Comparative Assessment of Biodiversity and Ecological Indicators in Three Forest Ecosystems of Southern Romania
Abstract
1. Introduction
2. Materials and Methods
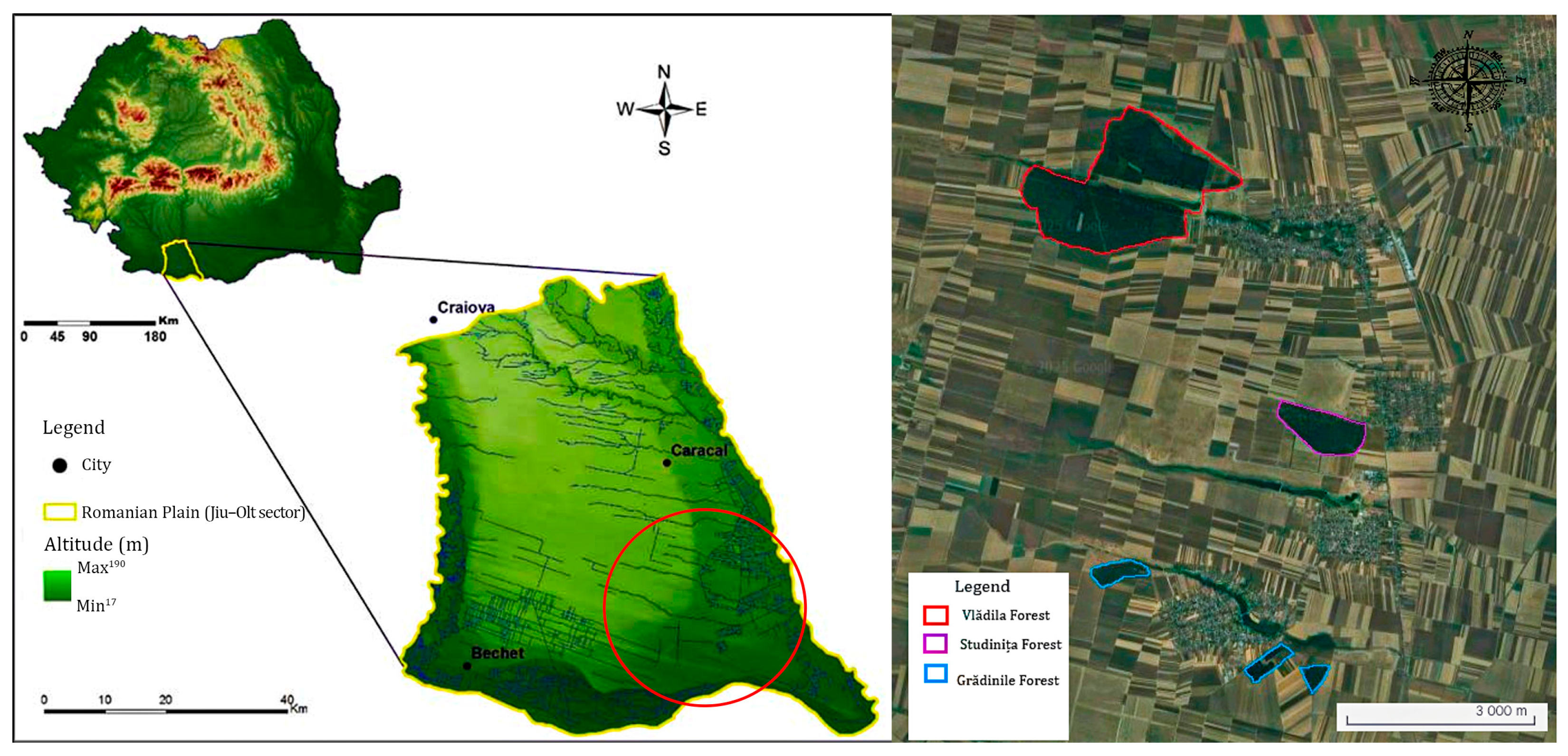
2.1. Study Area
2.2. Data Collecting
2.3. Structural Indices of the Community
2.4. Biodiversity Indices
2.5. Statistical Analysis
3. Results and Discussions
3.1. Analysis of Structural Indices of the Community for Woody Species
3.2. Analysis of Structural Indices of the Community for the Herbaceous Species
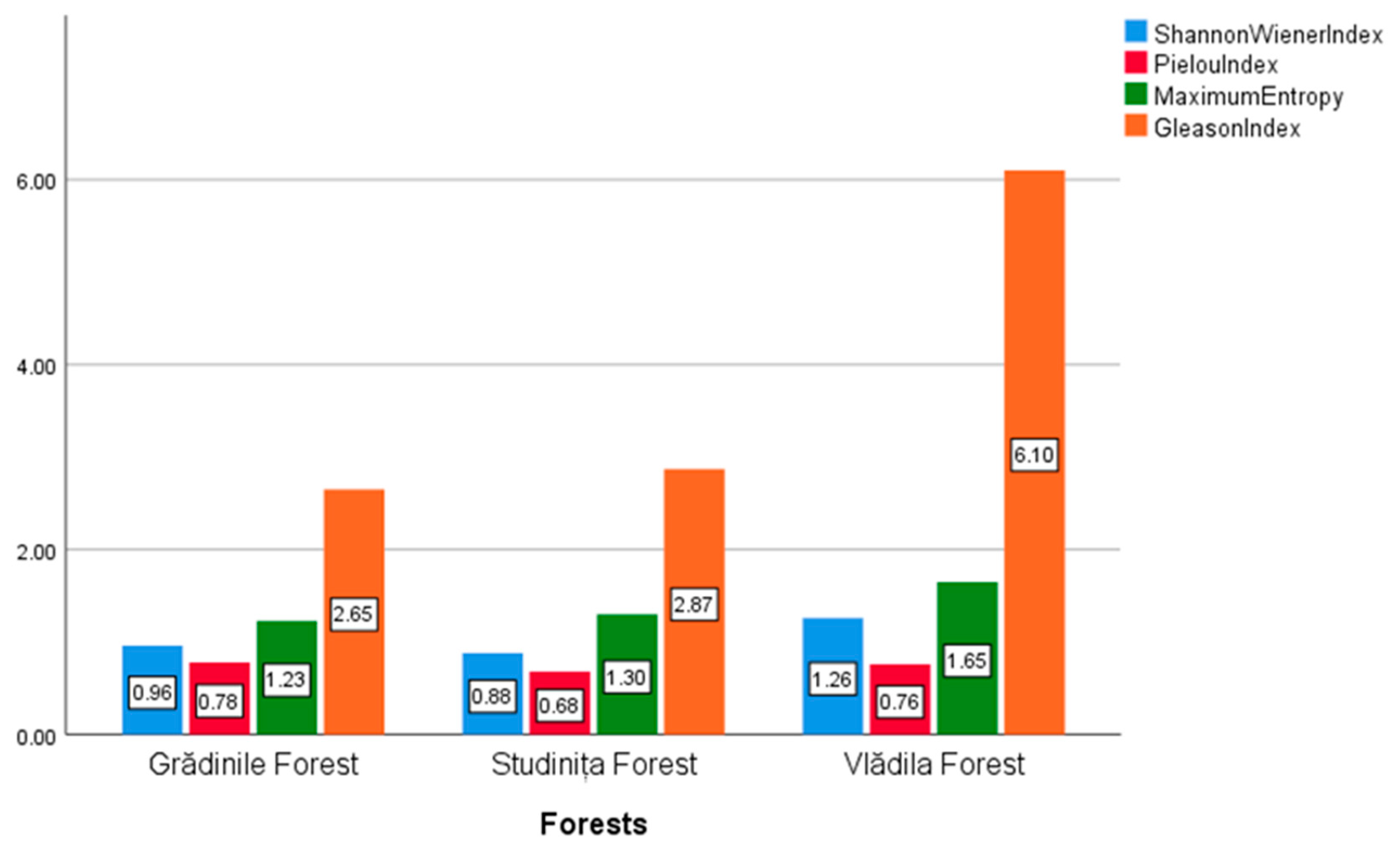
3.3. Analysis of Biodiversity Indices for the Three Forests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Threlfall, C.G.; Ossola, A.; Hahs, A.K.; Williams, N.S.; Wilson, L.; Livesley, S.J. Variation in vegetation structure and composition across urban green space types. Front. Ecol. Evol. 2016, 4, 66. [Google Scholar] [CrossRef]
- Lortie, C.J.; Brooker, R.W.; Choler, P.; Kikvidze, Z.; Michalet, R.; Pugnaire, F.I.; Callaway, R.M. Rethinking plant community theory. Oikos 2004, 107, 433–438. [Google Scholar] [CrossRef]
- Hodge, A.; Fitter, A.H. Microbial mediation of plant competition and community structure. Funct. Ecol. 2013, 27, 865–875. [Google Scholar] [CrossRef]
- Bayat, M.; Bettinger, P.; Heidari, S.; Hamidi, S.K.; Jaafari, A. A combination of biotic and abiotic factors and diversity determine productivity in natural deciduous forests. Forests 2021, 12, 1450. [Google Scholar] [CrossRef]
- Cheng, X.; Ping, T.; Li, Z.; Wang, T.; Han, H.; Epstein, H.E. Effects of environmental factors on plant functional traits across different plant life forms in a temperate forest ecosystem. New For. 2022, 53, 125–142. [Google Scholar] [CrossRef]
- Stoenescu, A.M.; Cosmulescu, S.N.; Gheorghiu, N. The influence of interannual climate variation on the phenology of some wild fruit species and their relation with avifauna. Sci. Pap. Ser. B Hortic. 2024, 68, 173–181. [Google Scholar]
- Smith, R.S.; Shiel, R.S.; Millward, D.; Corkhill, P. The interactive effects of management on the productivity and plant community structure of an upland meadow: An 8-year field trial. J. Appl. Ecol. 2000, 37, 1029–1043. [Google Scholar] [CrossRef]
- Hartel, T.; Hanspach, J.; Abson, D.J.; Máthé, O.; Moga, C.I.; Fischer, J. Bird communities in traditional wood-pastures with changing management in Eastern Europe. Basic Appl. Ecol. 2014, 15, 385–395. [Google Scholar] [CrossRef]
- Gharnit, Y.; Moujane, A.; Outourakhte, A.; Hassan, I.; El Amraoui, K.; Hasib, A.; Boulli, A. Plant Richness, Species Assessment, and Ecology in the M’goun Geopark Rangelands, High Atlas Mountains, Morocco. Rangel. Ecol. Manag. 2025, 98, 357–376. [Google Scholar] [CrossRef]
- Anitha, K.; Joseph, S.; Ramasamy, E.V.; Prasad, S.N. Changes in structural attributes of plant communities along disturbance gradients in a dry deciduous forest of Western Ghats, India. Environ. Monit. Assess. 2009, 155, 393–405. [Google Scholar] [CrossRef]
- Ferreira, P.M.; Andrade, B.O.; Podgaiski, L.R.; Dias, A.C.; Pillar, V.D.; Overbeck, G.E.; Mendonça, M.S., Jr.; Boldrini, I.I. Long-term ecological research in southern Brazil grasslands: Effects of grazing exclusion and deferred grazing on plant and arthropod communities. PLoS ONE 2020, 15, e0227706. [Google Scholar] [CrossRef] [PubMed]
- Vymazalová, P.; Košulič, O.; Hamřík, T.; Šipoš, J.; Hédl, R. Positive impact of traditional coppicing restoration on biodiversity of ground-dwelling spiders in a protected lowland forest. For. Ecol. Manag. 2021, 490, 119084. [Google Scholar] [CrossRef]
- Sabatini, F.M.; Burrascano, S.; Keeton, W.S.; Levers, C.; Lindner, M.; Pötzschner, F.; Verkerk, P.J.; Bauhus, J.; Buchwald, E.; Chaskovsky, O.; et al. Where are Europe’s last primary forests? Divers. Distrib. 2018, 24, 1426–1439. [Google Scholar] [CrossRef]
- Romeiro, J.M.N.; Eid, T.; Antón-Fernández, C.; Kangas, A.; Trømborg, E. Natural disturbances risks in European Boreal and Temperate forests and their links to climate change–A review of modelling approaches. For. Ecol. Manag. 2022, 509, 120071. [Google Scholar] [CrossRef]
- Chiarucci, A.; Piovesan, G. Need for a global map of forest naturalness for a sustainable future. Conserv. Biol. 2020, 34, 368–372. [Google Scholar] [CrossRef]
- Leidinger, J.; Blaschke, M.; Ehrhardt, M.; Fischer, A.; Gossner, M.M.; Jung, K.; Kienlein, S.; Kózak, J.; Michler, B.; Mosandl, R.; et al. Shifting tree species composition affects biodiversity of multiple taxa in Central European forests. For. Ecol. Manag. 2021, 498, 119552. [Google Scholar] [CrossRef]
- Johnson, D.J.; Magee, L.; Pandit, K.; Bourdon, J.; Broadbent, E.N.; Glenn, K.; Kaddoura, Y.; Machado, S.; Nieves, J.; Wilkinson, B.E.; et al. Canopy tree density and species influence tree regeneration patterns and woody species diversity in a longleaf pine forest. For. Ecol. Manag. 2021, 490, 119082. [Google Scholar] [CrossRef]
- Angessa, A.T.; Lemma, B.; Yeshitela, K.; Fischer, J.; May, F.; Shumi, G. Woody plant diversity, composition and structure in relation to environmental variables and land-cover types in Lake Wanchi watershed, central highlands of Ethiopia. Afr. J. Ecol. 2020, 58, 627–638. [Google Scholar] [CrossRef]
- Shumi, G.; Rodrigues, P.; Hanspach, J.; Haerdtle, W.; Hylander, K.; Senbeta, F.; Fischer, J.; Schultner, J. Woody plant species diversity as a predictor of ecosystem services in a social–ecological system of southwestern Ethiopia. Landsc. Ecol. 2021, 36, 373–391. [Google Scholar] [CrossRef]
- Ilie, D.; Cosmulescu, S. Spontaneous plant diversity in urban contexts: A review of its impact and importance. Diversity 2023, 15, 277. [Google Scholar] [CrossRef]
- Morin, X.; Toigo, M.; Fahse, L.; Guillemot, J.; Cailleret, M.; Bertrand, R.; Cateau, E.; de Coligny, F.; García-Valdés, R.; Ratcliffe, S.; et al. More species, more trees: The role of tree packing in promoting forest productivity. J. Ecol. 2025. [Google Scholar] [CrossRef]
- Tinya, F.; Kovács, B.; Bidló, A.; Dima, B.; Király, I.; Kutszegi, G.; Lakatos, F.; Mag, Z.; Márialigeti, S.; Nascimbene, J.; et al. Environmental drivers of forest biodiversity in temperate mixed forests–A multi-taxon approach. Sci. Total Environ. 2021, 795, 148720. [Google Scholar] [CrossRef] [PubMed]
- Hämäläinen, A.; Runnel, K.; Ranius, T.; Strengbom, J. Diversity of forest structures important for biodiversity is determined by the combined effects of productivity, stand age, and management. Ambio 2024, 53, 718–729. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kumar, A.; Sharma, M. Conservation of wildlife diversity in agroforestry systems in eastern Himalayan biodiversity hotspot. Proc. Zool. Soc. 2021, 74, 171–188. [Google Scholar] [CrossRef]
- Cosmulescu, S.; Ștefănescu, D.; Stoenescu, A.M. Variability of phenological behaviours of wild fruit tree species based on discriminant analysis. Plants 2021, 11, 45. [Google Scholar] [CrossRef]
- Hanberry, B.B.; Bragg, D.C.; Alexander, H.D. Open forest ecosystems: An excluded state. For. Ecol. Manag. 2020, 472, 118256. [Google Scholar] [CrossRef]
- Seidl, R.; Honkaniemi, J.; Aakala, T.; Aleinikov, A.; Angelstam, P.; Bouchard, M.; Boulanger, Y.; Burton, P.J.; De Grandpre, L.; Gauthier, S.; et al. Globally consistent climate sensitivity of natural disturbances across boreal and temperate forest ecosystems. Ecography 2020, 43, 967–978. [Google Scholar] [CrossRef]
- Li, T.; Kamran, M.; Chang, S.; Peng, Z.; Wang, Z.; Ran, L.; Jiang, W.Q.; Jin, Y.; Zhang, X.; Tu, Y.; et al. Climate-soil interactions improve the stability of grassland ecosystem by driving alpine plant diversity. Ecol. Indic. 2022, 141, 109002. [Google Scholar] [CrossRef]
- Yang, D.; Yang, Y.; Xia, J. Hydrological cycle and water resources in a changing world: A review. Geogr. Sustain. 2021, 2, 115–122. [Google Scholar] [CrossRef]
- Zhong, M.; Song, J.; Zhou, Z.; Ru, J.; Zheng, M.; Li, Y.; Hui, D.; Wan, S. Asymmetric responses of plant community structure and composition to precipitation variabilities in a semi-arid steppe. Oecologia 2019, 191, 697–708. [Google Scholar] [CrossRef]
- Yao, Z.; Xin, Y.; Yang, L.; Zhao, L.; Ali, A. Precipitation and temperature regulate species diversity, plant coverage and aboveground biomass through opposing mechanisms in large-scale grasslands. Front. Plant Sci. 2022, 13, 999636. [Google Scholar] [CrossRef] [PubMed]
- Stamin, F.D.; Vijan, L.E.; Topală, C.M.; Cosmulescu, S.N. The influence of genotype, environmental factors, and location on the nutraceutical profile of Rosa canina L. Fruits. Agronomy 2024, 14, 2847. [Google Scholar] [CrossRef]
- Battes, K. Ecologie Generală Ghid de Lucrări Practice; Editura Presa Universitară Clujeană: Cluj-Napoca, Romania, 2018. [Google Scholar]
- Ciocârlan, V. Flora Ilustrată a României: Pteridophyta et Spermatophyta; Editura Ceres: București, Romania, 2009. [Google Scholar]
- Sârbu, I.; Ștefan, N.; Oprea, A. Plante Vasculare din România Determinator ilustrat de teren; Editura Victor B Victor: București, Romania, 2013. [Google Scholar]
- Preda, C.; Ruști, D.M.; Cogălniceanu, D. Lucrări Practice de Ecologie Generală; Editura Universitară: București, Romania, 2020. [Google Scholar]
- Sîrbu, I.; Benedek, A.M. Ecologie Practică; Editura Universităţii “Lucian Blaga” din Sibiu: Sibiu, Romania, 2012. [Google Scholar]
- Botnariuc, N.; Vădineanu, A. Ecologie; Editura Didactică şi Pedagogică: București, Romania, 1982. [Google Scholar]
- McNaughton, S.J.; Wolf, L.L. Dominance and the Niche in Ecological Systems: Dominance is an expression of ecological inequalities arising out of different exploitation strategies. Science 1970, 167, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Cartozo, C.C.; Garlaschelli, D.; Ricotta, C.; Barthélemy, M.; Caldarelli, G. Quantifying the taxonomic diversity in real species communities. J. Phys. A Math. Theor. 2008, 41, 224012. [Google Scholar] [CrossRef]
- Heinen, R.; Hannula, S.E.; De Long, J.R.; Huberty, M.; Jongen, R.; Kielak, A.; Steinauer, K.; Zhu, F.; Bezemer, T.M. Plant community composition steers grassland vegetation via soil legacy effects. Ecol. Lett. 2020, 23, 973–982. [Google Scholar] [CrossRef]
- Stoenescu, A.M.; Cosmulescu, S. Variability of morphological characteristics in hawthorn (Crataegus monogyna L.) fruit genotypes. South-West J. Hortic. Biol. Environ. 2020, 11, 15–26. [Google Scholar]
- Stamin, F.D.; Cosmulescu, S.N. Morphological variability of some rosehip fruits (Rosa canina L.) from the spontaneous flora of Olt county, Romania. Sci. Pap. Ser. B Hortic. 2024, 68, 159–164. [Google Scholar]
- Leca, Ș.; Popa, I.; Chivulescu, Ș.; Popa, A.; Pitar, D.; Dobre, A.C.; Pascu, I.S.; Apostol, B.; Badea, O. Structure and diversity in a periurban forest of Bucharest, Romania. Ann. For. Res. 2023, 66, 139–153. [Google Scholar] [CrossRef]
- Fichtner, A.; Wissemann, V. Biological flora of the British isles: Crataegus monogyna. J. Ecol. 2021, 109, 541–571. [Google Scholar] [CrossRef]
- Altay, V. Ecology of Pinus sylvestris L. forests—A case study from Istanbul (Turkey). Pak. J. Bot. 2019, 51, 1711–1718. [Google Scholar] [CrossRef]
- Afifi, L.; Lapin, K.; Tremetsberger, K.; Konrad, H. A systematic review of threats, conservation, and management measures for tree species of the family Rosaceae in Europe. Flora 2023, 301, 152244. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, S.; Zhang, L.; Guo, Y.; Zhang, D.; Liu, S.; Ma, L.; Hu, J.; Lu, J.; Wang, X. Optimizing Pinus tabuliformis Forest Spatial Structure and Function in Beijing, China. Forests 2024, 15, 1963. [Google Scholar] [CrossRef]
| Structural Indices | X | SD | LC | DP | F | C | W | ID | |
|---|---|---|---|---|---|---|---|---|---|
| Species | |||||||||
| Grădinile Forest | |||||||||
| 1. Crataegus monogyna | 13.9 | 12.25 | 7.59 | 61.23 | 100 | Constant | 61.23 | 54.95 | |
| 2. Quercus robur | 5 | 3.89 | 2.41 | 64.10 | 90 | Constant | 57.69 | ||
| 3. Ulmus minor | 2.5 | 7.23 | 4.48 | 96.15 | 20 | Accidental | 19.23 | ||
| 4. Acer campestre | 0.9 | 2.85 | 1.76 | 3.96 | 10 | Accidental | 0.39 | ||
| 5. Gleditsia triacanthos | 0.2 | 0.63 | 0.39 | 0.88 | 20 | Accidental | 0.18 | ||
| 6. Acer tataricum | 0.1 | 0.32 | 0.20 | 0.44 | 10 | Accidental | 0.04 | ||
| 7. Rosa canina | 0.1 | 0.32 | 0.20 | 1.32 | 10 | Accidental | 0.13 | ||
| Studinița Forest | |||||||||
| 1. Acer campestre | 34.1 | 87.93 | 54.50 | 42.10 | 30 | Accessory | 12.63 | 77.53 | |
| 2. Crataegus monogyna | 28.7 | 19.41 | 12.03 | 35.43 | 100 | Constant | 35.43 | ||
| 3. Ligustrum vulgare | 7.9 | 19.02 | 11.79 | 9.75 | 20 | Accidental | 1.95 | ||
| 4. Quercus robur | 5.8 | 6.53 | 4.05 | 7.16 | 90 | Constant | 6.44 | ||
| 5. Rosa canina | 3.6 | 5.44 | 3.37 | 4.44 | 40 | Accessory | 1.78 | ||
| 6. Acer tataricum | 0.5 | 1.58 | 0.98 | 0.62 | 10 | Accidental | 0.06 | ||
| 7. Gleditsia triacanthos | 0.2 | 0.42 | 0.26 | 0.25 | 20 | Accidental | 0.05 | ||
| 8. Prunus cerasifera | 0.1 | 0.32 | 0.20 | 0.12 | 10 | Accidental | 0.01 | ||
| 9. Robinia pseudoacacia | 0.1 | 0.32 | 0.20 | 0.12 | 10 | Accidental | 0.01 | ||
| Vlădila Forest | |||||||||
| 1. Crataegus monogyna | 39.91 | 43.12 | 25.48 | 65.62 | 100 | Constant | 65.62 | 78.33 | |
| 2. Prunus spinosa | 7.73 | 17.89 | 10.57 | 12.71 | 54.54 | Constant | 6.93 | ||
| 3. Quercus robur | 2.64 | 2.69 | 1.59 | 4.33 | 63.64 | Constant | 2.76 | ||
| 4. Robinia pseudoacacia | 2.64 | 8.09 | 4.78 | 4.33 | 27.27 | Accessory | 1.18 | ||
| 5. Rosa canina | 2.45 | 2.07 | 1.22 | 4.04 | 27.73 | Accessory | 2.94 | ||
| 6. Acer campestre | 2.09 | 4.61 | 2.73 | 3.44 | 27.27 | Accessory | 0.94 | ||
| 7. Frangula alnus | 1.18 | 2.32 | 1.37 | 1.94 | 27.27 | Accessory | 0.53 | ||
| 8. Ligustrum vulgare | 0.73 | 1.85 | 1.09 | 1.20 | 18.18 | Accidental | 0.22 | ||
| 9. Ulmus minor | 0.55 | 1.04 | 0.61 | 0.90 | 27.27 | Accessory | 0.24 | ||
| 10. Prunus cerasifera | 0.45 | 0.82 | 0.48 | 0.75 | 27.27 | Accessory | 0.20 | ||
| 11. Acer tataricum | 0.09 | 0.30 | 0.18 | 0.15 | 9.09 | Accidental | 0.01 | ||
| 12. Euonymus europaeus | 0.09 | 0.30 | 0.18 | 0.15 | 9.09 | Accidental | 0.01 | ||
| 13. Fraxinus excelsior | 0.09 | 0.30 | 0.18 | 0.15 | 9.09 | Accidental | 0.01 | ||
| 14. Gleditsia triacanthos | 0.09 | 0.30 | 0.18 | 0.15 | 9.09 | Accidental | 0.01 | ||
| 15. Pyrus pyraster | 0.09 | 0.30 | 0.18 | 0.15 | 9.09 | Accidental | 0.01 | ||
| Structural Indices | X | SD | LC | DP | F | C | W | ID | |
|---|---|---|---|---|---|---|---|---|---|
| Species | |||||||||
| Grădinile Forest | |||||||||
| 1. Glechoma hederacea | 7.8 | 17.44 | 15.29 | 36.79 | 20 | Accidental | 7.36 | 58.49 | |
| 2. Geum urbanum | 4.6 | 10.29 | 9.02 | 21.70 | 20 | Accidental | 4.34 | ||
| 3. Viola canina | 4 | 8.94 | 7.84 | 18.87 | 20 | Accidental | 3.77 | ||
| 4. Carex sylvatica | 3.6 | 6.99 | 6.12 | 16.98 | 40 | Accessory | 6.79 | ||
| 5. Allium scorodoprasum | 0.6 | 1.34 | 1.18 | 2.83 | 20 | Accidental | 0.57 | ||
| 6. Fallopia convolvus | 0.6 | 1.34 | 1.18 | 2.83 | 20 | Accidental | 0.57 | ||
| Studinița Forest | |||||||||
| 1. Agrimonia eupatoria | 16.6 | 31.14 | 27.30 | 44.62 | 60 | Constant | 26.77 | 61.83 | |
| 2. Fragaria vesca | 6.4 | 7.16 | 6.28 | 17.20 | 60 | Constant | 10.32 | ||
| 3. Viola canina | 4.8 | 9.15 | 8.02 | 12.90 | 40 | Accessory | 5.16 | ||
| 4. Hypericum perforatum | 3.4 | 4.67 | 4.09 | 9.14 | 40 | Accessory | 3.66 | ||
| 5. Carex sylvatica | 2.4 | 3.91 | 3.43 | 6.45 | 40 | Accessory | 2.58 | ||
| 6. Geum urbanum | 1.8 | 2.49 | 2.18 | 4.84 | 40 | Accessory | 1.94 | ||
| 7. Mentha pulegium | 1.8 | 4.02 | 3.53 | 4.84 | 20 | Accidental | 0.97 | ||
| Vlădila Forest | |||||||||
| 1. Lithospermum purpurocaeruleum | 9.4 | 12.92 | 11.32 | 25.27 | 40 | Accessory | 10.11 | 20.81 | |
| 2. Fallopia convolvus | 6 | 9.19 | 8.06 | 16.13 | 60 | Constant | 9.68 | ||
| 3. Festuca arundinacea | 5.6 | 12.52 | 10.98 | 15.05 | 20 | Accidental | 3.01 | ||
| 4. Fragaria vesca | 3.4 | 4.10 | 3.59 | 9.14 | 60 | Constant | 5.48 | ||
| 5. Anthriscus cerefolium | 3.2 | 7.16 | 6.27 | 8.60 | 20 | Accidental | 1.72 | ||
| 6. Geranium pusillum | 2.2 | 4.92 | 4.31 | 5.91 | 20 | Accidental | 1.18 | ||
| 7. Prunella vulgaris | 2 | 3.46 | 3.04 | 5.38 | 40 | Accessory | 2.15 | ||
| 8. Geum urbanum | 1.8 | 2.68 | 2.35 | 4.84 | 40 | Accessory | 1.94 | ||
| 9. Rubus caesius | 1.6 | 3.58 | 3.14 | 4.30 | 20 | Accidental | 0.86 | ||
| 10. Viola canina | 1.6 | 3.58 | 3.14 | 4.30 | 20 | Accidental | 0.86 | ||
| 11. Asparagus tenuifolius | 0.4 | 0.89 | 0.78 | 100 | 20 | Accidental | 20 | ||
| Structural Indices | X | SD | LC | DP | F | C | W | ID | |
|---|---|---|---|---|---|---|---|---|---|
| Species | |||||||||
| Grădinile Forest | |||||||||
| 1. Geum urbanum | 11.8 | 8.64 | 7.575 | 21.38 | 80 | Constant | 17.10 | 42.39 | |
| 2. Carex sylvatica | 11.6 | 11.59 | 10.16 | 21.01 | 60 | Constant | 12.61 | ||
| 3. Glechoma hederacea | 11.6 | 25.94 | 22.74 | 21.01 | 20 | Accidental | 4.20 | ||
| 4. Torilis arvensis | 7.8 | 16.35 | 14.33 | 14.13 | 40 | Accessory | 5.65 | ||
| 5. Viola canina | 7.4 | 14.93 | 13.08 | 13.41 | 40 | Accessory | 5.36 | ||
| 6. Lamium purpureum | 2.4 | 5.37 | 4.70 | 4.35 | 20 | Accidental | 0.87 | ||
| 7. Fallopia convolvus | 1.4 | 3.13 | 2.74 | 2.54 | 20 | Accidental | 0.51 | ||
| 8. Malva sylvestris | 1 | 2.24 | 1.96 | 1.81 | 20 | Accidental | 0.36 | ||
| 9. Urtica dioica | 0.2 | 0.45 | 0.39 | 0.36 | 20 | Accidental | 0.07 | ||
| Studinița Forest | |||||||||
| 1. Viola canina | 3.4 | 3.58 | 3.14 | 25 | 60 | Constant | 2.94 | 41.18 | |
| 2. Fragaria vesca | 2.2 | 2.86 | 2.51 | 16.18 | 60 | Constant | 9.71 | ||
| 3. Ballota nigra | 1.8 | 2.49 | 2.18 | 13.24 | 60 | Constant | 7.94 | ||
| 4. Geum urbanum | 1.8 | 1.79 | 1.57 | 13.24 | 60 | Constant | 7.94 | ||
| 5. Agrimonia eupatoria | 1.6 | 2.30 | 2.02 | 11.76 | 40 | Accessory | 4.71 | ||
| 6. Astragalus glycyphyllos | 1.2 | 1.79 | 1.57 | 8.82 | 40 | Accessory | 3.53 | ||
| 7. Ornithogalum pyramidale | 1 | 1.41 | 1.24 | 7.35 | 40 | Accessory | 15 | ||
| 8. Filipendula vulgaris | 0.4 | 0.89 | 0.79 | 2.94 | 20 | Accidental | 0.59 | ||
| 9. Hypericum perforatum | 0.2 | 0.45 | 0.39 | 1.47 | 20 | Accidental | 0.29 | ||
| Vlădila Forest | |||||||||
| 1. Galium verum | 15 | 20.78 | 18.22 | 20.83 | 40 | Accessory | 8.33 | 27.78 | |
| 2. Lamium purpureum | 11.2 | 25.04 | 21.95 | 15.56 | 20 | Accidental | 3.11 | ||
| 3. Urtica dioica | 8.8 | 19.68 | 17.25 | 12.22 | 20 | Accidental | 2.44 | ||
| 4. Festuca arundinacea | 8.6 | 12.40 | 10.87 | 11.94 | 40 | Accessory | 4.78 | ||
| 5. Asparagus tenuifolius | 7.4 | 13.92 | 12.20 | 10.28 | 40 | Accessory | 4.11 | ||
| 6. Alkekengi officinarum | 4.6 | 10.29 | 9.02 | 6.39 | 20 | Accidental | 1.28 | ||
| 7. Rubus caesius | 4 | 5.87 | 5.15 | 5.56 | 40 | Accessory | 2.22 | ||
| 8. Sambucus ebulus | 2 | 4.47 | 3.92 | 2.78 | 20 | Accidental | 0.56 | ||
| 9. Geum urbanum | 1.8 | 4.02 | 3.53 | 2.50 | 20 | Accidental | 0.50 | ||
| 10. Ornithogalum pyramidale | 1.8 | 4.02 | 3.53 | 2.50 | 20 | Accidental | 0.50 | ||
| 11. Hypericum perforatum | 1.2 | 2.68 | 2.35 | 1.67 | 20 | Accidental | 0.33 | ||
| 12. Fragaria vesca | 1 | 1.41 | 1.24 | 1.39 | 40 | Accessory | 0.56 | ||
| 13. Mentha pulegium | 1 | 2.24 | 1.96 | 1.39 | 20 | Accidental | 0.28 | ||
| 14. Lithosperumum purpurocaeruleu | 0.8 | 1.79 | 1.57 | 1.11 | 20 | Accidental | 0.22 | ||
| 15. Allium scorodoprasum | 0.6 | 1.34 | 1.18 | 0.83 | 20 | Accidental | 0.17 | ||
| 16. Prunella vulgaris | 0.6 | 0.89 | 0.78 | 0.83 | 40 | Accessory | 0.33 | ||
| 17. Silene vulgaris | 0.6 | 1.34 | 1.18 | 0.83 | 20 | Accidental | 0.17 | ||
| 18. Ballota nigra | 0.4 | 0.89 | 0.78 | 0.56 | 20 | Accidental | 0.11 | ||
| 19. Moscari comosum | 0.4 | 0.89 | 0.78 | 0.56 | 20 | Accidental | 0.11 | ||
| 20. Viola canina | 0.2 | 0.45 | 0.39 | 0.28 | 20 | Accidental | 0.06 | ||
| Structural Indices | X | SD | LC | DP | F | C | W | ID | |
|---|---|---|---|---|---|---|---|---|---|
| Species | |||||||||
| 1. Fragaria vesca | 9.83 | 13.36 | 10.69 | 38.82 | 50 | Constant | 19.41 | 61.84 | |
| 2. Alkekengi officinarum | 5.83 | 10.85 | 8.68 | 23.03 | 33.33 | Accessory | 7.68 | ||
| 3. Rubus caesius | 3.33 | 5.85 | 4.68 | 13.16 | 50 | Constant | 6.58 | ||
| 4. Lamium purpureum | 1.83 | 4.49 | 3.59 | 7.24 | 16.67 | Accidental | 1.21 | ||
| 5. Geum urbanum | 1.50 | 2.81 | 2.25 | 5.92 | 33.33 | Accessory | 1.97 | ||
| 6. Matricaria recutita | 1.17 | 2.86 | 2.29 | 4.61 | 16.67 | Accidental | 0.77 | ||
| 7. Glechoma hederacea | 0.83 | 2.04 | 1.63 | 3.29 | 16.67 | Accidental | 0.55 | ||
| 8. Pentanema salicinum | 0.83 | 2.04 | 1.63 | 3.29 | 16.67 | Accidental | 0.55 | ||
| 9. Silene vulgaris | 0.17 | 0.41 | 0.33 | 0.66 | 16.67 | Accidental | 0.11 | ||
| Structural Indices | X | SD | LC | DP | F | C | W | ID | |
|---|---|---|---|---|---|---|---|---|---|
| Species | |||||||||
| 1. Ballota nigra | 15.6 | 19.91 | 6 | 60 | 60 | Constant | 36 | 70.77 | |
| 2. Filipendula vulgaris | 2.8 | 6.26 | 1.89 | 10.77 | 20 | Accidental | 2.15 | ||
| 3. Rubus caesius | 2 | 3.08 | 0.93 | 7.69 | 40 | Accessory | 3.08 | ||
| 4. Lithospermum purpurocaeruleum | 1.8 | 4.02 | 1.21 | 6.92 | 20 | Accidental | 1.38 | ||
| 5. Scrophularia nodosa | 1 | 1.41 | 0.43 | 3.85 | 40 | Accessory | 1.54 | ||
| 6. Astragalus glycyphyllos | 0.6 | 0.89 | 0.27 | 2.31 | 40 | Accessory | 0.92 | ||
| 7. Cannabis sativa subsp. Spontanea | 0.6 | 0.89 | 0.27 | 2.31 | 20 | Accidental | 0.46 | ||
| 8. Viola canina | 0.6 | 1.34 | 0.40 | 2.31 | 20 | Accidental | 0.46 | ||
| 9. Ornithogalum pyramidale | 0.4 | 0.55 | 0.17 | 1.54 | 40 | Accessory | 0.62 | ||
| 10. Allium scorodoprasum | 0.2 | 0.45 | 0.13 | 0.77 | 20 | Accidental | 0.15 | ||
| 11. Asparagus tenuifolius | 0.2 | 0.45 | 0.13 | 0.77 | 20 | Accidental | 0.15 | ||
| 12. Muscari comosum | 0.2 | 0.45 | 0.13 | 0.77 | 20 | Accidental | 0.15 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stamin, F.D.; Cosmulescu, S. Comparative Assessment of Biodiversity and Ecological Indicators in Three Forest Ecosystems of Southern Romania. Diversity 2025, 17, 277. https://doi.org/10.3390/d17040277
Stamin FD, Cosmulescu S. Comparative Assessment of Biodiversity and Ecological Indicators in Three Forest Ecosystems of Southern Romania. Diversity. 2025; 17(4):277. https://doi.org/10.3390/d17040277
Chicago/Turabian StyleStamin, Florin Daniel, and Sina Cosmulescu. 2025. "Comparative Assessment of Biodiversity and Ecological Indicators in Three Forest Ecosystems of Southern Romania" Diversity 17, no. 4: 277. https://doi.org/10.3390/d17040277
APA StyleStamin, F. D., & Cosmulescu, S. (2025). Comparative Assessment of Biodiversity and Ecological Indicators in Three Forest Ecosystems of Southern Romania. Diversity, 17(4), 277. https://doi.org/10.3390/d17040277






