Abstract
The dynamic behavior and biologically mediated transformation of microplastics (MPs) in crustaceans remain insufficiently explored in aquatic ecotoxicology. In this study, we employed the red swamp crayfish (Procambarus clarkii) as a model organism to systematically investigate the accumulation, distribution, fragmentation, and excretion kinetics of MPs within its digestive system under controlled conditions. We exposed crayfish to fluorescent polystyrene microplastics (50 μm) at a high concentration (100,000 particles/L), which exceeded typical environmental levels but was necessary to track accumulation and fragmentation dynamics within the experimental timeframe, and dissections were performed at 24, 48, and 96 h. Spatiotemporal patterns and morphological changes in MPs were analyzed using advanced microscopic imaging techniques. The results revealed a peak in MP accumulation at 48 h, followed by a decrease at 96 h, suggesting a dynamic equilibrium between ingestion and elimination. Over time, particle sizes decreased significantly, a result consistent with microplastic fragmentation. Additionally, feed supplementation during depuration was associated with increased fragmentation efficiency. Morphological analysis showed digestion-induced changes such as surface wrinkling, irregular edges, and particle shrinkage. These findings elucidate the transformation mechanisms of microplastics within crustaceans and provide crucial insights for assessing their potential ecological risks and fate as pollutants. Based on results from high-concentration short-term laboratory exposure studies, this paper further indicates the necessity for in-depth exploration into the long-term dynamics of microplastics within aquatic organisms and the potential for their transfer across trophic levels.
1. Introduction
The lightweight and durable nature of plastics has driven an exponential increase in global production, rising from 2 million tons in 1950 to 350 million tons in 2019, with projections reaching 700 million tons by 2040 [1]. This dramatic expansion has led many researchers to label the current era as the “Plastic Age” [2]. However, the same chemical stability that makes plastics useful also makes them highly resistant to degradation in natural environments, contributing to significant ecological harm [3,4]. For instance, a study by Mibrandt et al. (2019) found that only 5% of the 4400 tons of regulated plastic waste in the United States was recycled, while 86% was disposed of in landfills [5]. Over time, landfilled plastics fragment into microplastics (MPs), particles smaller than 5 mm, through processes such as weathering, photolysis, and mechanical abrasion [6]. The sizes of these can be divided into three categories: macroplastics range from 25 mm to 1 m, mesoplastics fall between 5 mm and 25 mm, microplastics range from 1 μm to 5 mm, and nanoplastics are defined as those smaller than 1 μm [7]. MPs exhibit high environmental persistence due to their strong molecular bonds, resulting in extremely slow degradation that can span centuries or longer [8]. Their small size not only complicates removal efforts but also enhances ecological risks [9,10]. These processes not only accelerate physical fragmentation but also modify surface characteristics (e.g., increased roughness or hydrophobicity) [11], thereby enhancing the leaching of toxic additives (e.g., PAE and PBDE) initially incorporated during manufacturing [12,13].
Microplastic pollution has emerged as a global environmental concern due to its pervasive presence across ecosystems. Aquatic environments, in particular, serve as major sinks for MPs, receiving substantial annual inputs of plastic debris [14,15]. Numerous studies have documented MP contamination in lakes [16,17], rivers [18], oceans [19], and soils [20]. For example, Sulistyowati et al. (2020) reported MP abundances ranging from 13.33 to 113.33 particles/m3 in the surface waters of Indonesia’s Cisadane River and its confluence with the Java Sea, with polyethylene, polystyrene, and polypropylene dominating polymer compositions [21]. Similarly, Eo et al. (2019) detected elevated concentrations (293–4760 particles/m3) in South Korea’s Nakdong River, demonstrating pronounced surface enrichment patterns [22].
Extensive research has demonstrated that microplastics are widely present within the bodies of various organisms in freshwater ecosystems. For instance, studies have detected microplastics within Mediterranean mussels (Mytilus galloprovincialis) [23]. Further research indicates that Litopenaeus vannamei shrimp can ingest microplastics from shrimp pond sediments through feeding behavior [24]. Furthermore, studies have demonstrated that microplastics can transfer along the food chain from the copper-rust snail (Bellamya aeruginosa) to the grass carp (Mylochthysgodon piceus) [25]. Freshwater economic species serve as a significant protein source for humans but also represent a key pathway for microplastics entering the human food chain. Research indicates a significant positive correlation between the concentration of small-sized microplastics in human feces and the consumption of aquatic products [26].
As the world’s largest plastic producer, China faces particularly severe challenges related to MP contamination [27]. In agricultural systems, long-term use of plastic mulching has led to significant microplastic accumulation in soils—rising from 80.3 ± 49.3 particles/kg after 5 years of use to 1075.6 ± 346.8 particles/kg after 24 years [28]. Aquatic ecosystems exhibit similarly concerning contamination levels. In Dongting Lake, MP concentrations (50–5000 μm) range from 900 to 2800 particles/m3 [29], while the Yangtze River estuary shows even higher levels (4137.3 ± 2461.5 particles/m3) [30]. Notably, MPs persist in remote environments, with sediments from Qinghai-Tibet Plateau lakes containing (8 ± 14)–(563 ± 1219) particles/m3 [31].
Crustaceans play vital ecological and economic roles in aquatic ecosystems and have recently gained attention as model organisms for microplastic research [32]. Species such as the brown shrimp (Crangon crangon) [33], Antarctic krill (Euphausia superba) [34], and green crab (Carcinus maenas) [35] have demonstrated the capacity to ingest, fragment, and even alter the morphology of MPs. These findings suggest that crustaceans may actively mediate the transformation of MPs, influencing their environmental fate and toxicity [36].
The red swamp crayfish (Procambarus clarkii), a globally farmed freshwater crustacean prized for its environmental resilience and commercial value [37], also serves as a critical bioindicator for assessing pollutant impacts on aquatic ecosystems [38,39]. However, the mechanisms governing MP fragmentation, transformation, and excretion in this species remain poorly characterized. Understanding these processes is essential to quantify MP fate in freshwater and evaluate their ecological risks. Furthermore, such knowledge could inform targeted mitigation strategies for plastic pollution.
In this study, we investigated the accumulation, distribution, and fragmentation kinetics of MPs in the digestive system of red swamp crayfish (Procambarus clarkii). A controlled exposure system was established using fluorescent polyethylene MPs (50 μm diameter, 105 particles/L). Individuals were dissected at 24, 48, and 96 h intervals post-exposure, with digestive compartments (stomach, midgut, and hindgut) isolated for analysis. Spatiotemporal distribution patterns were quantified through laser scanning confocal microscopy. To further resolve MP retention and elimination mechanisms, a 24 h depuration phase tracked residual particle dynamics. This work aims to perform the following: (1) delineate the compartment-specific accumulation kinetics of MPs in crustacean digestive systems; (2) identify the feeding behavior-mediated regulation of MP fragment efficiency and elimination rates; and (3) characterize the digestive tract-induced morphological transformation of MPs. The findings provide critical biokinetic parameters for assessing aquaculture contamination risks and establish a mechanistic framework to predict aquatic organism-mediated plastic fate, directly informing targeted mitigation strategies for freshwater ecosystems.
2. Materials and Methods
2.1. Materials
2.1.1. Preparation and Characterization of Microplastics
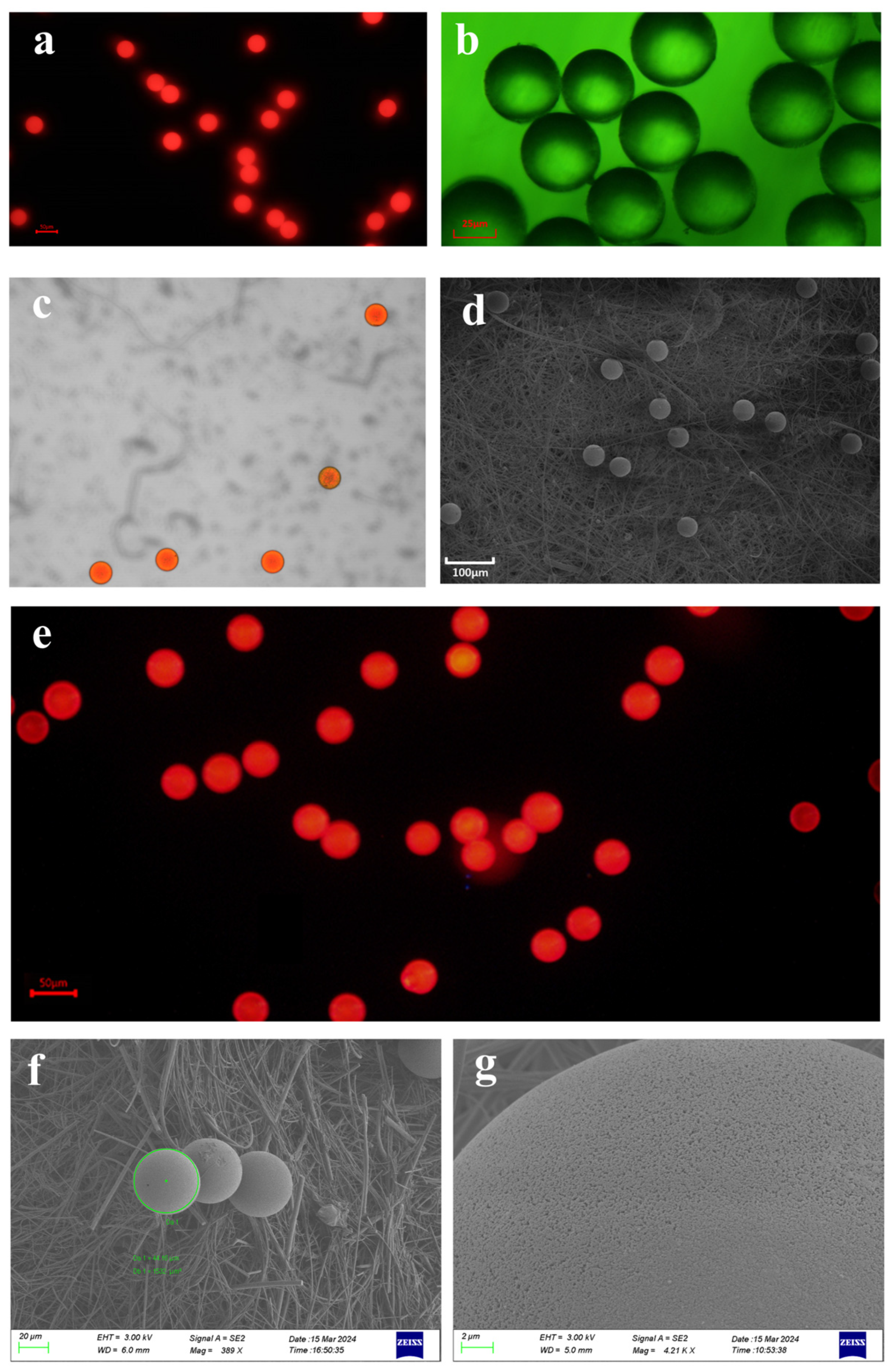
In this study, monodisperse red fluorescent polystyrene microspheres (50 μm, supplied by Jiangsu Zhichuan Technology Co., Ltd., Suzhou, China) were used as model MPs, with a density of 0.025 g/cm3, an excitation wavelength of 520 nm, and an emission wavelength of 588 nm (Figure 1a). The MPs were dispersed in ultrapure water via sonication for 20 min and diluted to an experimental concentration of 100,000 particles/L. This concentration setting is designed to elicit detectable biological effects under laboratory conditions within a finite observation period. However, as its level exceeds the concentrations reported in most natural environments today, the study’s findings present certain limitations for ecological extrapolation. Particle size distribution was characterized using a laser particle size analyzer (Malvern Mastersizer 3000, Malvern Panalytical, Malvern, UK) to confirm uniformity (Figure 1b). Morphological characteristics were observed using a laser confocal microscope (LSM710, Zeiss, Oberkochen, Germany) (Figure 1c) and a scanning electron microscope (SEM; S-4800, Hitachi, Tokyo, Japan) (Figure 1d).
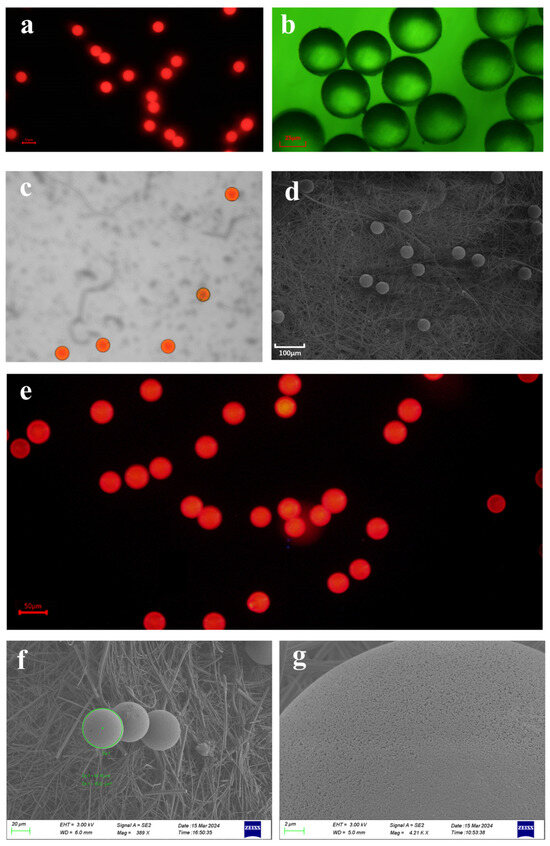
Figure 1.
The apparent characteristics of microplastics under different microscopes. (a) Fluorescent polystyrene microplastics of 50 μm were captured under a fluorescence microscope with dark-field illumination. Bar = 50 μm. (b) Fluorescent microscope images of microplastics. Bar = 25 μm. (c) Fluorescent polystyrene microplastics captured by laser confocal microscope. (d) Scanning electron microscopy (SEM) images of fluorescent-labeled polystyrene microplastics sized 50 μm. Bar = 100 µm. (e). Microscopic morphology of microplastic stock solutions prepared by the cryosectioning technique under the fluorescence microscope. Bar = 50 μm. No visibly broken microplastics were found in the frozen section mounts of the microplastic stock solution. (f,g) The morphology of microplastics following 48 h of digestion in a 30% potassium hydroxide solution was examined via scanning electron microscopy (SEM). Comparative analysis indicated that the 30% potassium hydroxide digestion treatment exerted no significant effect on either the integrity or surface characteristics of the microplastics.
Two methodological approaches—the frozen section technique and 30% KOH digestion—were applied to evaluate the potential impacts of sample processing on MPs. For the frozen section treatment, the stock solution of spherical MPs (50 μm particle size) was made into frozen sections (−20 °C, 10 μm thick), which were observed under an inverted fluorescence microscope (IX71, Olympus, Tokyo, Japan) for the presence of fragmented MPs and compared with unfrozen samples as a control group. The comparison showed no significant difference between microplastics and microplastic stock under frozen sections (Figure 1e). For the KOH digestion experiment, the MPs were divided into an experimental group (30% KOH, 25 °C, 48 h) and a control group (distilled water, 25 °C, 48 h), and after digestion, filtration was carried out. The filter membrane was sprayed with gold, and the morphological characteristics of the MPs on the filter membrane were observed and compared under a scanning electron microscope (SEM; S-4800, Hitachi, Tokyo, Japan). The results showed the following: no obvious broken MPs were found in the frozen section group; at 25 °C, the microplastics immersed in 30% KOH exhibited no apparent surface damage after 48 h; and there was no obvious difference with the MPs in the control group (Figure 1f,g).
2.1.2. Collection and Acclimation of Experimental Organisms
Healthy adult red swamp crayfish were collected from a farm in Zhejiang Province, China, with a sex ratio of approximately 1:1 (male:female), an average body weight of 25 ± 5 g, and a body length of 15 ± 5 cm. The crayfish were transported to the laboratory and acclimated in stainless steel tanks containing aerated ultrapure water for 1 week. During acclimation, they were fed a commercial diet twice daily at 3–5% of their body weight. Water parameters were maintained as follows: temperature 23–25 °C, dissolved oxygen 7.5 ± 0.6 mg/L, and pH 7.5 ± 0.2 [40], under a 12 h light–12 h dark cycle [41]. To stabilize water quality, 50% of the water volume was replaced daily. All crayfish were fasted for 24 h before the experiment to eliminate gut contents.
2.2. Methodology
2.2.1. Microplastic Exposure Experiment
This study employed a completely randomized experimental design. Eighty-four healthy adult crayfish (mean weight 25 ± 5 g, mean length 15 ± 5 cm, sex ratio 1:1) were randomly assigned to four treatment groups (three microplastic exposure groups and one control group). Each treatment group comprised three independent biological replicates (i.e., three separate glass tanks), totaling 12 experimental units. Each glass tank (5 L water volume) housed 7 crayfish. Exposure tanks contained microplastic solutions (100,000 particles/L), while the control tank held microplastic-free ultrapure water. Crayfish were not fed during the experiment, and the solution was replaced daily. Crayfish were not fed during the exposure period to ensure that MP ingestion was solely from the waterborne exposure and to standardize the initial MP load across individuals. Each aquarium housed seven crayfish. This number constituted a deliberate design choice to ensure sufficient individuals could be obtained from each biological replicate at the planned sampling time points (24, 48, 96 h) for the completion of all analytical procedures.
Sampling was conducted at 24, 48, and 96 h post-exposure. At each sampling time point, one crayfish was randomly sampled from each independent glass tank within each treatment group (i.e., n = 3 crayfish per treatment group at each time point, drawn from three independent biological replicates). The prawns were rinsed with ultrapure water, dissected, and the stomach, midgut, and hindgut were separated, where the dissections of the stomach, midgut, and hindgut referred to the study of Yang et al. [42]. After dissection, frozen sections of the tissues of the stomach, midgut, and hindgut were made, observed, and photographed under an inverted fluorescence microscope (IX71, Olympus, Tokyo, Japan) and a laser confocal microscope (LSM710, Zeiss, Oberkochen, Germany); the number of MPs was counted, and the particle size of the MPs was measured.
Additionally, after 48 h of exposure, one crayfish was additionally collected from each of three separate glass tanks within each exposure group (total n = 3); for dissection, digestive tract tissues were separated, and a 30% KOH solution was digested at 25 °C for 48 h. The filter membranes were collected by filtration and sprayed with gold, and the morphological characteristics of the MPs on the filter membranes were observed under a scanning electron microscope (SEM; S-4800, Hitachi, Tokyo, Japan).
2.2.2. Microplastic Depuration Experiment
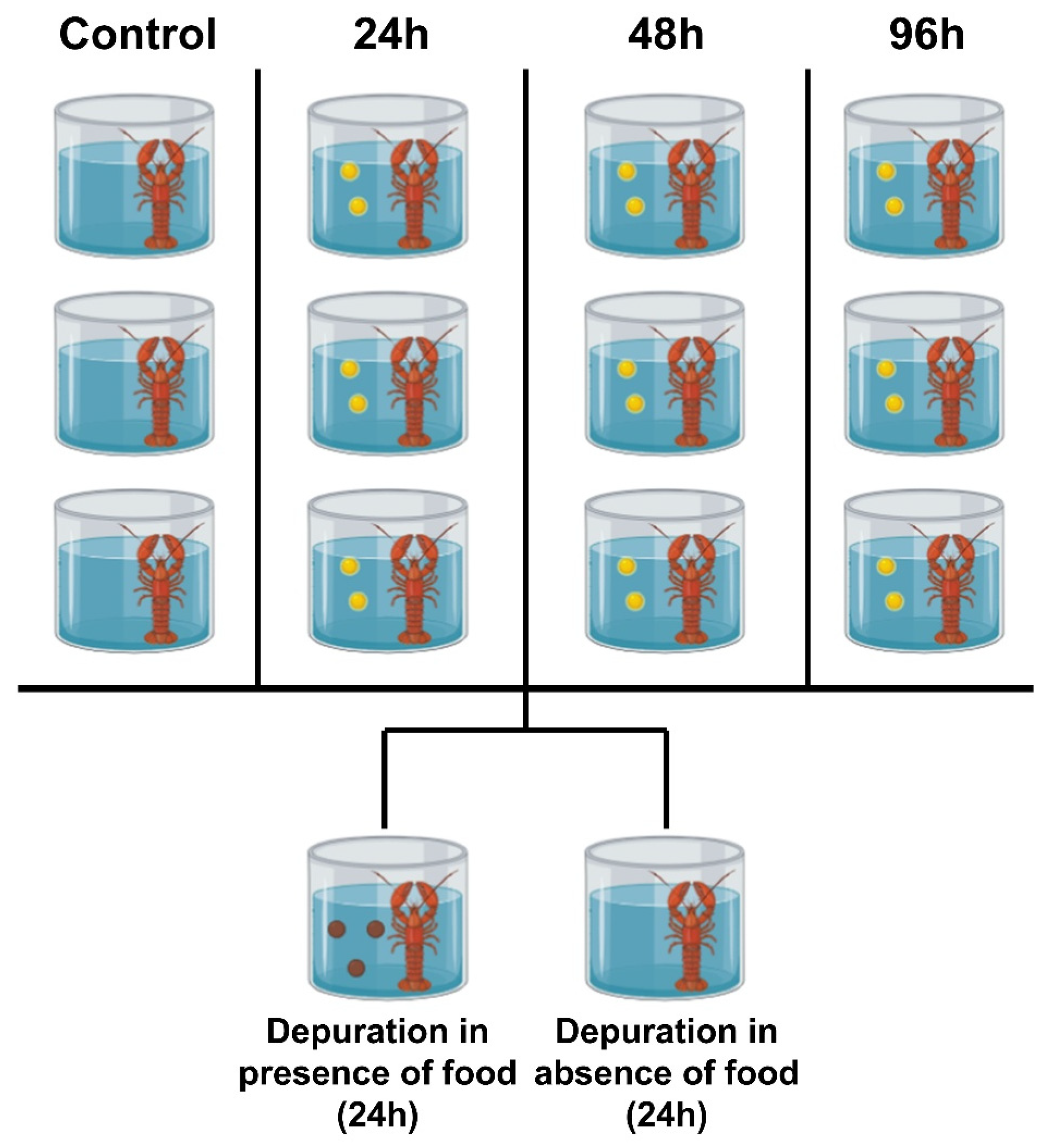
Following the conclusion of the exposure experiment, a 24 h purification experiment was immediately conducted. For the initial four treatment groups (control group, 24 h exposure group, 48 h exposure group, 96 h exposure group), the remaining crayfish from each individual glass tank were removed and pooled together. Subsequently, crayfish from the same initial treatment group were randomly assigned to two purification treatment groups: Group A (placed in microplastic-free ultrapure water and fed according to the same feeding regimen as during the acclimation period) and Group B (placed in microplastic-free ultrapure water but not fed). Each purification treatment group was housed in a separate glass tank containing three crayfish (Figure 2).
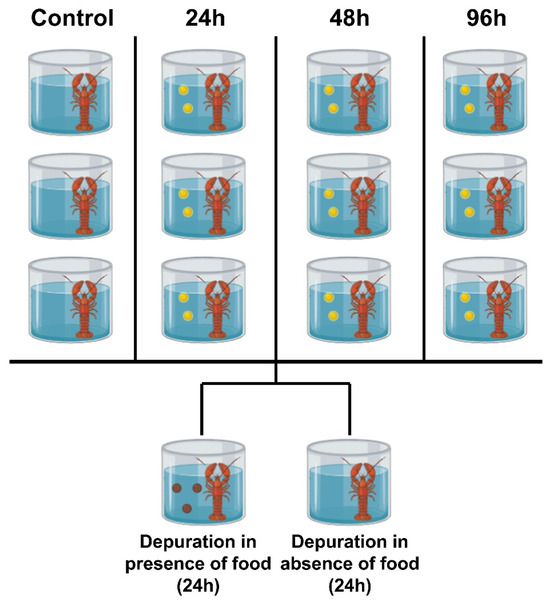
Figure 2.
The experimental design diagram illustrates an overview of the setup for a replicate experiment. The yellow spheres in the diagram represent microplastics, while the brown spheres represent feed diet. A total of three replicate experiments were conducted. All Procambarus clarkii crayfish were individually exposed to the experimental environment. This study involved 84 crayfish exposed to three distinct exposure durations and two detoxification cycles. Following exposure, three crayfish per exposure duration were dissected, while three additional specimens underwent scanning electron microscopy examination at 48 h. After the purification experiments, three specimens from each of the eight experimental groups were selected for dissection.
Thus, this purification experiment comprised 4 (initial treatment groups) × 2 (purification conditions) = 8 independent experimental groups.
At the conclusion of the 24 h purification period, three crayfish were retrieved from each tank within the aforementioned eight purification groups for analysis. Following sampling, specimens were rinsed with ultrapure water, dissected to isolate the stomach, midgut, and hindgut, and prepared as frozen tissue sections. These sections were examined and photographed under an inverted fluorescence microscope (IX71, Olympus, Tokyo, Japan) and a laser scanning confocal microscope (LSM710, Zeiss, Oberkochen, Germany) to quantify microplastic counts.
2.3. Statistical Analysis
One-way analysis of variance (ANOVA) was conducted to examine the effects of exposure duration (24, 48, and 96 h) on MP accumulation in red swamp crayfish (Procambarus clarkii). The dependent variable was the mean particle size calculated from all MPs measured within the digestive tract of each crayfish (with the number of particles measured per sample provided in the Results Section). Prior to ANOVA, the normality of the mean particle size data for each group was confirmed using the Shapiro–Wilk test (all p > 0.05), and the homogeneity of variances was verified using Levene’s test. Post hoc comparisons between groups were performed using Tukey’s HSD and Fisher’s LSD tests, with statistical significance set at p < 0.05. Among these, Tukey’s HSD test is more conservative, is employed to strictly control Type I errors, and is serving as the primary basis for this study; Fisher’s LSD test offers greater sensitivity and is used as a supplementary analysis to ensure no potential significant differences are overlooked.
The distribution patterns of MPs in the stomach, midgut, and hindgut were compared across exposure durations through ANOVA. Additionally, the abundance of fragmented MPs was quantified by particle size classes. For the analysis of fragmentation proportion data (which are expressed as percentages), arcsine square-root transformation was applied to better meet the assumptions of parametric tests. To evaluate the impact of the interaction between exposure duration and dietary treatment (fed vs. unfed) on MP fragmentation, a two-way ANOVA was applied, followed by comparisons of the proportions of fragmented and intact MPs between the fed and unfed groups. All data were visualized using standardized plots (e.g., bar graphs with error bars) and statistically analyzed in SPSS Statistics version 26.0 to ensure analytical rigor and reproducibility.
3. Results
3.1. Accumulation and Fragmentation of Microplastics in Procambarus clarkii
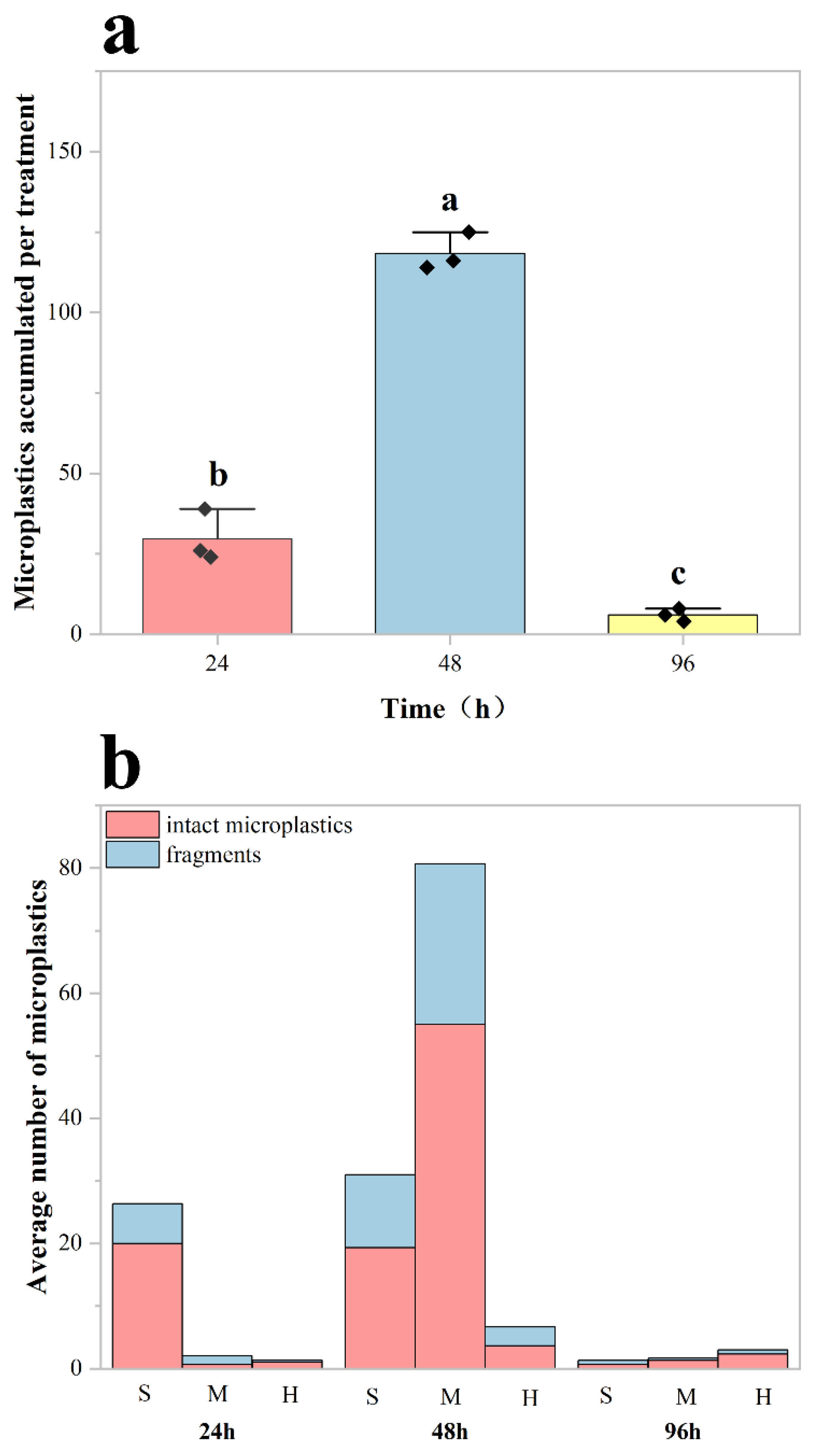
No microplastics were detected in the digestive organs of the control group of crayfish, whereas microplastics were identified and counted in the experimental group. The results demonstrated significant effects of exposure duration on MP accumulation in red swamp crayfish (Procambarus clarkii) (One-way ANOVA: df = 8, F = 301.54, p < 0.001; Figure 3a). Post hoc comparisons employed Tukey’s HSD test (more conservative) and Fisher’s LSD test (for exploratory analysis). The more robust Tukey’s HSD test revealed significant differences between all paired groups (24 vs. 48 h: MD = 88.67, SE = 4.82, p < 0.001; 48 vs. 96 h: MD = 112.33, SE = 4.82, p < 0.001; 24 vs. 96 h: MD = 23.67, SE = 4.82, p < 0.05). Specifically, the 48 h group exhibited the highest MP accumulation in their digestive organs, followed by the 24 h group, with the lowest levels observed in the 96 h group.
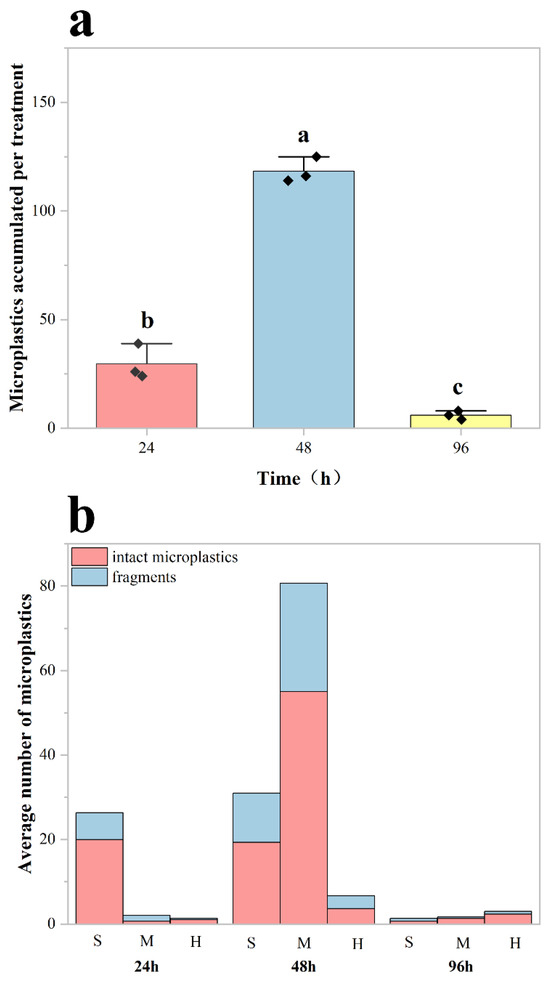
Figure 3.
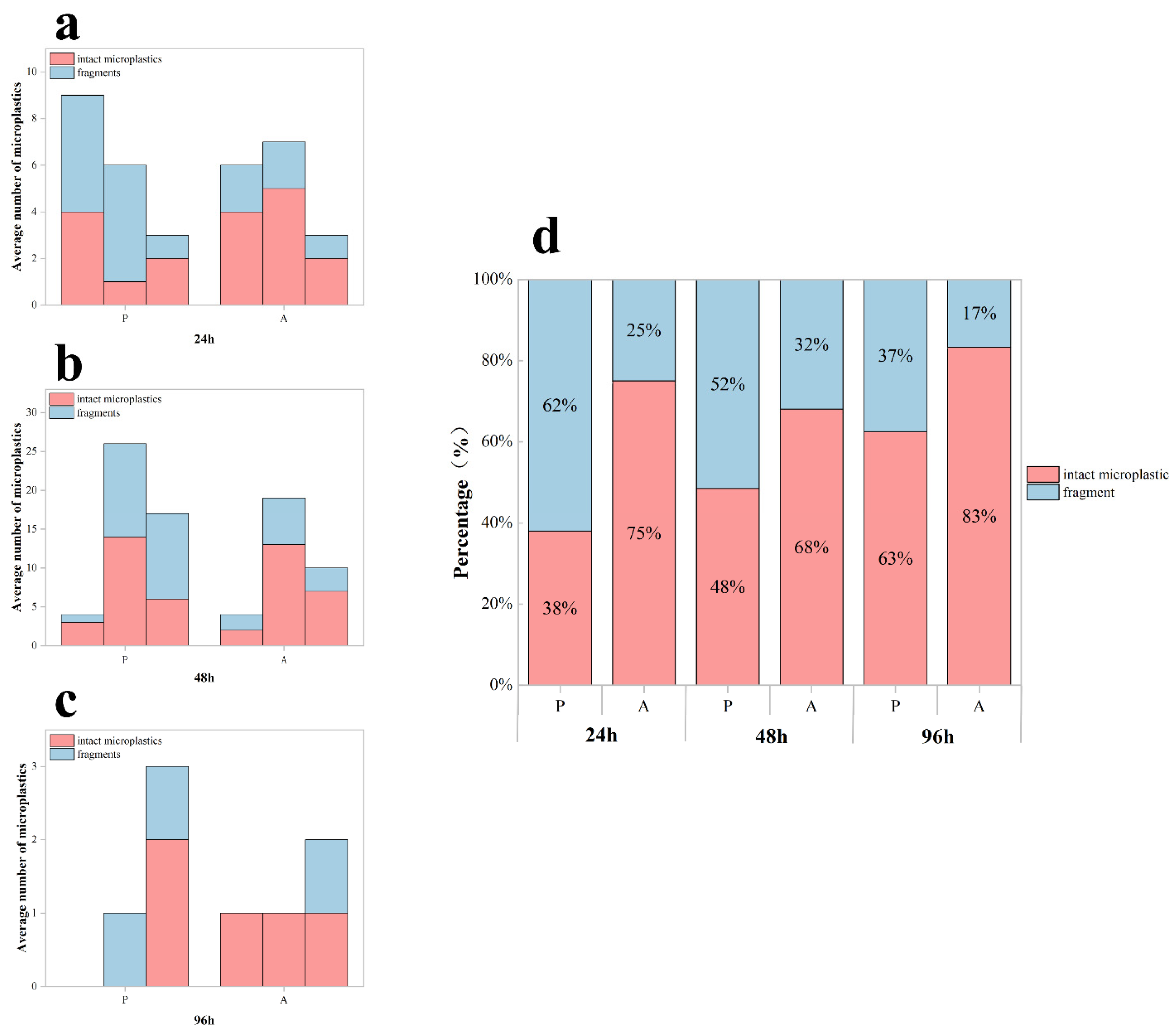
Quantification of microplastic accumulation in the digestive system of Procambarus clarkii. (a) Mean microplastic counts in the digestive tract of red swamp crayfish after 24, 48, and 96 h of exposure to microplastics (n = 3 per group). Error bars represent mean ± standard deviation. Each black diamond represents the total number of microplastics within the digestive system of a crayfish. Different lowercase letters (a, b, c) denote statistically significant differences among all exposure groups (p < 0.05). (b) Tissue-specific distribution of accumulated MPs across digestive tract compartments (stomach (S), midgut (M), and hindgut(H)) at each exposure time point. This shows the average number of intact and fragmented microplastics per crayfish in the digestive tract.
The distribution of MPs in the digestive system showed a strong correlation with exposure duration (p < 0.05; Figure 3b). The stomach served as the primary site of MP accumulation, particularly at shorter exposure durations (24 and 48 h). As exposure time increased, MPs progressively translocated to the midgut and hindgut, with midgut accumulation reaching its peak at 48 h in Procambarus clarkii. Although partial fragmentation occurred during digestion, intact MP particles constituted the majority (>65%) of total particles across all exposure groups.
3.2. Distribution of MP Fragments of Different Particle Sizes in Procambarus clarkii
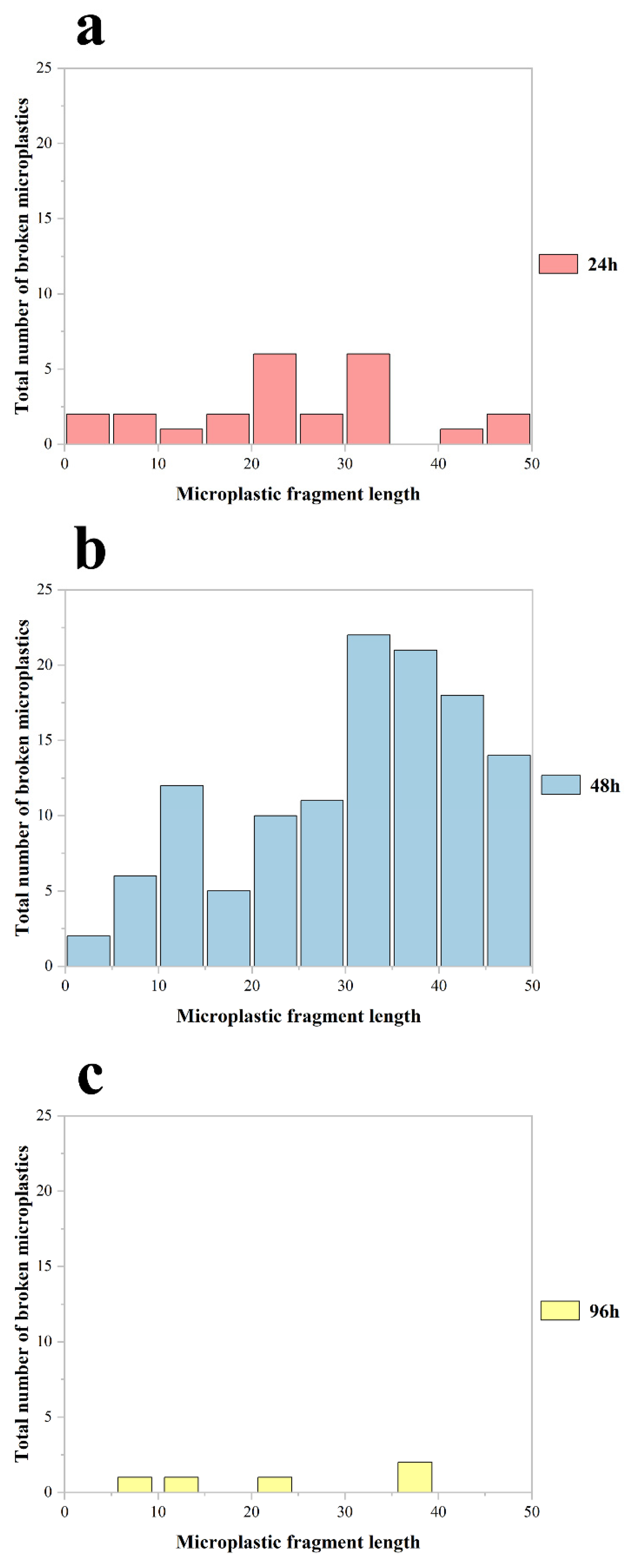
The abundance and size distribution of fragmented MPs in Procambarus clarkii exhibited significant temporal variations (Figure 4). Crayfish exposed for 48 h showed the highest abundance of fragmented particles, with the largest mean size (12.1 ± 2.14 μm; mean ± SD). In contrast, the 24 h exposure group displayed a lower abundance of smaller fragments (2.4 ± 0.64 μm), while the 96 h group had the lowest abundance and smallest particle size (0.5 ± 0.22 μm). Despite the 48 h group having the longest exposure duration among the short-term cohorts, their fragmented MPs retained the largest average size (p < 0.001 vs. 24 h group).
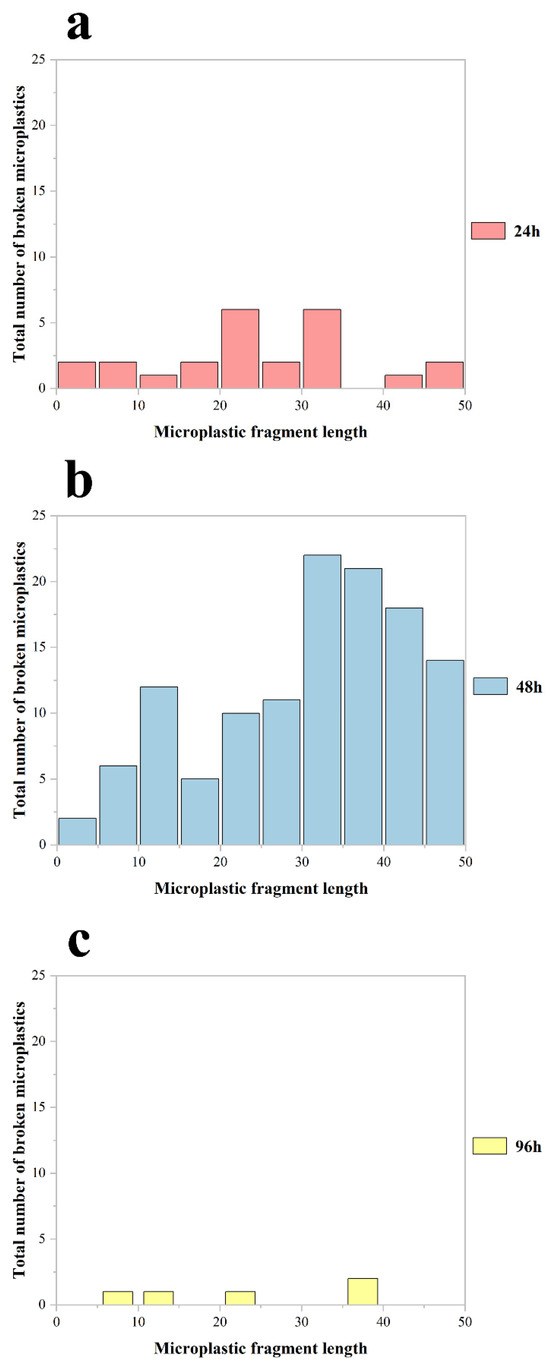
Figure 4.
The abundance of broken MPs of different particle sizes accumulated in the body of all Procambarus clarkii in the experiment at 24 h (a), 48 h (b), and 96 h (c) of exposure, respectively.
3.3. Effect of Food on the Ability of Procambarus clarkii to Fragment MPs
MP accumulation in distinct digestive compartments (stomach, midgut, hindgut) of Procambarus clarkii exhibited compartment-specific patterns following the depuration experiment (Figure 5a–c). A two-way ANOVA identified the significant main effects of exposure duration (df = 2, F = 70.383, p < 0.05), aligning with temporal accumulation trends from previous exposure trials. Critically, we observed a significant exposure time–purification treatment interaction (df = 2, F = 21.075, p < 0.001), though the main effect of purification treatment alone was non-significant (df = 1, F = 3.002, p > 0.05). Crayfish under purification treatment carried 32.8% higher MP loads than their unfed counterparts, with a higher proportion of fragmented particles.
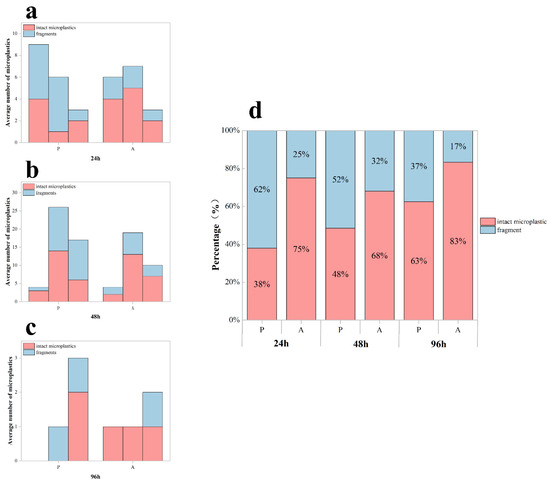
Figure 5.
Microplastic retention in the digestive system of Procambarus clarkii during depuration. (a–c) Tissue-specific microplastic counts (mean ± SD) per crayfish in different digestive compartments (stomach, midgut, hindgut) following 24 h depuration after exposure periods of 24 h, 48 h, and 96 h. (d) Proportional distribution of fragmented versus intact MPs retained after depuration. P: presence of food; A: absence of food.
The depuration process revealed a significant effect of feeding on the proportion of fragmented microplastics (df = 1, F = 19.709, p < 0.001; Figure 5d). Fed specimens exhibited a greater proportion of fragmented microplastics, with a fragmented particle ratio of 50.34 ± 12.29% (mean ± SD) versus 24.54 ± 7.66% in the unfed groups.
3.4. Morphological Transformation of MPs
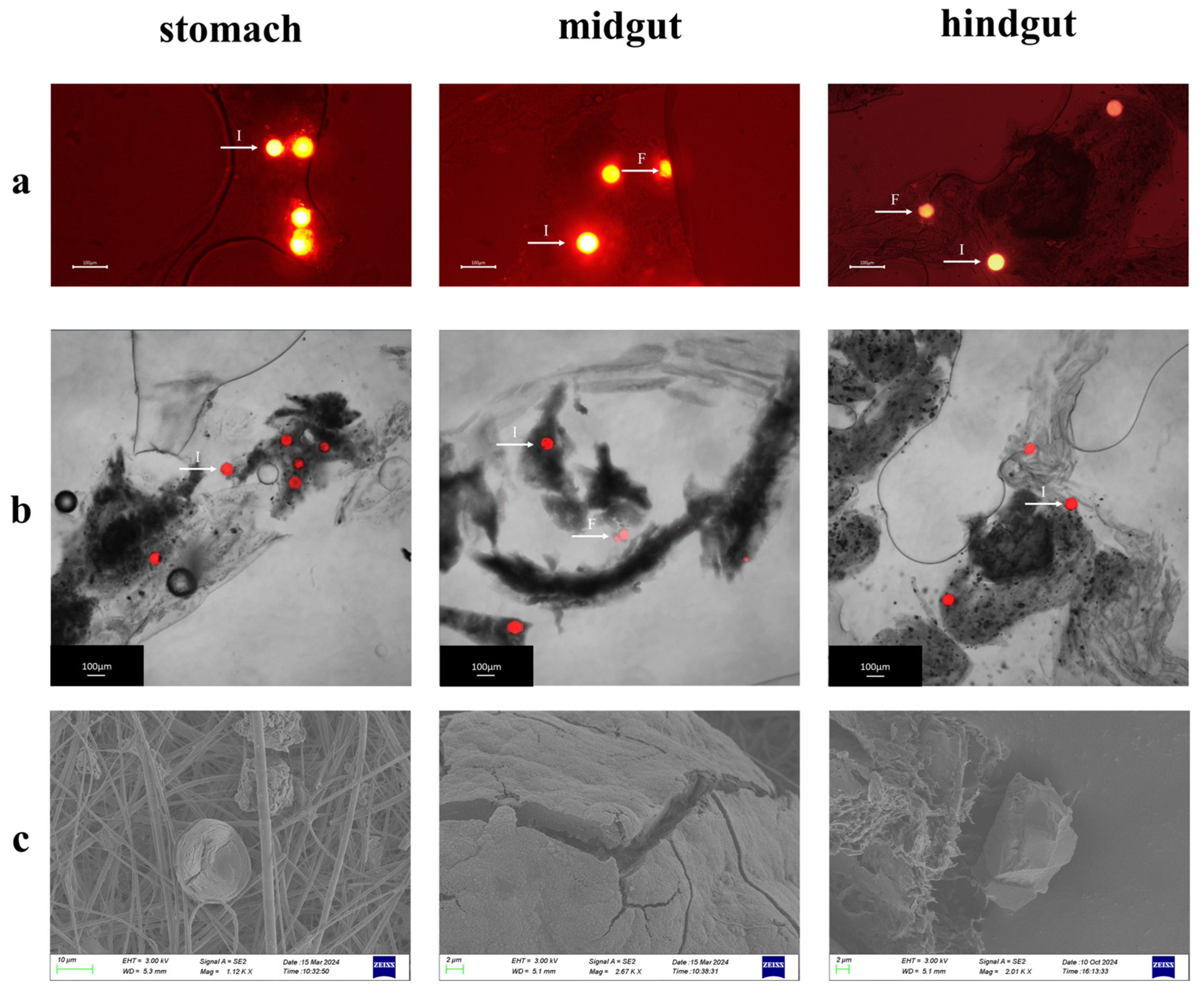
In the exposure experiments, MPs within the digestive organs of Procambarus clarkii were systematically characterized using a fluorescence microscope, a laser confocal microscope, and a scanning electron microscope (SEM)(Figure 6a–c). High-resolution imaging techniques revealed the spatial distribution patterns of MPs across gastrointestinal tissues. Fluorescence and CLSM imaging identified both intact particles retaining spherical morphology and fragmented particles with irregular geometries. The SEM analysis further demonstrated digestion-induced alterations of microplastics, including surface wrinkling, edge irregularization, and particle size reduction, which may correspond to the digestive process of Procambarus clarkii. These findings provide important morphological evidence to elucidate the fragmentation mechanism of MPs in the digestive system of crustaceans and their potential biological effects.
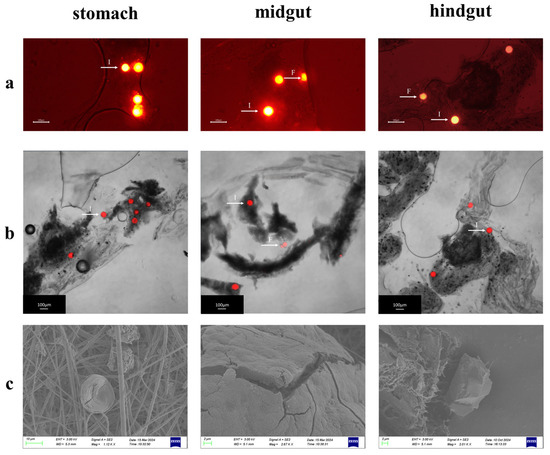
Figure 6.
Morphological characteristics of MPs in the digestive tract of Procambarus clarkii. (a) Fluorescence micrographs of MPs extracted from different digestive tract segments (stomach, midgut, and hindgut). (b) Confocal laser scanning microscopy images of MPs from distinct digestive regions. (c) Scanning electron microscopy images showing surface morphological alterations of MPs from various digestive compartments. Note: The bright spot pointed by the white arrow is the microplastic microsphere. I: intact microspheres; F: fragmented microspheres.
4. Discussion
Prior studies have confirmed MP ingestion in Procambarus clarkii [43,44]. Our results indicate that during short-term exposure (24–48 h), MPs primarily enter the digestive system via feeding and accumulate in the stomach (Figure 3b). Beyond 48 h, however, bioaccumulation decreased significantly (p < 0.05), likely due to efficient elimination through fecal egestion and/or branchial excretion, consistent with the ‘accumulation–elimination equilibrium’ observed in aquatic species [36].
This size-selective retention pattern aligns with reports in keystone taxa. For instance, Lymnaea stagnalis preferentially retains larger MPs (10–100 μm) in natural settings but accumulates particles as small as 1 μm under laboratory conditions [45]. Similarly, the trophic transfer of MPs from snails (Bellamya aeruginosa) to black carp (Mylopharyngodon piceus) [46] highlights cross-taxon commonalities in particle-size mediated bioaccumulation. The time-dependent size-sorting pattern suggests that peristaltic grinding in the stomach and selective hindgut retention may be underlying driving mechanisms. This could be interpreted as a potential adaptive strategy for mitigating microplastic toxicity.
The distribution of MPs in the digestive system of Procambarus clarkii was closely linked to their physical characteristics. Larger MP particles were more likely to be retained in the stomach, while smaller particles migrated to the midgut and hindgut during digestion. This observation aligns with prior studies on MP distribution in aquatic organisms [47]. Prolonged exposure to MPs may lead to increased contamination in crayfish, with longer durations potentially exacerbating the effects [44,48]. The size and shape of MPs likely influence their movement and retention within the digestive system, as larger particles may be physically trapped in the stomach due to digestive tract peristalsis, whereas smaller particles can more easily enter the intestines with digestive fluids.
Exposure duration not only modulated total MP bioaccumulation but also influenced particle size distribution in Procambarus clarkii. This size shift likely stems from digestive fragmentation mechanisms, where prolonged exposure (96 h) enables continuous breakdown via gastric mill grinding, enzymatic hydrolysis, and microbial activity [34]. It is important to note that the present study did not directly measure physiological or biochemical indicators. However, the fragmentation processes observed here are consistent with the mechanisms reported in prior research, wherein microplastic exposure has been shown to trigger antioxidant responses (e.g., the activation of SOD and CAT) [49,50], and correlate with metabolic impairment in this species [51,52].
The depuration experiments revealed a size-selective elimination mechanism in Procambarus clarkii, preferentially expelling larger MPs. Specifically, crustaceans regulate particle retention time through intestinal peristalsis: larger particles (>10 μm) are rapidly expelled due to physical exclusion at narrow digestive tract segments, while smaller particles are retained within intestinal mucosal folds or bound to mucus layers [53]. Our observations showed a significant reduction in fragmented MPs in crayfish after 96 h of exposure, indicating active excretion of these particles. This suggests a potential ecological pathway whereby Procambarus clarkii ingest MPs by grinding and crushing them through their own digestive organs and finally excreting the fragmented MPs into the environment through fecal pellets [54], and MPs smaller than 1 mm are more likely to be excreted [55].
Ingested MPs can bioaccumulate in aquatic organisms, potentially causing mechanical gut obstruction and suppressing feeding behavior through pseudo-satiety effects [56,57]. Our study found significantly higher MP abundance in fed Procambarus clarkii compared to unfed groups, with fragmented MPs dominating their composition. This difference likely stems from feeding-induced physiological mechanisms: Some literature indicates that food intake stimulates gastrointestinal peristalsis and digestive secretion while accelerating intestinal emptying, thereby enhancing mechanical fragmentation efficiency [58,59]. In contrast, unfed individuals exhibit reduced digestive enzyme activity and prolonged chyme retention [60], and this might limit fragmentation to passive physical interactions (e.g., random particle–stomach wall collisions). Notably, the lack of increased fragmented MP excretion in the fed groups suggests small particles may bind to intestinal mucus and accumulate at normally closed intestinal segments—a finding consistent with the proposed physiological retention mechanisms [53].
Notably, MPs may enter humans through dietary intake of commonly consumed species [61]. Existing evidence confirms the trophic transfer of MPs from crustaceans to humans via food chains [62], and our findings imply heightened risks of fragmented MP ingestion through seafood consumption. Internalized MPs can induce localized inflammation, cellular damage, and oxidative stress, posing threats to digestive, immune, and respiratory systems, with chronic exposure potentially elevating the risks of metabolic disorders [63]. Although this study was conducted under laboratory conditions involving high concentrations and short cycles, and its findings cannot be directly extrapolated to natural ecosystems, the observed microplastic accumulation and morphological transformation phenomena provide preliminary clues for exploring the potential risks of trophic transfer. The potential implications for human health remain highly speculative, necessitating urgent future research to elucidate these effects within realistic exposure scenarios.
The limitations of this study include sparse temporal sampling, precluding observation of dynamic MP accumulation/fragmentation patterns in Procambarus clarkii. Future work should establish time-resolved MP bioaccumulation curves to precisely quantify crustacean ingestion–fragmentation capacities and model the environmental fate of secondary MPs.
5. Conclusions
This study systematically elucidated the bioaccumulation, spatial distribution, fragmentation, and elimination dynamics of MPs in red swamp crayfish (Procambarus clarkii). Key findings revealed time-dependent accumulation patterns: peak MP loads occurred at 48 h of exposure, followed by a significant reduction at 96 h. MPs progressively translocated from the stomach to the midgut and hindgut during digestion, undergoing size-dependent fragmentation under prolonged exposure. We observed that the crayfish supplemented with feed exhibited a higher fragmentation rate of microplastics within their bodies; however, the underlying biological mechanisms—such as whether this is due to enhanced digestion or alterations in intestinal transit time—remain to be further investigated. The intact MPs retained a pristine spherical morphology, whereas fragmented particles exhibited irregular geometries with increased surface area-to-volume ratios.
Based on the aforementioned findings, future research should prioritize investigating the trophic transfer of microplastics through aquatic food webs, with particular emphasis on clarifying their bioaccumulation pathways from aquatic organisms to humans. To address this novel environmental pollutant, it is imperative to establish standardized microplastic detection methods and mitigation strategies, while simultaneously strengthening source control and comprehensive governance to mitigate potential risks to ecosystems and human health. This study provides crucial data for understanding the behavior and effects of microplastics in freshwater economic species, while also offering scientific grounds for formulating relevant environmental risk assessments and management strategies.
Author Contributions
Y.H.: Writing—original draft, Project administration, Methodology, Conceptualization. Q.L.: Software, Investigation, Data curation. X.X.: Validation, Supervision. J.J.: Software, Investigation. J.L.: Investigation, Formal analysis. H.C.: Writing—review and editing, Resources. M.Z.: Visualization, Validation. B.J.: Writing—review and editing, Supervision, Resources, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 31960254, the National Undergraduate Training Program for Innovation and Entrepreneurship, grant number 202410346014, and the Hangzhou Normal University Undergraduate Innovation Capability Enhancement Project, grant number CX2024137.
Institutional Review Board Statement
Ethical review and approval were not required for this study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Napper, I.E.; Thompson, R.C. Plastic Debris in the Marine Environment: History and Future Challenges. Glob. Chall. 2020, 4, 1900081. [Google Scholar] [CrossRef]
- Cózar, A.; Echevarría, F.; González-Gordillo, J.I.; Irigoien, X.; Úbeda, B.; Hernández-León, S.; Palma, Á.T.; Navarro, S.; García-de-Lomas, J.; Ruiz, A.; et al. Plastic debris in the open ocean. Proc. Natl. Acad. Sci. USA 2014, 111, 10239–10244. [Google Scholar] [CrossRef]
- Ermis, H.; Collins, C.; Kumar Saha, S.; Murray, P. Beyond Visibility: Microorganisms for tackling plastic and microplastic problems for cleaner future. Chem. Eng. J. 2024, 497, 154585. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, L. Microplastic migration and transformation pathways and exposure health risks. Environ. Pollut. 2025, 368, 125700. [Google Scholar] [CrossRef] [PubMed]
- Milbrandt, A.; Coney, K.; Badgett, A.; Beckham, G.T. Quantification and evaluation of plastic waste in the United States. Resour. Conserv. Recycl. 2022, 183. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; Anthony, W.G.; John McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Acot, F.T.; Sajorne, R.E.; Omar, N.-A.K.; Suson, P.D.; Rallos, L.E.E.; Bacosa, H.P. Unraveling Macroplastic Pollution in Rural and Urban Beaches in Sarangani Bay Protected Seascape, Mindanao, Philippines. J. Mar. Sci. Eng. 2022, 10, 1532. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef]
- Behera, S.; Das, S. Environmental impacts of microplastic and role of plastisphere microbes in the biodegradation and upcycling of microplastic. Chemosphere 2023, 334, 138928. [Google Scholar] [CrossRef]
- Liang, X.; Ye, J.; Cao, R.; Shuai, J.; Zhang, J.; Aimaiti, R.; Meng, S.; Wang, K.; Gomiero, A.; Wang, J.; et al. Microplastics and their interaction with microorganisms in Bosten Lake water. J. Clean. Prod. 2024, 481, 144157. [Google Scholar] [CrossRef]
- Helm, P.A. Improving microplastics source apportionment: A role for microplastic morphology and taxonomy? Anal. Methods 2017, 9, 1328–1331. [Google Scholar] [CrossRef]
- Paluselli, A.; Fauvelle, V.; Galgani, F.; Sempéré, R. Phthalate Release from Plastic Fragments and Degradation in Seawater. Environ. Sci. Technol. 2018, 53, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Hermabessiere, L.; Dehaut, A.; Paul-Pont, I.; Lacroix, C.; Jezequel, R.; Soudant, P.; Duflos, G. Occurrence and effects of plastic additives on marine environments and organisms: A review. Chemosphere 2017, 182, 781–793. [Google Scholar] [CrossRef] [PubMed]
- Pilechi, A.; Mohammadian, A.; Murphy, E. A numerical framework for modeling fate and transport of microplastics in inland and coastal waters. Mar. Pollut. Bull. 2022, 184, 114119. [Google Scholar] [CrossRef]
- Kushwaha, M.; Shankar, S.; Goel, D.; Singh, S.; Rahul, J.; Rachna, K.; Singh, J. Microplastics pollution in the marine environment: A review of sources, impacts and mitigation. Mar. Pollut. Bull. 2024, 209, 117109. [Google Scholar] [CrossRef]
- Anderson, P.J.; Warrack, S.; Langen, V.; Challis, J.K.; Hanson, M.L.; Rennie, M.D. Microplastic contamination in Lake Winnipeg, Canada. Environ. Pollut. 2017, 225, 223–231. [Google Scholar] [CrossRef]
- Bertoldi, C.; Lara, L.Z.; Mizushima, F.A.d.L.; Martins, F.C.G.; Battisti, M.A.; Hinrichs, R.; Fernandes, A.N. First evidence of microplastic contamination in the freshwater of Lake Guaíba, Porto Alegre, Brazil. Sci. Total Environ. 2021, 759, 143503. [Google Scholar] [CrossRef]
- Castañeda, R.A.; Avlijas, S.; Simard, M.A.; Ricciardi, A.; Smith, R. Microplastic pollution in St. Lawrence River sediments. Can. J. Fish. Aquat. Sci. 2014, 71, 1767–1771. [Google Scholar] [CrossRef]
- Everaert, G.; De Rijcke, M.; Lonneville, B.; Janssen, C.R.; Backhaus, T.; Mees, J.; van Sebille, E.; Koelmans, A.A.; Catarino, A.I.; Vandegehuchte, M.B. Risks of floating microplastic in the global ocean. Environ. Pollut. 2020, 267, 115499. [Google Scholar] [CrossRef]
- Corradini, F.; Meza, P.; Eguiluz, R.; Casado, F.; Huerta-Lwanga, E.; Geissen, V. Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci. Total Environ. 2019, 671, 411–420. [Google Scholar] [CrossRef]
- Sulistyowati, L.; Nurhasanah; Riani, E.; Cordova, M.R. The occurrence and abundance of microplastics in surface water of the midstream and downstream of the Cisadane River, Indonesia. Chemosphere 2022, 291, 133071. [Google Scholar] [CrossRef]
- Eo, S.; Hong, S.H.; Song, Y.K.; Han, G.M.; Shim, W.J. Spatiotemporal distribution and annual load of microplastics in the Nakdong River, South Korea. Water Res. 2019, 160, 228–237. [Google Scholar] [CrossRef]
- Nalbone, L.; Cincotta, F.; Giarratana, F.; Ziino, G.; Panebianco, A. Microplastics in fresh and processed mussels sampled from fish shops and large retail chains in Italy. Food Control 2021, 125, 108003. [Google Scholar] [CrossRef]
- Yan, M.; Li, W.; Chen, X.; He, Y.; Zhang, X.; Gong, H. A preliminary study of the association between colonization of microorganism on microplastics and intestinal microbiota in shrimp under natural conditions. J. Hazard. Mater. 2021, 408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Jin, Y.; Zhang, C.; Xu, Y.; Fan, C.; Pan, W.; Li, J.; Chen, H.; Jin, B. Impact of microplastic exposure on the antioxidant enzyme activity and intestimal microbiota composition of Bellamya aerugimosa. J. Hangzhou Norm. Univ. (Nat. Sci. Ed.) 2024, 23, 565–571. [Google Scholar] [CrossRef]
- Zhu, L.; Wu, Z.; Dong, J.; Zhao, S.; Zhu, J.; Wang, W.; Ma, F.; An, L. Unveiling Small-Sized Plastic Particles Hidden behind Large-Sized Ones in Human Excretion and Their Potential Sources. Environ. Sci. Technol. 2024, 58, 11901–11911. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Su, F.; Liu, C.; Guo, Z. Research progress for plastic waste management and manufacture of value-added products. Adv. Compos. Hybrid Mater. 2020, 3, 443–461. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Q.; Jia, W.; Yan, C.; Wang, J. Agricultural plastic mulching as a source of microplastics in the terrestrial environment. Environ. Pollut. 2020, 260, 114096. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, W.; Chen, Y.; Wang, J. Microplastics in surface waters of Dongting Lake and Hong Lake, China. Sci. Total Environ. 2018, 633, 539–545. [Google Scholar] [CrossRef]
- Zhao, S.; Zhu, L.; Wang, T.; Li, D. Suspended microplastics in the surface water of the Yangtze Estuary System, China: First observations on occurrence, distribution. Mar. Pollut. Bull. 2014, 86, 562–568. [Google Scholar] [CrossRef]
- Zhang, K.; Su, J.; Xiong, X.; Wu, X.; Wu, C.; Liu, J. Microplastic pollution of lakeshore sediments from remote lakes in Tibet plateau, China. Environ. Pollut. 2016, 219, 450–455. [Google Scholar] [CrossRef]
- Christie, A.E. Crustacean neuroendocrine systems and their signaling agents. Cell Tissue Res. 2011, 345, 41–67. [Google Scholar] [CrossRef]
- Devriese, L.I.; van der Meulen, M.D.; Maes, T.; Bekaert, K.; Paul-Pont, I.; Frère, L.; Robbens, J.; Vethaak, A.D. Microplastic contamination in brown shrimp (Crangon crangon, Linnaeus 1758) from coastal waters of the Southern North Sea and Channel area. Mar. Pollut. Bull. 2015, 98, 179–187. [Google Scholar] [CrossRef]
- Dawson, A.L.; Kawaguchi, S.; King, C.K.; Townsend, K.A.; King, R.; Huston, W.M.; Bengtson Nash, S.M. Turning microplastics into nanoplastics through digestive fragmentation by Antarctic krill. Nat. Commun. 2018, 9, 1001. [Google Scholar] [CrossRef]
- Watts, A.J.R.; Urbina, M.A.; Corr, S.; Lewis, C.; Galloway, T.S. Ingestion of Plastic Microfibers by the Crab Carcinus maenas and Its Effect on Food Consumption and Energy Balance. Environ. Sci. Technol. 2015, 49, 14597–14604. [Google Scholar] [CrossRef]
- Mateos-Cárdenas, A.; O’Halloran, J.; van Pelt, F.N.A.M.; Jansen, M.A.K. Rapid fragmentation of microplastics by the freshwater amphipod Gammarus duebeni (Lillj.). Sci. Rep. 2020, 10, 12799. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Xu, X.; Chen, Y.; Xie, H.; Zhang, T.; Mao, X. Red Swamp Crayfish (Procambarus clarkii) as a Growing Food Source: Opportunities and Challenges in Comprehensive Research and Utilization. Foods 2024, 13, 3780. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Z.; Kholodkevich, S.; Sharov, A.; Feng, Y.; Ren, N.; Sun, K. Cadmium-induced oxidative stress, histopathology, and transcriptome changes in the hepatopancreas of freshwater crayfish (Procambarus clarkii). Sci. Total Environ. 2019, 666, 944–955. [Google Scholar] [CrossRef] [PubMed]
- Osuna-Jiménez, I.; Abril, N.; Vioque-Fernández, A.; Gómez-Ariza, J.L.; Prieto-Álamo, M.-J.; Pueyo, C. The environmental quality of Doñana surrounding areas affects the immune transcriptional profile of inhabitant crayfish Procambarus clarkii. Fish Shellfish Immunol. 2014, 40, 136–145. [Google Scholar] [CrossRef]
- Zeng, H.; Zhong, Y.; Wei, W.; Luo, M.; Xu, X. Combined exposure to microplastics and copper elicited size-dependent uptake and toxicity responses in red swamp crayfish (Procambarus clarkia). J. Hazard. Mater. 2025, 487, 137263. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, Y.; Song, Z.; Liang, H.; Zhong, S.; Yu, Y.; Liu, T.; Sha, H.; He, L.; Gan, J. Mercury Induced Tissue Damage, Redox Metabolism, Ion Transport, Apoptosis, and Intestinal Microbiota Change in Red Swamp Crayfish (Procambarus clarkii): Application of Multi-Omics Analysis in Risk Assessment of Hg. Antioxidants 2022, 11, 1944. [Google Scholar] [CrossRef]
- Yang, Z.; Hao, J.; Liu, Y.; Wu, X.; Chen, X. Histological morphology and gene expression in the digestive system of Procambarus clarkii. Aquac. Fish. 2025, 10, 596–607. [Google Scholar] [CrossRef]
- Zhang, D.; Fraser, M.A.; Huang, W.; Ge, C.; Wang, Y.; Zhang, C.; Guo, P. Microplastic pollution in water, sediment, and specific tissues of crayfish (Procambarus clarkii) within two different breeding modes in Jianli, Hubei province, China. Environ. Pollut. 2021, 272, 115939. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Liu, X.; Mei, T.; Xu, M.; Lu, Z.; Dai, H.; Pi, F.; Wang, J. Estimation of contamination level in microplastic-exposed crayfish by laser confocal micro-Raman imaging. Food Chem. 2022, 397, 133844. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.; von Randow, M.; Voigt, A.-L.; von der Au, M.; Fischer, E.; Meermann, B.; Wagner, M. Ingestion and toxicity of microplastics in the freshwater gastropod Lymnaea stagnalis: No microplastic-induced effects alone or in combination with copper. Chemosphere 2021, 263, 128040. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Jin, Y.; Fan, C.; Xu, Y.; Li, J.; Pan, W.; Lou, Z.; Chen, H.; Jin, B. Exploring the trophic transfer and effects of microplastics in freshwater ecosystems: A focus on Bellamya aeruginosa to Mylopharyngodon piceus. Environ. Pollut. 2024, 357, 124426. [Google Scholar] [CrossRef]
- Watts, A.J.R.; Lewis, C.; Goodhead, R.M.; Beckett, S.J.; Moger, J.; Tyler, C.R.; Galloway, T.S. Uptake and Retention of Microplastics by the Shore Crab Carcinus maenas. Environ. Sci. Technol. 2014, 48, 8823–8830. [Google Scholar] [CrossRef]
- Han, M.; Gao, T.; Liu, G.; Zhu, C.; Zhang, T.; Sun, M.; Li, J.; Ji, F.; Si, Q.; Jiang, Q. The effect of a polystyrene nanoplastic on the intestinal microbes and oxidative stress defense of the freshwater crayfish, Procambarus clarkii. Sci. Total Environ. 2022, 833, 155722. [Google Scholar] [CrossRef]
- Silveyra, G.R.; Silveyra, P.; Brown, M.; Poole, S.; Vatnick, I.; Medesani, D.A.; Rodríguez, E.M. Oxidative stress and histopathological effects by microplastic beads, in the crayfish Procambarus clarkii, and fiddler crab Leptuca pugilator. Chemosphere 2023, 343, 140260. [Google Scholar] [CrossRef]
- Zeng, Q.; Yang, Q.; Chai, Y.; Wei, W.; Luo, M.; Li, W. Polystyrene microplastics enhanced copper-induced acute immunotoxicity in red swamp crayfish (Procambarus clarkii). Ecotoxicol. Environ. Saf. 2023, 249, 114432. [Google Scholar] [CrossRef]
- Woods, M.N.; Hong, T.J.; Baughman, D.; Andrews, G.; Fields, D.M.; Matrai, P.A. Accumulation and effects of microplastic fibers in American lobster larvae (Homarus americanus). Mar. Pollut. Bull. 2020, 157, 111280. [Google Scholar] [CrossRef]
- Umamaheswari, S.; Priyadarshinee, S.; Kadirvelu, K.; Ramesh, M. Polystyrene microplastics induce apoptosis via ROS-mediated p53 signaling pathway in zebrafish. Chem.-Biol. Interact. 2021, 345, 109550. [Google Scholar] [CrossRef]
- Welden, N.A.C.; Cowie, P.R. Long-term microplastic retention causes reduced body condition in the langoustine, Nephrops norvegicus. Environ. Pollut. 2016, 218, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Yan, Z.; Qiao, L.; Gui, F.; Li, T.; Yang, Q.; Zhang, X.; Ren, C. Biological effects on the migration and transformation of microplastics in the marine environment. Mar. Environ. Res. 2023, 185, 105875. [Google Scholar] [CrossRef] [PubMed]
- Nebil Yücel, E.K. Occurrence and human exposure risk of microplastics in commercially important shrimp species from Northeastern Mediterranean Sea. Mar. Pollut. Bull. 2025, 214, 117796. [Google Scholar] [CrossRef]
- Wang, W.; Gao, H.; Jin, S.; Li, R.; Na, G. The ecotoxicological effects of microplastics on aquatic food web, from primary producer to human: A review. Ecotoxicol. Environ. Saf. 2019, 173, 110–117. [Google Scholar] [CrossRef]
- Avio, C.G.; Gorbi, S.; Milan, M.; Benedetti, M.; Fattorini, D.; d’Errico, G.; Pauletto, M.; Bargelloni, L.; Regoli, F. Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ. Pollut. 2015, 198, 211–222. [Google Scholar] [CrossRef]
- Lygre, E.F.; Gomes, A.S.; Hess-Erga, O.-K.; Norberg, B.; Nilsson, J.; Perrichon, P.; Rønnestad, I. Gastrointestinal evacuation and return of appetite in Atlantic halibut (Hippoglossus hippoglossus L.). Aquaculture 2025, 599, 742147. [Google Scholar] [CrossRef]
- Das, S.K.; Noor, N.M.; Kai, K.S.; Juan, Q.Z.; Mohd Iskandar, N.S.; De, M. Effects of temperature on the growth, gastric emptying time, and oxygen consumption rate of mahseer (Tor tambroides) under laboratory conditions. Aquac. Rep. 2018, 12, 20–24. [Google Scholar] [CrossRef]
- Busti, S.; Bonaldo, A.; Diana, A.; Perfetti, S.; Viroli, C.; Fontanillas, R.; Eriksen, T.B.; Gatta, P.P.; Parma, L. The incidence of different pellet size on growth, gut evacuation, feed digestibility and feed waste in gilthead sea bream (Sparus aurata). Aquaculture 2022, 555, 738204. [Google Scholar] [CrossRef]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef]
- Carbery, M.; O’Connor, W.; Palanisami, T. Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environ. Int. 2018, 115, 400–409. [Google Scholar] [CrossRef]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).