Are Painted Turtles (Chrysemys picta) Resilient to the Potential Impact of Climate Change on Vitamin D via Overgrown Floating Vegetation?
Abstract
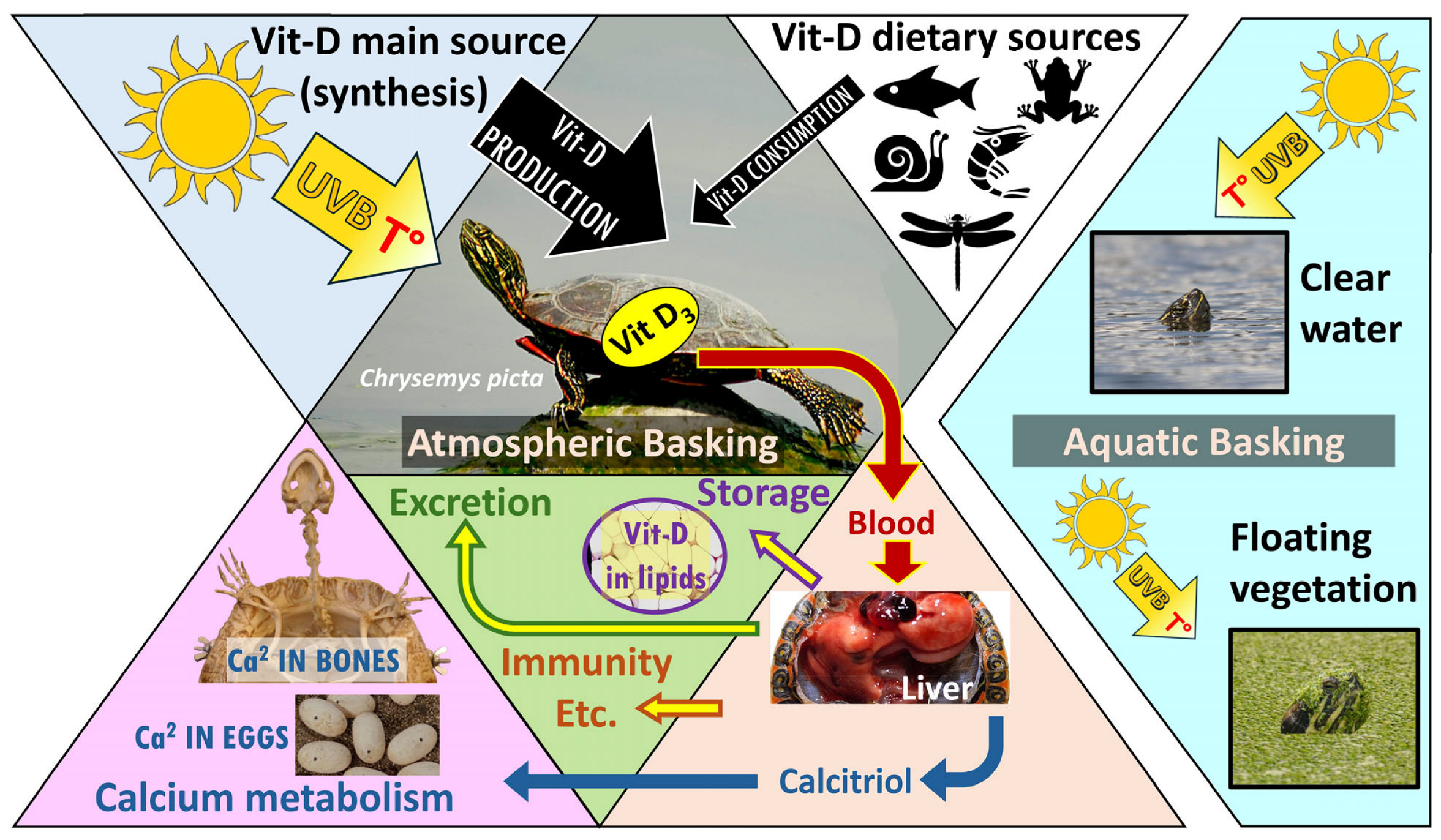
1. Introduction
2. Materials and Methods
2.1. 2022 Vitamin D Experiment
2.1.1. Animal Procurement and Experimental Design
2.1.2. Vitamin D Sample Quantification
2.1.3. Vitamin D Statistical Analysis
2.2. 2023–2024 Habitat Choice Experiments
2.2.1. 2023 Outdoor Group Choice Experiment
2.2.2. 2024 Individual-Choice Experiments
2.2.3. Habitat Choice Statistical Analysis
3. Results
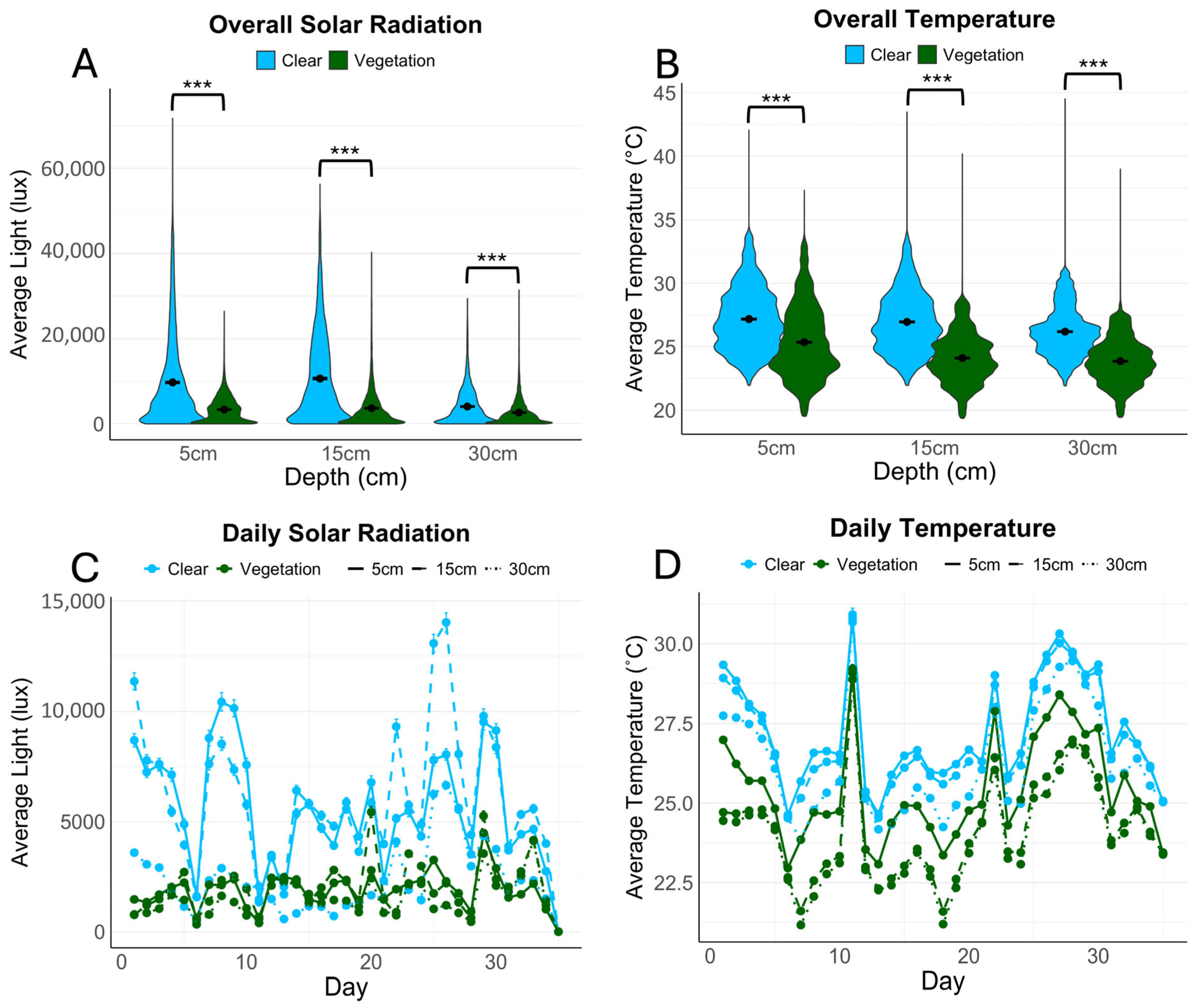
3.1. Temperature and Sunlight
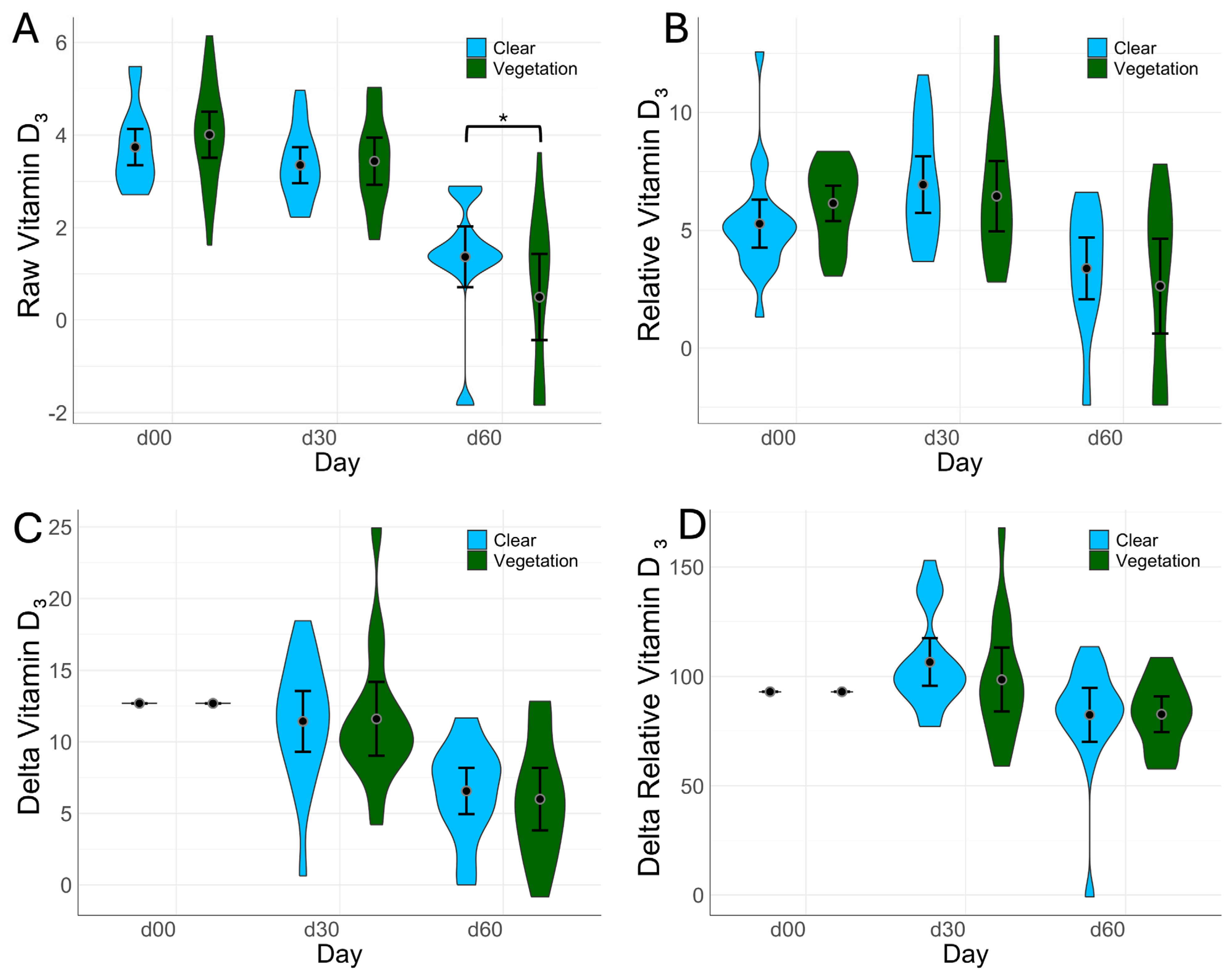
3.2. Circulating Vit-D Levels
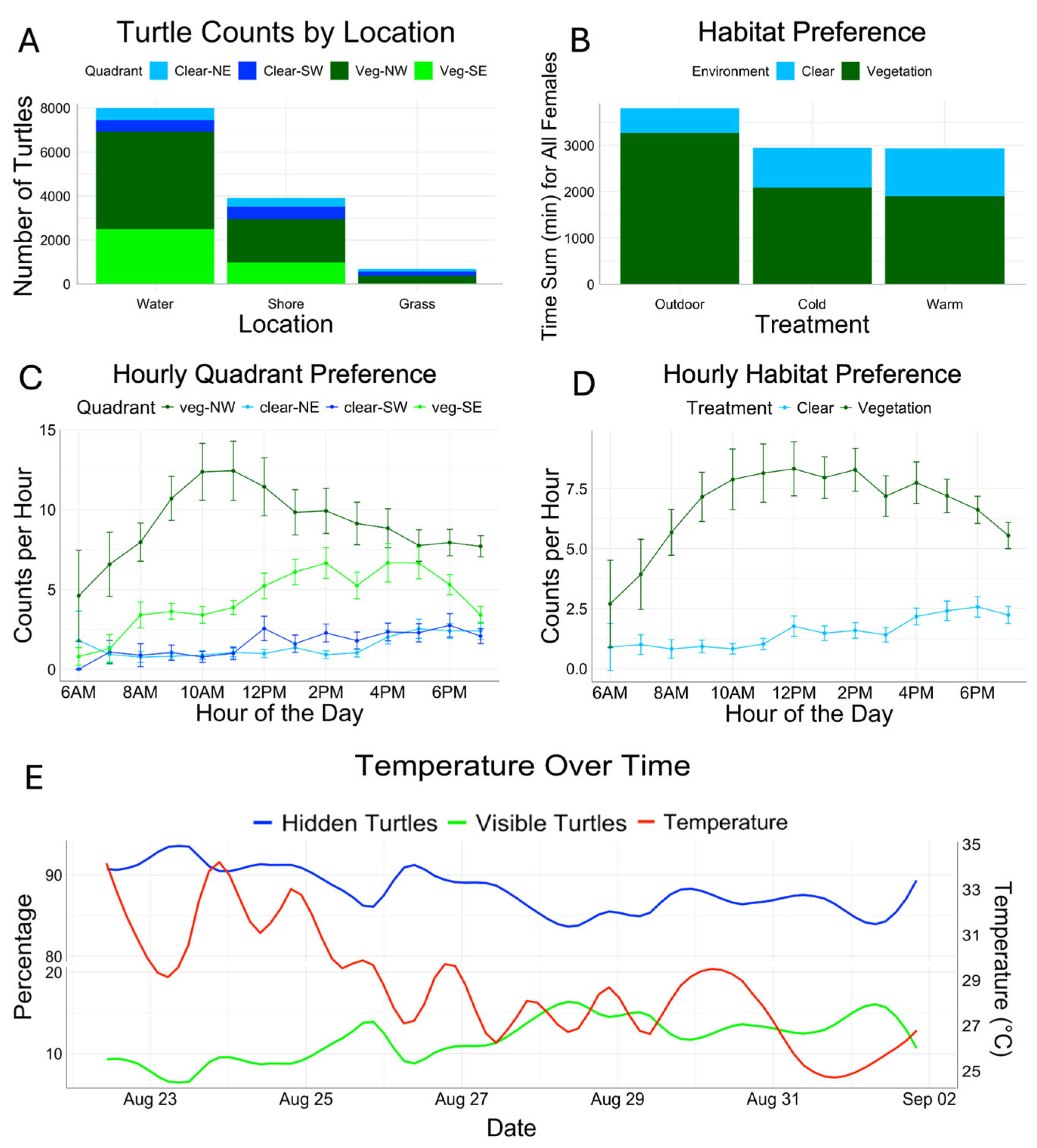
3.3. Habitat Choice Experiments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, R.V.; Gutierrez, M.L.; Romano, M.A. Turtle habitat use in a reach of the upper Mississippi River. J. Freshw. Ecol. 2002, 17, 171–177. [Google Scholar] [CrossRef]
- Lopez, C.B.; Jewett, E.B.; Dortch, Q.; Walton, B.T.; Hudnell, H.K. Scientific Assessment of Freshwater Harmful Algal Blooms; Interagency Working Group on Harmful Algal Blooms, Hypoxia, and Human Health of the Joint Subcommittee on Ocean Science and Technology: Washington, DC, USA, 2008.
- Short, F.T.; Kosten, S.; Morgan, P.A.; Malone, S.; Moore, G.E. Impacts of climate change on submerged and emergent wetland plants. Aquat. Bot. 2016, 135, 3–17. [Google Scholar] [CrossRef]
- Barko, J.W.; Smart, R.M. Comparative influences of light and temperature on the growth and metabolism of selected submersed freshwater macrophytes. Ecol. Monogr. 1981, 51, 219–236. [Google Scholar] [CrossRef]
- Pielke, R., Jr.; Burgess, M.G.; Ritchie, J. Most plausible 2005–2040 emissions scenarios project less than 2.5 degrees C of warming by 2100. Environ. Res. Lett. 2022, 17, 024027. [Google Scholar] [CrossRef]
- Schwantes, A.M.; Swenson, J.J.; González-Roglich, M.; Johnson, D.M.; Domec, J.C.; Jackson, R.B. Measuring canopy loss and climatic thresholds from an extreme drought along a fivefold precipitation gradient across Texas. Glob. Change Biol. 2017, 23, 5120–5135. [Google Scholar] [CrossRef]
- Alahuhta, J.; Heino, J.; Luoto, M. Climate change and the future distributions of aquatic macrophytes across boreal catchments. J. Biogeogr. 2011, 38, 383–393. [Google Scholar] [CrossRef]
- Lind, L.; Eckstein, R.L.; Relyea, R.A. Direct and indirect effects of climate change on distribution and community composition of macrophytes in lentic systems. Biol. Rev. 2022, 97, 1677–1690. [Google Scholar] [CrossRef]
- Angoh, S.Y.J.; Freeland, J.; Paterson, J.; Rupasinghe, P.A.; Davy, C.M. Effects of invasive wetland macrophytes on habitat selection and movement by freshwater turtles. Biol. Invasions 2021, 23, 2271–2288. [Google Scholar] [CrossRef]
- Carrière, M.A.; Blouin-Demers, G. Habitat selection at multiple spatial scales in Northern Map Turtles (Graptemys geographica). Can. J. Zool. 2010, 88, 846–854. [Google Scholar] [CrossRef]
- Winchell, K.M.; Gibbs, J.P. Golf courses as habitat for aquatic turtles in urbanized landscapes. Landsc. Urban Plan. 2016, 147, 59–70. [Google Scholar] [CrossRef]
- Semlitsch, R.D.; Bodie, J.R. Biological criteria for buffer zones around wetlands and riparian habitats for amphibians and reptiles. Conserv. Biol. 2003, 17, 1219–1228. [Google Scholar] [CrossRef]
- Stanford, C.B.; Iverson, J.B.; Rhodin, A.G.J.; van Dijk, P.P.; Mittermeier, R.A.; Kuchling, G.; Berry, K.H.; Bertolero, A.; Bjorndal, K.A.; Blanck, T.E.G.; et al. Turtles and tortoises are in trouble. Curr. Biol. 2020, 30, R721–R735. [Google Scholar] [CrossRef] [PubMed]
- Peterman, W.E.; Ryan, T.J. Basking Behavior of Emydid Turtles (Chysemys picta, Graptemys geographica, and Trachemys scripta) in an Urban Landscape. Northeast. Nat. 2009, 16, 629–636. [Google Scholar] [CrossRef]
- Reese, D.A.; Welsh, H.H., Jr. Habitat use by western pond turtles in the Trinity River, California. J. Wildl. Manag. 1998, 62, 842–853. [Google Scholar] [CrossRef]
- Spotila, J.R.; Foley, R.E.; Schubauer, J.P.; Semlitsch, R.D.; Crawford, K.M.; Standora, E.A.; Gibbons, J.W. Opportunistic behavioral thermoregulation of turtles, Pseudemys scripta, in response to microclimatology of a nuclear reactor cooling reservoir. Herpetologica 1984, 40, 299–308. [Google Scholar]
- Vignoli, L.; Bologna, M.A.; Manzini, S.; Rugiero, L.; Luiselli, L. Attributes of basking sites of the European pond turtle (Emys orbicularis) in central Italy. Amphibia-Reptilia 2015, 36, 125–131. [Google Scholar] [CrossRef]
- Topping, N.E.; Valenzuela, N. Turtle nest-site choice, anthropogenic challenges, and evolutionary potential for adaptation. Front. Ecol. Evol. 2021, 9, 808621. [Google Scholar] [CrossRef]
- Capula, M.; Luiselli, L.; Rugiero, L.; Filippi, E. A field experiment on the selection of basking sites by Emys orbicularis (Linnaeus, 1758). Herpetozoa 1994, 7, 91–94. [Google Scholar]
- Crawford, K.M.; Spotila, J.R.; Standora, E.A. Operative environmental temperatures and basking behavior of the turtle Pseudemys scripta. Ecology 1983, 64, 989–999. [Google Scholar] [CrossRef]
- Moll, E.; Legler, J. The Life History of a Neotropical Slider Turtle. J. Herpetol. 1971, 24, 191–196. [Google Scholar]
- Chessman, B.C. The conundrum of turtle and tortoise basking: A critical review. J. Zool. 2024, 323, 263–283. [Google Scholar] [CrossRef]
- McKnight, D.T.; Wirth, W.; Schwarzkopf, L.; Nordberg, E.J. Leech removal is not the primary driver of basking behavior in a freshwater turtle. Ecol. Evol. 2021, 11, 10936–10946. [Google Scholar] [CrossRef]
- Nordberg, E.J.; McKnight, D.T. Nocturnal basking behavior in a freshwater turtle. Ecology 2020, 101, e03048. [Google Scholar] [CrossRef] [PubMed]
- Tucker, A.D.; Limpus, C.J.; McCallum, H.I.; McDonald, K.R. Ontogenetic dietary partitioning by Crocodylus johnstoni during the dry season. Copeia 1996, 1996, 978–988. [Google Scholar] [CrossRef]
- Webb, G.J.W.; Manolis, S.C.; Buckworth, R. Crocodylus johnstoni in the McKinlay River Area, N.T. I. variation in the diet, and a new method of assessing the relative importance of prey. Aust. J. Zool. 1982, 30, 877–899. [Google Scholar] [CrossRef]
- Boyer, D.R. Ecology of the basking habit in turtles. Ecology 1965, 46, 99–118. [Google Scholar] [CrossRef]
- Cann, J.; Sadlier, R.A. Freshwater Turtles of Australia; ECO Wear & Publishing: Animas, NM, USA, 2017. [Google Scholar]
- Neil, W.T.; Allen, E.R. Algae on turtles: Some additional considerations. Ecology 1954, 35, 581–584. [Google Scholar] [CrossRef]
- Edwards, A.L.; Blouin-Demers, G. Thermoregulation as a function of thermal quality in a northern population of painted turtles, Chrysemys picta. Can. J. Zool. 2007, 85, 526–535. [Google Scholar] [CrossRef]
- Ernst, C.H. Temperature-activity relationship in the painted turtle, Chrysemys picta. Copeia 1972, 1972, 217–222. [Google Scholar] [CrossRef]
- Chessman, B.C. Behavioural thermoregulation by Australian freshwater turtles: Interspecific differences and implications for responses to climate change. Aust. J. Zool. 2019, 67, 94–105. [Google Scholar] [CrossRef]
- Chessman, B.C. Atmospheric and aquatic basking of the Australian fresh-water turtle Emydura-macquarii (Gray) (Testudines, Chelidae). Herpetologica 1987, 43, 301–306. [Google Scholar]
- Grayson, K.L.; Dorcas, M.E. Seasonal temperature variation in the painted turtle (Chrysemys picta). Herpetologica 2004, 60, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Conley, D.A.; Lattanzio, M.S. Active regulation of ultraviolet light exposure overrides thermal preference behaviour in eastern fence lizards. Funct. Ecol. 2022, 36, 2240–2250. [Google Scholar] [CrossRef]
- Acierno, M.J.; Mitchell, M.A.; Roundtree, M.K.; Zachariah, T.T. Effects of ultraviolet radiation on 25-hydroxyvitamin D3 synthesis in red-eared slider turtles (Trachemys scripta elegans). Am. J. Vet. Res. 2006, 67, 2046–2049. [Google Scholar] [CrossRef]
- Chou, S.-J.; Huang, C.-H. Ultraviolet influences growth, tissue vitamin D status, and carapace strength of Chinese soft-shelled turtle, Pelodiscus sinensis. J. Fish. Soc. Taiwan 2013, 40, 71–77. [Google Scholar] [CrossRef]
- Geisler, G.; Leineweber, C.; Pees, M.; Öfner, S.; Marschang, R.E. The effects of sex, season, and natural sunlight on plasma vitamin D3 levels in two chelonian species (Testudo hermanni, Trachemys scripta) and their interaction with calcium, phosphate, and magnesium as associated plasma compounds. Front. Amphib. Reptile Sci. 2023, 1, 1268801. [Google Scholar] [CrossRef]
- Hetényi, N.; Lang, Z.; Sátorhelyi, T.; Hullár, I. Effects of different vitamin d sources on blood biochemistry of bearded dragons (Pogona spp.) and Hermann’s tortoises (Testudo hermanni). Berl. Und Munch. Tierarztl. Wochenschr. 2020, 133, 149–158. [Google Scholar] [CrossRef]
- Hoskins, A.; Thompson, D.; Mitchell, M.A. Effects of artificial ultraviolet B radiation on plasma 25-hydroxyvitamin D3 concentrations in juvenile Blanding’s turtles (Emydoidea blandingii). J. Herpetol. Med. Surg. 2022, 32, 225–229. [Google Scholar] [CrossRef]
- Rawski, M.; Mans, C.; Kierończyk, B.; Świątkiewicz, S.; Barc, A.; Józefiak, D. Freshwater turtle nutrition—A review of scientific and practical knowledge. Ann. Anim. Sci. 2018, 18, 17–37. [Google Scholar] [CrossRef]
- Acierno, M.J.; Mitchell, M.A.; Zachariah, T.T.; Roundtree, M.K.; Kirchgessner, M.S.; Guzman, D.S.-M. Effects of ultraviolet radiation on plasma 25-hydroxyvitamin D3 concentrations in corn snakes (Elaphe guttata). Am. J. Vet. Res. 2008, 69, 294–297. [Google Scholar] [CrossRef]
- Bos, J.H.; Klip, F.C.; Oonincx, D.G.A.B. Artificial ultraviolet B radiation raises plasma 25-hydroxyvitamin D3 concentrations in Burmese pythons (Python bivittatus). J. Zoo Wildl. Med. 2018, 49, 810–812. [Google Scholar] [CrossRef] [PubMed]
- Carman, E.N.; Ferguson, G.W.; Gehrmann, W.H.; Chen, T.C.; Holick, M.F. Photobiosynthetic opportunity and ability for UV-B generated vitamin D synthesis in free-living house geckos (Hemidactylus turcicus) and Texas spiny lizards (Sceloporus olivaceous). Copeia 2000, 2000, 245–250. [Google Scholar] [CrossRef]
- Holick, M.F.; Tian, X.Q.; Allen, M. Evolutionary importance for the membrane enhancement of the production of vitamin D3 in the skin of poikilothermic animals. Proc. Natl. Acad. Sci. USA 1995, 92, 3124–3126. [Google Scholar] [CrossRef]
- Garefino, V.E.; Milton, S.L. Influence of sunlight on vitamin D and health status in green (Chelonia mydas) sea turtles with fibropapillomatosis. Animals 2022, 12, 488. [Google Scholar] [CrossRef] [PubMed]
- Lemire, J.M. Immunomodulatory role of 1, 25-dihydroxyvitamin D3. J. Cell. Biochem. 1992, 49, 26–31. [Google Scholar] [CrossRef]
- McWILLIAMS, D. Nutrition research on calcium homeostasis. II. Freshwater turtles (with recommendations). Int. Zoo Yearb. 2005, 39, 77–85. [Google Scholar] [CrossRef]
- Purgley, H.; Jewell, J.; Deacon, J.E.; Winokur, R.M.; Tripoli, V.M. Vitamin D3 in captive green sea turtles (Chelonia mydas). Chelonian Conserv. Biol. 2009, 8, 161–167. [Google Scholar] [CrossRef]
- Carr, J.L.; Holcomb, S.R.; Ray, M.J. Basking in the alligator snapping turtle, Macrochelys temminckii (Testudines: Chelydridae). Reptiles Amphib. 2011, 18, 2–5. [Google Scholar] [CrossRef]
- Hill, S.K.; Vodopich, D.S. Habitat use and basking behavior of a freshwater turtle community along an urban gradient. Chelonian Conserv. Biol. 2013, 12, 275–282. [Google Scholar] [CrossRef]
- Lindeman, P.V. Resource use of five sympatric turtle species: Effects of competition, phylogeny, and morphology. Can. J. Zool. 2000, 78, 992–1008. [Google Scholar] [CrossRef]
- Picard, G.; Carrière, M.-A.; Blouin-Demers, G. Common musk turtles (Sternotherus odoratus) select habitats of high thermal quality at the northern extreme of their range. Amphibia-Reptilia 2011, 32, 83–92. [Google Scholar] [CrossRef]
- Roper, A.M.; Parks, S.L.I. Phosphorus removal from wastewater. In Proceedings of the World Environmental and Water Resources Congress 2019: Water, Wastewater, and Stormwater; Urban Water Resources; and Municipal Water Infrastructure, Pittsburgh, PA, USA, 19–23 May 2019; pp. 222–226. [Google Scholar]
- Hedley, J. Metabolic bone disease in reptiles: Part 1. Companion Anim. 2012, 17, 52–54. [Google Scholar] [CrossRef]
- Hoby, S.; Wenker, C.; Robert, N.; Jermann, T.; Hartnack, S.; Segner, H.; Aebischer, C.-P.; Liesegang, A. Nutritional metabolic bone disease in juvenile veiled chameleons (Chamaeleo calyptratus) and its prevention. J. Nutr. 2010, 140, 1923–1931. [Google Scholar] [CrossRef]
- Kawazu, I.; Kino, M.; Yanagisawa, M.; Maeda, K.; Nakada, K.; Yamaguchi, Y.; Sawamukai, Y. Signals of vitellogenesis and estrus in female hawksbill turtles. Zool. Sci. 2015, 32, 114–118. [Google Scholar] [CrossRef]
- Scott, G.N.; Nollens, H.H.; Schmitt, T.L. Evaluation of plasma 25-hydroxyvitamin D, ionized calcium, and parathyroid hormone in green sea turtles (Chelonia mydas) exposed to different intensities of ultraviolet B radiation. J. Zoo Wildl. Med. 2019, 50, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Cagle, F.R. A system of marking turtles for future identification. Copeia 1939, 1939, 170–173. [Google Scholar] [CrossRef]
- Hernandez-Divers, S.M.; Hernandez-Divers, S.J.; Wyneken, J. Angiographic, anatomic and clinical technique descriptions of a subcarapacial venipuncture site for chelonians. J. Herpetol. Med. Surg. 2002, 12, 32–37. [Google Scholar] [CrossRef]
- Makowski, A.J.; Rathmacher, J.A.; Horst, R.L.; Sempos, C.T. Simplified 25-hydroxyvitamin D standardization and optimization in dried blood spots by LC-MS/MS. J. AOAC Int. 2017, 100, 1328–1336. [Google Scholar] [CrossRef]
- Mans, C. Venipuncture techniques in chelonian species. Lab Anim. 2008, 37, 303–304. [Google Scholar] [CrossRef]
- Team, R.C. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Collyer, M.L.; Adams, D.C. RRPP: An r package for fitting linear models to high-dimensional data using residual randomization. Methods Ecol. Evol. 2018, 9, 1772–1779. [Google Scholar] [CrossRef]
- Escalona, T.; Valenzuela, N.; Adams, D.C. Nesting ecology in the freshwater turtle Podocnemis unifilis: Spatiotemporal patterns and inferred explanations. Funct. Ecol. 2009, 23, 826–835. [Google Scholar] [CrossRef]
- Escalona, T.; Valenzuela, N.; Adams, D.C. Do Local Environmental Factors and Lunar Cycle Influence Timing and Synchrony of Oviposition of a Turtle with Strict Nocturnal Nesting? Diversity 2019, 11, 78. [Google Scholar] [CrossRef]
- Rstudio Team. RStudio: Integrated Development for R; Rstudio Team, PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com (accessed on 5 June 2024).
- Heaney, R.P.; Recker, R.R.; Grote, J.; Horst, R.L.; Armas, L.A.G. Vitamin D3 is more potent than vitamin D2 in humans. J. Clin. Endocrinol. Metab. 2011, 96, E447–E452. [Google Scholar] [CrossRef]
- Blechschmidt, J.; Wittmann, M.J.; Blüml, C. Climate change and green sea turtle sex ratio—Preventing possible extinction. Genes 2020, 11, 588. [Google Scholar] [CrossRef]
- Grayson, K.L.; Mitchell, N.J.; Monks, J.M.; Keall, S.N.; Wilson, J.N.; Nelson, N.J. Sex ratio bias and extinction risk in an isolated population of tuatara (Sphenodon punctatus). PLoS ONE 2014, 9, e94214. [Google Scholar] [CrossRef]
- Jensen, M.P.; Allen, C.D.; Eguchi, T.; Bell, I.P.; LaCasella, E.L.; Hilton, W.A.; Hof, C.A.M.; Dutton, P.H. Environmental warming and feminization of one of the largest sea turtle populations in the world. Curr. Biol. 2018, 28, 154–159.e4. [Google Scholar] [CrossRef]
- Valenzuela, N.; Literman, R.; Neuwald, J.L.; Mizoguchi, B.; Iverson, J.B.; Riley, J.L.; Litzgus, J.D. Extreme thermal fluctuations from climate change unexpectedly accelerate demographic collapse of vertebrates with temperature-dependent sex determination. Sci. Rep. 2019, 9, 4254. [Google Scholar] [CrossRef] [PubMed]
- Vincent, E.C.; Fayette, M.A.; Griffioen, J.A.; Litwiler, G.; Adamovicz, L.; Ospina, E.; Allender, M.C. Health assessment of painted turtles (Chrysemys picta) in a restored wetland habitat in northwestern Indiana, USA. J. Wildl. Dis. 2023, 59, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Stringer, E.M.; Harms, C.A.; Beasley, J.F.; Anderson, E.T. Comparison of ionized calcium, parathyroid hormone, and 25-hydroxyvitamin D in rehabilitating and healthy wild green sea turtles (Chelonia mydas). J. Herpetol. Med. Surg. 2010, 20, 122–127. [Google Scholar] [CrossRef]
- Eatwell, K. Plasma concentrations of 25-hydroxycholecalciferol in 22 captive tortoises (Testudo species). Vet. Rec. 2008, 162, 342–345. [Google Scholar] [CrossRef]
- Selleri, P.; Di Girolamo, N. Plasma 25-hydroxyvitamin D3 concentrations in Hermann’s tortoises (Testudo hermanni) exposed to natural sunlight and two artificial ultraviolet radiation sources. Am. J. Vet. Res. 2012, 73, 1781–1786. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.K.; Byrd, J.; Phillips, C.A.; Allender, M.C. Characterizing the 25-hydroxyvitamin D status of two populations of free-ranging eastern box turtles (Terrapene carolina carolina). J. Zoo Wildl. Med. 2017, 48, 742–747. [Google Scholar] [CrossRef]
- Friedrichs, K.R.; Harr, K.E.; Freeman, K.P.; Szladovits, B.; Walton, R.M.; Barnhart, K.F.; Blanco-Chavez, J. ASVCP reference interval guidelines: Determination of de novo reference intervals in veterinary species and other related topics. Vet. Clin. Pathol. 2012, 41, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.; Holick, M.F. Sunlight and Vitamin D: A global perspective for health. Dermato-Endocrinology 2013, 5, 51–108. [Google Scholar] [CrossRef]
- Lindgren, J. UV-lamps for terrariums: Their spectral characteristics and efficiency in promoting vitamin D synthesis by UVB irradiation. Herpetomania 2004, 13, 13–20. [Google Scholar]
- Laing, C.J.; Trube, A.; Shea, G.M.; Fraser, D.R. The requirement for natural sunlight to prevent vitamin D deficiency in iguanian lizards. J. Zoo Wildl. Med. 2001, 32, 342–348. [Google Scholar] [CrossRef]
- Borisjuk, N.; Peterson, A.A.; Lv, J.; Qu, G.; Luo, Q.; Shi, L.; Chen, G.; Kishchenko, O.; Zhou, Y.; Shi, J. Structural and biochemical properties of duckweed surface cuticle. Front. Chem. 2018, 6, 317. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D: An ancient hormone. Exp. Dermatol. 2011, 20, 7–13. [Google Scholar] [CrossRef]
- Oonincx, D.; Stevens, Y.; Van den Borne, J.; Van Leeuwen, J.; Hendriks, W.H. Effects of vitamin D3 supplementation and UVB exposure on the growth and plasma concentration of vitamin D3 metabolites in juvenile bearded dragons (Pogona vitticeps). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2010, 156, 122–128. [Google Scholar] [CrossRef]
- Ferguson, G.W.; Gehrmann, W.H.; Peavy, B.; Painter, C.; Hartdegen, R.; Chen, T.C.; Holick, M.F.; Pinder Iii, J.E. Restoring vitamin D in monitor lizards: Exploring the efficacy of dietary and UVB sources. J. Herpetol. Med. Surg. 2009, 19, 81–88. [Google Scholar] [CrossRef]
- Tetzlaff, S.J.; Sperry, J.H.; DeGregorio, B.A. Captive-reared juvenile box turtles innately prefer naturalistic habitat: Implications for translocation. Appl. Anim. Behav. Sci. 2018, 204, 128–133. [Google Scholar] [CrossRef]
- Case, B.C.; Lewbart, G.A.; Doerr, P.D. The physiological and behavioural impacts of and preference for an enriched environment in the eastern box turtle (Terrapene carolina carolina). Appl. Anim. Behav. Sci. 2005, 92, 353–365. [Google Scholar] [CrossRef]
- Webster, C. Substrate preference and activity in the turtle, Kinosternon flavescens flavescens. J. Herpetol. 1986, 20, 477–482. [Google Scholar] [CrossRef]
- Hartwig, T.S.; Kiviat, E. Microhabitat association of Blanding’s turtles in natural and constructed wetlands in southeastern New York. J. Wildl. Manag. 2007, 71, 576–582. [Google Scholar] [CrossRef]
- Wieten, A.C.; Cooper, M.J.; Parker, A.D.; Uzarski, D.G. Great Lakes coastal wetland habitat use by seven turtle species: Influences of wetland type, vegetation, and abiotic conditions. Wetl. Ecol. Manag. 2012, 20, 47–58. [Google Scholar] [CrossRef]
- Stemle, L.R.; Searcy, C.A. Habitat characteristics favoring native freshwater turtles in the highly invaded urban turtle community of Miami-Dade County. Biol. Invasions 2024, 26, 1181–1194. [Google Scholar] [CrossRef]
- Lin, S.-M.; Lee, Y.; Chen, T.-H.; Lin, J.-W. Habitat preference and management of a Chinese pond turtle population protected by the demilitarized Kinmen Islands. J. Herpetol. 2015, 49, 399–404. [Google Scholar] [CrossRef]
- Petrozzi, F.; Ajong, S.N.; Pacini, N.; Dendi, D.; Bi, S.G.; Fa, J.E.; Luiselli, L. Spatial niche expansion at multiple habitat scales of a tropical freshwater turtle in the absence of a potential competitor. Diversity 2021, 13, 55. [Google Scholar] [CrossRef]
- Kosten, S.; Jeppesen, E.; Huszar, V.L.M.; Mazzeo, N.; Van Nes, E.H.; Peeters, E.T.H.M.; Scheffer, M. Ambiguous climate impacts on competition between submerged macrophytes and phytoplankton in shallow lakes. Freshw. Biol. 2011, 56, 1540–1553. [Google Scholar] [CrossRef]
- Le Bagousse-Pinguet, Y.; Liancourt, P.; Gross, N.; Straile, D. Indirect facilitation promotes macrophyte survival and growth in freshwater ecosystems threatened by eutrophication. J. Ecol. 2012, 100, 530–538. [Google Scholar] [CrossRef]
- Pannard, A.; Massé, S.; Llopis, S.; Leitao, M.; Morata, S.; Bouger, G.; Gillier, J.-M.; Piscart, C. Rooted floating-leaf macrophytes structure the coexistence of different phytoplankton assemblages within a shallow lake. Hydrobiologia 2024, 851, 915–939. [Google Scholar] [CrossRef]
- Phillips, G.; Willby, N.; Moss, B. Submerged macrophyte decline in shallow lakes: What have we learnt in the last forty years? Aquat. Bot. 2016, 135, 37–45. [Google Scholar] [CrossRef]
- Yuan, L.L. Continental-scale effects of phytoplankton and non-phytoplankton turbidity on macrophyte occurrence in shallow lakes. Aquat. Sci. 2021, 83, 14. [Google Scholar] [CrossRef]
- Jeppesen, E.; Kronvang, B.; Meerhoff, M.; Søndergaard, M.; Hansen, K.M.; Andersen, H.E.; Lauridsen, T.L.; Liboriussen, L.; Beklioglu, M.; Özen, A.; et al. Climate change effects on runoff, catchment phosphorus loading and lake ecological state, and potential adaptations. J. Environ. Qual. 2009, 38, 1930–1941. [Google Scholar] [CrossRef]
- Jackson, D.C. Hibernating without oxygen: Physiological adaptations of the painted turtle. J. Physiol. 2002, 543, 731–737. [Google Scholar] [CrossRef]
- Ultsch, G.R.; Carwile, M.E.; Crocker, C.E.; Jackson, D.C. The physiology of hibernation among painted turtles: The eastern painted turtle Chrysemys picta picta. Physiol. Biochem. Zool. 1999, 72, 493–501. [Google Scholar] [CrossRef]


| Treatment | Day | n | Vitamin D3 (ng/mL) | ||
|---|---|---|---|---|---|
| Mean | Min | Max | |||
| Clear | 0 | 21 | 7.84 | 5.30 | 12.70 |
| Vegetation | 0 | 21 | 8.61 | 3.20 | 14.90 |
| Clear | 30 | 18 | 6.86 | 4.30 | 11.10 |
| Vegetation | 30 | 15 | 7.19 | 3.40 | 11.30 |
| Clear | 60 | 18 | 3.26 | 1.06 | 5.70 |
| Vegetation | 60 | 15 | 2.48 | 1.06 | 7.40 |
| (A) Between Treatments at Each Day | (B) Overall Compared to Day 0 | ||||
|---|---|---|---|---|---|
| Variable | Day 0 | Day 30 | Day 60 | Day 30 | Day 60 |
| Untransformed values | |||||
| Raw Vit-D3 | 0.15 | 0.627 | 0.055 | 0.325 | 0.001 |
| Relative Vit-D3 | 0.291 | 0.533 | 0.985 | 0.011 | 0.005 |
| Delta Vit-D3 | 1 | 0.813 | 0.368 | 0.302 | 0.001 |
| Delta Relative Vit-D3 | 1 | 0.102 | 0.913 | 0.012 | 0.007 |
| Box–Cox-transformed values | |||||
| BC Vit-D3 | 0.274 | 0.795 | 0.008 | 0.426 | 0.001 |
| BC relative Vit-D3 | 0.134 | 0.451 | 0.272 | 0.084 | 0.001 |
| BC delta Vit-D3 | 1 | 0.815 | 0.458 | 0.318 | 0.001 |
| BC delta relative Vit-D3 | 1 | 0.101 | 0.944 | 0.009 | 0.008 |
| Untransformed values, female size covariate | |||||
| Size-covariate Vit-D3 | 0.183 | 0.403 | 0.963 | 0.224 | 0.001 |
| Size-covariate relative Vit-D3 | 0.377 | 0.629 | 0.863 | 0.032 | 0.002 |
| Size-covariate delta Vit-D3 | 0.921 | 0.845 | 0.437 | 0.318 | 0.001 |
| Size-covariate delta relative Vit-D3 | 0.773 | 0.21 | 0.579 | 0.011 | 0.016 |
| Box-Cox-transformed values, female size covariate | |||||
| Size-cov BC Vit-D3 | 0.147 | 0.276 | 0.979 | 0.264 | 0.001 |
| Size-cov BC relative Vit-D3 | 0.129 | 0.57 | 0.779 | 0.356 | 0.001 |
| Size-cov BC delta Vit-D3 | 0.923 | 0.859 | 0.483 | 0.333 | 0.001 |
| Size-cov BC delta relative Vit-D3 | 0.777 | 0.212 | 0.578 | 0.009 | 0.017 |
| (A) Total Female Count 2023 Experiment | (B) Time Spent (min) 2024 Experiments | |||||||
|---|---|---|---|---|---|---|---|---|
| Zone | Habitat | |||||||
| Habitat | Quadrant | Water | Shore | Grass | Experiment | Vegetation | Clear | Chi-sq p |
| Clear | NE | 540 | 291 | 112 | Outdoor | 3266.66 | 530 | <2.2 × 10−16 |
| Clear | SW | 526 | 561 | 202 | Indoor Cold | 2090 | 860 | <2.2 × 10−16 |
| Vegetation | NW | 4444 | 1978 | 331 | Indoor Warm | 1900 | 1033.33 | <2.2 × 10−16 |
| Vegetation | SE | 2485 | 331 | 42 | ||||
| Chi-sq p | <2.2 × 10−16 | <2.2 × 10−16 | <2.2 × 10−16 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Topping, N.E.; Valenzuela, N. Are Painted Turtles (Chrysemys picta) Resilient to the Potential Impact of Climate Change on Vitamin D via Overgrown Floating Vegetation? Diversity 2025, 17, 414. https://doi.org/10.3390/d17060414
Topping NE, Valenzuela N. Are Painted Turtles (Chrysemys picta) Resilient to the Potential Impact of Climate Change on Vitamin D via Overgrown Floating Vegetation? Diversity. 2025; 17(6):414. https://doi.org/10.3390/d17060414
Chicago/Turabian StyleTopping, Nicholas E., and Nicole Valenzuela. 2025. "Are Painted Turtles (Chrysemys picta) Resilient to the Potential Impact of Climate Change on Vitamin D via Overgrown Floating Vegetation?" Diversity 17, no. 6: 414. https://doi.org/10.3390/d17060414
APA StyleTopping, N. E., & Valenzuela, N. (2025). Are Painted Turtles (Chrysemys picta) Resilient to the Potential Impact of Climate Change on Vitamin D via Overgrown Floating Vegetation? Diversity, 17(6), 414. https://doi.org/10.3390/d17060414







