s Largest Carnivores. Science 2014, 343, 1241484. [Google Scholar] [CrossRef]
- Monterroso, P.; Díaz-Ruiz, F.; Lukacs, P.M.; Alves, P.C.; Ferreras, P. Ecological traits and the spatial structure of competitive coexistence among carnivores. Ecology 2020, 101, e03059. [Google Scholar] [CrossRef]
- HilleRisLambers, J.; Adler, P.B.; Harpole, W.S.; Levine, J.M.; Mayfield, M.M. Rethinking Community Assembly Through the Lens of Coexistence Theory. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 227–248. [Google Scholar] [CrossRef]
- Dayan, T.; Simberloff, D.; Tchernov, E.; Yom-tov, Y. Canine carnassials: Character displacement in the wolves, jackals and foxes of Israel. Biol. J. Linn. Soc. 1992, 45, 315–331. [Google Scholar] [CrossRef]
- Karanth, K.U.; Srivathsa, A.; Vasudev, D.; Puri, M.; Parameshwaran, R.; Kumar, N.S. Spatio-temporal interactions facilitate large carnivore sympatry across a resource gradient. Proc. R. Soc. B Biol. Sci. 2017, 284, 20161860. [Google Scholar] [CrossRef]
- Ritchie, E.G.; Johnson, C.N. Predator interactions, mesopredator release and biodiversity conservation. Ecol. Lett. 2009, 12, 982–998. [Google Scholar] [CrossRef] [PubMed]
- Fedriani, J.M.; Fuller, T.K.; Sauvajot, R.M.; York, E.C. Competition and intraguild predation among three sympatric carnivores. Oecologia 2000, 125, 258–270. [Google Scholar] [CrossRef]
- Vanak, A.T.; Fortin, D.; Thaker, M.; Ogden, M.; Owen, C.; Greatwood, S.; Slotow, R. Moving to stay in place: Behavioral mechanisms for coexistence of African large carnivores. Ecology 2013, 94, 2619–2631. [Google Scholar] [CrossRef]
- Swanson, A.; Caro, T.; Davies-Mostert, H.; Mills, M.G.L.; Macdonald, D.W.; Borner, M.; Masenga, E.; Packer, C. Cheetahs and wild dogs show contrasting patterns of suppression by lions. J. Anim. Ecol. 2014, 83, 1418–1427. [Google Scholar] [CrossRef] [PubMed]
- Kneitel, J.M.; Chase, J.M. Trade-offs in community ecology: Linking spatial scales and species coexistence. Ecol. Lett. 2004, 7, 69–80. [Google Scholar] [CrossRef]
- Palomares, F.; Caro, T.M. Interspecific Killing Among Mammalian Carnivores. Am. Nat. 1999, 153, 492–508. [Google Scholar] [CrossRef] [PubMed]
- Creel, S. Dominance, aggression, and glucocorticoid levels in social carnivores. J. Mammal. 2005, 86, 255–264. [Google Scholar] [CrossRef]
- Allen, M.L.; Wilmers, C.C.; Elbroch, L.M.; Golla, J.M.; Wittmer, H.U. The importance of motivation, weapons, and foul odors in driving encounter competition in carnivores. Ecology 2016, 97, 1905–1912. [Google Scholar] [CrossRef]
- Sivy, K.J.; Pozzanghera, C.B.; Grace, J.B.; Prugh, L.R. Fatal Attraction? Intraguild Facilitation and Suppression Among Predators. Am. Nat. 2017, 190, 663–679. [Google Scholar] [CrossRef]
- Allen, M.L.; Avrin, A.C.; Wittmer, H.U.; Wang, Y.; Wilmers, C.C. Mesocarnivores vary in their spatiotemporal avoidance strategies at communications hubs of an apex carnivore. Oecologia 2024, 204, 805–813. [Google Scholar] [CrossRef]
- López-Bao, J.V.; Palomares, F.; Rodríguez, A.; Ferreras, P. Intraspecific interference influences the use of prey hotspots. Oikos 2011, 120, 1489–1496. [Google Scholar] [CrossRef]
- Dorning, J.; Harris, S. Dominance, gender, and season influence food patch use in a group-living, solitary foraging canid. Behav. Ecol. 2017, 28, 1302–1313. [Google Scholar] [CrossRef]
- Cronk, N.E.; Pillay, N. Food choice and feeding on carrion in two African mongoose species in an urban environment. Acta Ethol. 2018, 21, 127–136. [Google Scholar] [CrossRef]
- Garvey, P.M.; Glen, A.S.; Pech, R.P. Foraging Ermine Avoid Risk: Behavioural responses of a mesopredator to its interspecific competitors in a mammalian guild. Biol. Invasions 2015, 17, 1771–1783. [Google Scholar] [CrossRef]
- Zevgolis, Y.G.; Christopoulos, A.; Kalargalis, I.I.; Zannetos, S.P.; Botetzagias, I.; Dimitrakopoulos, P.G. An (Un)Expected Threat for a Regionally Near-Threatened Species: A Predation Case of a Persian Squirrel on an Insular Ecosystem. Animals 2022, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Garvey, P.M.; Glen, A.S.; Clout, M.N.; Nichols, M.; Pech, R.P. Niche partitioning in a guild of invasive mammalian predators. Ecol. Appl. 2022, 32, e2566. [Google Scholar] [CrossRef]
- Peers, M.J.L.; Thornton, D.H.; Murray, D.L. Evidence for large-scale effects of competition: Niche displacement in Canada lynx and bobcat. Proc. R. Soc. B Biol. Sci. 2013, 280, 20132495. [Google Scholar] [CrossRef] [PubMed]
- Sanglas, A.; Palomares, F. Response of a mesocarnivore community to a new food resource: Recognition, exploitation, and interspecific competition. Eur. J. Wildl. Res. 2022, 68, 51. [Google Scholar] [CrossRef]
- Kutsukake, N.; Clutton-Brock, T.H. Aggression and submission reflect reproductive conflict between females in cooperatively breeding meerkats Suricata suricatta. Behav. Ecol. Sociobiol. 2006, 59, 541–548. [Google Scholar] [CrossRef]
- Kutsukake, N.; Clutton-Brock, T.H. Do meerkats engage in conflict management following aggression? Reconciliation, submission and avoidance. Anim. Behav. 2008, 75, 1441–1453. [Google Scholar] [CrossRef]
- Baan, C.; Bergmüller, R.; Smith, D.W.; Molnar, B. Conflict management in free-ranging wolves, Canis lupus. Anim. Behav. 2014, 90, 327–334. [Google Scholar] [CrossRef]
- Reddon, A.R.; Ruberto, T.; Reader, S.M. Submission signals in animal groups. Behaviour 2021, 159, 1–20. [Google Scholar] [CrossRef]
- McCormick, S.K.; Holekamp, K.E. Aggressiveness and submissiveness in spotted hyaenas: One trait or two? Anim. Behav. 2022, 186, 179–190. [Google Scholar] [CrossRef]
- Rowell, T.E. The concept of social dominance. Behav. Biol. 1974, 11, 131–154. [Google Scholar] [CrossRef] [PubMed]
- Mitchell-Jones, A.J.; Amori, G.; Bogdanowicz, W.; Krystufek, B.; Reijnders, P.J.H.; Spitzenberger, F.; Stubbe, M.; Thissen, J.B.M.; Vohralik, V.; Zima, J. The Atlas of European Mammals; Poyser: London, UK, 1999; ISBN 9780856611308. [Google Scholar]
- Hoffmann, M.; Arnold, J.; Duckworth, J.W.; Jhala, Y.; Kamler, J.F.; Krofel, M. Canis aureus. In IUCN Red List Threatened Species; IUCN: Gland, Switzerland, 2018. [Google Scholar]
- Lloyd, H.G. The Red Fox; B.T. Batsford: London, UK, 1980; ISBN 9780713411904. [Google Scholar]
- Macdonald, D.W. The ecology of carnivore social behaviour. Nature 1983, 301, 379–384. [Google Scholar] [CrossRef]
- Gittleman, J.L. Carnivore body size: Ecological and taxonomic correlates. Oecologia 1985, 67, 540–554. [Google Scholar] [CrossRef]
- Hoffmann, M.; Sillero-Zubiri, C. Vulpes vulpes. In IUCN Red List Threatened Species; IUCN: Gland, Switzerland, 2016. [Google Scholar]
- Tsunoda, H.; Raichev, E.G.; Newman, C.; Masuda, R.; Georgiev, D.M.; Kaneko, Y. Food niche segregation between sympatric golden jackals and red foxes in central Bulgaria. J. Zool. 2017, 303, 64–71. [Google Scholar] [CrossRef]
- Scheinin, S.; Yom-Tov, Y.; Motro, U.; Geffen, E. Behavioural responses of red foxes to an increase in the presence of golden jackals: A field experiment. Anim. Behav. 2006, 71, 577–584. [Google Scholar] [CrossRef]
- Farkas, A.; Jánoska, F.; Fodor, J.-T.; Náhlik, A. The high level of nutritional niche overlap between red fox (Vulpes vulpes) and sympatric golden jackal (Canis aureus) affects the body weight of juvenile foxes. Eur. J. Wildl. Res. 2017, 63, 46. [Google Scholar] [CrossRef]
- Lanszki, J.; Kurys, A.; Heltai, M.; Csányi, S.; Ács, K. Diet Composition of the Golden Jackal in an Area of Intensive Big Game Management. Ann. Zool. Fenn. 2015, 52, 243–255. [Google Scholar] [CrossRef]
- Lanszki, J.; Kurys, A.; Szabó, L.; Nagyapáti, N.; Porter, L.B.; Heltai, M. Diet composition of the golden jackal and the sympatric red fox in an agricultural area (Hungary). Folia Zool. 2016, 65, 310–322. [Google Scholar] [CrossRef]
- Macdonald, D.W. “Helpers” in fox society. Nature 1979, 282, 69–71. [Google Scholar] [CrossRef]
- Yom-Tov, Y.; Ashkenazi, S.; Viner, O. Cattle predation by the golden jackal Canis aureus in the Golan Heights, Israel. Biol. Conserv. 1995, 73, 19–22. [Google Scholar] [CrossRef]
- Chapron, G.; Kaczensky, P.; Linnell, J.D.C.; von Arx, M.; Huber, D.; Andrén, H.; López-Bao, J.V.; Adamec, M.; Álvares, F.; Anders, O.; et al. Recovery of large carnivores in Europe’s modern human-dominated landscapes. Science 2014, 346, 1517–1519. [Google Scholar] [CrossRef] [PubMed]
- Giannatos, G.; Karypidou, A.; Legakis, A.; Polymeni, R. Golden jackal (Canis aureus L.) diet in Southern Greece. Mamm. Biol. 2010, 75, 227–232. [Google Scholar] [CrossRef]
- Borkowski, J.; Zalewski, A.; Manor, R. Diet Composition of Golden Jackals in Israel. Ann. Zool. Fenn. 2011, 48, 108–118. [Google Scholar] [CrossRef]
- Ćirović, D.; Penezić, A.; Krofel, M. Jackals as cleaners: Ecosystem services provided by a mesocarnivore in human-dominated landscapes. Biol. Conserv. 2016, 199, 51–55. [Google Scholar] [CrossRef]
- Singh, A.; Mukherjee, A.; Dookia, S.; Kumara, H.N. High resource availability and lack of competition have increased population of a meso-carnivore—A case study of Golden Jackal in Keoladeo National Park, India. Mammal Res. 2016, 61, 209–219. [Google Scholar] [CrossRef]
- Torretta, E.; Riboldi, L.; Costa, E.; Delfoco, C.; Frignani, E.; Meriggi, A. Niche partitioning between sympatric wild canids: The case of the golden jackal (Canis aureus) and the red fox (Vulpes vulpes) in north-eastern Italy. BMC Ecol. Evol. 2021, 21, 129. [Google Scholar] [CrossRef]
- Raichev, E.G.; Tsunoda, H.; Newman, C.; Masuda, R.; Georgiev, D.M.; Kaneko, Y. The Reliance of the Golden Jackal (Canis aureus) on Anthropogenic Foods in winter in Central Bulgaria. Mammal Study 2013, 38, 19–27. [Google Scholar] [CrossRef]
- Böcker, F.; Weber, H.; Arnold, J.; Collet, S.; Hatlauf, J. Interspecific social interaction between golden jackal (Canis aureus) and red fox (Vulpes vulpes). Mammal Res. 2024, 69, 319–324. [Google Scholar] [CrossRef]
- Zevgolis, Y.G.; Kotselis, C.; Kouris, A.D.; Christopoulos, A. A New Aspect of Predator–Prey Dynamics: The Case of a Livestock Guardian Dog Predating upon an Invasive Coypu in Lake Kerkini, Greece. Conservation 2024, 4, 609–616. [Google Scholar] [CrossRef]
- Tsiobani, E.T.; Yiakoulaki, M.D.; Hasanagas, N.D.; Menexes, G.; Papanikolaou, K. Water Buffaloes Grazing Behaviour at the Lake Kerkini National Park, Northern Greece. Hacquetia 2016, 15, 133–142. [Google Scholar] [CrossRef]
- European Environment Agency (EEA). EUNIS Habitat Classification. Available online: https://www.eea.europa.eu/data-and-maps/data/eunis-habitat-classification (accessed on 15 March 2025).
- Stanton, L.A.; Bridge, E.S.; Huizinga, J.; Johnson, S.R.; Young, J.K.; Benson-Amram, S. Variation in reversal learning by three generalist mesocarnivores. Anim. Cogn. 2021, 24, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Mettler, A.E.; Shivik, J.A. Dominance and neophobia in coyote (Canis latrans) breeding pairs. Appl. Anim. Behav. Sci. 2007, 102, 85–94. [Google Scholar] [CrossRef]
- Windberg, L.A. Coyote responses to visual and olfactory stimuli related to familiarity with an area. Can. J. Zool. 1996, 74, 2248–2253. [Google Scholar] [CrossRef]
- Friard, O.; Gamba, M. BORIS: A free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 2016, 7, 1325–1330. [Google Scholar] [CrossRef]
- Allen, M.L.; Elbroch, L.M.; Wittmer, H.U. Can’t bear the competition: Energetic losses from kleptoparasitism by a dominant scavenger may alter foraging behaviors of an apex predator. Basic Appl. Ecol. 2021, 51, 1–10. [Google Scholar] [CrossRef]
- Haswell, P.M.; Kusak, J.; Jones, K.A.; Hayward, M.W. Fear of the dark? A mesopredator mitigates large carnivore risk through nocturnality, but humans moderate the interaction. Behav. Ecol. Sociobiol. 2020, 74, 62. [Google Scholar] [CrossRef]
- Bell, E.; Fisher, J.T.; Darimont, C.; Hart, H.; Bone, C. Influence of heterospecifics on mesocarnivore behaviour at shared scavenging opportunities in the Canadian Rocky Mountains. Sci. Rep. 2023, 13, 11026. [Google Scholar] [CrossRef] [PubMed]
- Wikenros, C.; Ståhlberg, S.; Sand, H. Feeding under high risk of intraguild predation: Vigilance patterns of two medium-sized generalist predators. J. Mammal. 2014, 95, 862–870. [Google Scholar] [CrossRef]
- Tsunoda, H. Niche Overlaps and Partitioning Between Eurasian Golden Jackal Canis aureus and Sympatric Red Fox Vulpes vulpes. Proc. Zool. Soc. 2022, 75, 143–151. [Google Scholar] [CrossRef]
- Searle, C.E.; Smit, J.B.; Cusack, J.J.; Strampelli, P.; Grau, A.; Mkuburo, L.; Macdonald, D.W.; Loveridge, A.J.; Dickman, A.J. Temporal partitioning and spatiotemporal avoidance among large carnivores in a human-impacted African landscape. PLoS ONE 2021, 16, e0256876. [Google Scholar] [CrossRef]
- Nakabayashi, M.; Kanamori, T.; Matsukawa, A.; Tangah, J.; Tuuga, A.; Malim, P.T.; Bernard, H.; Ahmad, A.H.; Matsuda, I.; Hanya, G. Temporal activity patterns suggesting niche partitioning of sympatric carnivores in Borneo, Malaysia. Sci. Rep. 2021, 11, 19819. [Google Scholar] [CrossRef]
- Castelló, J.R.; Claudio, S.-Z. Canids of the World: Wolves, Wild Dogs, Foxes, Jackals, Coyotes, and Their Relatives; Princeton University Press: Princeton, NJ, USA, 2018. [Google Scholar]
- Hernandez-Puentes, C.; Torre, I.; Vilella, M. Spatio-temporal interactions within a Mediterranean community of Mesocarnivores. Mamm. Biol. 2022, 102, 357–373. [Google Scholar] [CrossRef]
- Zevgolis, Y.G.; Zotou, S.; Iliou, A.; Christopoulos, A. Subterranean to submarine: Stress-induced locomotor repertoire expansion and aquatic escape in the Anatolian mole rat (Nannospalax xanthodon) under risk of predation. J. Ethol. 2024, 43, 39–43. [Google Scholar] [CrossRef]
- Klauder, K.J.; Borg, B.L.; Sivy, K.J.; Prugh, L.R. Gifts of an enemy: Scavenging dynamics in the presence of wolves (Canis lupus). J. Mammal. 2021, 102, 558–573. [Google Scholar] [CrossRef]
- Brown, J.H.; Maurer, B.A. Body size, ecological dominance and Cope’s rule. Nature 1986, 324, 248–250. [Google Scholar] [CrossRef]
- Woodward, G.; Hildrew, A.G. Body-size determinants of niche overlap and intraguild predation within a complex food web. J. Anim. Ecol. 2002, 71, 1063–1074. [Google Scholar] [CrossRef]
- Hansen, K.W.; Ranc, N.; Morgan, J.; Jordan, N.R.; McNutt, J.W.; Wilson, A.; Wilmers, C.C. How territoriality and sociality influence the habitat selection and movements of a large carnivore. Ecol. Evol. 2024, 14, e11217. [Google Scholar] [CrossRef]
- Brown, M.B.; Schlacher, T.A.; Schoeman, D.S.; Weston, M.A.; Huijbers, C.M.; Olds, A.D.; Connolly, R.M. Invasive carnivores alter ecological function and enhance complementarity in scavenger assemblages on ocean beaches. Ecology 2015, 96, 2715–2725. [Google Scholar] [CrossRef]
- Newsome, T.; Cairncross, R.; Cunningham, C.X.; Spencer, E.E.; Barton, P.S.; Ripple, W.J.; Wirsing, A.J. Scavenging with invasive species. Biol. Rev. 2024, 99, 562–581. [Google Scholar] [CrossRef]
- Wooster, E.; Wallach, A.D.; Ramp, D. The Wily and Courageous Red Fox: Behavioural Analysis of a Mesopredator at Resource Points Shared by an Apex Predator. Animals 2019, 9, 907. [Google Scholar] [CrossRef]
- Morton, F.B.; Gartner, M.; Norrie, E.-M.; Haddou, Y.; Soulsbury, C.D.; Adaway, K.A. Urban foxes are bolder but not more innovative than their rural conspecifics. Anim. Behav. 2023, 203, 101–113. [Google Scholar] [CrossRef]
- Olson, L.E.; Squires, J.R.; Roberts, E.K.; Ivan, J.S.; Hebblewhite, M. Sharing the same slope: Behavioral responses of a threatened mesocarnivore to motorized and nonmotorized winter recreation. Ecol. Evol. 2018, 8, 8555–8572. [Google Scholar] [CrossRef] [PubMed]
- Rossa, M.; Lovari, S.; Ferretti, F. Spatiotemporal patterns of wolf, mesocarnivores and prey in a Mediterranean area. Behav. Ecol. Sociobiol. 2021, 75, 32. [Google Scholar] [CrossRef]
- Gómez-Ortiz, Y.; Monroy-Vilchis, O.; Mendoza-Martínez, G.D. Feeding interactions in an assemblage of terrestrial carnivores in central Mexico. Zool. Stud. 2015, 54, 16. [Google Scholar] [CrossRef]
- Arnold, J.; Humer, A.; Heltai, M.; Murariu, D.; Spassov, N.; Hackländer, K. Current status and distribution of golden jackals Canis aureus in Europe. Mamm. Rev. 2012, 42, 1–11. [Google Scholar] [CrossRef]
- Rutkowski, R.; Krofel, M.; Giannatos, G.; Ćirović, D.; Männil, P.; Volokh, A.M.; Lanszki, J.; Heltai, M.; Szabó, L.; Banea, O.C.; et al. A European Concern? Genetic Structure and Expansion of Golden Jackals (Canis aureus) in Europe and the Caucasus. PLoS ONE 2015, 10, e0141236. [Google Scholar] [CrossRef]
- Karamanlidis, A.; de Gabriel Hernando, M.; Avgerinou, M.; Bogdanowicz, W.; Galanis, K.; Kalogeropoulou, S.; Krambokoukis, L.; Panagiotopoulos, N.; Taklis, C. Rapid expansion of the golden jackal in Greece: Research, management and conservation priorities. Endanger. Species Res. 2023, 51, 1–13. [Google Scholar] [CrossRef]
- Jiménez, J.; Nuñez-Arjona, J.C.; Mougeot, F.; Ferreras, P.; González, L.M.; García-Domínguez, F.; Muñoz-Igualada, J.; Palacios, M.J.; Pla, S.; Rueda, C.; et al. Restoring apex predators can reduce mesopredator abundances. Biol. Conserv. 2019, 238, 108234. [Google Scholar] [CrossRef]
- Ferreiro-Arias, I.; Isla, J.; Jordano, P.; Benítez-López, A. Fine-scale coexistence between Mediterranean mesocarnivores is mediated by spatial, temporal, and trophic resource partitioning. Ecol. Evol. 2021, 11, 15520–15533. [Google Scholar] [CrossRef]
- Prugh, L.R.; Sivy, K.J. Enemies with benefits: Integrating positive and negative interactions among terrestrial carnivores. Ecol. Lett. 2020, 23, 902–918. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).