Changes in the Soil Bacterial Community Across Fairy Rings in Grasslands Using Environmental DNA Metabarcoding
Abstract
:1. Introduction
2. Materials and Methods
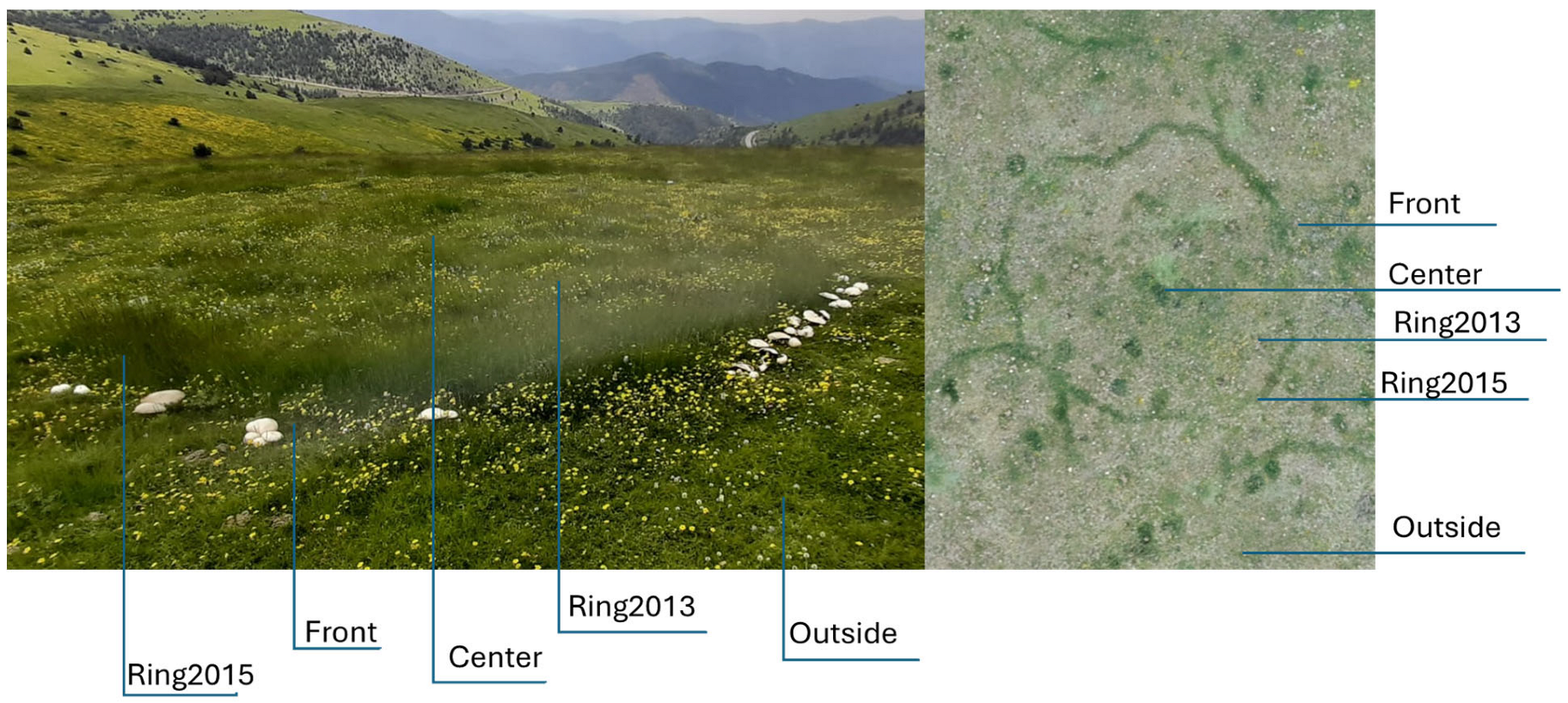
2.1. Study Site and Experimental Design
2.2. Soil Physicochemical Properties Analysis
2.3. Next Generation Sequencing and Bioinformatic Analysis
2.4. Statistical Analyses
3. Results
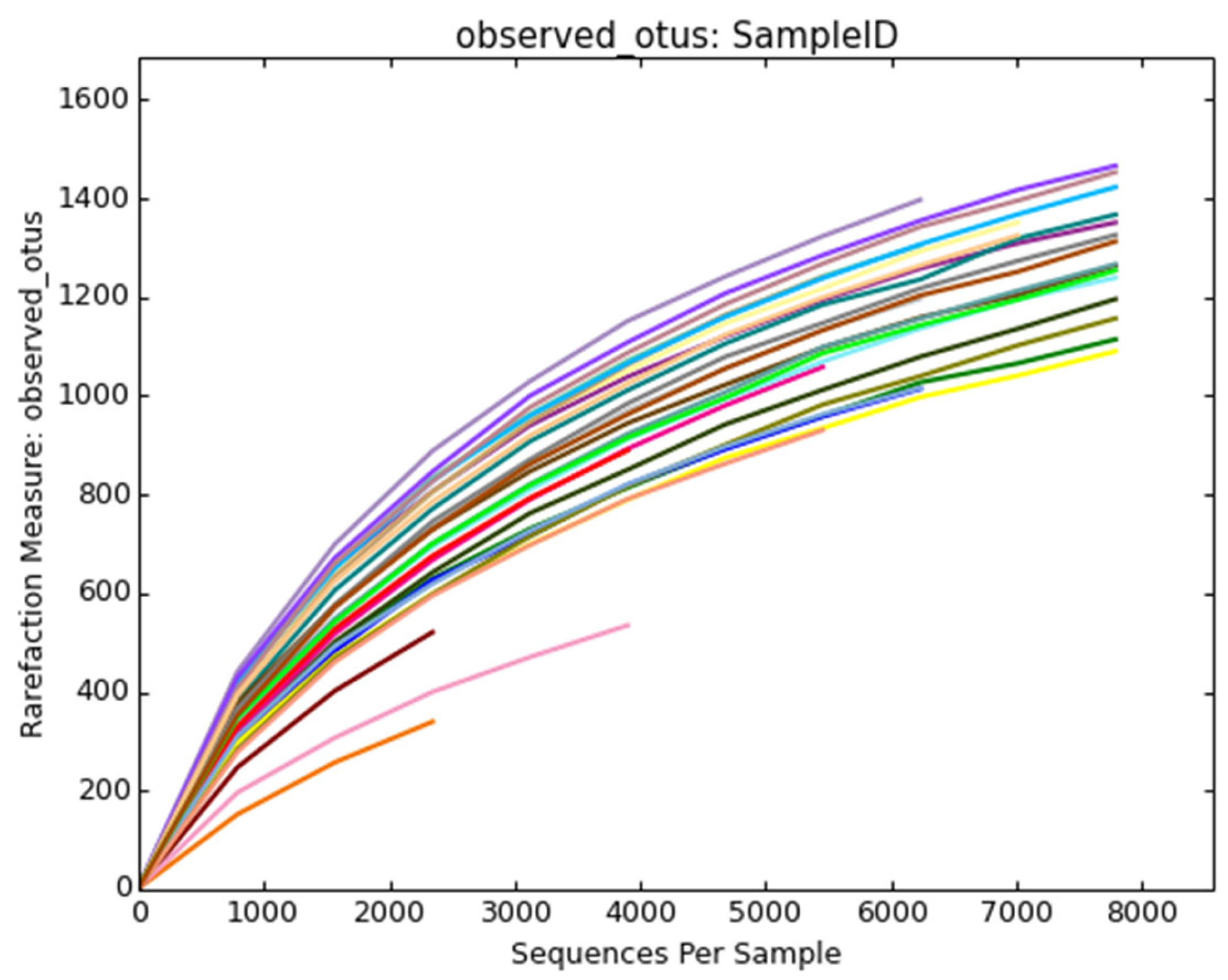
3.1. Sequencing Results of Bacterial Communities
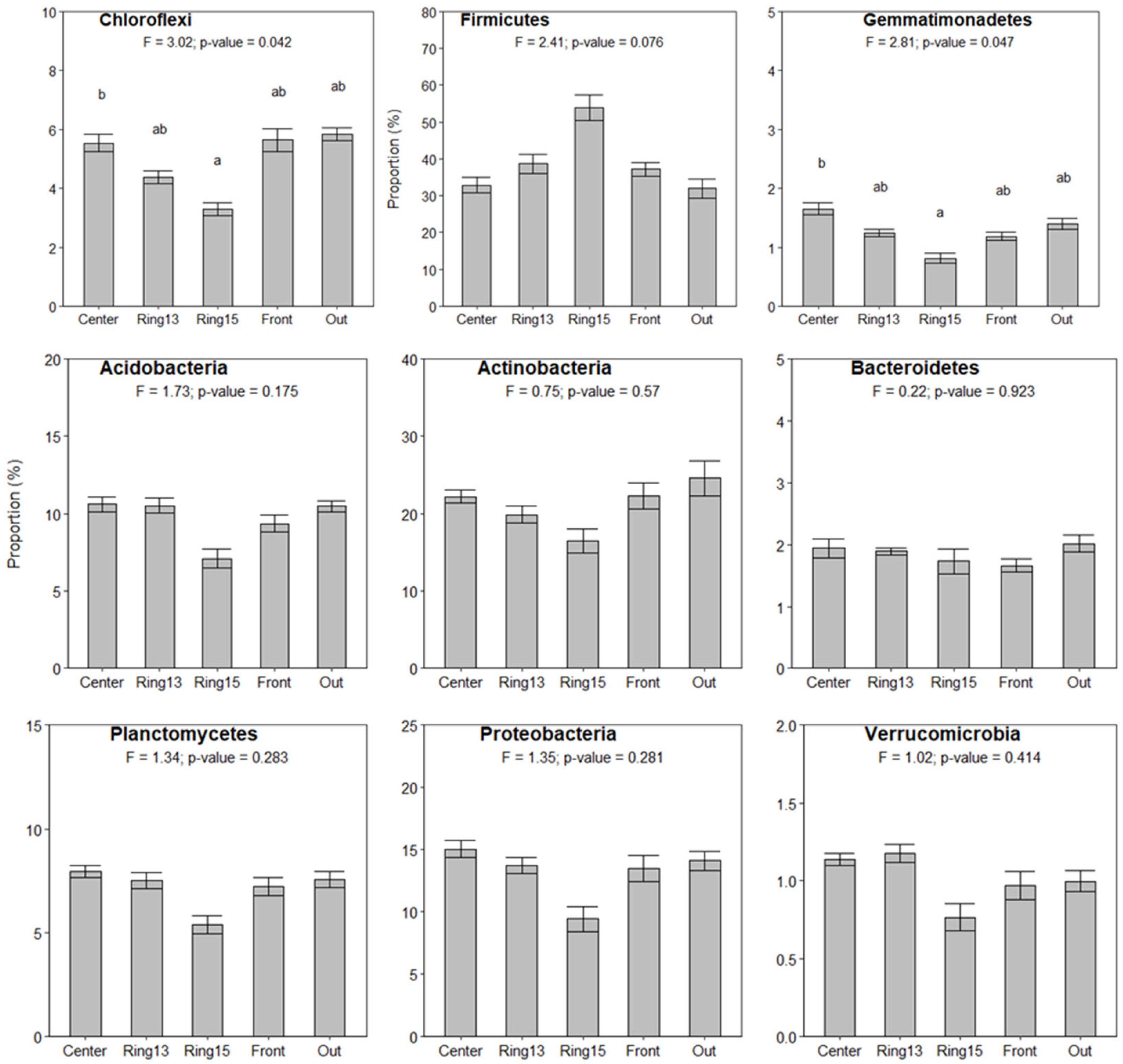
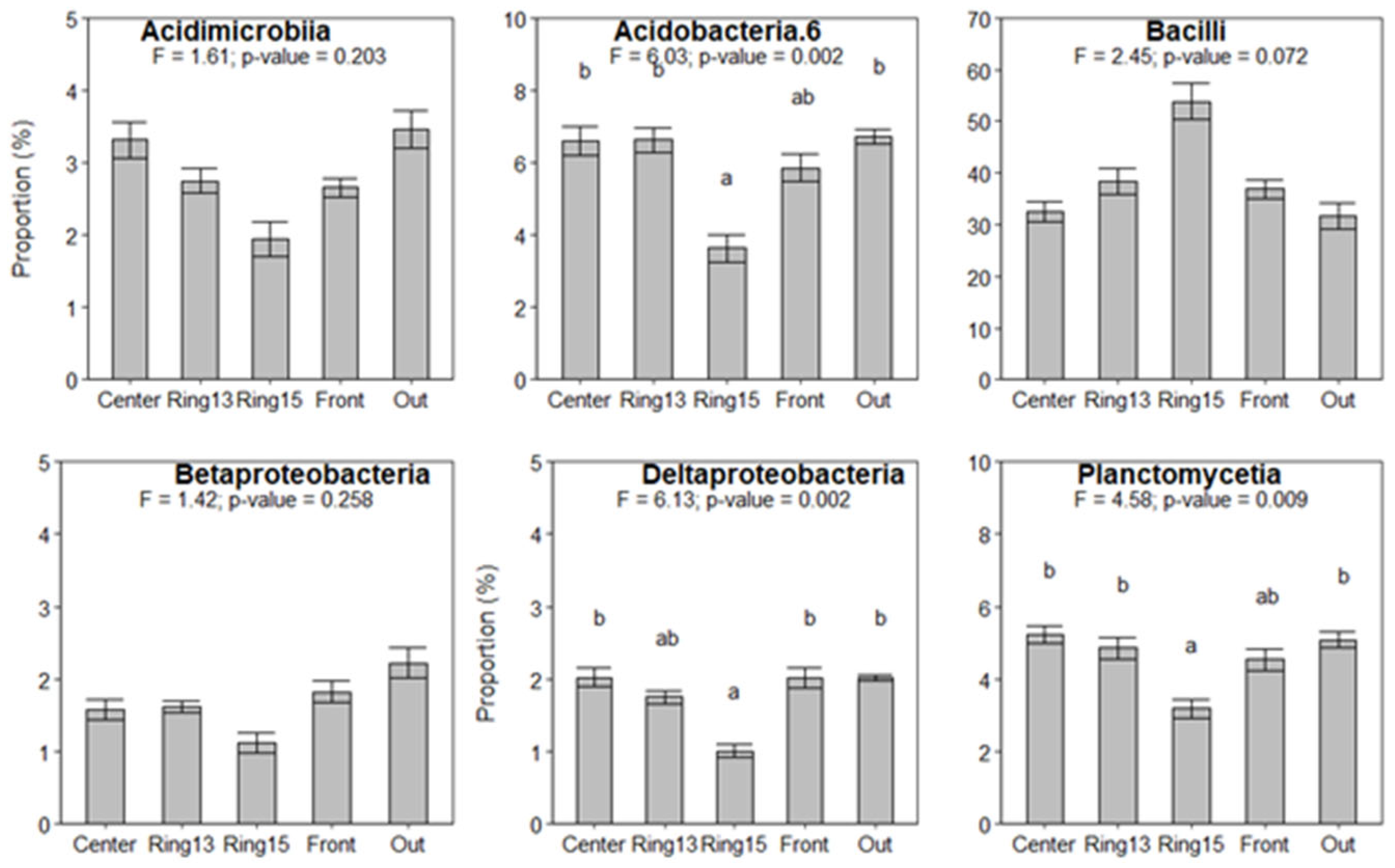
3.2. Bacterial Community Composition in La Bertolina
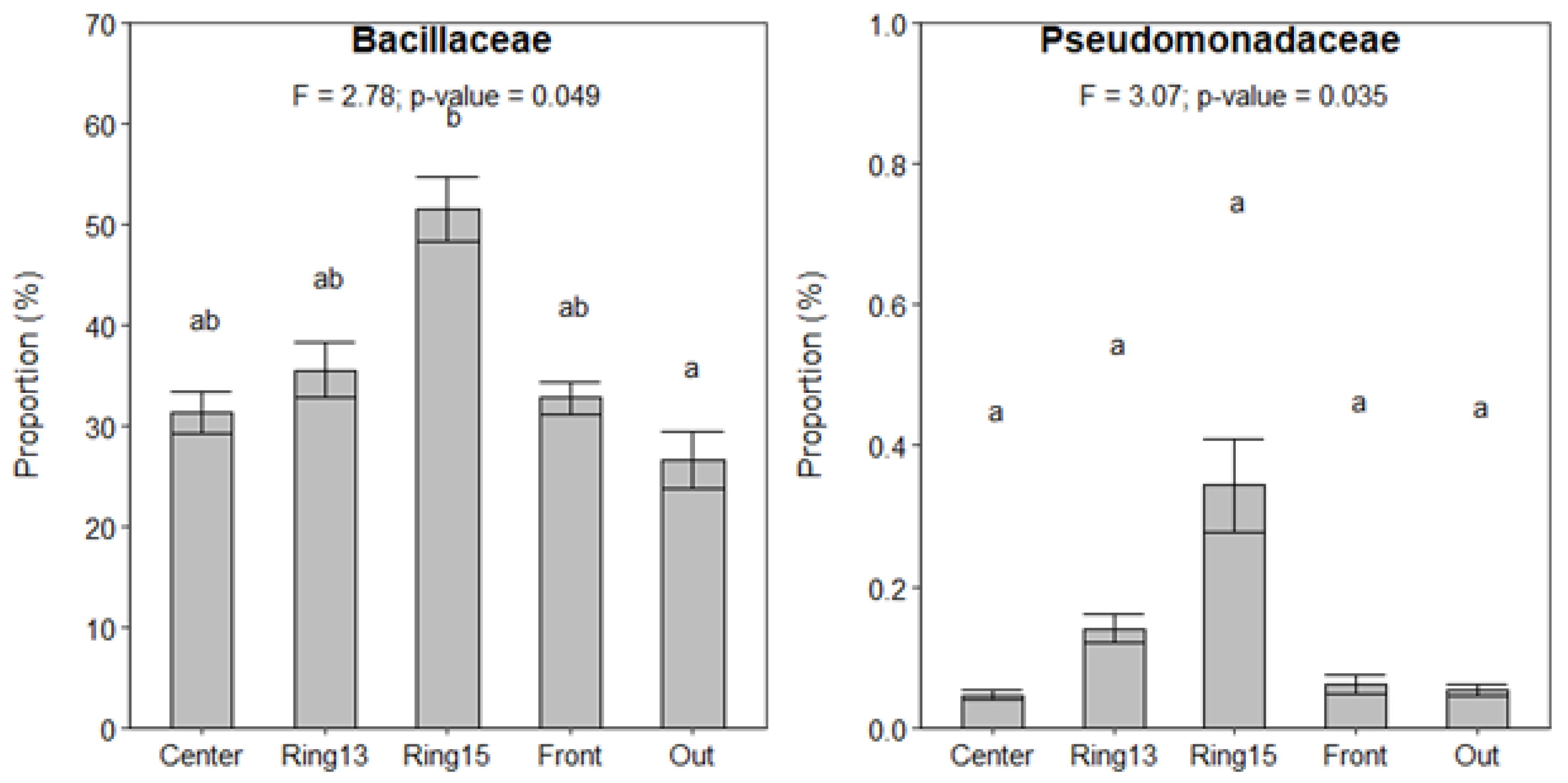
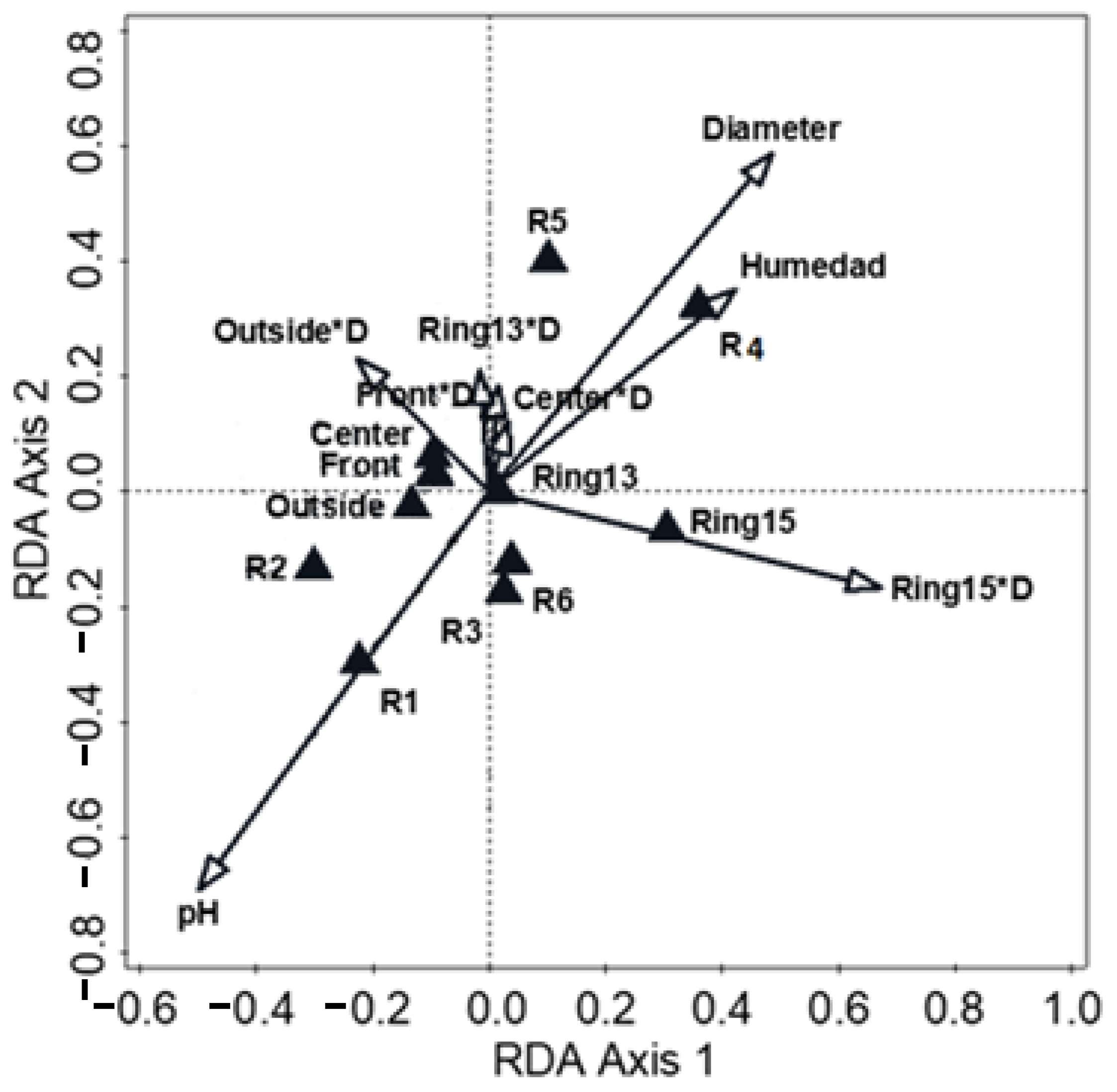
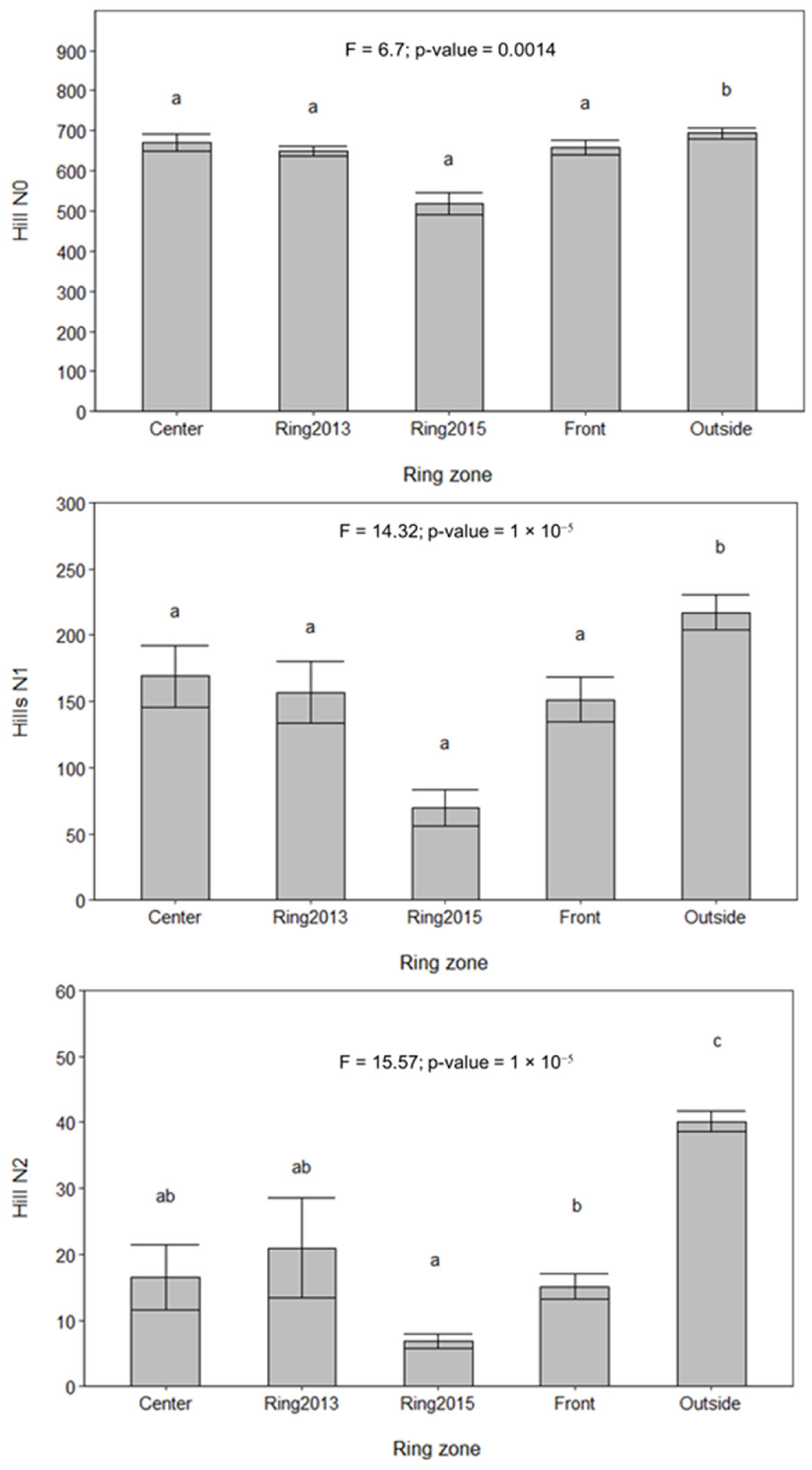
3.3. Changes in Bacterial Community Across Fairy Rings and Environmental Gradients
4. Discussion
4.1. Bacterial Community Composition in La Bertolina Grassland
4.2. Soil Physicochemical Changes Across Fairy Rings
4.3. Effects of the Fairy Ring Fungi on the Soil Bacterial Diversity
4.4. Changes in the Soil Bacterial Community Composition Across Fairy Rings
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OTU | Operational Taxonomic Unit |
| RZ | Ring Zone |
| LSU | Livestock Unit |
| LME | Linear Mixed Effect Model |
References
- Blair, J.; Nippert, J.; Briggs, J. Grassland Ecology. In Ecology and the Environment; Monson, R.K., Ed.; Springer: New York, NY, USA, 2014; pp. 389–423. ISBN 978-1-4614-7500-2. [Google Scholar]
- Rodríguez, A.; de Lamo, X.; Sebastià, M.-T. Interactions between Global Change Components Drive Plant Species Richness Patterns Within Communities in Mountain Grasslands Independently of Topography. J. Veg. Sci. 2018, 29, 1029–1039. [Google Scholar] [CrossRef]
- Bonanomi, G.; Mingo, A.; Incerti, G.; Mazzoleni, S.; Allegrezza, M. Fairy Rings Caused by a Killer Fungus Foster Plant Diversity in Species-Rich Grassland. J. Veg. Sci. 2012, 23, 236–248. [Google Scholar] [CrossRef]
- Bürgi, M.; Silbernagel, J.; Wu, J.; Kienast, F. Linking Ecosystem Services with Landscape History. Landsc. Ecol. 2015, 30, 11–20. [Google Scholar] [CrossRef]
- Wilson, J.B.; Peet, R.K.; Dengler, J.; Pärtel, M. Plant Species Richness: The World Records. J. Veg. Sci. 2012, 23, 796–802. [Google Scholar] [CrossRef]
- Schirpke, U.; Kohler, M.; Leitinger, G.; Fontana, V.; Tasser, E.; Tappeiner, U. Future Impacts of Changing Land-Use and Climate on Ecosystem Services of Mountain Grassland and Their Resilience. Ecosyst. Serv. 2017, 26, 79–94. [Google Scholar] [CrossRef]
- Maron, P.-A.; Sarr, A.; Kaisermann, A.; Lévêque, J.; Mathieu, O.; Guigue, J.; Karimi, B.; Bernard, L.; Dequiedt, S.; Terrat, S.; et al. High Microbial Diversity Promotes Soil Ecosystem Functioning. Appl. Environ. Microbiol. 2018, 84, e02738-17. [Google Scholar] [CrossRef] [PubMed]
- Bonanomi, G.; Incerti, G.; Allegrezza, M. Assessing the Impact of Land Abandonment, Nitrogen Enrichment and Fairy-Ring Fungi on Plant Diversity of Mediterranean Grasslands. Biodivers. Conserv. 2013, 22, 2285–2304. [Google Scholar] [CrossRef]
- Van der Wal, A.; Geydan, T.D.; Kuyper, T.W.; De Boer, W. A Thready Affair: Linking Fungal Diversity and Community Dynamics to Terrestrial Decomposition Processes. FEMS Microbiol. Rev. 2013, 37, 477–494. [Google Scholar] [CrossRef]
- Shantz, H.L.; Piemeisel, R.L. Fungus Fairy Rings in Eastern Colorado and Their Effect on Vegetation. J. Agric. Res. 1917, 11, 191–245. [Google Scholar]
- Dernoeden, P.H. About Fairy Rings and Their Management. Int. Newsl. Curr. Dev. Turfgrass 2002, 10, 1–5. [Google Scholar]
- Edwards, P.J. Effects of the Fairy Ring Fungus Agaricus Arvensis on Nutrient Availability in Grassland. New Phytol. 1988, 110, 377–381. [Google Scholar] [CrossRef]
- Xu, X.; Ouyang, H.; Cao, G.; Richter, A.; Wanek, W.; Kuzyakov, Y. Dominant Plant Species Shift Their Nitrogen Uptake Patterns in Response to Nutrient Enrichment Caused by a Fungal Fairy in an Alpine Meadow. Plant Soil 2011, 341, 495–504. [Google Scholar] [CrossRef]
- Yang, C.; Li, J.; Zhang, F.; Liu, N.; Zhang, Y. The Optimal Redfield N: P Ratio Caused by Fairy Ring Fungi Stimulates Plant Productivity in the Temperate Steppe of China. Fungal Ecol. 2018, 34, 91–98. [Google Scholar] [CrossRef]
- Caesar-TonThat, T.C.; Espeland, E.; Caesar, A.J.; Sainju, U.M.; Lartey, R.T.; Gaskin, J.F. Effects of Agaricus Lilaceps Fairy Rings on Soil Aggregation and Microbial Community Structure in Relation to Growth Stimulation of Western Wheatgrass (Pascopyrum smithii) in Eastern Montana Rangeland. Microb. Ecol. 2013, 66, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, F.; Liu, N.; Hu, J.; Zhang, Y. Changes in Soil Bacterial Communities in Response to the Fairy Ring Fungus Agaricus Gennadii in the Temperate Steppes of China. Pedobiol.-J. Soil Ecol. 2018, 69, 34–40. [Google Scholar] [CrossRef]
- Vaario, L.; Fritze, H.; Spetz, P.; Heinonsalo, J.; Hanajík, P.; Pennanen, T. Tricholoma matsutake Dominates Diverse Microbial Communities in Different Forest Soils. Appl. Environ. Microbiol. 2011, 77, 8523–8531. [Google Scholar] [CrossRef]
- Ohara, H.; Hamada, M. Disappearance of Bacteria from the Zone of Active Mycorrhizas in Tricholoma matsutake (S. Ito et Imai) Singer. Nature 1967, 213, 528–529. [Google Scholar] [CrossRef]
- Wang, J.T.; Zheng, Y.M.; Hu, H.W.; Zhang, L.M.; Li, J.; He, J.Z. Soil pH Determines the Alpha Diversity but Not Beta Diversity of Soil Fungal Community along Altitude in a Typical Tibetan Forest Ecosystem. J. Soils Sediments 2015, 15, 1224–1232. [Google Scholar] [CrossRef]
- Miller, G.L.; Grand, L.F.; Tredway, L.P. Identification and Distribution of Fungi Associated with Fairy Rings on Golf Putting Greens. Plant Dis. 2011, 95, 1131–1138. [Google Scholar] [CrossRef]
- Simberloff, D.; Martin, J.L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; García-Berthou, E.; Pascal, M.; et al. Impacts of Biological Invasions: What’s What and the Way Forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef]
- Hassan, A.; Ricciardi, A. Are Non-Native Species More Likely to Become Pests? Influence of Biogeographic Origin on the Impacts of Freshwater Organisms. Front. Ecol. Environ. 2014, 12, 218–223. [Google Scholar] [CrossRef]
- Pivello, V.R.; Vieira, M.V.; Grombone-Guaratini, M.T.; Matos, D.M.S. Thinking About Super-Dominant Populations of Native Species—Examples from Brazil. Perspect. Ecol. Conserv. 2018, 16, 74–82. [Google Scholar] [CrossRef]
- Duan, M.; Bau, T. Grassland Fairy Rings of Leucocalocybe mongolica Represent the Center of a Rich Soil Microbial Community. Braz. J. Microbiol. 2021, 52, 1357–1369. [Google Scholar] [CrossRef] [PubMed]
- Marí, T.; Castaño, C.; Rodríguez, A.; Ibáñez, M.; Lobo, A.; Sebastià, M.-T. Fairy Rings Harbor Distinct Soil Fungal Communities and High Fungal Diversity in a Montane Grassland. Fungal Ecol. 2020, 47, 100962. [Google Scholar] [CrossRef]
- Zotti, M.; De Filippis, F.; Cesarano, G.; Ercolini, D.; Tesei, G.; Allegrezza, M.; Giannino, F.; Mazzoleni, S.; Bonanomi, G. One Ring to Rule Them All: An Ecosystem Engineer Fungus Fosters Plant and Microbial Diversity in a Mediterranean Grassland. New Phytol. 2020, 227, 884–898. [Google Scholar] [CrossRef]
- Zotti, M.; Bonanomi, G.; Mancinelli, G.; Barquero, M.; De Filippis, F.; Giannino, F.; Mazzoleni, S.; González-Andrés, F. Riding the Wave: Response of Bacterial and Fungal Microbiota Associated with the Spread of the Fairy Ring Fungus Calocybe gambosa. Appl. Soil Ecol. 2021, 163, 103963. [Google Scholar] [CrossRef]
- Gramss, G.; Voigt, K.D.; Bergmann, H. Factors Influencing Water Solubility and Plant Availability of Mineral Compounds in the Tripartite Fairy Rings of Marasmius Oreades (BOLT.: FR.) FR. J. Basic Microbiol. 2005, 45, 41–54. [Google Scholar] [CrossRef]
- Fidanza, M.A. Characterization of Soil Properties Associated with Type-I Fairy Ring Symptoms in Turfgrass. Sect. Bot. 2007, 625, 533–536. [Google Scholar] [CrossRef]
- Rillig, M.C. A Connection Between Fungal Hydrophobins and Soil Water Repellency? Pedobiologia 2005, 49, 395–399. [Google Scholar] [CrossRef]
- Rodríguez, A.; Ibáñez, M.; Bol, R.; Brüggemann, N.; Lobo, A.; Jimenez, J.J.; Ruess, L.; Sebastià, M.-T. Fairy Ring-Induced Soil Potassium Depletion Gradients Reshape Microbial Community Composition in a Montane Grassland. Eur. J. Soil Sci. 2022, 73, e13239. [Google Scholar] [CrossRef]
- Marí Marí, T. Changes in Soil Biodiversity and Activity Along Management and Climatic Gradients. Ph.D. Thesis, Universitat de Lleida, Lleida, Spain, 2017. [Google Scholar]
- Oh, S.-Y.; Fong, J.J.; Park, M.S.; Lim, Y.W. Distinctive Feature of Microbial Communities and Bacterial Functional Profiles in Tricholoma Matsutake Dominant Soil. PLoS ONE 2016, 11, e0168573. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Yoon, H.; You, Y.H.; Kim, Y.E.Y.J.; Woo, J.R.; Seo, Y.; Lee, G.M.; Kim, Y.E.Y.J.; Kong, W.S.; Kim, J.G. Metagenomic Analysis of Fungal Communities Inhabiting the Fairy Ring Zone of Tricholoma matsutake. J. Microbiol. Biotechnol. 2013, 23, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; U.S. Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- Herlemann, D.P.; Labrenz, M.; Jürgens, K.; Bertilsson, S.; Waniek, J.J.; Andersson, A.F. Transitions in Bacterial Communities Along the 2000 km Salinity Gradient of the Baltic Sea. ISME J. 2011, 5, 1571–1579. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast Length Adjustment of Short Reads to Improve Genome Assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pẽa, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME Improves Sensitivity and Speed of Chimera Detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a Chimera-Checked 16S rRNA Gene Database and Workbench Compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Good, I.J.; Toulmin, G.H. The Number of New Species, and the Increase in Population Coverage, When a Sample Is Increased. Biometrika 1956, 43, 45. [Google Scholar] [CrossRef]
- Rohlf, F.; Sokal, R. Statistical Tables; Macmillan: New York, NY, USA, 1995. [Google Scholar]
- Hill, M.O. Diversity and Evenness: A Unifying Notation and Its Consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and Extrapolation with Hill Numbers: A Framework for Sampling and Estimation in Species Diversity Studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Lenth, R. Emmeans Package: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.11.0-004. 2019. Available online: https://rvlenth.github.io/emmeans/authors.html (accessed on 19 September 2019).
- Janssen, P.H. Identifying the Dominant Soil Bacterial Taxa in Libraries of 16S rRNA and 16S rRNA Genes. Appl. Environ. Microbiol. 2006, 72, 1719–1728. [Google Scholar] [CrossRef] [PubMed]
- Huhe; Jiang, C.; Wu, Y.; Cheng, Y. Bacterial and Fungal Communities and Contribution of Physicochemical Factors During Cattle Farm Waste Composting. MicrobiologyOpen 2017, 6, e00518. [Google Scholar] [CrossRef]
- Kim, M.; Yoon, H.; Kim, Y.E.; Kim, Y.J.; Kong, W.S.; Kim, J.G. Comparative Analysis of Bacterial Diversity and Communities Inhabiting the Fairy Ring of Tricholoma matsutake by Barcoded Pyrosequencing. J. Appl. Microbiol. 2014, 117, 699–710. [Google Scholar] [CrossRef]
- Pan, Y.; Cassman, N.; de Hollander, M.; Mendes, L.W.; Korevaar, H.; Geerts, R.H.E.M.; van Veen, J.A.; Kuramae, E.E. Impact of Long-Term N, P, K, and NPK Fertilization on the Composition and Potential Functions of the Bacterial Community in Grassland Soil. FEMS Microbiol. Ecol. 2014, 90, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Kuramae, E.E.; Gamper, H.A.; Yergeau, E.; Piceno, Y.M.; Brodie, E.L.; DeSantis, T.Z.; Andersen, G.L.; van Veen, J.A.; Kowalchuk, G.A. Microbial Secondary Succession in a Chronosequence of Chalk Grasslands. ISME J. 2010, 4, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Lienhard, P.; Terrat, S.; Prévost-Bouré, N.C.; Nowak, V.; Régnier, T.; Sayphoummie, S.; Panyasiri, K.; Tivet, F.; Mathieu, O.; Levêque, J.; et al. Pyrosequencing Evidences the Impact of Cropping on Soil Bacterial and Fungal Diversity in Laos Tropical Grassland. Agron. Sustain. Dev. 2014, 34, 525–533. [Google Scholar] [CrossRef]
- Li, H.Y.; Yao, T.; Zhang, J.G.; Gao, Y.M.; Ma, Y.C.; Lu, X.W.; Zhang, H.R.; Yang, X.L. Relationship between soil bacterial community and environmental factors in the degraded alpine grassland of eastern Qilian Mountains, China. Ying Yong Sheng Tai Xue Bao 2018, 29, 3793–3801. [Google Scholar] [CrossRef]
- French, K.E.; Tkacz, A.; Turnbull, L.A. Conversion of Grassland to Arable Decreases Microbial Diversity and Alters Community Composition. Appl. Soil Ecol. 2017, 110, 43–52. [Google Scholar] [CrossRef]
- Govaerts, B.; Mezzalama, M.; Unno, Y.; Sayre, K.D.; Luna-Guido, M.; Vanherck, K.; Dendooven, L.; Deckers, J. Influence of Tillage, Residue Management, and Crop Rotation on Soil Microbial Biomass and Catabolic Diversity. Appl. Soil Ecol. 2007, 37, 18–30. [Google Scholar] [CrossRef]
- Scavino, A.F.; Ji, Y.; Pump, J.; Klose, M.; Claus, P.; Conrad, R. Structure and Function of the Methanogenic Microbial Communities in Uruguayan Soils Shifted Between Pasture and Irrigated Rice Fields. Environ. Microbiol 2013, 15, 2588–2602. [Google Scholar] [CrossRef] [PubMed]
- Grönemeyer, J.L.; Burbano, C.S.; Hurek, T.; Reinhold-Hurek, B. Isolation and Characterization of Root-Associated Bacteria from Agricultural Crops in the Kavango Region of Namibia. Plant Soil 2012, 356, 67–82. [Google Scholar] [CrossRef]
- Filippidou, S.; Wunderlin, T.; Junier, T.; Jeanneret, N.; Dorador, C.; Molina, V.; Johnson, D.R.; Junier, P. A Combination of Extreme Environmental Conditions Favor the Prevalence of Endospore-Forming Firmicutes. Front. Microbiol. 2016, 7, 1707. [Google Scholar] [CrossRef] [PubMed]
- Druce, R.G.; Thomas, S.B. An Ecological Study of the Psychrotrophic Bacteria of Soil, Water, Grass and Hay. J. Appl. Bacteriol. 1970, 33, 420–435. [Google Scholar] [CrossRef]
- Stackebrandt, E.; Rainey, F.A.; Ward-Rainey, N.L. Proposal for a New Hierarchic Classification System, Actinobacteria classis Nov. Int. J. Syst. Evol. Microbiol. 1997, 47, 479–491. [Google Scholar] [CrossRef]
- Zotti, M.; Bonanomi, G.; Mazzoleni, S. Fungal Fairy Rings: History, Ecology, Dynamics and Engineering Functions. IMA Fungus 2025, 16, e138320. [Google Scholar] [CrossRef]
- de Vries, F.T.; Manning, P.; Tallowin, J.R.B.; Mortimer, S.R.; Pilgrim, E.S.; Harrison, K.A.; Hobbs, P.J.; Quirk, H.; Shipley, B.; Cornelissen, J.H.C.; et al. Abiotic Drivers and Plant Traits Explain Landscape-Scale Patterns in Soil Microbial Communities. Ecol. Lett. 2012, 15, 1230–1239. [Google Scholar] [CrossRef]
- Kirk, G.J.d.; Bellamy, P.H.; Lark, R.M. Changes in Soil pH across England and Wales in Response to Decreased Acid Deposition. Glob. Change Biol. 2010, 16, 3111–3119. [Google Scholar] [CrossRef]
- Yang, Y.; Ashworth, A.J.; DeBruyn, J.M.; Willett, C.; Durso, L.M.; Cook, K.; Philip, A.; Moore, J.; Owens, P.R. Soil Bacterial Biodiversity Is Driven by Long-Term Pasture Management, Poultry Litter, and Cattle Manure Inputs. PeerJ 2019, 7, e7839. [Google Scholar] [CrossRef]
- Nagendra, H. Opposite Trends in Response for the Shannon and Simpson Indices of Landscape Diversity. Appl. Geogr. 2002, 22, 175–186. [Google Scholar] [CrossRef]
- Allison, S.D.; Martiny, J.B.H. Resistance, Resilience, and Redundancy in Microbial Communities. Proc. Natl. Acad. Sci. USA 2008, 105, 11512–11519. [Google Scholar] [CrossRef]
- Shade, A.; Peter, H.; Allison, S.; Baho, D.; Berga, M.; Buergmann, H.; Huber, D.; Langenheder, S.; Lennon, J.; Martiny, J.; et al. Fundamentals of Microbial Community Resistance and Resilience. Front. Microbiol. 2012, 3, 417. [Google Scholar] [CrossRef] [PubMed]
- Edwards, P.J. The Growth of Fairy Rings of Agaricus Arvensis and Their Effect upon Grassland Vegetation and Soil. J. Ecol. 1984, 72, 505–513. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an Ecological Classification of Soil Bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Leff, J.W.; Jones, S.E.; Prober, S.M.; Barberán, A.; Borer, E.T.; Firn, J.L.; Harpole, W.S.; Hobbie, S.E.; Hofmockel, K.S.; Knops, J.M.H.; et al. Consistent Responses of Soil Microbial Communities to Elevated Nutrient Inputs in Grasslands Across the Globe. Proc. Natl. Acad. Sci. USA 2015, 112, 10967–10972. [Google Scholar] [CrossRef] [PubMed]
- Fidanza, M.A.; Cisar, J.L.; Kostka, S.J.; Gregos, J.S.; Schlossberg, M.J.; Franklin, M. Preliminary Investigation of Soil Chemical and Physical Properties Associated with Type-I Fairy Ring Symptoms in Turfgrass. Hydrol. Process. 2007, 21, 2285–2290. [Google Scholar] [CrossRef]
- Lee, S.-M.; Kong, H.G.; Song, G.C.; Ryu, C.-M. Disruption of Firmicutes and Actinobacteria Abundance in Tomato Rhizosphere Causes the Incidence of Bacterial Wilt Disease. ISME J. 2021, 15, 330–347. [Google Scholar] [CrossRef]
- Artursson, V.; Finlay, R.D.; Jansson, J.K. Interactions between Arbuscular Mycorrhizal Fungi and Bacteria and Their Potential for Stimulating Plant Growth. Environ. Microbiol. 2006, 8, 1–10. [Google Scholar] [CrossRef]
- van Overbeek, L.; van Elsas, J.D. Adaptation of Bacteria to Soil Conditions: Applications of Molecular Physiology in Soil Microbiology. In Modern Soil Microbiology; Marcel Dekker: New York, NY, USA, 1997. [Google Scholar]
- Garbaye, J. Tansley Review No. 76 Helper Bacteria: A New Dimension to the Mycorrhizal Symbiosis. New Phytol. 1994, 128, 197–210. [Google Scholar] [CrossRef]
- Young, L.-S.; Chu, J.-N.; Young, C.-C. Beneficial Bacterial Strains on Agaricus blazei Cultivation. Pesqui. Agropecuária Bras. 2012, 47, 815–821. [Google Scholar] [CrossRef]
| Ring Zone | |||||
|---|---|---|---|---|---|
| Center | Ring13 | Ring15 | Front | Out | |
| pH | 7.8 ± 0.22 | 7.9 ± 0.18 | 7.9 ± 0.17 | 7.9 ± 0.17 | 8.0 ± 0.13 |
| Moisture (%) | 7.2 ± 0.92 | 6.6 ± 1.40 | 6.5 ± 1.80 | 6.7 ± 1.47 | 7.5 ± 1.30 |
| Tsoil (°C) | 19.6 ± 4.51 | 23.2 ± 3.58 | 20.9 ± 4.31 | 22.3 ± 5.29 | 20.9 ± 3.28 |
| Ca (mg·Kg−1) | 5933.3 ± 1028.91 | 5750 ± 1169.2 | 5150 ± 1602.2 | 5633.3 ± 1209.40 | 5733.3 ± 920.14 |
| P (mg·Kg−1) | 13 ± 3.16 | 20 ± 12.1 | 17.8 ± 10.30 | 20.5 ± 10.65 | 22.7 ± 5.99 |
| Mg (mg·Kg−1) | 205 ± 48.4 | 180 ± 36.9 | 176.7 ± 70.33 | 193.3 ± 56.45 | 183.3 ± 32.66 |
| K (mg·Kg−1) | 305 ± 102.1 | 280 ± 100.6 | 271.7 ± 167.50 | 296.7 ± 122.09 | 315 ± 105.9 |
| NO3− (ppm) | 0.16 ± 0.032 | 0.56 ± 0.478 | 0.23 ± 0.121 | 0.65 ± 0.826 | 0.8 ± 1.43 |
| NH4+ (ppm) | 9.9 ± 1.24 | 7.1 ± 3.77 | 9.0 ± 2.70 | 6.9 ± 4.52 | 8.0 ± 2.45 |
| Litter (g·cm−2) | 5.6 ± 4.00 | 6.1 ± 1.07 | 5.5 ± 2.00 | 4.3 ± 2.76 | 5.6 ± 3.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marí, T.; Manjón-Cabeza, J.; Rodríguez, A.; San Emeterio, L.; Ibáñez, M.; Sebastià, M.-T. Changes in the Soil Bacterial Community Across Fairy Rings in Grasslands Using Environmental DNA Metabarcoding. Diversity 2025, 17, 322. https://doi.org/10.3390/d17050322
Marí T, Manjón-Cabeza J, Rodríguez A, San Emeterio L, Ibáñez M, Sebastià M-T. Changes in the Soil Bacterial Community Across Fairy Rings in Grasslands Using Environmental DNA Metabarcoding. Diversity. 2025; 17(5):322. https://doi.org/10.3390/d17050322
Chicago/Turabian StyleMarí, Teresa, José Manjón-Cabeza, Antonio Rodríguez, Leticia San Emeterio, Mercedes Ibáñez, and M.-Teresa Sebastià. 2025. "Changes in the Soil Bacterial Community Across Fairy Rings in Grasslands Using Environmental DNA Metabarcoding" Diversity 17, no. 5: 322. https://doi.org/10.3390/d17050322
APA StyleMarí, T., Manjón-Cabeza, J., Rodríguez, A., San Emeterio, L., Ibáñez, M., & Sebastià, M.-T. (2025). Changes in the Soil Bacterial Community Across Fairy Rings in Grasslands Using Environmental DNA Metabarcoding. Diversity, 17(5), 322. https://doi.org/10.3390/d17050322






