Ecological Factors Associated with Burrow System Occupancy by Great Desert Skinks (Liopholis kintorei)
Abstract
1. Introduction
2. Methods
3. Results
3.1. Demographics
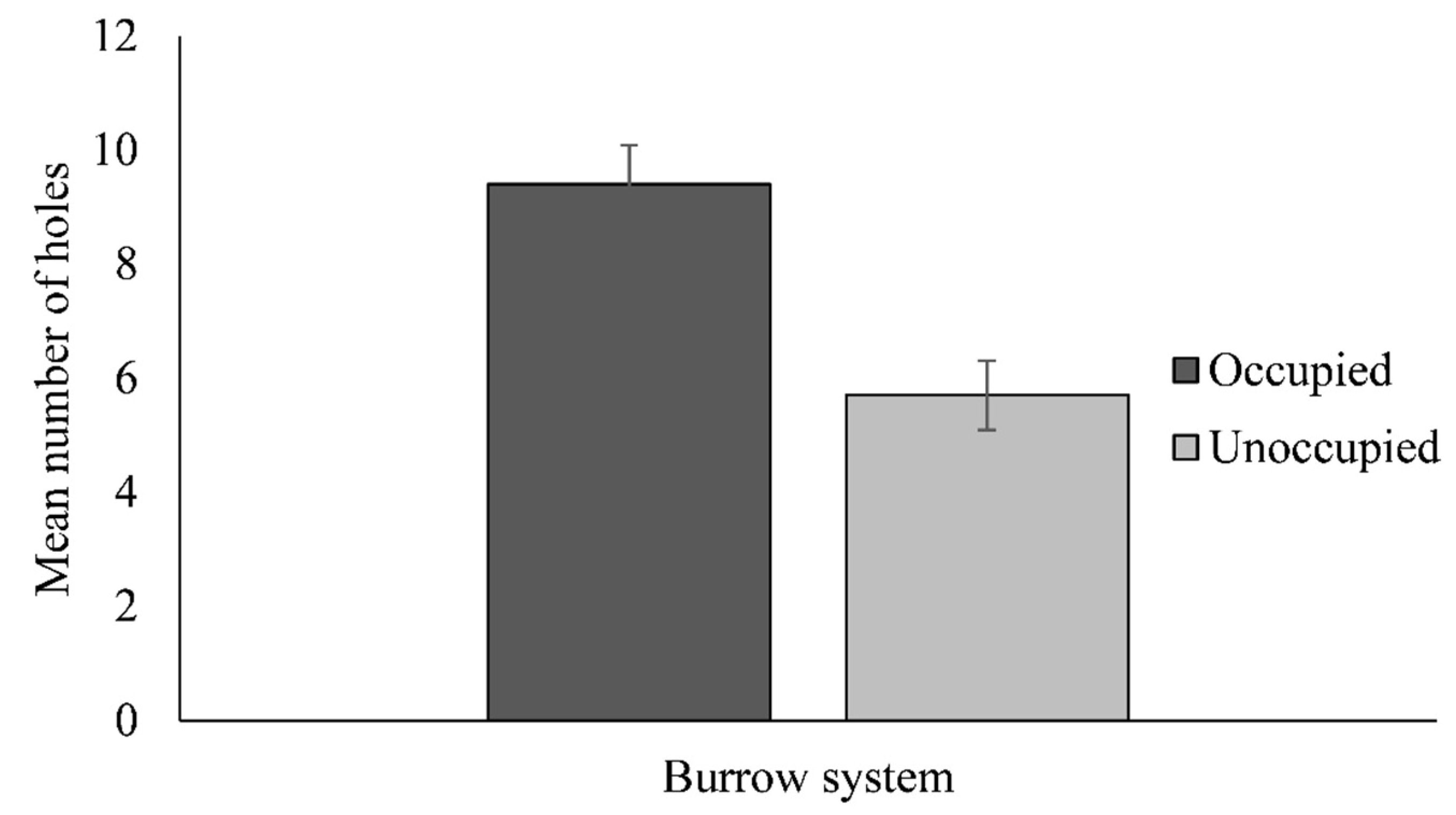
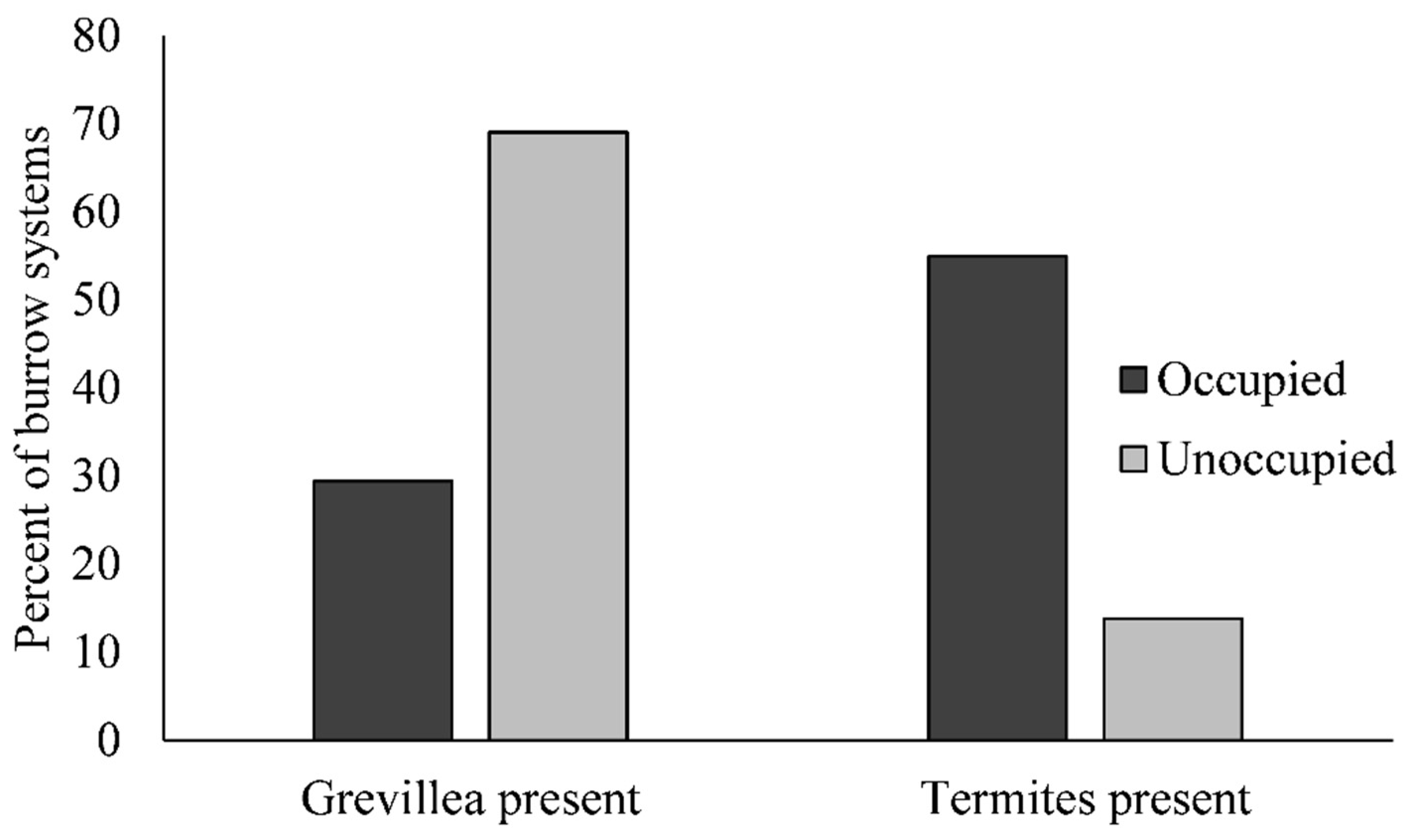
3.2. Habitat
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kinlaw, A. A review of borrowing by semi-fossorial vertebrates in arid environments. J. Arid. Environ. 1999, 41, 127–145. [Google Scholar] [CrossRef]
- Moore, D.; Kearney, M.R.; Paltridge, R.; McAlpin, S.; Stow, A. Is fire a threatening process for Liopholis kintorei, a nationally listed threatened skink? Wildl. Res. 2015, 42, 207–216. [Google Scholar] [CrossRef]
- Hodgson, M.J.; Ritchie, D. Strange bedfellows: Mammal burrow disturbances may provide thermoregulatory microsites for fossorial reptiles in densely vegetated dunes. Austral Ecol. 2023, 48, 1473–1478. [Google Scholar] [CrossRef]
- Morris, S.D.; Johnson, C.N.; Brook, B.W.; Kearney, M.R. Seasonal and depth-dependent thermoregulatory benefits of burrows for wombats—The largest burrowing marsupials. J. Therm. Biol. 2024, 125, 103961. [Google Scholar] [CrossRef] [PubMed]
- Cadenhead, N.C.R.; Kearney, M.R.; Moore, D.; McAlpin, S.; Wintle, B.A. Climate and fire scenario uncertainty dominate the evaluation of options for conserving the Great Desert Skink. Conserv. Lett. 2016, 9, 181–190. [Google Scholar] [CrossRef]
- Robb, G.N.; Harrison, A.; Woodborne, S.; Bennett, N.C. Diet composition of two common mole-rat populations in arid and mesic environments in South Africa as determined by stable isotope analysis. J. Zool. 2016, 300, 257–264. [Google Scholar] [CrossRef]
- Hoogland, J.L. The Black-Tailed Prairie Dog: Social Life of a Burrowing Mammal; Chicago University Press: Chicago, IL, USA, 1995. [Google Scholar]
- Spinks, A.C.; Bennett, N.C.; Jarvis, J.U.M. A comparison of the ecology of two populations of the common mole-rat, Cryptomys hottentotus hottentotus: The effect of aridity on food, foraging and body mass. Oecologia 2000, 125, 341–349. [Google Scholar] [CrossRef]
- Ebensperger, L.A.; Chesh, A.S.; Castro, R.A.; Tolhuysen, L.O.; Quirici, V.; Burger, J.R.; Sobrero, R.; Hayes, L.D. Burrow limitations and group living in the communally rearing rodent, Octodon degus. J. Mammal. 2011, 92, 21–30. [Google Scholar] [CrossRef]
- Chapple, D.G. Ecology, life-history, and behavior in the Australian scincid genus Egernia, with comments on the evolution of complex sociality in lizards. Herpetol. Monogr. 2003, 17, 145–180. [Google Scholar] [CrossRef]
- Moore, D.; Stow, A.; Kearney, M.R. Under the weather?—The direct effects of climate warming on a threatened desert lizard are mediated by their activity phase and burrow system. J. Anim. Ecol. 2017, 87, 660–671. [Google Scholar] [CrossRef]
- Du, W.-G.; Li, S.-R.; Sun, B.-J.; Shine, R. Can nesting behavior allow reptiles to adapt to climate change? Philos. Trans. R. Soc. B 2022, 278, 20220153. [Google Scholar]
- Hradsky, B.A.; Mildwaters, C.; Ritchie, E.G.; Christie, F.; Di Stefano, J. Responses of invasive predators and natural prey to a prescribed forest fire. J. Mammal. 2017, 98, 835–847. [Google Scholar] [CrossRef]
- Davidson, A.D.; Lightfoot, D.C.; McIntyre, J.L. Engineering rodents create key habitat for lizards. J. Arid. Environ. 2008, 72, 2142–2149. [Google Scholar] [CrossRef]
- Molyneux, J.; Pavey, C.R.; James, A.I.; Carthew, S.M. Habitat use by the brush-tailed mulgara (Dasycercus blythi). Aust. J. Zool. 2017, 65, 335–345. [Google Scholar] [CrossRef]
- Pianka, E.R. Convexity, desert lizards, and spatial heterogeneity. Ecology 1966, 47, 1055–1059. [Google Scholar] [CrossRef]
- Paltridge, R.; Catt, G.; Cowan, M.; Gaikhorst, G.; How, R.; Zichy-Woinarski, J.; Cogger, H.; Teale, R. Liopholis kintorei. IUCN Red List of Threatened Species; International Union for Conservation of Nature and Natural Resources: Gland, Switzerland, 2018. [Google Scholar] [CrossRef]
- Indigenous Desert Alliance. Looking After Tjakura, Tjalapa, Mulyamiji, Warrarna, Nampu: National Recovery Plan for the Great Desert Skink (Liopholis kintorei) 2023–2033; Department of Climate Change, Energy, the Environment and Water: Canberra, Australia, 2023.
- McAlpin, S.; Duckett, P.; Stow, A. Lizards cooperatively tunnel to construct a long-term home for family members. PLoS ONE 2011, 6, e19041. [Google Scholar] [CrossRef]
- McAlpin, S. A Recovery Plan for the Great Desert Skink (Egernia kintorei); Arid Lands Environment Centre: Alice Springs, Australia, 2001. [Google Scholar]
- Thuo, D.; Macgregor, N.A.; Merson, S.D.; Scopel, D.; Keogh, J.S.; Kenny, J.; Williams, J.L.; Guest, T.; Swan, S.; McAlpin, S.; et al. Metabarcoding clarifies the diet of the elusive and vulnerable Australian tjakuṟa (great desert skink, Liopholis kintorei). Front. Ecol. Evol. 2024, 12, 1354138. [Google Scholar] [CrossRef]
- Abensperg-Traun, M.; Steven, D. Ant- and termite-eating in Australian mammals and lizards: A comparison. Aust. J. Ecol. 1997, 22, 9–17. [Google Scholar] [CrossRef]
- McGregor, H.W.; Legge, S.; Jones, M.E.; Johnson, C.N. Landscape management of fire and grazing regimes alters fine-scale habitat utilisation by feral cats. PLoS ONE 2014, 9, e109097. [Google Scholar] [CrossRef]
- Moore, D.; Kearney, M.R.; Paltridge, R.; McAlpin, S.; Stow, A. Feeling the pressure at home: Predator activity at the burrow entrance of an endangered arid-zone skink. Austral. Ecol. 2018, 43, 102–109. [Google Scholar] [CrossRef]
- Ridley, J.C.H.; Schlesinger, C.A.; Bull, C.M. Location of long-term communal burrows of a threatened arid-zone lizard in relation to soil and vegetation. Austral. Ecol. 2020, 45, 444–453. [Google Scholar] [CrossRef]
- Ridley, J.C.H.; Schlesinger, C.A. Activity of tjakuṟa (great desert skinks) at burrows in relation to plant cover and predators. Ecol. Evol. 2023, 13, e10391. [Google Scholar] [CrossRef]
- Halliwell, B.; Uller, T.; Wapstra, E.; While, G.M. Resource distribution mediates social and mating behavior in a family living lizard. Behav. Ecol. 2017, 28, 145–153. [Google Scholar] [CrossRef]
- Halliwell, B.; Uller, T.; Chapple, D.G.; Gardner, M.G.; Wapstra, E.; While, G.M. Habitat saturation promotes delayed dispersal in a social reptile. Behav. Ecol. 2017, 28, 515–522. [Google Scholar] [CrossRef]
- Pavey, C.R.; Burwell, C.J.; Nano, C.E.M. Foraging ecology and habitat use of Slater’s skink (Egernia slateri): An endangered Australian desert skink. J. Herpetol. 2010, 44, 563–571. [Google Scholar] [CrossRef]
- Greenville, A.C.; Dickman, C.R. Factors affecting habitat selection in a specialist fossorial skink. Biol. J. Linn. Soc. 2009, 97, 531–544. [Google Scholar] [CrossRef]
- Masters, P.; Dickman, C.R.; Crowther, M. Effects of cover reduction on mulgara Dasycercus cristicauda (Marsupialia: Dasyuridae), rodent and invertebrate populations in central Australia: Implications for land management. Austral. Ecol. 2003, 28, 658–665. [Google Scholar] [CrossRef]
- Moseby, K.E.; McGregor, H.M. Feral cats use fine scale prey cues and microhabitat patches of dense vegetation when hunting prey in arid Australia. Glob. Ecol. Conserv. 2022, 35, e02093. [Google Scholar] [CrossRef]
- Griffiths, A.D.; Christian, K.A. Diet and habitat use of frillneck lizards in a seasonal tropical environment. Oecologia 1996, 106, 39–48. [Google Scholar] [CrossRef]
- Loveridge, J.P.; Moe, S.R. Termitaria as browsing hotspots for African megaherbivores in miombo woodland. J. Trop. Ecol. 2004, 20, 337–343. [Google Scholar] [CrossRef]
- Fleming, P.A.; Loveridge, J.P. Miombo woodland termite mounds: Resource islands for small vertebrates? J. Zool. Soc. Lond. 2003, 259, 161–168. [Google Scholar] [CrossRef]
- Vitt, L.J.; Shepard, D.B.; Caldwell, J.P.; Vieira, G.H.C.; França, F.G.R.; Colli, G.R. Living with your food: Geckos in termitaria in Cantão. J. Zool. 2007, 272, 321–328. [Google Scholar] [CrossRef]
- Moro, D.; Cullen, P.; Fletcher, J. Vertebrate fauna in termite mounds compared to surrounding vegetation on Barrow Island. Pac. Conserv. Biol. 2014, 20, 296–301. [Google Scholar] [CrossRef]
- Thompson, G.G.; Thompson, S.A. Termitaria are an important refuge for reptiles in the Pilbara of Western Australia. Pac. Conserv. Biol. 2015, 21, 226–233. [Google Scholar] [CrossRef]
- Whittingham, M.J.; Evans, K.L. The effects of habitat structure on predation risk of birds in agricultural landscapes. Ibis 2004, 146, 210–220. [Google Scholar] [CrossRef]
- Risbey, D.A.; Calver, M.C.; Short, J.; Bradley, J.S.; Wright, I.W. The impact of cats and foxes on the small vertebrate fauna of Heirisson Prong, Western Australia. II. A field experiment. Wildl. Res. 2000, 27, 223–235. [Google Scholar] [CrossRef]
- Denny, E.A.; Dickman, C.R. Review of Cat Ecology and Management Strategies in Australia; Invasive Animals Cooperative Research Centre: Canberra, Australia, 2010. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eifler, M.A.; Eifler, D.A. Ecological Factors Associated with Burrow System Occupancy by Great Desert Skinks (Liopholis kintorei). Diversity 2025, 17, 134. https://doi.org/10.3390/d17020134
Eifler MA, Eifler DA. Ecological Factors Associated with Burrow System Occupancy by Great Desert Skinks (Liopholis kintorei). Diversity. 2025; 17(2):134. https://doi.org/10.3390/d17020134
Chicago/Turabian StyleEifler, Maria A., and Douglas A. Eifler. 2025. "Ecological Factors Associated with Burrow System Occupancy by Great Desert Skinks (Liopholis kintorei)" Diversity 17, no. 2: 134. https://doi.org/10.3390/d17020134
APA StyleEifler, M. A., & Eifler, D. A. (2025). Ecological Factors Associated with Burrow System Occupancy by Great Desert Skinks (Liopholis kintorei). Diversity, 17(2), 134. https://doi.org/10.3390/d17020134





