Revisiting the Invasion: A Success Story of Crayfish Species in Piedmont Plain Lakes (NW Italy)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field and Laboratory Procedures
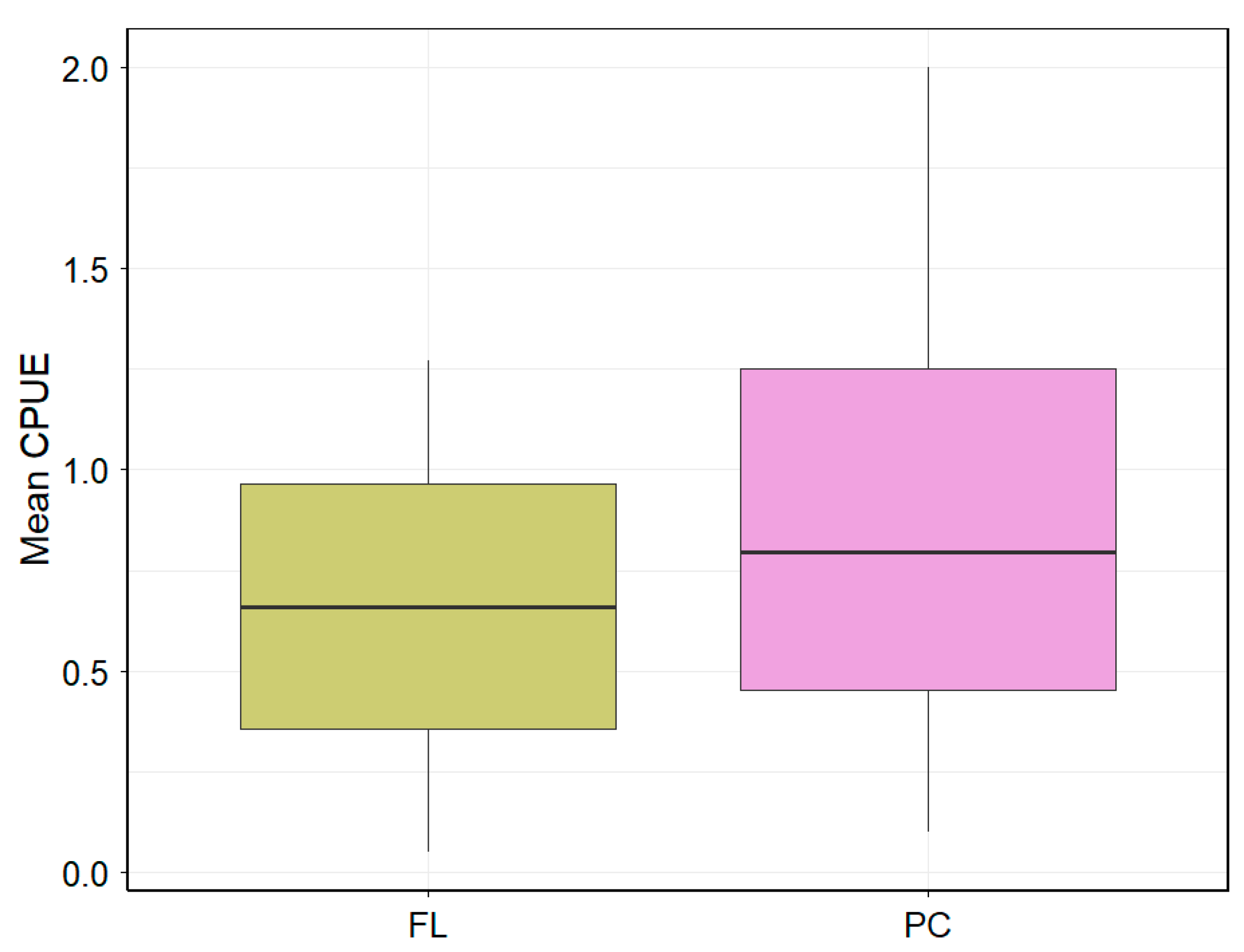
3. Results
4. Discussion
Historical Context
| Cited Taxon | Data Type | Sampling | Sites | Reference |
|---|---|---|---|---|
| O. limosus | O | traps | Baldissero pond | Delmastro, 1999 [40] |
| O. limosus | A | fishing net, traps | lakes Maggiore, Orta | Bazzoni, 2006 [41] |
| O. limosus | O | literature, observations | multiple localities | Morpurgo et al., 2010 [42] |
| O. limosus | D | traps | Lake Maggiore | Garzoli et al., 2020 [17] |
| F. limosus | O | active search, traps | lakes Maggiore, Mergozzo, Orta | Boggero et al., 2023 [39] |
| F. limosus | D | traps | Lake Orta | Boggero et al., 2025a [43] |
| F. limosus | D | observation, traps | Lake Maggiore | Boggero et al., 2025b [44] |
| F. limosus | D | traps | Lake Mergozzo | Kamburska et al., 2025 [45] |
| P. clarkii | A, O | dip net, electrofishing, observations | Venesima stream | Delmastro, 1992 [46] |
| P. clarkii | O | observations | multiple localities | Delmastro, 1994 [47] |
| P. clarkii | O | observations | multiple localities | Delmastro, 1999 [40] |
| P. clarkii | O | literature, observations | multiple localities | Morpurgo et al., 2010 [42] |
| P. clarkii | O | observations | Lake Orta | Piscia et al., 2011a [48] |
| P. clarkii | O | observations | Lake Orta | Piscia et al., 2011b [49] |
| P. clarkii | D | traps | Lake Candia | Donato, 2016 [50] |
| P. clarkii | O | observations | lakes Bertignano, Viverone | Regione Piemonte, 2017a [51] |
| P. clarkii | O | observations | Lake Viverone | Regione Piemonte, 2017b [52] |
| P. clarkii | O | dip net, electrofishing, fishing net, observations, torches, traces, traps | lakes Avigliana G., Campagna, Candia, Gay-Stroppiana, Maggiore, del Malpasso, Orta, Viverone | Delmastro, 2017 [53] |
| P. clarkii | D | traps | Lake Candia | Donato et al., 2018 [37] |
| P. clarkii | O | observations | Lake Candia | Città Metropolitana Torino, 2019 [54] |
| P. clarkii | O | literature | multiple localities | Lo Parrino et al., 2020 [55] |
| P. clarkii | A | traps | Lake Candia | Pastorino et al. 2023 [56] |
| P. clarkii | A | active search | Lake Candia | Scoparo et al., 2023 [57] |
| P. clarkii | D | traps | Lake Orta | Kamburska et al., 2024 [58] |
| P. clarkii | A | traps | lakes Avigliana, tributaries | Maganza et al., 2025 [59] |
| P. clarkii | D | traps | Lake Orta | Boggero et al., 2025a [43] |
| P. clarkii | D | observations, traps | Lake Orta | Boggero et al., 2025b [44] |
| P. leniusculus | O | active search, observations | Valla stream | Candiotto et al., 2010 [60] |
| P. leniusculus | O | observations | Lake Maggiore | Boggero et al., 2018 [21] |
| P. leniusculus | D | active search, traps | rivers Valla, Erro | Larson et al., 2022 [38] |
| P. leniusculus | D | observations, taps | Lake Maggiore | Boggero et al., 2025b [44] |
5. Conclusions
- An inventory of non-native crayfish for Piedmont lakes had never been conducted prior to 2025, making this the first comprehensive effort to document the occurrence and to assess the distribution of non-native crayfish species within the region. Extensive baited-trap monitoring allowed us to confirm the occurrence of two non-native species of North American origin (F. limosus, and P. clarkii) and to record P. clarkii for the first time ever in three lakes that had never been monitored before (Pistono, San Michele, and Sirio).
- Isolated lakes or those with complex predator–prey networks or with illegal activities host few or lack non-native crayfish because limited connectivity restricts dispersal while high predator and human pressures and biotic interactions lower population density and thus detectability.
- Standardized monitoring of crayfish populations is not only crucial for assessing the ecological health of freshwater ecosystems but also to enable comparison of data and trend analyses.
- This baseline is crucial for informing managers to develop effective strategies to prevent further spread and to protect Piedmont’s freshwaters. Therefore, coordinated measures are required to prevent and control the ecological threats posed by invasive species.
- Moreover, improving information exchange across stakeholders and water managers is necessary to halt illegal harvesting for food and pet trade, since all these activities highly contribute to crayfish spread.
- The data we obtained help fill a knowledge gap not only in our region but across Italian lakes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mathers, K.L.; White, J.C.; Fornaroli, R.; Chadd, R. Flow regimes control the establishment of invasive crayfish and alter their effects on lotic macroinvertebrate communities. J. Appl. Ecol. 2020, 57, 886–902. [Google Scholar] [CrossRef]
- Soto, I.; Ahmed, D.A.; Beidas, A.; Oficialdegui, F.J.; Tricarico, E.; Angeler, D.G.; Haubrock, P.J. Long-term trends in crayfish invasions across European rivers. Sci. Total Environ. 2023, 867, 161537. [Google Scholar] [CrossRef]
- Giordano, J.; Battisti, C. The non native red swamp crayfish Procambarus clarkii as prey for waterbirds: A note from Torre Flavia wetland (Central Italy). Alula 2023, 30, 194–199. [Google Scholar] [CrossRef]
- Reynolds, C.; Miranda, N.A.F.; Cumming, G.S. The role of waterbirds in the dispersal of aquatic alien and invasive species. Divers. Distrib. 2015, 21, 744–754. [Google Scholar] [CrossRef]
- Lodge, D.M.; Taylor, C.M.; Holdich, D.M.; Skurdal, J. Non-indigenous crayfishes threaten North American freshwater biodiversity: Lessons from Europe. Fisheries 2000, 25, 7–20. [Google Scholar] [CrossRef]
- Emery-Butcher, H.E.; Beatty, S.J.; Robson, B.J. The impacts of invasive ecosystem engineers in freshwaters: A review. Freshw. Biol. 2020, 65, 999–1015. [Google Scholar] [CrossRef]
- Crandall, K.A.; de Grave, S. An updated classification of the freshwater crayfishes (Decapoda: Astacidea) of the world, with a complete species list. J. Crustac. Biol. 2017, 37, 615–653. [Google Scholar] [CrossRef]
- Faulkes, Z. Prohibiting pet crayfish does not consistently reduce their availability online. Nauplius 2018, 26, e2018023. [Google Scholar] [CrossRef]
- Patoka, J.; Kocánová, B.; Kalous, L. Crayfish in Czech cultural space: The longest documented relationship between humans and crayfish in Europe. Knowl. Manag. Aquat. Ecosyst. 2016, 417, 5. [Google Scholar] [CrossRef]
- Lindqvist, O.V.; Huner, J.V. Life history characteristics of crayfish: What makes some of them good colonizers? In Crayfish in Europe as Alien Species. How to Make the Best of a Bad Situation? Gherardi, F., Holdich, D.M., Eds.; Balkema: Rotterdam, The Netherland, 1999; pp. 23–30. [Google Scholar] [CrossRef]
- Moorhouse, T.P.; Macdonald, D.W. Are invasives worse in freshwater than terrestrial ecosystems? WIREs Water 2015, 2, 1–8. [Google Scholar] [CrossRef]
- Simberloff, D. Maintenance management and eradication of established aquatic invaders. Hydrobiologia 2021, 848, 2399–2420. [Google Scholar] [CrossRef]
- Lodge, D.M.; Deines, A.; Gherardi, F.; Yeo, D.C.J.; Arcella, T.; Baldridge, A.K.; Barnes, M.A.; Chadderton, W.L.; Feder, J.L.; Gantz, C.A.; et al. Global Introductions of Crayfishes: Evaluating the Impact of Species Invasions on Ecosystem Services. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 449–472. [Google Scholar] [CrossRef]
- O’Hea-Miller, S.B.; Davis, A.R.; Wong, M.Y.L. The impacts of invasive crayfish and other non-native species on native freshwater crayfish: A review. Biology 2024, 13, 610. [Google Scholar] [CrossRef]
- Olden, J.D.; Whattam, E.; Wood, S.A. Online auction marketplaces as a global pathway for aquatic invasive species. Hydrobiologia 2021, 848, 1967–1979. [Google Scholar] [CrossRef] [PubMed]
- Haubrock, P.J.; Cuthbert, R.N.; Haase, P. Long-term trends and drivers of biological invasion in Central European streams. Sci. Total Environ. 2023, 876, 162817. [Google Scholar] [CrossRef] [PubMed]
- Garzoli, L.; Mammola, S.; Ciampittiello, M.; Boggero, A. Alien crayfish species in the deep subalpine Lake Maggiore (NW-Italy), with a focus on the biometry and habitat preferences of the spiny-cheek crayfish. Water 2020, 12, 1391. [Google Scholar] [CrossRef]
- Demers, A.; Reynolds, J.D. Water quality requirements of the white-clawed crayfish, Austropotamobius pallipes, a field study. Freshw. Crayfish 2006, 15, 283–291. [Google Scholar]
- Gray, A.; Mayer, K.; Gore, B.; Gaesser, M.; Ferguson, N. Microplastic burden in native (Cambarus appalachiensis) and non-native (Faxonius cristavarius) crayfish along semi-rural and urban streams in southwest Virginia, USA. Environ. Res. 2024, 258, 119494. [Google Scholar] [CrossRef]
- Souty-Grosset, C.; Holdich, D.M.; Noël, P.Y.; Reynolds, J.D.; Haffner, P. Atlas of Crayfish in Europe; Patrimoines Naturels: Paris, France, 2006; Volume 64, 187p. [Google Scholar]
- Boggero, A.; Dugaro, M.; Migliori, L.; Garzoli, L. Prima segnalazione del gambero invasivo Pacifastacus leniusculus (Dana 1852) nel Lago Maggiore (Cantone Ticino, Svizzera). Boll. Della STSN 2018, 106, 103–106. [Google Scholar]
- Welch, B.L. The significance of the difference between two means when the population variances are unequal. Biometrika 1938, 29, 350–362. [Google Scholar] [CrossRef]
- Regulation (EU). No. 1143/2014 of the European Parliament and of the Council on the Prevention and Management of the Introduction and Spread of Invasive Alien Species. Available online: https://eur-lex.europa.eu/eli/reg/2014/1143/2019-12-14 (accessed on 16 October 2025).
- Caffrey, J.M.; Baars, J.-R.; Barbour, J.H.; Boets, P.; Boon, P.; Davenport, K.; Dick, J.T.A.; Early, J.; Edsman, L.; Gallagher, C.; et al. Tackling Invasive Alien Species in Europe: The Top 20 Issues. Manag. Biol. Invasion 2014, 5, 1–20. [Google Scholar] [CrossRef]
- Holdich, D.M.; Reynolds, J.D.; Souty-Grosset, C.; Sibley, P.J. A review of the ever increasing threat to European crayfish from non-indigenous crayfish species. Knowl. Manag. Aquat. Ecosyst. 2009, 11, 394–395. [Google Scholar] [CrossRef]
- Aquiloni, L.; Tricarico, E.; Gherardi, F. Crayfish in Italy: Distribution, threats and management. Int. Aquat. Res. 2010, 2, 1–14. [Google Scholar]
- Manenti, R.; Ghia, D.; Fea, G.; Ficetola, G.F.; Padoa-Schioppa, E.; Canedoli, C. Causes and consequences of crayfish extinction: Stream connectivity, habitat changes, alien species and ecosystem services. Freshw. Biol. 2019, 64, 284–293. [Google Scholar] [CrossRef]
- Siesa, M.E.; Manenti, R.; Padoa-Schioppa, E.; De Bernardi, F.; Ficetola, G.F. Spatial autocorrelation and the analysis of invasion processes from distribution data: A study with the crayfish Procambarus clarkii. Biol. Invasions 2011, 13, 2147–2160. [Google Scholar] [CrossRef]
- Correia, C.; Borges, L. Spanish Crayfish, Procambarus Clarkii, with Fyke Nets & Traps in Andalusia and Extremadura; Fith Report of the implementation of the FIP; 2024; p. 19. Available online: https://fisheryprogress.org/sites/default/files/documents_actions/FishFix%20Crayfish%20FIP%20report%20FINAL%209%20MAR%202022.pdf (accessed on 16 October 2025).
- Holdich, D.M. Biology of Freshwater Crayfish; Blackwell Science Ltd.: Oxford, UK, 2002. [Google Scholar]
- Englund, G.; Krupa, J.J. Habitat use by crayfish in stream pools: Influence of predators, depth and body size. Freshw. Biol. 2000, 43, 75–83. [Google Scholar] [CrossRef]
- Pérez-Santigosa, N.; Hidalgo-Vila, J.; Florencio, M.; Díaz-Paniagua, C. Does the exotic invader turtle, Trachemys scripta elegans, compete for food with coexisting native turtles? Amphib.-Reptil. 2011, 32, 167–175. [Google Scholar] [CrossRef]
- Ferreira, M.; Gago, J.; Ribeiro, F. Diet of European Catfish in a Newly Invaded Region. Fishes 2019, 4, 58. [Google Scholar] [CrossRef]
- David, P.; Thebault, E.; Anneville, O.; Duyck, P.F.; Chapuis, E.; Loeuille, N. Impacts of invasive species on food webs: A review of empirical data. Adv. Ecol. Res. 2017, 56, 1–60. [Google Scholar] [CrossRef]
- Móréh, Á.; Scheuring, I. Invaders’ trophic position and their direct and indirect relationship influence on resident food webs. Ecol. Model. 2025, 508, 111238. [Google Scholar] [CrossRef]
- Gherardi, F.; Aquiloni, L.; Diéguez-Uribeondo, J.; Tricarico, E. Managing invasive crayfish: Is there a hope? Aquat. Sci. 2011, 73, 185–200. [Google Scholar] [CrossRef]
- Donato, R.; Rollandin, M.; Favaro, L.; Ferrarese, A.; Pessani, D.; Ghia, D. Habitat use and population structure of the invasive red swamp crayfish Procambarus clarkii (Girard, 1852) in a protected area in northern Italy. Knowl. Manag. Aquat. Ecosyst. 2018, 419, 12. [Google Scholar] [CrossRef]
- Larson, C.E.; Bo, T.; Candiotto, A.; Fenoglio, S.; Doretto, A. Predicting invasive signal crayfish (Pacifastacus leniusculus) spread using a traditional survey and river network simulation. River Res. Appl. 2022, 38, 1424–1435. [Google Scholar] [CrossRef]
- Boggero, A.; Croci, C.; Zanaboni, A.; Zaupa, S.; Paganelli, D.; Garzoli, L.; Bras, T.; Busiello, A.; Orrù, A.; Beatrizzutti, S.; et al. New records of the spinycheek crayfish Faxonius limosus (Rafinesque, 1817): Expansion in subalpine lakes in north-western Italy. Bioinvasions Rec. 2023, 12, 445–456. [Google Scholar] [CrossRef]
- Delmastro, G.B. Annotazioni sulla storia naturale del Gambero della Louisiana Procambarus clarkii (Girard, 1852) in Piemonte centrale e prima segnalazione regionale del Gambero americano Orconectes limosus (Rafinesque, 1817) (Crustacea: Decapoda: Astacidea: Cambaridae). Rivista Piemont. Storia Nat. 1999, 20, 65–92. [Google Scholar]
- Bazzoni, P. Censimento e Studio delle Popolazioni di Gambero D’acqua dolce Nell’area del Verbano-Cusio-Ossola; Azienda Agricola Ossolana Acque: Verbania, Italy, 2006; p. 25. [Google Scholar]
- Morpurgo, M.; Aquiloni, L.; Bertocchi, S.; Brusconi, S.; Tricarico, E.; Gherardi, F. Distribuzione dei gamberi d’acqua dolce in Italia. STSN 2010, 87, 125–132. [Google Scholar]
- Boggero, A.; Croci, C.; Orlandi, M.; Paganelli, D.; Zaupa, S.; Kamburska, L. Invasive Crayfish Occurrence in the Subalpine Lake Orta (NW Italy) in 2021–2022, Version 1.10; Occurrence Dataset; Consiglio Nazionale delle Ricerche—Istituto di Ricerca sulle Acque: Verbania Pallanza, Italy, 2025. [Google Scholar] [CrossRef]
- Boggero, A.; Garzoli, L.; Orlandi, M.; Zaupa, S.; Kamburska, L. Invasive Crayfish Occurrence in the Subalpine Lake Maggiore (NW Italy) in 2017–2018, Version 1.10; Occurrence Dataset; Consiglio Nazionale delle Ricerche—Istituto di Ricerca sulle Acque: Verbania Pallanza, Italy, 2025. [Google Scholar] [CrossRef]
- Kamburska, L.; Croci, C.; Orlandi, M.; Paganelli, D.; Zaupa, S.; Boggero, A. Occurrence of Invasive Faxonius Limosus in the Subalpine Lake Mergozzo (NW-Italy) in 2021–2022, Version 1.8; Occurrence Dataset; Consiglio Nazionale delle Ricerche—Istituto di Ricerca sulle Acque: Verbania Pallanza, Italy, 2025. [Google Scholar] [CrossRef]
- Delmastro, G.B. Sull’acclimatazione del gambero della Louisiana Procambarus clarkii (Girard, 1852) nelle acque dolci italiane (Crustacea: Decapoda: Cambaridae). Pianura—Suppl. Prov. Nuova Cremona 1992, 4, 5–10. [Google Scholar]
- Delmastro, G.B. Il gambero della Louisiana, un nuovo abitatore delle nostre acque. Il Notiziario, Pro Natura Carmagnola 1994, 19. [Google Scholar]
- Piscia, R.; Volta, P.; Boggero, A.; Manca, M. The invasion of Lake Orta (Italy) by the red swamp crayfish Procambarus clarkii (Girard, 1852): A new threat to an unstable environment. Aquat. Invasions 2011, 6, S45–S48. [Google Scholar] [CrossRef]
- Piscia, R.; Volta, P.; Boggero, A.; Manca, M. Segnalazione di Procambarus clarkii (Girard 1852) una specie aliena invasiva, nel Lago d’Orta (Piemonte). Biol. Ambient. 2011, 25, 71–73. [Google Scholar]
- Donato, R. Life history e Struttura di Popolazione di Procambarus clarkii (Girard, 1852) (Crustacea, Cambaridae) nel Parco Naturale del Lago di Candia (TO). Master’s Thesis, Università degli Studi di Torino, Turin, Italy, 2016; p. 122. [Google Scholar]
- Regione Piemonte. Piano di Gestione: Zona Speciale di Conservazione IT1130004—Lago di Bertignano e Stagni di Roppolo. 2017, p. 191. Available online: https://www.regione.piemonte.it/giscartografia/Parchi/Piani/IT1130004_PdG_Relazione.pdf (accessed on 16 October 2025).
- Regione Piemonte. Piano di Gestione: Zona Speciale di Conservazione e Zona di Protezione Speciale IT1110020—Lago di Viverone. 2017, p. 184. Available online: https://www.regione.piemonte.it/giscartografia/Parchi/Piani/IT1110020_PdG_Relazione.pdf (accessed on 16 October 2025).
- Delmastro, G.B. Il gambero della Louisiana Procambarus clarkii (Girard, 1852) in Piemonte: Nuove osservazioni su distribuzione, biologia, impatto e utilizzo (Crustacea: Decapoda: Cambaridae). Rivista Piemont. Storia Nat. 2017, 38, 61–129. [Google Scholar]
- Città Metropolitana Torino—Parco Naturale Lago di Candia. Piano di Gestione: Zona Speciale di Conservazione (ZSC) Zona di Protezione Speciale (ZPS) IT1110036—Lago di Candia. 2019, p. 204. Available online: http://www.cittametropolitana.torino.it/cms/risorse/natura/dwd/pdf/aree_protette/aree/candia/PdG/PdG__Relazione.pdf (accessed on 16 October 2025).
- Lo Parrino, E.; Ficetola, G.F.; Manenti, R.; Falaschi, M. Thirty years of invasion: The distribution of the invasive crayfish Procambarus clarkii in Italy. Biogeographia 2020, 35, 43–50. [Google Scholar] [CrossRef]
- Pastorino, P.; Anselmi, S.; Zanoli, A.; Esposito, G.; Bondavalli, F.; Dondo, A.; Pucci, A.; Pizzul, E.; Faggio, C.; Barceló, D.; et al. The invasive red swamp crayfish (Procambarus clarkii) as a bioindicator of microplastic pollution: Insights from Lake Candia (northwestern Italy). Ecol. Indic. 2023, 150, 110200. [Google Scholar] [CrossRef]
- Scoparo, M.; Cardinali, I.; La Porta, G.; Caldaroni, B.; Magara, G.; Dörr, A.J.M.; Elia, A.C.; Lancioni, H. Phylogenetic Diversity of the Red Swamp Crayfish Procambarus clarkii and Its Dispersal Pattern in Northern and Central Italy. Biology 2023, 12, 313. [Google Scholar] [CrossRef] [PubMed]
- Kamburska, L.; Sabatino, R.; Schiavetta, D.; De Santis, V.; Ferrari, E.; Mor, J.-R.; Zaupa, S.; Garzoli, L.; Boggero, A. A new misleading colour morph: Is Marmorkrebs the only “marbled” crayfish? BioInvasions Rec. 2024, 13, 949–961. [Google Scholar] [CrossRef]
- Maganza, A.; Gabetti, A.; Mossotto, C.; Pastorino, P.; Esposito, G.; Di Nicola, M.R.; Rizzioli, B.; Elia, A.C.; Prearo, M. Assessing the distribution and habitat suitability of Austropotamobius pallipes complex in proximity of invasive Procambarus clarkii in the Avigliana Lakes (northwest Italy): An integrated approach to ecosystem health and conservation. Aquat. Sci. 2025, 87, 50. [Google Scholar] [CrossRef]
- Candiotto, A.; Delmastro, G.B.; Dotti, L.; Sindaco, R. Pacifastacus leniusculus (Dana, 1852), un nuovo gambero esotico naturalizzato in Piemonte (Crustacea: Decapoda: Astacidae). Rivista Piemont. Storia Nat. 2010, 31, 73–82. [Google Scholar]
- Kim, J.-H.; Nam, S.-H.; Kim, M.-K.; Serrano-Notivoli, R.; Tejedor, E. The 2022 record-high heat waves over southwestern Europe and their underlying mechanism. Weather Clim. Extrem. 2024, 46, 100729. [Google Scholar] [CrossRef]
- Lake, P.S.; Palmer, M.A.; Biro, P.; Cole, J.; Covich, A.P.; Dahm, C.; Verhoeven, J.O.S. Global change and the biodiversity of freshwater ecosystems: Impacts on linkages between above-sediment and sediment biota: All forms of anthropogenic disturbance—Changes in land use, biogeochemical processes, or biotic addition or loss—Not only damage the biota of freshwater sediments but also disrupt the linkages between above-sediment and sediment-dwelling biota. BioScience 2000, 50, 1099–1107. [Google Scholar] [CrossRef]
- Waters, M.N.; Piehler, M.F.; Rodriguez, A.B.; Smoak, J.M.; Bianchi, T.S. Shallow lake trophic status linked to late Holocene climate and human impacts. J. Paleolimnol. 2009, 42, 51–64. [Google Scholar] [CrossRef]
- Tricarico, E.; Aquiloni, L. How Behaviour Has Helped Invasive Crayfish to Conquer Freshwater Ecosystems. In Biological Invasions and Animal Behaviour; Weis, J.S., Sol, D., Eds.; Cambridge University Press: Cambridge, UK, 2016; pp. 291–308. [Google Scholar] [CrossRef]



| Lake | State-Province | Protection | Altitude | Lat N | Long E | Lake Area | Max Depth |
|---|---|---|---|---|---|---|---|
| Viverone | BI/TO/VC | SAC/SPA | 230 | 45°24′59″ | 08°02′08″ | 5.7 | 50 |
| Alice Superiore | TO | SAC | 575 | 45°27′44″ | 07°47′43″ | 0.1 | 11 |
| Avigliana Grande | TO | SAC/SPA | 352 | 45°03′57″ | 07°23′13″ | 0.9 | 28 |
| Avigliana Piccolo | TO | SAC/SPA | 356 | 45°03′13″ | 07°23′30″ | 0.6 | 12 |
| Bertignano | TO | SAC/SPA | 377 | 45°25′56″ | 08°03′44″ | 0.1 | 11 |
| Campagna | TO | SAC/SPA | 238 | 45°29′02″ | 07°53′42″ | 0.1 | 5 |
| Candia | TO | SAC/SPA | 226 | 45°19′25″ | 07°54′43″ | 1.5 | 8 |
| Meugliano | TO | SAC | 715 | 45°28′36″ | 07°47′23″ | 0.03 | 11 |
| Nero | TO | SAC/SPA | 342 | 45°30′17″ | 07°52′24″ | 0.1 | 27 |
| Pistono | TO | SAC/SPA | 280 | 45°29′34″ | 07°52′28″ | 0.1 | 16 |
| San Michele | TO | SAC/SPA | 238 | 45°28′37″ | 07°53′16″ | 0.1 | 19 |
| Sirio | TO | SAC/SPA | 266 | 45°29′13″ | 07°53′02″ | 0.3 | 44 |
| Mergozzo | VCO | 204 | 45°57′20″ | 08°28′00″ | 1.8 | 73 | |
| Moncrivello | VC | SAC/SPA | 263 | 45°20′24″ | 07°59′32″ | 0.03 | 2 |
| Maglione | VC | SAC/SPA | 251 | 45°20′43″ | 07°59′44″ | 0.1 | 2 |
| Orta | VCO/NO | 290 | 45°49′02″ | 08°24′24″ | 18.2 | 143 | |
| Maggiore | CH-VCO/NO/VA | 193 | 46°05′53″ | 08°42′53″ | 212.5 | 372 |
| Lake | Crayfish | Mean CPUE |
|---|---|---|
| Viverone | PC | 0.44 |
| Alice Superiore | -- | -- |
| Avigliana Grande | PC | 0.49 |
| Avigliana Piccolo | -- | -- |
| Bertignano | PC | 1.27 |
| Campagna | PC | 1.19 |
| Candia | PC | 2.00 |
| Meugliano | -- | -- |
| Nero | -- | -- |
| Pistono | PC | 1.50 |
| San Michele | PC | 1.10 |
| Sirio | PC | 0.10 |
| Mergozzo | -- | -- |
| Moncrivello | -- | -- |
| Maglione | -- | -- |
| Orta | FL, PC | 0.05, 0.49 |
| Maggiore | FL, PC | 1.27, 0.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boggero, A.; Orlandi, M.; Zaupa, S.; Kamburska, L. Revisiting the Invasion: A Success Story of Crayfish Species in Piedmont Plain Lakes (NW Italy). Diversity 2025, 17, 868. https://doi.org/10.3390/d17120868
Boggero A, Orlandi M, Zaupa S, Kamburska L. Revisiting the Invasion: A Success Story of Crayfish Species in Piedmont Plain Lakes (NW Italy). Diversity. 2025; 17(12):868. https://doi.org/10.3390/d17120868
Chicago/Turabian StyleBoggero, Angela, Marco Orlandi, Silvia Zaupa, and Lyudmila Kamburska. 2025. "Revisiting the Invasion: A Success Story of Crayfish Species in Piedmont Plain Lakes (NW Italy)" Diversity 17, no. 12: 868. https://doi.org/10.3390/d17120868
APA StyleBoggero, A., Orlandi, M., Zaupa, S., & Kamburska, L. (2025). Revisiting the Invasion: A Success Story of Crayfish Species in Piedmont Plain Lakes (NW Italy). Diversity, 17(12), 868. https://doi.org/10.3390/d17120868









