Abstract
In order to establish the influence of grazing regime on soil fauna communities, a complex study was conducted on eight mountain grasslands in Romania. The grassland sites were grouped by management regime: ungrazed or intensely grazed by sheep. Eight environmental factors were measured, both abiotic (soil acidity, soil resistance at penetration, soil and air humidity, soil and air temperature, and soil electrical conductivity) and biotic (vegetation coverage). There was significant variability in the average values of these factors at the microhabitat level (between all grasslands investigated). Analysis of eighty soil samples allowed for the identification of sixteen soil fauna taxa, which constituted the database for statistical processing. The community status of these soil invertebrate faunas was mainly evaluated using three parameters: numerical abundance, taxa richness, and Shannon–Wiener index of diversity. Collembola and Oribatida were the most dominant taxa. The numerical abundance and taxa diversity recorded high values in ungrazed grasslands. Soil resistance at penetration, vegetation coverage, and soil pH influenced the numerical abundance of soil fauna communities significantly. Grassland management influenced the composition of soil invertebrates in both regimes, with Chilopoda, Staphylinidae, Diplopoda, and Enchytraeidae clearly preferring ungrazed ecosystems, whilst Mesostigmata was much commoner in grazed sites. The study revealed that correlations between the species composition of soil communities and environmental parameters under differing management regimes (ungrazed vs. grazed), demonstrated that these invertebrates can be used as bioindicators in such terrestrial ecosystems.
1. Introduction
Understanding the relationship between soil bioindicators and ecosystem services has become a growing trend in soil science, with increased visibility to the public, stakeholders, and the political environment. The first step in examining this relationship is to establish the soil bioindicators; their structural and functional characteristics; and their correlation to those environmental parameters that are specific for a certain type of terrestrial ecosystem, such as grasslands. From an ecological point of view, grassland is one of the most heavily exploited ecosystems in Europe, providing important ecosystem services, such as (a) provision of forage for livestock; (b) habitat for wildlife and overall biodiversity; (c) contribution to the attractiveness and aesthetic value of the landscape; (d) pest control; and (e) conservation of soil and water resources [1,2]. Maintaining these services depends on developing a balanced human–nature system which is based on biodiversity protection. Research has established a simple scheme that should be followed for grassland biodiversity conservation, maintaining its stability, continuity, heterogeneity, and diversity. All of these features depend upon the genetic, species, and habitat diversity of such grasslands. A further objective for grassland protection is the maintenance of species dispersal and coexistence [3].
According to Article 17 of the EU Habitats Directive (92/43/EEC), the main pressures on grasslands requiring reporting are habitat management, habitat conversion, pollution, and natural processes [4]. The most frequently reported pressure is habitat management, which includes mowing, overgrazing, undergrazing, or even the abandonment of grasslands. Most pastures have been converted into cropland, causing overgrazing in the remaining grasslands. Intensive land use has led to the homogenization of ecological communities, with an increase in generalist species and a decrease in habitat specialists [2,3,5,6]. Overgrazing, in particular, is a major threat to grasslands, especially in Eastern Europe, causing biodiversity decline [7,8].
Soil faunas are an important link to biodiversity and provide a huge reservoir of information that has been little investigated scientifically. In terms of ecosystem services, soil faunas are a component that contributes to provisioning, supporting, and regulating services. Through grazing on microbial communities, organic matter transformation, and translocation, soil biotas are important participants in the processes of soil formation and nutrient cycling [9,10]. Other research stated that soil ecological status can be evaluated using some indicators of its quality, such as chemical, physical, and biological attributes, e.g., soil fauna [11]. Soil faunas are an important indicator of the biological quality of edaphic habitats, and they are very sensitive to any environmental stress [12,13,14,15,16,17]. However, which are the structural parameters that have the best quality as bioindicators? The answer was given by many studies from all over the world: abundance, richness, diversity of animal taxa, and the specific community structures could indicate a level of faunal maturity [12,13,16,17,18,19,20,21]. The most commonly used quantitative metric of invertebrate community is its species richness (i.e., number of species), but more complex measurements of species heterogeneity (i.e., species evenness or the Shannon index) are often used, especially in Western Europe, sometimes revealing different trends in their ecology [22].
Another bioindicator tool is the establishment of correlations between environmental variables to characterize both ecosystem disturbance and the population parameters of soil taxa [13,15,16,18,20,23,24,25,26]. International studies have demonstrated that grazing regime directly influences soil faunal structure and indirectly functional processes of soils (as soil structure, the decomposition of soil organic matter, and nutrient cycling) [17,23,24]. On the other hand, from a taxonomical point of view, species-level identifications take time and human resources. Using higher taxa to predict species richness and community composition constitutes a solution. Different studies have shown that the performance of higher taxa as surrogates for species is variable, being influenced by stochastic factors [15,26,27,28].
In Romania, studies on the impact of grazing intensity on soil fauna are few. A majority of the papers which address the faunistic and ecological aspects of soil fauna are focused on particular taxonomic groups, e.g., mites, nematodes, enchytraeids, millipedes, etc. [16,29,30,31]. Only a few studies investigated soil fauna as a complex biological system in grassland [15,32,33,34,35,36,37].
In the light of all of these investigations, the present study of Romanian grasslands hypothesized that the grazing regime influences the local environmental factors and soil fauna communities. In order to clarify this hypothesis, three objectives were established:
- To analyze the structure of soil fauna communities from two grassland types (ungrazed vs. intense grazed);
- To measure environmental variables that are characteristic of the investigated ecosystems;
- To establish any correlations between the type of grassland management, environmental variables, and characteristic structures of soil fauna communities.
We believe that the present study will collect actual and original data on the ecology of edaphic fauna from a European country where studies in this field are very few, especially due to the lack of specialists and the fact that such investigations are time-consuming.
2. Material and Methods
2.1. Study Area
Our study was located in the Făgăraş Mountains. They are the highest mountains in Romania (with Moldoveanu peak reaching 2544 m) and are part of the Meridional Carpathians. Among Romanian mountain ranges, they represent the most extensive block of alpine habitat, extending over an area of 2400 km2. Our research was conducted in August 2018 in four grasslands with ungrazed and sheep-grazed sections: Cocorâciu, Galbena, Sterminoasa, and Vemeşoaia. The grasslands were located at altitudes between 1710 and 1910 m, with slopes between 5° and 20°, and southerly or westerly exposures. The dominant plant species were Agrostis capillaris L., Festuca rubra L., Deschampsia cespitosa (L.) P. Beauv., Alchemilla glabra Neygenf. (incl. A. boleslai Pawl.), Achillea distans subsp. stricta Janch., Nardus stricta L., Plantago gentianoides Sm., Poa annua L., Poa media Schur, Veratrum album L., Trifolium pratense L., Trifolium repens L., and Vaccinium myrtillus L. (Supplementary Table S1). The geographical localization of investigated ecosystems is presented in Figure 1 [16]. Focusing on vegetation, the dominant plant species from ungrazed grasslands were Agrostis capillaris L., Deschampsia cespitosa (L.) P. Beauv., Festuca rubra L., Achillea distans subsp. stricta Janch., Veratrum album L., Poa media (L.) Cav., Alchemilla glabra Neygenf. (incl. A. boleslai Pawl.), Nardus stricta L., Plantago gentianoides Sm., Poa annua L., and Trifolium repens L. On grazed grasslands, the dominant plant species were Agrostis capillaris L., Deschampsia cespitosa (L.) P. Beauv., Festuca rubra L., Nardus stricta L., Poa media (L.), and Trifolium pratense L. A detailed description of vegetation was made by [38], with the classification of soil made after [39].

Figure 1.
Geographical characterization of ungrazed and grazed grasslands in the Făgăraş Mountains, Romania, 2018. Map created using ArcGIS software by Esri. (ArcGIS and ArcMap are the intellectual property of Esri and are used herein under license. Version number: 10.4.0554. Copyright Esri. All rights reserved. For more information about Esri software, please visit www.esri.com (accessed on 27 March 2023). Base-map service layer credits: Esri, HERE, DeLorme, Intermap, increment P Corp., GEBCO, USGS, FAO, NPS, NRCAN, GeoBase, IGN, Kadaster NL, Ordnance Survey, Esri Japan, METI, Esri China, swisstopo, Map-myIndia, OpenStreetMap contributors, and the GIS.)
2.2. Soil Fauna Samples
In order to reflect any environmental heterogeneity, the edaphic fauna samples were collected randomly. Soil fauna samples were collected in a single campaign, from 28 July to 3 August 2018. A MacFadyen soil core was used for fauna samples, with a length of 10 cm and a diameter of 5 cm. Eighty samples from five grasslands were collected in total (10 samples/grassland). For soil fauna extraction, we used the Berlese–Tullgren method, with natural light and heat [40]; the extraction lasted 10–14 days. Identification and counting of taxa were undertaken using a Zeiss stereomicroscope and published identification keys [14,41,42,43,44]. All specimens were analyzed at the higher taxonomic level (orders and families), and classification of soil fauna was made while taking into account their ecological and trophic requirements [14,45,46].
2.3. Environmental Variables
Eight environmental variables were investigated, measured from 80 soil samples, i.e., (1) air temperature, Tair (°C), measured at 5 cm above the soil level; (2) air relative humidity, Rhair (%); (3) soil temperature, Tsoil (°C); (4) soil moisture content, Rh soil (%); (5) soil electrical conductivity, CE (μS/cm); (6) soil acidity, pH; (7) soil penetration resistance, RP (MPa); and (8) vegetation coverage, VegCov (%). For these parameters, three types of measuring equipment were used: (a) C532 Jasco Consort pH-meter (for the pH and CE); (b) digital thermo-hygrometer PCE-310 (for soil moisture content, soil temperature air relative humidity, and air temperature); and (c) soil penetrometer, Step System GmbH, 41,010 (for soil resistance at penetration) [15,16,26]. The environmental parameters were measured for each soil sample at the same time as the collection of soil fauna. The average values of each environmental variable (with standard error) from ungrazed and grazed grasslands are presented in Table 1 and Table 2.

Table 1.
Abiotic parameters measured in ungrazed grasslands (average value ± standard error; p = significance level).

Table 2.
Abiotic parameters measured in intensely grazed grasslands (average value ± standard error; p = significance level).
2.4. Data Analysis
All statistical analyses were conducted in R (version 4.0.3), R Foundation for Statistical Computing, Vienna, Austria. Available online: http://www.R-project.org/ (accessed 10 December 2024) [47]. Data visualizations were created using the ggplot2 package [48]. To evaluate the completeness of the faunal survey and the representation of functional groups across grazed and ungrazed grasslands, we constructed taxon accumulation curves. Diversity patterns were initially assessed by plotting abundance distribution curves for functional groups. Subsequently, we applied generalized linear mixed-effects models (GLMMs) combined with analysis of variance (ANOVA) to explore how grassland management and environmental factors influenced species abundance, richness, and the Shannon–Wiener diversity index (H′). When modeling abundance and richness, we specified a Poisson distribution with a log link, whereas H′ was modeled using a Gaussian distribution with an identity link. To account for the paired sampling framework and reduce the risk of pseudoreplication, grassland identity was included as a random effect. Explanatory variables were first screened for multicollinearity by calculating variance inflation factors (VIFs) with the corvif function [49]. Variables with VIF values exceeding 3, notably air temperature (Tair) and relative humidity (Rh air), were excluded from the final models. The BiodiversityR package [50] was employed to produce the accumulation and distribution curves, while the GLMMs were fitted using the glmer function from the lme4 package [51]. Continuous predictors were centered and scaled using the scale function from R’s base package [52] to mitigate convergence issues. The effects package [53] was used to visualize the predicted influence of environmental factors on abundance, richness, and H′. To assess whether functional group composition was more homogeneously distributed within grazed or ungrazed grasslands, we applied the betadisper test. Differences in composition between the two grassland types were tested using PERMANOVA (Adonis function). We also used non-metric multidimensional scaling (NMDS) based on Bray–Curtis dissimilarities to visualize compositional differences. Finally, redundancy analysis (RDA) was conducted to determine the relationship between environmental variables and functional group composition. All composition-based analyses were carried out using the vegan package [54].
3. Results
We found that taxon accumulation curve of ungrazed grasslands approached an asymptote (Figure 2). However, an asymptote was not reached for grazed grasslands, indicating that, from a theoretical point of view, a higher number of samples are required to detect all soil fauna groups. In total, 16 soil invertebrate groups were identified within the soil fauna, with 2863 individuals. Comparing ungrazed and grazed ecosystems, we recorded the same number of taxa (14) in both types, but with different community compositions (Supplementary Table S2). The abundance distributions of soil invertebrates indicate variation in taxon numbers between grazed and ungrazed grasslands (Figure 2). The number of individuals was greater in ungrazed grasslands (1615) than in grazed ecosystems (1248). The most dominant soil taxa for both types of grassland were Collembola and Oribatida. For the ungrazed ecosystems, Chilopoda, Diplopoda, and Coleoptera–Staphylinidae were numerically dominant, whilst for grazed grasslands, Mesostigmata and Insect larvae were most abundant (Figure 3; Supplementary Table S2).
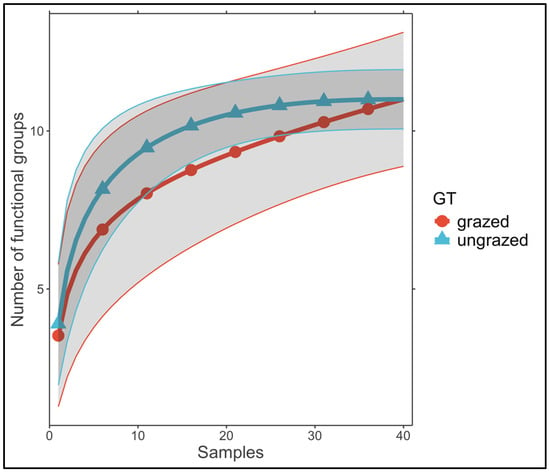
Figure 2.
Accumulation curves for comparing taxon richness in the two grassland types (GTs): grazed and ungrazed. The shaded area represents the 95% confidence intervals.
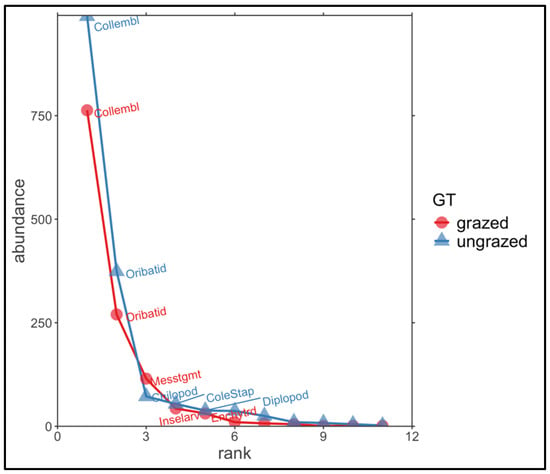
Figure 3.
Abundance distributions for the two different grassland types (GTs), i.e., intensely grazed and ungrazed.
Analysis of the abiotic factors for the four ungrazed grasslands showed significant local variability, especially at Tair, R air, Tsoil, Rh soil, Rp, and CE (Table 1). An almost-identical pattern was obtained for the environmental parameters in intensely grazed ecosystems, but with a small modification: pH and RP differed between the areas studied (Table 2).
Abundance significantly increased with VegCov, RP, and pH (Table 3 and Figure 4A–C). None of the environmental variables significantly affected richness and Shannon–Wiener diversity index, H′ (Table 3).

Table 3.
Results of the analysis of variance (ANOVA) based on generalized linear mixed-effects models (GLMMs), which were used to evaluate the influence of grassland type and environmental factors on soil taxon abundance, richness, and the Shannon–Wiener diversity index (H′). Statistically significant findings are shown in bold. Abbreviations: Df—degrees of freedom; χ2—chi-square statistic; p-value for significance. T soil—soil temperature (°C); Rh soil—soil moisture content (%); RP—soil penetration resistance (Map); pH—soil acidity; CE-soil electrical conductivity; VegCov—Vegetation cover (%); GT—grassland type.
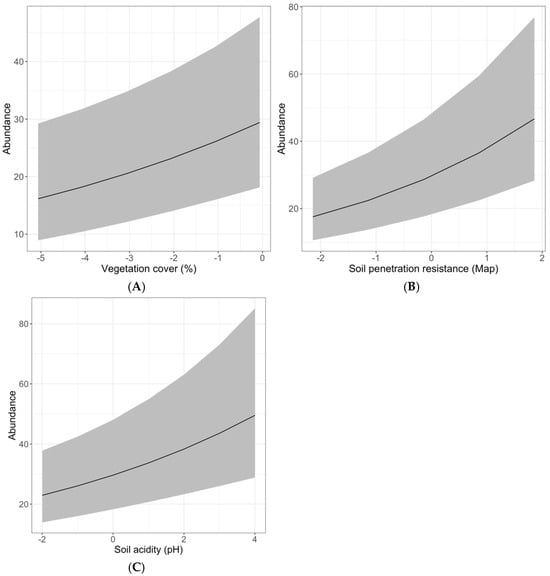
Figure 4.
Effect of vegetation cover (A), soil penetration resistance (B), and soil acidity (C) on abundance based on values predicted from generalized linear mixed models fitted assuming a Poisson distribution for the residuals and a log-link function.
The betadisper test showed that the dispersion of the two types of grasslands was not significantly different (p = 0.459) (Figure 5). Non-metric multidimensional scaling showed distinct soil fauna compositions between intensely grazed and ungrazed grasslands (Figure 6). This result was also supported by the Adonis test, indicating that the β-diversity of Bray–Curtis’s distance between grazed and ungrazed grasslands was significant (F = 12.1654, R2 = 0.139, p = 0.001). Soil taxa such as Chilopoda, Coleoptera–Staphylinidae, Diplopoda, and Enchytraeidae were strongly associated with ungrazed grasslands. Mesostigmata mites and insect larvae were strongly associated with grazed ecosystems.
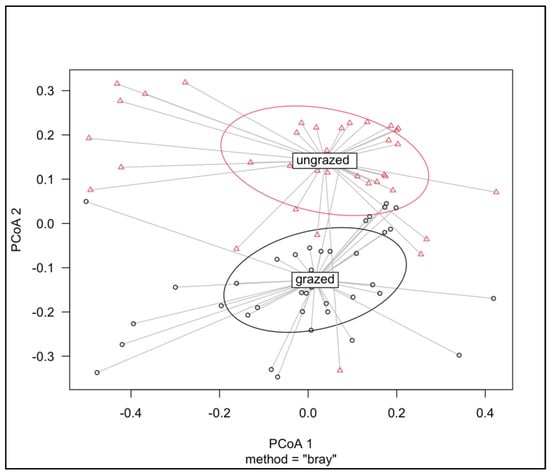
Figure 5.
Results of principal coordinate analysis (PCoA) based on Bray–Curtis dissimilarity, illustrating patterns of community structure (β-diversity) across the two grassland types. Distinct colors and shapes are used to distinguish grazed (red triangles) from ungrazed sites (black circles). Adonis analysis was used to test the significance between groups (i.e., grazed and ungrazed).
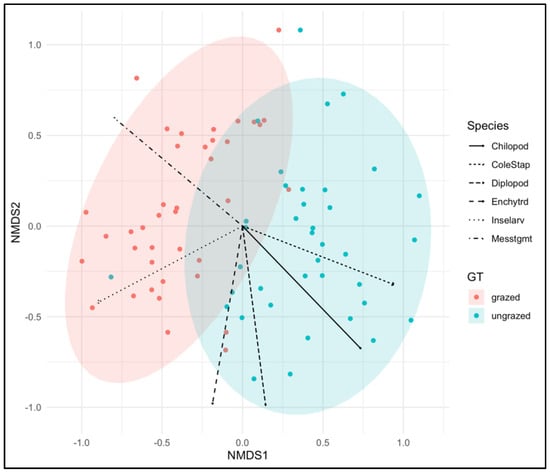
Figure 6.
Results of non-metric multidimensional scaling (NMDS) using Bray–Curtis distances to visualize differences in soil fauna composition between grazed and ungrazed grasslands. Ellipses represent 95% confidence intervals for each grassland type. Soil taxa were passively fitted onto the NMDS ordination and are shown as vectors. The stress value of the NMDS was 0.228.
The adjusted R2 value from the RDA model was 0.31, indicating that 31% of the variation in community structure across the grazed and ungrazed grasslands could be attributed to the measured environmental variables. Among these factors, Tair and Rh air were the primary drivers of variation, with VegCov, Rh soil, and RP also contributing notably (Figure 7).
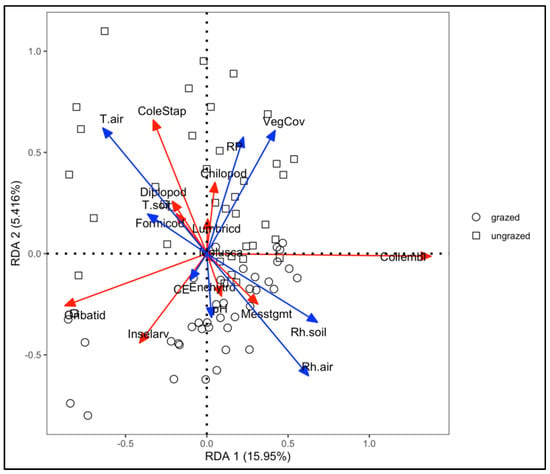
Figure 7.
Redundancy analysis of the environmental variables on the composition of soil fauna. Blue arrows refer to environmental variables, red solid lines refer to soil taxa, and open squares and circles refer to sampling points.
Tair was positively corelated with Coleoptera–Staphylinidae and negatively correlated with Oribatida and insect larvae. A positive relationship was found between both Rh air and Rh soil and Collembola.
4. Discussion
In the two types of mountain grassland (ungrazed and grazed), 16 soil fauna groups were identified, with a few dominant taxa (Collembola, Oribatida, Mesostigmata, Enchytraeidae, and insect larvae). The dominance and equitability indices showed small differences between ungrazed and intensely grazed grasslands. Higher dominance (but lower equitability and evenness) was obtained in ungrazed grasslands due to the greater representation of Collembola and Oribatida. In terms of specific soil invertebrate taxa, Isopoda were characteristic of ungrazed grasslands, whilst Coleoptera–Staphylinidae and Gastropoda (Mollusca) was typical of grazed types.
From these results, two questions arise: (a) Does the number of invertebrate groups show significant patterns in response to grassland management? (b) How do these results compare with similar studies elsewhere in Romania and Europe? In Romania, three ecological studies are relevant: (a) pastures from the Ponto-Sarmatic steppes revealed the presence of 24 soil fauna groups; (b) within mountain grasslands treated with inorganic and organic fertilizers, 17 invertebrate groups were identified; and (c) results from meadows in the Southern Carpathians showed 13 taxa [15,16,26,36,37,55]. In these studies, Collembola and mites (especially Oribatida) were the dominant taxa, with greatest numbers in grasslands found in the flood zone (totals double those in unflooded areas), and characterized by high floristic diversity. The researchers found that the quantitative and qualitative features depend to a great extent on the particular biopedoclimatic conditions of a site (especially soil moisture content, pH, and salinity). These patterns were influenced by the degree of environmental anthropization, such as livestock grazing [32,33,34,35].
A detailed literature review of studies in Europe found 368 published articles presenting research focused on “biodiversity indicator species groups” in permanent and rotational grasslands [22]. From 1984 to 2022, the number of studies focused on this topic increased exponentially, especially in Western Europe (United Kingdom, Germany, France, Spain, Sweden, Switzerland, Poland, Norway, and Ireland), which provided 64% of the studies. This review showed that soil fauna and ground-dwelling arthropods were especially powerful as biodiversity indicator species groups (ISGs) at the edaphic level. A wide spectrum of soil fauna (12 taxa) was identified in European grassland ecosystems, including annelids, springtails, nematodes, mites, slugs, snails, beetles (carabids and staphylinids), spiders, ants, millipedes, and centipedes. In all of these studies, mites and springtails were often studied together and were numerically dominant [56,57]. The effects of agri-environment schemes were tested on belowground soil invertebrate biodiversity in 90 agricultural grassland fields in Northern Ireland [58]. They identified 13 taxa of soil fauna, with 992 individuals and different percentages representation: Collembola represents 39% of all invertebrates, 18% is Enchytraeidae, 13% is Nematoda, 11% is Oribatida, 9% is Mesostigmata, 6% is other insects, and 2% is Prostigmata.
The results of our study are quite similar to those obtained in other highland grasslands in the southern part of Romania, and elsewhere in Europe, with the same dominance of Collembola and Oribatida, and shaped by the varied environmental parameters. The numerical abundance of the soil fauna groups that were investigated was significantly influenced by the soil resistance at penetration/compactation, soil pH, and vegetation coverage. In ungrazed ecosystems, soil resistance upon penetration is a little higher, possibly due to the greater vegetation coverage. A higher resistance to soil penetration means a higher bulk density and lower soil moisture content, and it represents a physical constraint to the volume of soil explored by the roots, hindering water and nutrient uptake [20,59]. Although some studies revealed that grazing generally slightly increases soil penetration resistance, in the ecosystems that we investigated, the opposite trend was revealed, possibly due to average soil moisture content being lower in the grazed grasslands that we studied.
However, intense grazing not only influences soil penetration resistance but also the entire local microclimatic conditions, with higher average values of both air humidity (due to the lack of aboveground biomass) and soil moisture content. Grazing is one of the controlling factors of the local microclimate. It is necessary also to consider the heterogeneity of the grasslands, which are affected by intensive grazing, leading to deterioration of the specific microhabitats [3]. A more detailed investigation of the environmental parameters was presented in [16].
According to our study, the common dominant species in both types of grassland were Collembola and Oribatida, representing 61.22% and 21.90% of all soil taxa identified. The type of grassland management influenced the composition of soil invertebrates’ communities in grazed and ungrazed grasslands. Intensively grazed areas have a reduced representation of soil fauna. Other studies have also demonstrated that different land-use intensities can change the numerical structure of specific taxa [15,21,22,57]. In our analysis, we observed positive relationships in grazed grasslands between Collembola communities and soil moisture content or air humidity. Development of Oribatida communities was favored by the decreased air temperature from the same type of ecosystems. Similar results were obtained by other researchers, observing that soil moisture content is the main abiotic parameter which influences the Collembola community. At the same time, these studies revealed that the climate change will further strongly modify the Collembola community structure [60]. The extensive pastures characterized by humid soils and by native vegetation were preferred by Oribatid mites. In contrast, more intensively managed, drier pastures, reseeded with Lolium perenne L., were preferred by Collembola communities [58].
In the grassland ecosystems of the Făgărăş Mountains, Enchytraeidae was poorly represented numerically, in comparison with the study from Northern Ireland, where these annelids and Nematoda were more representative. The Romanian grasslands investigated by our study are situated in a mountain area, at over 1700 m altitude, on steep slopes (often attaining a gradient of 20°), with a soil layer that is very thin and no more than 40 cm [38]. These topographical features, together with the recent climatic warming (as much as 31.24 °C in the soil when we collected samples), do not allow the soil to retain much water, and a high moisture content represents optimal conditions for enchytraeid and nematode survival [61]. Such numerical dominance of Collembola and Oribatida was also observed in grasslands treated with inorganic and organic fertilizers from another region of Romania, the Bucegi Mountains [15].
Statistical analysis demonstrated that Chilopoda, Coleoptera–Staphylinidae, and Diplopoda preferred ungrazed grasslands. Chilopoda are predominantly predators feeding on other soil invertebrates, while Diplopoda (millipedes) are detritivores, feeding on dead plant material and fungi [62]. Vegetation structure influenced the millipede abundance and composition [62]. It is probable that the greater biomass found in ungrazed ecosystems produces a higher quantity of organic matter and carbon content, and hence a favorable environment for soil fauna, especially detritivores. A high value of soil electrical conductivity found in ungrazed ecosystems determined an increased availability of water-soluble salts for plants. This high availability leads to development of high plant biomass [15]. Temperate grasslands typically have a thick A horizon, in which humus provides a habitat for other soil fauna that contribute to litter decomposition [63]. Factors such as plant aboveground biomass, soil pH, carbon concentration, soil moisture, and bulk density were the most important determinants for soil fauna in ungrazed grasslands [57]. Seeber et al. (2005) discovered that Diplopoda were among the most abundant soil taxa from abandoned pastures in Austria [64].
Mesostigmata mites and insect larvae preferred grazed grasslands. Mesostigmata are mainly represented by predatory mites, free living and mobile species [9]. Intensively grazed pastures are covered with a great quantity of dung, which can constitute a trophic source for these invertebrates. Species richness, abundance, and diversity of beetles and flies (and their larvae) are correlated with soil moisture, dung availability, and altitude, but not with soil hardness or land-use heterogeneity [65]. Other studies have shown that insect larvae are also found in managed (mown and fertilized) grasslands [15,64]. Our study on the effect of grazing management on predator edaphic mites in the same ecosystem revealed the numerical dominance of mesostigmatid species Alliphis halleri (G. & R. Canestrini, 1881), in intensely grazed grasslands [16]. The numerical dominance of this species led to the high total abundance of Mesostigmata mites in the grazed grasslands of the Făgăraş Mountains.
As a generalization, anthropogenic pressures influence the life traits of invertebrates, with r-strategists becoming dominant [66]. Testing the proposed hypothesis of our study, we established that grazing regime influenced local environmental factors and determined the distinct compositions of soil taxa, confirming their value as bioindicators of ecosystem management and health.
5. Conclusions
A scientific study in Romania, conducted across eight grasslands, demonstrated that soil fauna taxa could be precious bioindicators for two types of grazing management. The investigated environmental parameters recorded specific characteristics for each investigated type of grassland. Grazing regime influenced the composition of the communities, and was correlated with certain environmental parameters (especially soil acidity and resistance to penetration, as well as vegetation coverage). Using diversity metrics on sixteen soil fauna taxa, we discovered that the studied grasslands were dominated by Collembola and Oribatida, the main decomposers engineering in soil. Comparing the two types of ecosystems, the authors demonstrated that ungrazed grasslands were characterized by Chilopoda, Staphylinidae, Diplopoda, and Enchytraeidae, while grazed grasslands favored Mesostigmata and insect larvae development. Grassland management influenced the composition of soil invertebrates in both regimes, revealing specific patterns. The dominance of specific soil fauna groups in anthropogenically disturbed grasslands (e.g., intense grazing) reflects the ecological traits of species with wide ecological tolerance capable of adapting to challenging conditions. These findings open avenues for future research on the ecological adaptations and traits of soil fauna under varying grassland management regimes.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/d17050323/s1, Table S1: Detailed description of the investigated grassland ecosystems in Romania. Table S2: Numerical abundance and other population parameters of the soil fauna taxa investigated from the ungrazed (un) and grazed (g) grasslands in Romania (V= Vemeşoaia; S= Sterminoasa; C= Cocorâciu; G= Galbena).
Author Contributions
Conceptualization, M.M. and M.O.; data curation, M.M., R.I.B. and M.O.; formal analysis, M.M., R.I.B. and M.O.; funding acquisition, M.O.; investigation, M.M. and M.O.; methodology, M.M., R.I.B. and M.O.; project administration, M.M. and M.O.; resources, M.M. and M.O.; software, R.I.B.; supervision, M.M.; validation, M.M., R.I.B. and M.O.; visualization, M.M., R.I.B., and M.O.; writing—original draft, M.M., R.I.B. and M.O.; writing—review and editing, M.M., R.I.B. and M.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no funding, but the work was supported in the frame of the project number RO1567-IBB01/2025, Institute of Biology, Bucharest, Romanian Academy.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article or Supplementary Materials.
Acknowledgments
We thank Lazăr Dumitru, Rodica Iosif, and Simona Plumb, for their assistance in the lab and the field. We also thank J. Owen Mountford (UKCEH) for English checking.
Conflicts of Interest
The authors have no competing interests to declare that are relevant to the content of this article.
References
- Carlier, L.; Rotar, I.; Vlahova, M.; Vidican, R. Importance and Functions of Grasslands. Not. Bot. Horti Agrobot. Cluj-Napoca 2009, 37, 25–30. [Google Scholar]
- Kachler, J.; Benra, F.; Bolliger, R.; Isaac, R.; Bonn, A.; Felipe-Lucia, M.R. Can we have it all? The role of grassland conservation in supporting forage production and plant diversity. Landsc. Ecol. 2023, 38, 4451–4465. [Google Scholar] [CrossRef]
- Pärtel, M.; Bruun, H.H.; Sammul, M. Biodiversity in temperate European grasslands: Origin and conservation. Grassl. Sci. Eur. 2005, 10, 1–14. [Google Scholar]
- European Commision. Pressure Reported by Member States Under Article 17 Reporting (Period 2013–2018). Conservation Status of Habitat Types and Species: Datasets from Article 17, Habitats Directive 92/43/EEC Reporting. 2020. Available online: https://www.eea.europa.eu/data-and-maps/data/article-17-database-habitats-directive-92-43-eec-2/article-17-2020-dataset (accessed on 20 December 2024).
- Habel, J.C.; Dengler, J.; Janišová, M.; Török, P.; Wellstein, C.; Wiezik, M. European grassland ecosystems:threatened hotspots of biodiversity. Biodiv. Conserv. 2013, 22, 2131–2138. [Google Scholar] [CrossRef]
- Tavşanoğlu, Ç.; Bernardi, R. Old-growth grasslands of Central Anatolia (Türkiye) require better conservation and management. Environ. Conserv. 2024, 51, 242–244. [Google Scholar] [CrossRef]
- Nita, A.; Hartel, T.; Manolache, S.; Ciocanea, C.M.; Miu, I.V.; Rozylowicz, L. Who is researching biodiversity hotspots in Eastern Europe? A case study on the grasslands in Romania. PLoS ONE 2019, 14, e0217638. [Google Scholar] [CrossRef]
- Török, P.; Dembicz, I.; Dajic-Stevanovic, Z.; Kuzemko, A. Grasslands of Eastern Europe. Encyclopedia of World’s Biomes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 703–713. [Google Scholar]
- Potapov, A.M.; Beaulieu, F.; Birkhofer, K.; Bluhm, S.L.; Degtyarev, M.I.; Devetter, M.; Goncharov, A.A.; Klarner, B.; Korobushkin, D.I.; Liebke, D.F.; et al. Feeding habits and multifunctional classification of soil-associated consumers from protists to vertebrates. Biol. Rev. 2022, 97, 1057–1117. [Google Scholar] [CrossRef] [PubMed]
- Angst, G.; Potapov, A.; Joly, F.X.; Angst, S.; Frouz, J.; Ganault, P.; Eisenhauer, N. Conceptualizing soil fauna effects on labile and stabilized soil organic matter. Nat. Commun. 2024, 15, 5005. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; de Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P.; et al. Soil quality—A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Cole, L.; Mark, A.; Bradford, M.A.; Shaw, P.J.A.; Bardgett, R.D. The abundance, richness and functional role of soil meso- and macrofauna in temperate grassland—A case study. Appl. Soil Ecol. 2006, 33, 186–198. [Google Scholar] [CrossRef]
- Menta, C. Soil Fauna Diversity—Function, Soil Degradation, Biological Indices, Soil Restoration. In Biodiversity Conservation and Utilization in a Diverse World; Lameed, G.A., Ed.; IntechOpen: London, UK, 2012; pp. 59–94. [Google Scholar]
- Coleman, D.C.; Wall, D.H. Soil Fauna: Occurrence, Biodiversity, and Roles in Ecosystem Function. In Soil Microbiology, Ecology and Biochemistry, 4th ed.; Eldor, A.P., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 1–41. [Google Scholar]
- Manu, M.; Băncilă, R.I.; Mountford, O.J.; Onete, M. Soil Invertebrate Communities as Indicator of Ecological Conservation Status of Some Fertilised Grasslands from Romania. Diversity 2022, 14, 1031. [Google Scholar] [CrossRef]
- Manu, M.; Băncilă, R.I.; Onete, M. Effect of Grazing Management on Predator Soil Mite Communities (Acari: Mesotigmata) in Some Subalpine Grasslands from the Făgăraş Mountains—Romania. Insects 2023, 14, 626. [Google Scholar] [CrossRef]
- Fusco, T.; Fortini, L.; Casale, F.; Di Giulio, A. Assessing soil quality of Italian Western Alps protected areas by QBS-ar: Impact of management and habitat type on soil microarthropods. Environ. Monit. Assess. 2023, 195, 1287. [Google Scholar] [CrossRef] [PubMed]
- Menta, C.; Leoni, A.; Gardi, C.; Conti, F.D. Are grasslands important habitats for soil microarthropod conservation? Biodivers. Conserv. 2011, 20, 1073–1087. [Google Scholar] [CrossRef]
- Menta, C.; Conti, F.D.; Pinto, S. Microarthropods biodiversity in natural, seminatural and cultivated soils—QBS-ar approach. Appl. Soil Ecol. 2017, 123, 740–743. [Google Scholar] [CrossRef]
- Potapov, A.M.; Goncharov, A.A.; Semenina, E.E.; Korotkevich, A.Y.; Tsurikov, S.M.; Rozanova, O.L.; Anichkin, A.E.; Zuev, A.G.; Samoylova, E.S.; Semenyuk, I.I.; et al. Arthropods in the subsoil: Abundance and vertical distribution as related to soil organic matter, microbial biomass and plant roots. Eur. J. Soil Biol. 2017, 82, 88–97. [Google Scholar] [CrossRef]
- Mioulet, C.; Schrama, M.; Berg, M.P.; Hannula, S.E. Comparison of metrics to reveal the role of soil fauna in soil health assessment in peat meadow restoration. Eur. J. Soil Sci. 2024, 75, e70018. [Google Scholar] [CrossRef]
- Triquet, C.; Perennes, M.; Séchaud, R.; van der Meer, M.; Fabian, Y.; Jeanneret, P. What evidence exists on the effect of the main European lowland crop and grassland management practices on biodiversity indicator species groups? A systematic map. Environ. Evid. 2024, 13, 20. [Google Scholar] [CrossRef]
- Miller, J.J.; Battigelli, J.P.; Willms, W.D. Grazing Protection Influences Soil Mesofauna in Ungrazed and Grazed Riparian and Upland Pastures. Rangel. Ecol. Manag. 2014, 67, 429–434. [Google Scholar] [CrossRef]
- Siebert, J.; Thakur, M.P.; Reitz, T.; Schaedler, M.; Schulz, E.; Yin, R.; Weigelt, A.; Eisenhauer, N. Extensive grassland-use sustains high levels of soil biological activity, but does not alleviate detrimental climate change effects. In Resilience in Complex Socio-Ecological Systems; Advances in Ecological Research; Academic Press: Cambridge, MA, USA, 2019; Volume 60, pp. 25–58. [Google Scholar]
- Johnston, A.S.A.; Sibly, R.M. Multiple environmental controls explain global patterns in soil animal communities. Oecologia 2020, 192, 1047–1056. [Google Scholar] [CrossRef]
- Manu, M.; Băncilă, R.I.; Mountford, O.J.; Maruşca, T.; Blaj, V.A.; Onete, M. Soil Mite (Acari: Mesostigmata) Communities and Their Relationship with Some Environmental Variables in Experimental Grasslands from Bucegi Mountains in Romania. Insects 2022, 13, 285. [Google Scholar] [CrossRef]
- Báldi, A. Using higher taxa as surrogates of species richness: A study based on 3700 Coleoptera, Diptera, and Acari species in Central-Hungarian reserves. Basic Appl. Ecol. 2003, 4, 589–593. [Google Scholar] [CrossRef]
- Rosser, N. Shortcuts in biodiversity research: What determines the performance of higher taxa as surrogates for species? Ecol. Evol. 2017, 7, 2595–2603. [Google Scholar] [CrossRef]
- Popovici, I.; Ciobanu, M. Diversity and distribution of nematode communities in grasslands from Romania in relation to vegetation and soil characteristics. Appl. Soil Ecol. 2000, 14, 27–36. [Google Scholar] [CrossRef]
- Korsós, Z.; Lazányi, E. Millipedes (Diplopoda) of Maramureş (Romania). Stud. Univ. Vasile Goldiş Arad Ser. Ştiinţ. Vieţii. 2008, 18, 199–209. [Google Scholar]
- Boros, G.; Dózsa-Farkas, K. The Enchytraeid fauna of Romania. Soil Org. 2015, 87, 137–147. [Google Scholar]
- Călugăr, A. Qualitative and quantitative studies upon the edaphic microarthropods fauna in some grassland ecosystems from Moldavian Plain. Universitatea de Ştiinţe Agricole şi Medicină Veterinară Iaşi (Romania). Studii şi Comunicări Compl. Muz. Şt. Nat. Ion Borcea Bacău 2006, 21, 230–231. [Google Scholar]
- Călugăr, A. Researches on the edaphic mesofauna from some agro-ecosystems from North-Eastern part of Romania. Rom. Biol. Sci. 2007, 5, 92–100. [Google Scholar]
- Călugăr, A. Researches on the edaphic mesofauna from some grassland ecosystems from the inferior section of Prut riverside (Romania). Lucr. Stiinţ. Univ. Agron. Ion Ionescu de la Brad Iaşi Ser. Agron. 2010, 53, 84–88. [Google Scholar]
- Călugăr, A.; Ivan, O. Saline grasslands, a suitable environment for maintaining the functional relationships of the edaphic mesofauna? Lucr. Stiinţ. Univ. Agron. Ion Ionescu de la Brad Iaşi Ser. Agron. 2018, 61, 123–129. [Google Scholar]
- Băncilă, R.I.; Cogălniceanu, D.; Manu, M.; Plăiaş̧u, R.; Stănescu, F.; Memedemin, D.; Skolka, M.; Tofan, L.; Lăcătuşu, A. A Multi-Community Approach in Biodiversity Assessment of a Peat Bog in the Southern Carpathians (Romania) and Implications for Conservation. Environ. Entomol. 2023, 52, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Manu, M.; Bîrsan, C.C.; Mountford, O.; Lăcătuşu, A.R.; Onete, M. Preliminary study on soil fauna as a tool for monitoring of the “Springs Complex of Corbii Ciungi” protected area—Romania. Sci. Papers Ser. D Anim. Sci. 2020, 63, 272–280. [Google Scholar]
- Onete, M.; Zaharia, D.; Nicoară, G.R.; Manu, M. Studii Privind Aprecierea Valorii Pastorale şi a Capacităţii de Păşunat în Unele Pajişti Din Zona Sud-Vestică a Masivului Făgăraş; Editura Ars Docendi, Universitatea Bucureşti: Bucureşti, Romania, 2021; pp. 1–124. [Google Scholar]
- Florea, N.; Munteanu, I. Sistemul Român de Taxonomie a Solurilor (SRTS-2003); Editura ESTFALIA: Bucureşti, Romania, 2003; pp. 1–182. [Google Scholar]
- Balogh, J. The Oribatid Genera of the World; Akademiai Kiado: Budapest, Hungary, 1972; pp. 1–188. [Google Scholar]
- Gîdei, P.; Popescu, I.E. Îndrumator Pentru Cunoas, terea Coleopterelor; Editura Pim: Iaşi, Romania, 2009; pp. 5–419. [Google Scholar]
- Krantz, G.W.; Walter, D.E. A Manual of Acarology, 3rd ed.; Texas Tech University Press: Lubbock, TX, USA, 2009; pp. 1–807. [Google Scholar]
- Ceuca, T. Diplopoda. In Determinatorul Ilustrat al Florei si Faunei Romaniei—Mediul Terestru; Godeanu, S.P., Ed.; Vasile Goldiş University Press: Arad, România, 2010; Volume 3, pp. 290–300. [Google Scholar]
- Platnick, N.I. World Spider Catalog. World Spider Catalog. Version 19.5. Natural History Museum Bern. 2018. Available online: http://wsc.nmbe.ch (accessed on 5 February 2022).
- Brussaard, L.; Behan-Pelletier, V.M.; Bignell, D.E.; Brown, V.K.; Didde, W.; Folgaria, P.; Fragoso, C.; Freckman, D.W.; Gupta, V.V.S.R.; Hattori, T.; et al. Biodiversity and ecosystem functioning in soil. Ambio. J. Hum. Environ. 1997, 26, 563–570. [Google Scholar]
- Chahartaghi, M.; Langel, R.; Scheu, S.; Ruess, L. Feeding guilds in Collembola based on nitrogen stable isotope ratios. Soil Biol. Biochem. 2005, 37, 1718–1725. [Google Scholar] [CrossRef]
- The R Project for Statistical Computing. Available online: http://www.R-project.org/ (accessed on 10 December 2024).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; pp. 1–276. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009; pp. 1–574. [Google Scholar]
- Kindt, R.; Coe, R. Tree Diversity Analysis: A Manual and Software for Common Statistical Methods for Ecological and Biodiversity Studies; World Agroforestry Centre (ICRAF): Nairobi, Kenya, 2005; pp. 1–196. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Bolker, B. GLMM FAQ. Available online: http://bbolker.github.io/mixedmodels-misc/glmmFAQ.html (accessed on 15 June 2022).
- Fox, J.; Hong, J. Effect Displays in R for Multinomial and Proportional-Odds Logit Models: Extensions to the effects Package. J. Stat. Softw. 2009, 32, 1–24. [Google Scholar] [CrossRef]
- Oksanen, J.F.; Blanchet, G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-7. Available online: https://CRAN.R-project.org/package=vegan (accessed on 20 June 2022).
- Manu, M.; Lăcătuşu, A.; Bodescu, F.; Nicoară, R.; Chiriac, L.S.; Onete, M. Are habitats with Campanula romanica Săvul. preferred by the soil fauna? Sci. Papers Ser. D Anim. Sci. 2023, 46, 105–112. [Google Scholar]
- Curry, J.P. The invertebrate fauna of grassland and its influence on productivity. The composition of the fauna. Grass Forage Sci. 1987, 42, 103–120. [Google Scholar] [CrossRef]
- Schrama, M.; Casper, W.; Quist, C.W.; de Groot, A.; Cieraad, E.; Ashworth, D.; Laros, I.; Hestbjerg Hansen, L.; Leff, J.; Fierer, N.; et al. Cessation of grazing causes biodiversity loss and homogenization of soil food webs. Proc. R. Soc. B. 2023, 290, 20231345. [Google Scholar] [CrossRef]
- Arnott, A.; Riddell, G.; Emmerson, M.; Caruso, T.; Reid, N. Upland grassland habitats and agri-environment schemes change soil microarthropod abundance. J. Appl. Ecol. 2021, 58, 2256–2265. [Google Scholar] [CrossRef]
- Souza, R.; Hartzell, S.; Ferraz, A.P.F.; de Almeida, A.Q.; de Sousa Lima, R.S.; Antonino, A.C.D.; de Souza, E.S. Dynamics of soil penetration resistance in water-controlled environments. Soil Till. Res. 2021, 205, 104768. [Google Scholar] [CrossRef]
- Aupic-Samain, A.; Baldy, V.; Delcourt, N.; Krogh, P.H.; Gauquelin, T.; Fernandez, C.; Santonja, M. Water availability rather than temperature control soil fauna community structure and prey–predator interactions. Funct. Ecol. 2020, 35, 1550–1559. [Google Scholar] [CrossRef]
- Amossé, J.; Dózsa-Farkas, K.; Boros, G.; Rochat, G.; Sandoz, G.; Fournier, B.; Mitchell, E.A.D.; Le Bayon, R.C. Patterns of earthworm, enchytraeid and nematode diversity and community structure in urban soils of different ages. Eur. J. Soil Biol. 2016, 73, 46–58. [Google Scholar] [CrossRef]
- Bogyó, D.; Magura, T.; Nagy, D.D.; Tóthmérész, B. Distribution of millipedes (Myriapoda, Diplopoda) along a forest interior—forest edge—grassland habitat complex. ZooKeys 2015, 510, 181–195. [Google Scholar] [CrossRef]
- Heděnec, P.; Jiménez, J.J.; Moradi, J.; Domene, X.; Hackenberger, D.; Barot, S.; Frossard, A.; Oktaba, L.; Filser, J.; Kindlmann, P.; et al. Global distribution of soil fauna functional groups and their estimated litter consumption across biomes. Sci. Rep. 2022, 12, 17362. [Google Scholar] [CrossRef]
- Seeber, J.; Seeber, G.U.H.; Kössler, W.; Langel, R.; Scheu, S.; Meyer, E. Abundance and trophic structure of macro-decomposers on alpine pastureland (Central Alps, Tyrol): Effects of abandonment of pasturing. Pedobiologia 2005, 49, 221–228. [Google Scholar] [CrossRef]
- Ríos-Díaz, C.L.; Moreno, C.E.; Ortega-Martínez, I.J.; Zuria, I.; Escobar, F.; Castellanos, I. Sheep herding in small grasslands promotes dung beetle diversity in a mountain forest landscape. J. Insect. Conserv. 2021, 25, 13–26. [Google Scholar] [CrossRef]
- Feketeová, Z.; Mangová, B.; Cierniková, M. The Soil Chemical Properties Influencing the Oribatid Mite (Acari; Oribatida) Abundance and Diversity in Coal Ash Basin Vicinage. Appl. Sci. 2021, 11, 3537. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).