Abstract
Although the effects of human disturbance on population genetic variation in plants have been widely studied, little attention has been paid to the impact of environmental changes on genetic dynamics after the implementation of conservation measures. Previously, freshwater Caohai Lake, famous for its abundant aquatic plants and birds, was strongly disturbed by tourism and other human activities; however, strict protective measures have been implemented since 2019. Therefore, the lake provides a suitable natural sampling ecosystem for investigating genetic variation changes following the implementation of conservation measures. Samples of cosmopolitan aquatic Ceratophyllum demersum were collected in 2019 and 2021, and they were analyzed using microsatellite primers. Our results show the presence of considerable genetic diversity in the C. demersum Caohai population. Although human disturbance decreased, the impact of natural disturbances, such as water flow and bird activities, persisted and may have increased. For C. demersum, water flow may cause vegetative propagules of different genotypes to pool in the downstream area of the lake. At sites with a very rich diversity of birds, increasing bird activities may augment the advantage of competitive clones in communities. Therefore, the continuous monitoring of the population’s genetic variation and the impact of related environmental factors is required for the efficient management of the lake ecosystem.
1. Introduction
Although the effects of human disturbance on population genetic variation in plants have been widely studied, little attention has been paid to the impact of environmental changes on genetic dynamics after the implementation of conservation measures. In recent decades, many freshwater lake ecosystems worldwide have been disturbed by human activities, which have reduced their stability [1,2]. For the conservation of lakes and the restoration of ecological functions, reducing human disturbance is a frequently used measure and specifically includes reducing sewage emissions, prohibiting fishing, and reducing boating and tourism activities on lakes [3,4,5]. The implementation of protective measures may cause abrupt environmental changes in lake ecosystems. To determine whether lake ecosystems will move toward becoming increasingly healthy, the responses of critical components within the ecosystems need to be tracked and monitored. Aquatic plants, especially submerged macrophytes, as important components of lake ecosystems, will be able to recover and increase their populations. Submerged macrophytes play a key role in aquatic food webs and biochemical cycles, and they provide both habitats for aquatic animals and multiple benefits for humans [6,7]. In order to maintain the ecological services of lake ecosystems, the genetic variation in submerged macrophytes needs to be monitored and effectively managed, as the resilience of ecosystems to changing environments increases with genetic diversity [7,8,9,10].
Caohai Lake, famous for its abundant aquatic plants, is located on the Yunnan–Guizhou Plateau, southwest China [11]. The rich resources of food and habitats attract more than 100,000 migratory birds, which rest or winter in the lake area every year [9,12,13]. The beautiful scenery, comprising water, plants, and birds, attracts a large number of birdwatchers and other tourists to the lake, reaching more than 10 million per year [3]. Thus, the lake has been strongly disturbed by tourism, boating, fishing, and other human activities. To conserve the lake ecosystem, the Caohai Nature Reserve has implemented stringent protection measures since 2019, including a comprehensive ban on fishing activities, the prohibition of fish farming practices, the closure of all tourist boat docks, the suspension of boating tourism activities, the prohibition of visitors entering water areas, and a strict ban on sewage discharge into the lake [14]. Therefore, Caohai Lake provides a suitable natural sampling ecosystem for investigating the changes in genetic variation after the implementation of conservation measures.
Ceratophyllum demersum L. is a cosmopolitan submerged macrophyte [15], dominant in the communities of Caohai Lake [16,17]. This species is hydrophilous and inefficient in terms of pollen dispersal via the aquatic medium; thus, it has low seed production [18]. However, even a small amount of successful sexual reproduction can have a non-negligible impact on the genetic diversity and structure of the population [19], thereby affecting its development. The sexual reproduction of water-mediated species is highly dependent on aquatic environmental conditions, and it may be more sensitive to environmental changes than that of non-hydrophilous plants [20]. Furthermore, this species has the ability to tolerate pollution and disturbance, and it is frequently used in the restoration of polluted waterbodies [21,22]; thus, its growth and distribution area are increasing [16]. C. demersum has diverse modes of vegetative reproduction and dispersal, including shoot fragments, turions, and rootless plants [23]. Vegetative propagules can migrate by flowing in water and attaching externally to the bodies of waterbirds [24]. Thus, it is generally considered that the dispersion of C. demersum mainly relies on vegetative propagules rather than on pollen or seeds [9,25,26]. Recent studies on C. demersum in different spatially interconnected aquatic habitats [9,26] have focused on the spatial variation in genetic diversity, and they have confirmed that the main mode of propagule spread of this species is hydrochory. However, more research into the temporal dynamics of genetic diversity is required. The aim of this study was to assess how the genetic diversity of the C. demersum population changes under conservation, which is important for predicting the development of the population in changing environments.
2. Materials and Methods
2.1. Sample Collection
Shoot samples of Ceratophyllum demersum were collected from Caohai Lake (26°49′–26°53′ N, 104°12′–104°18′ E). The lake is a plateau freshwater lake in a subtropical monsoon climate, with a height of 2170.7 m above sea level, an average temperature of 10.4 °C, and an average annual rainfall of 950.9 mm [11]. The Caohai Reserve covers an area of 96 km2. The water area of the lake is 25 km2, with an average water depth of 2.4 m and a maximum depth of 5.0 m [11]. The precipitation from May to October accounts for 88% of the annual total, with the highest water level occurring in summer and the lowest occurring in spring each year. In the last ten years, the trophic level of the lake water has changed from mesotrophic to slightly eutrophic, the total nitrogen (TN) has increased from 0.55 to 2.05 mg L−1 [14], and the Secchi depth has decreased from 0.55 to 0.44 m [27]. In summer 2019, the first year that the strict prohibition of human activities was implemented, we collected samples from five sites, covering wide areas of the lake. In summer 2021, we planned to collect samples for the second time at the same five sites, but C. demersum plants were only observed and collected at three of them. In fact, in this year, all submerged plants in the lake experienced a decline [27]. In the following three years, few subpopulations of C. demersum were observed [personal communication]. Therefore, we compared the genetic variation at the three sites (subpopulations) where the samples were collected in 2019 and 2021 (Figure 1). Although the time scale is only two years, this study may provide some exploratory guidance for subsequent studies. In particular, regarding the drivers of the submerged plant decline in the lake, while existing research has examined water level regulation [27], our results may provide novel insights into plant population genetics.
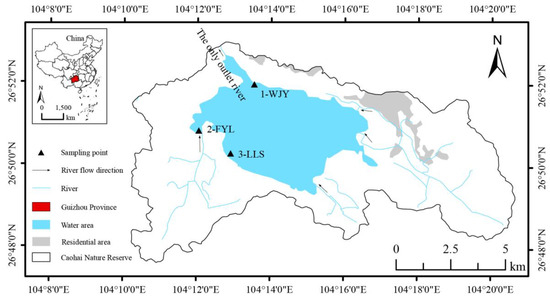
Figure 1.
Map of sampling locations (1-WJY, 2-FYL, and 3-LLS) of Ceratophyllum demersum from Caohai Lake on the Yunnan–Guizhou Plateau, southwest China, in 2019 and 2021.
The three sampling sites were located in the western half of the lake, as no visible distribution of C. demersum was observed in the eastern part in 2021. At each site, we recorded the latitude and longitude using GPS, measured the water depth, and collected water samples to measure the total nitrogen (TN, alkaline potassium persulfate ultraviolet spectrophotometry) and phosphorus (TP, ammonium molybdate spectrophotometry) (Table 1). The 1-WJY subpopulation (local name “Wujiayan”) was located in the northwestern area adjacent to the only outlet river of the lake (Figure 1). At the 1-WJY site, the water was relatively deep, boating activity was relatively frequent, and the bird population was relatively low. The 2-FYL subpopulation (local name “Fuyelin”) was located at a famous birdwatching site situated in the westernmost area of the lake, with a very rich diversity of bird species in both winter and summer [28]. The 3-LLS subpopulation (local name “Louluoshan”) was located in the southern area, which has a rich diversity of bird species in winter but a lower diversity in summer. These latter two sites were in shallow water and, thus, experienced rare boating activity. Since the year of the implementation of strict protective measures (2019), many environmental factors have changed. For instance, boating and fishing activities have almost ceased, tourists are prohibited from entering the water area of the lake, the water quality has decreased (the trophic level index ranged from 48.1 in 2018 to 53.9 in 2021 [14]), and the bird population size has increased. The spatial coverage of C. demersum was investigated at each sampling site. In areas with a concentrated distribution, sampling was conducted with approximately 2.5 m intervals between adjacent samples, aiming to collect about 30 samples. The sampling points were preferentially arranged in a long transect pattern. If the initial transect yielded insufficient samples, then additional parallel transects were established. Only one shoot was sampled at each point. The shoot samples were desiccated with allochroic silica gel and stored until DNA extraction.

Table 1.
Water characteristics of sampling sites/subpopulations (1-WJY, 2-FYL, and 3-LLS) in Caohai Lake.
2.2. DNA Extraction and PCR Amplification
The total genomic DNA of the dry shoot samples was extracted following a modified cetyl-trimethyl ammonium bromide (CTAB) protocol [29]. We developed nine loci of microsatellite (i.e., simple sequence repeats, SSRs) primers for C. demersum (Table S1) using simplified genome sequencing at Tianyi Huiyuan Biotechnology Co., Ltd. (Wuhan, China).
The amplification of the microsatellites was completed in a 20 μL sample containing a mix of Taq polymerase (0.1 U/μL), dATP, dCTP, dGTP, dTTP (0.4 mM each), and a buffer (ComWin Biotech, Beijing, China). The amplification of reactions was completed in a C1000 Thermal Cycler (Bio-Rad Laboratories, Hercules, CA, USA) with the following thermal profile: initial denaturation at 94 °C for 5 min, followed by 34 cycles of 30 s at 94 °C; 30 s at the optimum annealing temperature; 45 s at 72 °C; and, for the final extension, 7 min at 72 °C. PCR products were analyzed by Tianyi Huiyuan Biotechnology Co., Ltd. (Wuhan, China), and the length of the DNA fragments was determined using GeneMarker software (version 1.3; SoftGenetics, State College, PA, USA).
2.3. Data Analysis
The allele data used were the DNA fragment lengths of the amplification products. Using GenAlEx 6.5 [30], the following were examined: (1) the parameters of genetic diversity, including the percentage of polymorphic loci (P), average number of different alleles per locus (Na), total number of alleles across all loci (Nt), number of private alleles (Np), observed heterozygosity (Ho), and unbiased expected heterozygosity (He); (2) the frequency of private alleles for each subpopulation compared between the two years; (3) pairwise subpopulation matrix of Nei unbiased genetic distance; (4) principal coordinate analysis (PCoA) (all individuals studied were analyzed together); and (5) the estimated gene flow (Nm, the theoretical number of migrants per generation) among subpopulations. In addition, from SPAGeDi 1.5 [31], the rarefied allelic richness (Ar) was obtained.
Using GenoDive 3.06 [32] on a Mac (Apple Inc., Cupertino, CA, USA), clones were assigned. Individuals (ramets) with the same multi-locus genotype (MLG) were treated as clones (genet). The parameters of clonal diversity contained the number of MLGs (G) and the effective number of MLGs (Ge). Then, we calculated the genotypic richness (R), where R = (G − 1)/(N − 1), with N being the number of samples [33]. Additionally, the individual heterozygosity of each MLG was calculated (all homozygous loci were recorded as 0, and all heterozygous loci were recorded as 9) [34].
Owo-way ANOVA tests and post hoc multiple comparisons (Duncan) were used to analyze the differences in the diversity parameters (Na, Ar, Ho, and He) among the subpopulations in each year. Independent sample t-tests were used to analyze the differences in the diversity parameters between the two years for each subpopulation. When p < 0.05, the differences were considered significant.
3. Results
3.1. Genetic Diversity
At the total population level, the genetic diversity of C. demersum in Caohai Lake was almost unchanged (p > 0.05) from 2019 to 2021, based on the allelic parameters of Na, Nt, and Ar (Table 2); however, at the ramet level, a weak increase was observed in terms of the heterozygosity parameters (He: 0.452 in 2019 and 0.591 in 2021). Among the three subpopulations, the 1-WJY subpopulation slightly increased in allelic diversity from 2019 to 2021, but it somewhat decreased in heterozygosity. Both the 2-FYL and 3-LLS subpopulations weakly increased in heterozygosity at the ramet level. However, the allelic parameters were maintained in the 2-FYL subpopulation but decreased significantly (p < 0.05) in the 3-LLS subpopulation from 2019 to 2021. Among the three subpopulations, 2-FYL had the lowest genetic diversity (Na, Ar, and He) in each year.

Table 2.
Parameters of genetic and clonal diversity of Ceratophyllum demersum from Caohai Lake in 2019 and 2021.
When comparing the private alleles at each site between the two years, at the 1-WJY site, we observed seven private alleles at six loci in 2021 but only one private allele at one locus in 2019 (Table 2 and Table 3). On the contrary, at the 3-LLS site, no private alleles were found in 2021, but a large number (12 alleles at seven loci) were found in 2019. At the 2-FYL site, private alleles were observed at most loci in each year: 9 and 10 private alleles in 2019 and 2021, respectively. Furthermore, the sum of the frequencies of private alleles at the 2-FYL site in each year was equal to 1 at each of three loci (CdL034, CdL042, and CdL093), meaning that the alleles at these loci were completely different between the two years.

Table 3.
Private alleles (frequency in parentheses) in the sampled individuals of each subpopulation of Ceratophyllum demersum from Caohai Lake, compared between 2019 and 2021.
The individual heterozygosity of all MLGs ranged from 4 to 8 (out of 9 microsatellite loci). The MLGs were divided into two classes: low (4–5 heterozygous loci) and high (6–8) heterozygosity. At the 2-FYL and 3-LLS sites, the numbers of high-heterozygosity individuals were greater in 2021 than in 2019, while the opposite was true for low-heterozygosity individuals (Figure 2). Correspondingly, at the two sites, the observed heterozygosity (Ho) at the ramet level was greater in 2021 than in 2019 (Table 2). However, this was not the case at the 1-WJY site.
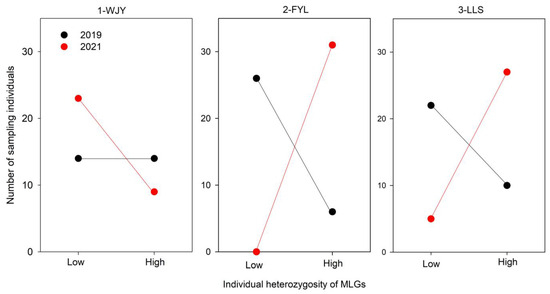
Figure 2.
Individual heterozygosity (low, 4–5 heterozygous loci; high, 6–8 heterozygous loci) of MLGs (genets) of Ceratophyllum demersum from three subpopulations (1-WJY, 2-FYL, and 3-LLS) of Caohai Lake in 2019 and 2021.
3.2. Clonal Diversity
At the total population level, the clonal diversity of C. demersum exhibited little change from 2019 (total G = 19; R = 0.198) to 2021 (total G = 17; R = 0.170) (Table 2). Among the three subpopulations, 2-FYL and 3-LLS showed decreasing clonal diversities from 2019 to 2021. In contrast, the clonal diversity of the 1-WJY subpopulation increased within the same period.
Considering all three subpopulations in the two years, four MLGs (clone codes 1–4) were shared among the sites, and five MLGs (clone codes 1, 2, 4, 5, and 17) were shared between the two years at the same sites (Figure 3 and Figure 4). In 2021, the 2-FYL subpopulation had only four MLGs, which were fewer in number than and genetically different from those in 2019 (seven MLGs) (Table 2 and Figure 3, Figure 4 and Figure 5). A similarly low number of MLGs was observed in the 3-LLS subpopulation (three MLGs) in 2021. However, in the 3-LLS subpopulation, all three MLGs in 2021 genetically included those in 2019 (nine MLGs). In the 1-WJY subpopulation, three MLGs were shared between the two years; additionally, the other nine MLGs in 2021 were genetically different from those in 2019.
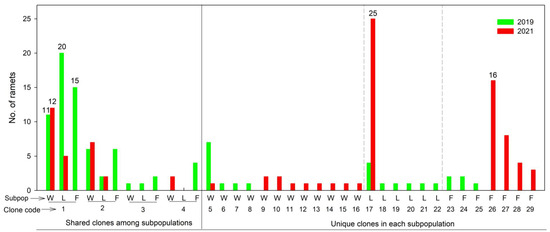
Figure 3.
Frequency distribution of clonal size of Ceratophyllum demersum from Caohai Lake in 2019 and 2021. Clonal size is represented by the number of ramets (clone replicates) belonging to one MLG (clone or genet) at the subpopulation level. Subpopulation codes: W, 1-WJY; F, 2-FYL; L, 3-LLS. The numbers above the bars are the number of ramets.
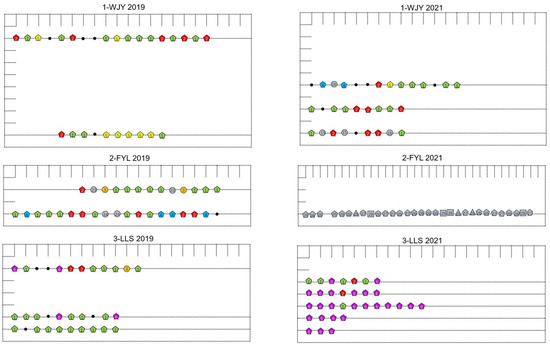
Figure 4.
The distribution of samples and genotypes of Ceratophyllum demersum at/in the three sites/subpopulations (1-WJY, 2-FYL, and 3-LLS) from Caohai Lake in 2019 and 2021. The distance between two adjacent small lines is 2.5 m. The number in each shape is the clone code of the multi-locus genotype (MLG). The MLGs shared between years and/or subpopulations are shown in colorful pentagons. The MLGs unique to each subpopulation are shown in gray shapes. Circles, triangles, squares, and pentagons indicate that the clone has 2, 3, 4, and more than 5 ramets/replicates, respectively; and black points indicate single-ramet MLGs.
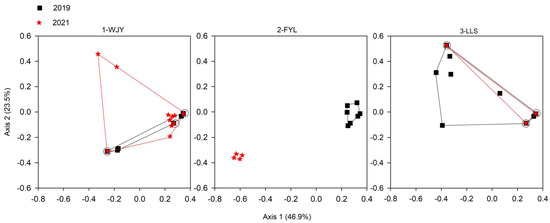
Figure 5.
Scatter plots of principle coordinate analysis (PCoA) of MLGs (genets) of Ceratophyllum demersum from three subpopulations (1-WJY, 2-FYL, and 3-LLS) from Caohai Lake in 2019 and 2021. The circles emphasize the MLGs shared between the two years.
The clone size (the number of ramets/clone replicates belonging to one MLG/genet) was the greatest in the 3-LLS and 2-FYL subpopulations, being up to 20 and 15 ramets (clone code 1), respectively, in 2019 and up to 25 (clone code 17) and 16 ramets (clone code 26) in 2021 (Figure 3). However, at each of the two sites, the clones with the greatest number of ramets were different between the two years. At the 1-WJY site, the clone size was relatively smaller.
3.3. Genetic Distance and Estimated Gene Flow Among Subpopulations
In terms of the Nei unbiased genetic distance calculated from allele frequencies, between the subpopulation pairs, the three subpopulations were more genetically distant from each other in 2021 (with a genetic distance of 0.277–0.773) than in 2019 (0.012–0.060) (Table 4). At each site, the genetic distance between the two years was the largest for 2-FYL (0.847), moderate for 3-LLS (0.178), and the smallest for 1-WJY (0.034).

Table 4.
Genetic distance between subpopulation pairs of Ceratophyllum demersum from Caohai Lake in 2019 and 2021.
Among the subpopulations, the mean estimated gene flow (Nm) across all loci was 1.173 in 2021, which was much lower than that (9.767) in 2019 (Table 5). On a per locus basis, the Nm values were lower in 2021 than in 2019 for eight out of nine loci.

Table 5.
Estimated gene flow (Nm *) at each locus among subpopulations of Ceratophyllum demersum from Caohai Lake in 2019 and 2021.
4. Discussion
4.1. Genetic and Clonal Diversity
Two years after the implementation of strict conservation measures at Caohai Lake in 2019, the genetic and clonal diversity of the C. demersum population was primarily maintained at the total population level, with a weak increase in heterogeneity (from 2019 to 2021: Ar, from 4.28 to 4.17; ramet He, from 0.452 to 0.591; R, from 0.198 to 0.170). The constant diversity of C. demersum indicates that, overall, the population is resilient to environmental changes, including reduced human activities of boating, fishing, and birdwatching. Alternatively, the change in environments has not yet demonstrated a noticeable impact on the genetic aspects of the C. demersum population within the short span of two years.
Compared with recent studies on C. demersum, it was found that the genetic diversity of the Caohai population is higher than that of the river and connected oxbow populations of this species in Hungary (total Ar, 2.905 and 3.611; total He, 0.436), detected by Engloner et al. [9,26], who similarly used newly developed microsatellite primers. Moreover, the spatial distance between the two adjacent samples was notably larger in the study conducted by Engloner et al. [9,26] (at least 30 m) than in our study (about 2.5 m), thus further indicating the high genetic diversity of C. demersum in Caohai Lake. The difference in genetic diversity between the studies is presumably due to the different contributions of sexual reproduction. Engloner et al. [9,26] observed a clear relationship between the genetic diversity of populations and the hydrological types of the habitats, indicating mainly the hydrochorous spread of vegetative propagules. In our previous study [19], we found that sexual dispersal (mainly seeds for the hydrophilous species) contributed more than vegetative dispersal in some C. demersum subpopulations in Caohai Lake. In addition, the relatively high genetic diversity of C. demersum in Caohai Lake might benefit from its natural aquatic communities having evolved over a long period of time. Compared with other previous studies, the heterogeneity (He, 0.452–0.591) of the Caohai C. demersum population is also higher than that of other populations detected using other molecular techniques (Nei gene diversity, HS = 0.258, using the amplified fragment polymorphism length (AFLP) [35]; mean genetic diversity within lake populations, HS = 0.169, using inter-simple sequence repeat (ISSR) markers [36]). Therefore, our results indicate the presence of considerable genetic diversity in the C. demersum population in Caohai Lake with abundant aquatic plants.
However, among the three C. demersum subpopulations studied, there were different change tendencies of genetic and clonal diversity with the temporal changes in environments. Both the 2-FYL and 3-LLS subpopulations showed notably decreased clonal diversity; moreover, the latter also showed significantly decreased allelic richness (Ar). On the contrary, the 1-WJY subpopulation showed increased clonal diversity and allelic richness. The similarities between the 2-FYL and 3-LLS sites are shallow water habitats and abundant bird populations. Following the implementation of strict protective measures, avian populations have increased significantly [13], with birdwatcher access to shallow-water zones being prohibited. When human interference decreased at Caohai Lake, bird activity (as a natural disturbance) increased [13], especially at the 2-FYL and 3-LLS sites, which have large bird populations. Bird activities may enhance propagule dispersal. In vigorous vegetative growth seasons, birds trample on and eat the shoots of C. demersum, and, at the same time, shoot fragments can be produced and carried within subpopulations [37]. It is possible that the most competitive clones have plenty of opportunities to be spread by birds. The ramet number per MLG reached 20 (at the 3-LLS site) and 15 (at the 2-FYL site) in 2019, and reached 25 (at the 3-LLS site) and 16 (at the 2-FYL site) in 2021. The environmental changes in human and bird activities may be associated with the dynamics of genetic diversity. In subsequent studies, it is imperative to monitor the influences of avian species and human activities on the dispersal of C. demersum propagules.
Nevertheless, the observed heterozygosity (Ho) at the ramet level in the 2-FYL and 3-LLS subpopulations of C. demersum did not decrease with the environmental changes, which might be attributed to the increased frequency of high-heterozygosity individuals at the microsatellite loci. In 2021, high-heterozygosity MLGs had more sampling individuals than low-heterozygosity ones, with the opposite being found in 2019. Hämmerli and Reusch [34] found that individual heterozygosity could predict the competitive superiority of clones. Therefore, environmental changes may facilitate high-heterozygosity clones in exhibiting their competitive advantage over low-heterozygosity clones.
Different from the 2-FYL and 3-LLS subpopulations, the 1-WJY subpopulation demonstrated increased clonal diversity and allelic richness to some extent when the environment changed. A considerable number of MLGs and alleles detected in 2021 differed from those detected in 2019 at this site. The difference between 1-WJY and the other two subpopulations is that the 1-WJY subpopulation was located near the only outlet river of the lake, where the persistent and single-directional water flow (as a natural mechanical disturbance) may gather a large quantity of C. demersum propagules from all sides of the lake. Therefore, in subsequent studies, it is necessary to monitor the impact of water flow on C. demersum propagules. Previous studies have shown that hydrochory is a very important mode of propagule spread for this species [9,19,26]. Diverse vegetative propagules, including shoot fragments, turions, and rootless ramet plants, can float in the water column and be easily spread [38].
4.2. Genetic Relationships
At the 2-FYL site, all four MLGs in 2021 were genetically different from those in 2019, with a high frequency of private alleles in each year, a relatively large genetic distance (0.847), and no shared MLGs between the two years. Different from the 2-FYL site, no immigratory MLGs were observed at the 3-LLS site in 2021. All three MLGs of the 3-LLS subpopulation in 2021, without private alleles, genetically included those in 2019 without exception. The differences in environmental conditions between the 2-FYL and 3-LLS sites include different bird populations. At the 2-FYL site, birds are very common both in summer and winter, while at the 3-LLS site, birds are frequently observed in winter but less so in summer [28]. Therefore, summer birds may have more opportunities for transporting C. demersum fruits maturing in late summer and early autumn [15] from other areas to the 2-FYL site than to the 3-LLS site. Although the low seed production and long spines of C. demersum fruits [15] seemingly imply few chances for birds to transport them internally among subpopulations, analyses of sexual gene dispersal and the relative contribution of vegetative and sexual dispersal show that sexual dispersal occurs substantially [19]. However, more studies on sexual dispersal and fruit/seed transportation are needed.
The genetic distance between the sites exhibited an increasing tendency after the implementation of strict conservation measures, in the ranges of 0.012 to 0.060 in 2019 and 0.277 to 0.773 in 2021. The protective measures reduced human activities and may simultaneously decrease the gene flow (Nm) among subpopulations, thus increasing genetic differentiation. C. demersum subpopulations unaffected by human disturbance may be less genetically connected with each other. Less connectivity is commonly accompanied by less gene exchange and more genetic differentiation among populations, such as in other aquatic [39,40] and terrestrial species [41,42].
5. Conclusions
Our results show the presence of considerable genetic diversity in the C. demersum population in Caohai Lake with abundant aquatic plants. Two years after the implementation of strict conservation measures in 2019, the genetic and clonal diversity of the C. demersum population was primarily maintained at the total population level. Under stringent conservation, the impact of natural disturbances, such as water flow and bird activities, has persisted and may have increased. For submerged and hydrophilous C. demersum, whose main dispersal mode is hydrochory at the local scale, water flow may cause vegetative propagules of different genotypes to pool in the downstream area of the lake (such as the 1-WJY subpopulation). At sites with very large bird populations, such as the sites of the 2-FYL and 3-LLS subpopulations, an increase in bird activities may augment the advantage of competitive clones in these communities. Reducing anthropological disturbance decreases the gene flow via human activities among subpopulations, which may result in an increase in genetic differentiation to some extent. Therefore, the continuous monitoring of population genetic variation, as well as the impact of related environmental factors, including human activities, bird activities, and water flow, is required for the efficient management of the lake ecosystem.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d17060413/s1, Table S1: Information of microsatellite (i.e., simple sequence repeats, SSR) primers of Ceratophyllum demersum.
Author Contributions
Conceptualization, software, writing—original draft preparation, writing—review and editing, visualization, supervision, project administration, funding acquisition, Q.-J.C.; methodology, validation, formal analysis, investigation, resources, data curation, Q.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (31600325).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data on the microsatellite (i.e., SSRs) primer pairs used for DNA amplification in this study are included in the Supplementary Materials. Data on the allele information generated and analyzed in the current study, are available from the corresponding author upon reasonable request.
Acknowledgments
We are sincerely grateful to the Caohai Nature Reserve Management Committee for their help in our sampling process. We thank Shenglei Tu for their help during sampling and for providing information on subsequent field observations.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SSR | Simple sequence repeats |
| MLG | Multi-locus genotype |
| PCoA | Principal coordinate analysis |
References
- Turner, M.G. Disturbance and landscape dynamics in a changing world. Ecology 2010, 91, 2833–2849. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.J.; Wu, D.; Niu, L.L.; Ma, X.; Li, Y.; Hillman, A.L.; Abbott, M.B.; Zhou, A. Contrasting ecosystem responses to climatic events and human activity revealed by a sedimentary record from Lake Yilong, southwestern China. Sci. Total Environ. 2021, 783, 146922. [Google Scholar] [CrossRef]
- Cai, F.R. Study on Influencing Factors of Pro-Environmental Behavior of Bird-Watching Tourists in Caohai, Weining. Master’s Thesis, Yunnan University, Kunming, China, 2021. (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Liu, X.; Qiu, Y.; Zheng, Z.L.; Hong, Q.; Zhang, Y.; Qian, Q.; Wan, B.; Chen, Q. Spatiotemporal changes in waterfowl habitat suitability in the Caohai Lake wetland and responses to human activities. Sustainability 2022, 14, 14409. [Google Scholar] [CrossRef]
- Wang, R.L.; Han, Y.; Fan, F.; Molinos, J.G.; Xu, J.; Wang, K.; Wang, D.; Mei, Z. Need to shift in river-lake connection scheme under the “ten-year fishing ban” in the Yangtze River, China. Ecol. Indic. 2022, 143, 109434. [Google Scholar] [CrossRef]
- Bornette, G.; Puijalon, S. Response of aquatic plants to abiotic factors: A review. Aquat. Sci. 2011, 73, 1–14. [Google Scholar] [CrossRef]
- Thomaz, S.M. Ecosystem services provided by freshwater macrophytes. Hydrobiologia 2023, 850, 2757–2777. [Google Scholar] [CrossRef]
- Hughes, A.R.; Stachowicz, J.J. Genetic diversity enhances the resistance of a seagrass ecosystem to disturbance. Proc. Natl. Acad. Sci. USA 2004, 101, 8998–9002. [Google Scholar] [CrossRef]
- Engloner, A.I.; Németh, K.; Kós, P.B.; Meglecz, E.; Bereczki, J. Genetic diversity of the submerged macrophyte Ceratophyllum demersum depends on habitat hydrology and habitat fragmentation. Front. Plant Sci. 2023, 14, 1277916. [Google Scholar] [CrossRef]
- Vásquez, C.; Quinones, R.A.; Brante, A.; Hernandez-Miranda, E. Genetic diversity and resilience in benthic marine populations. Rev. Chil. Hist. Nat. 2023, 96, 4. [Google Scholar] [CrossRef]
- Wang, S.M.; Dou, H.S. Memoirs of Lakes in China; Science Press: Beijing, China, 1998. (In Chinese) [Google Scholar]
- Peng, F.C.; He, T.R.; Li, Z.J.; Chen, M.; Qian, X.; Zeng, L.; Xu, Y. Enrichment characteristics and risk assessment of Hg in bird feathers from Caohai wetland in Guizhou Province, China. Acta Geochim. 2018, 37, 526–536. [Google Scholar] [CrossRef]
- Tu, S.L. Effects of Reed (Phragmites australis) Expansion on Wintering Behavior of Black-Necked Crane (Grus nigricollis) in Caohai Lake Reserve, Guizhou Province. Master’s Thesis, Guizhou Normal University, Guiyang, China, 2023. (In Chinese with English abstract). [Google Scholar]
- Xu, L.; Guo, Y.M.; Liu, D.; Li, R.; Du, M. Temporal and influencing factors of Caohai water quality in recent ten years. Environ. Prot. Sci. 2024, 50, 127–133, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Flora of China Editorial Committee. Flora of China. 2024. Available online: http://www.iplant.cn (accessed on 29 March 2024). (In Chinese).
- Lou, J. The Quantitative Study of Aquatic Vegetation Community and Human Disturbance in Caohai, Guizhou. Master’s Thesis, Guizhou University, Guiyang, China, 2006. (In Chinese with English abstract). [Google Scholar]
- Dai, L.L.; Chen, X.; Li, L.; Liu, C.; Yuan, G. Aquatic plant diversity and community succession in Caohai wetland, Guizhou Province. Acta Hydrobiol. Sin. 2020, 44, 869–876, (In Chinese with English abstract). [Google Scholar]
- Triest, L.; Tran Thi, V.; Le Thi, D.; Sierens, T.; Geert, A.V. Genetic differentiation of submerged plant populations and taxa between habitats. Hydrobiologia 2010, 656, 15–27. [Google Scholar] [CrossRef]
- Cao, W.; Wang, Q.; Cao, Q.J. Fine-scale spatial genetic structure and gene dispersal in lake populations of submerged species. J. Oceanol. Limnol. 2025, 43, 831–847. [Google Scholar] [CrossRef]
- Les, D.H. Breeding systems, population structure, and evolution in hydrophilous angiosperms. Ann. Mo. Bot. Gard. 1988, 75, 819–835. [Google Scholar] [CrossRef]
- Foroughi, M.; Najafi, P.; Toghiani, A.; Honarjoo, N. Analysis of pollution removal from wastewater by Ceratophyllum demersum. Afr. J. Biotechnol. 2010, 9, 2125–2128. [Google Scholar]
- Dai, Y.; Jia, C.; Liang, W.; Hu, S.; Wu, Z. Effects of the submerged macrophyte Ceratophyllum demersum L. on restoration of a eutrophic waterbody and its optimal coverage. Ecol. Eng. 2012, 40, 113–116. [Google Scholar] [CrossRef]
- Les, D.H. Genetic diversity in the monoecious hydrophile Ceratophyllum (Ceratophyllaceae). Am. J. Bot. 1991, 78, 1070–1082. [Google Scholar] [CrossRef]
- Reynolds, C.; Cumming, G.S. Seed dispersal by waterbirds in southern Africa: Comparing the roles of ectozoochory and endozoochory. Freshw. Biol. 2016, 61, 349–361. [Google Scholar] [CrossRef]
- Capers, R.S. Macrophyte colonization in a freshwater tidal wetland (Lyme, CT, USA). Aquat. Bot. 2003, 77, 325–338. [Google Scholar] [CrossRef]
- Engloner, A.I.; Németh, K.; Bereczki, J. The genetic diversity of the macrophyte Ceratophyllum demersum in Backwaters reflects differences in the hydrological connectivity and water flow rate of habitats. Plants 2024, 13, 2220. [Google Scholar] [CrossRef] [PubMed]
- Chao, F.; Jiang, X.; Wang, X.; Lu, B.; Liu, J.; Xia, P. Water level fluctuation rather than eutrophication induced the extinction of submerged plants in Guizhou’s Caohai Lake: Implications for lake management. Water 2024, 16, 772. [Google Scholar] [CrossRef]
- Zhu, Y. Dynamics of Waterbirds Community and Their Exposure Risk of Heavy Metal Exposure in Caohai Wetland, Guizhou Province, China. Master’s Thesis, Guizhou University, Guiyang, China, 2020. (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Saghai-Maroof, M.A.; Soliman, K.M.; Jorgensen, R.A.; Allard, R.W. Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosomal location, and population dynamics. Proc. Natl. Acad. Sci. USA 1984, 81, 8014–8018. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in excel. Population genetic software for teaching and research-an update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [PubMed]
- Hardy, O.J.; Vekemans, X. SPAGeDi: A versatile computer program to analyse spatial genetic structure at the individual or population levels. Mol. Ecol. Notes 2002, 2, 618–620. [Google Scholar] [CrossRef]
- Meirmans, P.G. GENODIVE version 3.0: Easy-to-use software for the analysis of genetic data of diploids and polyploids. Mol. Ecol. Resour. 2020, 20, 1126–1131. [Google Scholar] [CrossRef]
- Dorken, M.E.; Eckert, C.G. Severely reduced sexual reproduction in northern populations of a clonal plant, Decodon verticillatus (Lythraceae). J. Ecol. 2001, 89, 339–350. [Google Scholar] [CrossRef]
- Hämmerli, A.; Reusch, T.B.H. Inbreeding depression influences genet size distribution in a marine angiosperm. Mol. Ecol. 2003, 12, 619–629. [Google Scholar] [CrossRef]
- Li, Y.X.; Zhao, X.Y.; Xia, M.L.; Wei, X.; Hou, H. Temperature is a cryptic factor shaping the geographical pattern of genetic variation in Ceratophyllum demersum across a subtropical freshwater lake. Plant Divers. 2024, 46, 630–639. [Google Scholar] [CrossRef]
- Cao, Q.J.; Mei, F.; Wang, L. Population genetic structure in six sympatric and widespread aquatic plants inhabiting diverse lake environments in China. Ecol. Evol. 2017, 7, 5713–5723. [Google Scholar] [CrossRef]
- Hidding, B.; Meirmans, P.G.; Klaassen, M.; Ouborg, T.d.B.N.J.; Wagemaker, C.A.M.; Nolet, B.A. The effect of herbivores on genotypic diversity in a clonal aquatic plant. Oikos 2014, 123, 1112–1120. [Google Scholar] [CrossRef]
- Wu, Z.B.; Zuo, J.C.; Ma, J.M.; Wu, J.; Cheng, S.; Liang, W. Establishing submersed macrophytes via sinking and colonization of shoot fragments clipped off manually. Wuhan Univ. J. Nat. Sci. 2007, 12, 553–557. [Google Scholar] [CrossRef]
- Bilton, D.T.; Paula, J.; Bishop, J.D.D. Dispersal, genetic differentiation and speciation in estuarine organisms. Estuar. Coast. Shelf Sci. 2002, 55, 937–952. [Google Scholar] [CrossRef]
- Cao, Q.J.; Liu, B.; Hu, F. Effects of hydrological connection and human disturbance on genetic variation of submerged Vallisneria natans populations in four lakes in China. J. Oceanol. Limnol. 2021, 39, 1403–1416. [Google Scholar] [CrossRef]
- Young, A.; Boyle, T.; Brown, T. The population genetic consequences of habitat fragmentation for plants. Trends Ecol. Evol. 1996, 11, 413–418. [Google Scholar] [CrossRef]
- Soares, L.; Cazetta, E.; Santos, L.R.; Franca, D.d.S.; Gaiotto, F.A. Anthropogenic disturbances eroding the genetic diversity of a threatened palm tree: A multiscale approach. Front. Genet. 2019, 10, 1090. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).