Abstract
South Mediterranean forests are known for their spatial and temporal variability in fauna and flora species abundance. Using acoustic surveys and generalized linear models, we investigated the nocturnal activity of bats in five forest sites in northeast Algeria from March to November. A total of 12 species were detected: Rhinolophus blasii, R. hipposideros, Tadarida teniotis, Miniopterus schreibersii, Eptesicus isabellinus, Hypsugo savii, Pipistrellus kuhlii, P. pipistrellus, Plecotus gaisleri, Myotis cappaccinii, M. emarginatus, and M. punicus. Activity levels varied significantly among months and sites. Overall bat activity and P. kuhlii activity increased from spring to summer, peaked in August, and slightly decreased in early autumn. Activity levels also differed markedly among forest sites, with low activity levels in the urban forest site, and E. isabellinus was mostly active in only two forest sites. These results suggest that both environmental conditions and location-specific factors shape the activity patterns.
1. Introduction
The Mediterranean area is recognized as one of the 36 global biodiversity hotspots [1], and also as one of the most threatened [2]. With an area of 8779 million km2, the Mediterranean region is shared by 26 countries, five of which are countries on the southern side. Within this region, forests cover 85 million ha, about 9.7% of the total area, amounting to 2% of the world’s forest area [3]. South Mediterranean forests are known for their high diversity of fauna and flora species, and for their distinctive climate characterized by cold and wet winters, as well as hot and dry summers [4]. In northern Algeria, with approximately 800,000 ha [5], the Aleppo pine (Pinus halepensis) covers 35% of the wooded areas [6]. With a potential area estimated to be 1,807,000 ha [7], the holm oak (Quercus ilex) is another significant component of Algeria’s forest ecosystems. The Algerian climax forests of this species are located in the semi-arid and lower sub-humid bioclimatic stages [8,9].
Bats represent a species-rich mammalian order comprising more than 1400 species worldwide [10]. Forests are a key habitat for bats throughout the world [11,12,13], and almost half of known bat species worldwide use trees as roosts for at least part of the year [14]. Forests provide both foraging and roosting resources for bats, and in return, bats provide significant ecosystem services to forests [15], including control of phytophagous insects [16,17]. Thus, bat conservation in forests is crucial not only for maintaining biodiversity, but also for a sustainable forest management.
In the Mediterranean area, bats are moderately diversified, with 63 species belonging to eight families, and only 22 species belonging to six families in Northwestern Africa [18]. While significant interest in bats of this region recently arose (e.g., [19,20,21]), studies on bats in Mediterranean forests were mainly concentrated in European areas. In Italy, high bat activity was reported in wooded areas, particularly in broad-leaved woodlands (oaks Quercus spp., beech Fagus sylvatica, and chestnut Castanea sativa) [22], rather than in conifer plantations. In southern Portugal, riparian habitats surrounded by autochthonous broad-leaved forests provided optimal foraging areas for bats [23]. In southern Spain, assemblages of forest bats were driven primarily by forest composition and structure rather than by climate [24].
There is currently limited information available about spatial and temporal patterns of bat activity in south Mediterranean forests. In central Tunisia, several pine forests of the Jebel Mghilla National Park were surveyed only once [25]. In the Near East, bats were studied in planted pine forests [26]. In Morocco, different forest types, including several argane (Argania spinosa) woodlands, were sampled in the coastal area of the Safi–Essaouira provinces [27]. In northern Algeria, the winter activity of two bat species was investigated in five different habitats, near and inside the forests of Bejaia region [28]. Comparing bat richness and activity in urban and rural habitats in the Constantine area, only two forest sites were sampled [29]. However, to date, no research on bat activity has occurred in Northwestern African forests.
Acoustic monitoring is a highly effective method to survey bat assemblages and understand behavior and habitat use of different bat species [30]. This technique enables annual surveys and provides insights into spatial and temporal aspects of bat behavior [31]. Herein, we investigated the seasonal variation in bat activity in Mediterranean forests using acoustic detection in five sites in northeast Algeria and generalized linear models. (1) We expected to find seasonal variations in measured bat activity, with significant interactions among sites, months, temperature and hygrometry, for either the overall bat community or for the most active species. (2) We predicted that activity would increase during spring because of increased energy requirements due to gestation and lactation, and that a secondary peak of activity in autumn would be observed, due to resource acquisition before the wintering period.
2. Material and Methods
2.1. Study Areas
Field work was conducted in two areas in northeast Algeria, approximately 160 km apart: Bordj Bou Arreridj (36°04′03.8″ N 4°46′02.7″ E), and Chettaba, in the vicinity of Constantine (36°21′16.3″ N 6°38′43.4″ E) (Figure 1, Table 1).
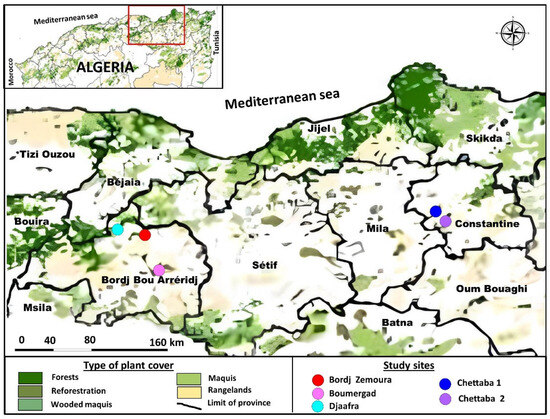
Figure 1.
Location of the five sites surveyed for bat activity in forests in northeast Algeria.

Table 1.
Main characteristics of the five sites surveyed for bat activity in forests in northeast Algeria.
Forests in Bordj Bou Arreridj cover an area of 80,799 ha and are mainly composed of Aleppo pine (76%), with some holm oak (19%) and Atlas cedar Cedrus atlanticus (1%). The bioclimate is semi-arid, characterized by an average annual thermal amplitude of 21.3 °C and an average annual rainfall less than 400 mm [8].
Forests of the Chettaba massif cover an area of 2398 ha [32], and are characterized by a semi-arid bioclimate, with an average annual rainfall between 670 and 800 mm [33]. These forests are composed either of holm oak (1127 ha) or Aleppo pine [34,35].
Three sites were selected from the first region, namely Bordj Zemoura, Boumergad, and Djaafra, and two from the second, an Aleppo pine stand and a holm oak stand.
2.2. Acoustic Sampling
We conducted acoustic sampling at each site from March to November 2023 when climatic conditions were favorable (without rain or strong winds) each month for two hours, starting at sunset. Bat calls were recorded along a transect walked at a slow and constant speed using a Batlogger M (Elekon AG, Lucerne, Switzerland) equipped with firmware version 2.6.2 in Bordj Bou Arreridj forests. The acoustic data were recorded at a frequency of 312.5 kHz, with a recording duration of 5 s, the default setting of the Batlogger. In Chettaba forests, we used a time-expansion D240X ultrasonic detector (Petterson Electronik Uppsala, AB, Sweden) with a frequency range from 10 to 128 kHz, connected by cable to a digital recorder (Edirol R-09HR, Roland Corporation, Shanghai, China), fitted with a memory card. For both devices, only 5 s sequences containing bat calls were manually analyzed using BatSound, v.3.10 (Petterson Elektronik AB) for spectrogram analyses. We used a simple frequency of 44.100 samples/s, 16 bits/sample and selected 512 pt FFT with a Hamming window for analysis [36].
Recorded calls were identified at the species level, based on signal shape and call parameters [37], and on published data from Northwest Africa [21,38,39,40]. Rhinolophus blasii and R. hipposideros are easily identifiable as they produce specific FM/CF/FM-type signals, featuring a long constant frequency (CF) portion and differing in the maximum energy frequency, ranging from 95.4 to 98.4 kHz for R. blasii, and 115.9 to 121.0 kHz for R. hipposideros [21,40]. Tadarida teniotis, Miniopterus schreibersii, Pipistrellus pipistrellus, P. kuhlii, Hypsugo savii and Eptesicus isabellinus were identified by their FM/QCF-type signals with maximum energy frequencies at 12.83 ± 1.14 kHz, 56.51 ± 0.6 kHz, 47.72 ± 3.71 kHz, 38.71 ± 1.03 kHz, 32.40 ± 1.71 kHz and 25.97 ± 1.50 kHz, respectively [39]. Plecotus gaisleri produce FM calls, which can be identified by their shape and their starting frequency, which is 31.5–58.9 kHz [39]. Myotis capaccinii, M. emarginatus and M. punicus emit abrupt FM-type signals with terminal frequencies ranging from 30 to 35 kHz, 34 to 48 kHz, and 21.4 to 28.5 kHz, respectively [38,40].
Additionally, during the two-hour survey, air temperature and hygrometry were measured every 30 min, using an IHM6150SI pocket thermo-hygrometer type K digital (precision ± 1 °C and ±5% RH). We also recorded the cloud cover and the wind speed.
2.3. Data Analysis
To estimate bat activity levels, we summed up bat passes for periods of 30 min over the 2 h surveys. Then, we used generalized linear models to investigate bat activity variation and to identify the key factors influencing either the activity of all bat species pulled together, or solely P. kuhlii activity and E. isabellinus activity separately. All analyses were conducted using the R software version 4.4.0 [41]. The factors that might influence bat activity include study site, month, air temperature, hygrometry and the 30 min period of sampling. The latter we included either as a random effect to control for variability in the recording across the four periods of 30 min, or as fixed effect in some models to evaluate its impact on mean bat activity. We used a negative binomial distribution with the glmmTMB R package (version 1.1.11) to model overdispersion [42] in all models (i.e., for the overall bat activity, as well as for P. kuhlii and E. isabellinus separate activities). We constructed candidate models with two-way interactions to reflect our hypotheses, and we included the null model in our comparisons to evaluate the plausibility of all candidate models. We selected the best models using the Akaike’s information criterion for small sample size (AICc) [43,44] with the aictab function from the AICcmodavg package in R (version 2.3.3) [45]. During the model selection process of E. isabellinus, the inclusion of hygrometry and periods led to convergence errors, which forced their exclusion from the final selection.
To check whether the residuals of the models were uniformly distributed and for under- or over-dispersion [46], we used the DHARMa R package (version 0.4.7). This package uses a simulation-based approach to generate readily interpretable residuals for generalized linear (mixed) models. These residuals are standardized for values between 0 and 1, allowing for a clear interpretation when comparing to residuals from a linear model (see Supplementary Materials for more details).
Furthermore, multicollinearity among predictor variables was investigated using the performance R package (version 0.13.0) [47], by calculating the variance inflation factor (VIF). All selected best models were checked and variables included exhibited acceptable VIFs between 1.34 and 2.37 (see Supplementary Materials for more details).
3. Results
A total of 1738 bat passes were recorded across the five sites: 527 in Bordj Zemoura, 152 in Boumergad, 536 in Djaafra, 287 in Chettaba forest of Aleppo pine, 236 in Chettaba forest of holm oak, for a total of 12 species: Rhinolophus blasii, R. hipposideros, Tadarida teniotis, Miniopterus schreibersii, Eptesicus isabellinus, Hypsugo savii, Pipistrellus kuhlii, P. pipistrellus, Plecotus gaisleri, Myotis capaccini, M. emarginatus, and M. punicus (Table 2). The most active species in four sites were P. kuhlii and E. isabellinus; in Djaafra, a third species, H. savii, was very active. Interestingly, T. teniotis was particularly active in the Chettaba forests.

Table 2.
Percentages of bat species activity recorded in five forests in northeast Algeria between March and November 2023.
The model including the site and the month as fixed effects with no interaction and the sampling period as a random effect (AICcWt = 0.85, Table 3), provided the best explanation for the overall bat activity than the model with temperature. Bats were most active at Bordj Zemoura (p < 0.001), followed by Djaafra (p = 0.001), where bats were significantly more active than at the reference site Boumergad (p = 0.04) (Figure 2). On the other hand, bat activity in the forests of Chettaba (Aleppo pine and holm oak forests) was not significantly different from bat activity in Boumergad. In terms of temporal variations, most of the activity was recorded between July and October. The peak of activity occurred in August (p < 0.001).

Table 3.
Candidate generalized linear models to investigate predictors of bat activity (month, region, site, temperature and period of the night) in five forests in northeast Algeria from March to November 2023. Model classification is based on the corrected Akaike’s Information Criterion (ΔAICc) and Akaike weights (AICcWt). K refers to the number of parameters. The retained model is in bold.
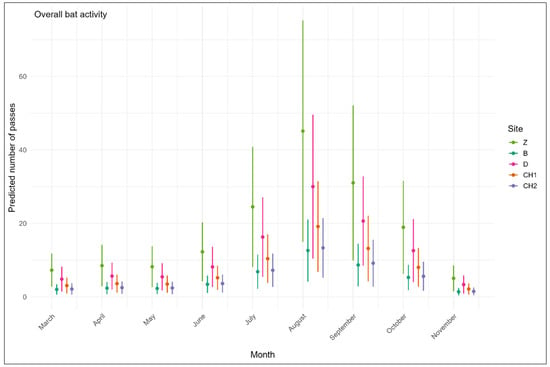
Figure 2.
Monthly variations in overall bat activity across five forests in northeast Algeria according to the best models (Table 3 and Table 4) using the number of passes as a measure of activity level. Z: Bordj Zemoura, B: Boumergad, D: Djaafra, CH1: Chettaba Aleppo pine forest, CH2: Chettaba holm oak forest. The period was included as a random effect in the predictions.
For P. kuhlii activity, similarly to the overall bat activity, we selected the model including the month and the site with no interaction (AICcWt = 0.37, Table 4) as the most appropriate as it included only two variables versus the two-way interaction between site and month and period for the model with the lower AICc, according to the principle of parsimony [48]. Moreover, the AICc difference between these models was small (ΔAICc = 0.82), and the model including the region is possibly biassed by the use of different detector types (Batlogger/Petterson). Month was a better predictor then temperature. Among the sites, only the holm oak forest of Chettaba was significantly different (p = 0.03) from the reference site Boumergad (Figure 3). Bat activity was significantly higher from June to October, with a peak in August (p < 0.001), and a secondary peak in October.

Table 4.
Candidate generalized linear models to explain Pipistrellus kuhlii activity (month, region, site, temperature and period of the night) in five forests in northeast Algeria from March to November 2023. Model classification is based on the corrected Akaike’s Information Criterion (ΔAICc) and Akaike weights (AICcWt). K refers to the number of parameters. The retained model, according to the principle of parsimony and an AICc < 2, is in bold.
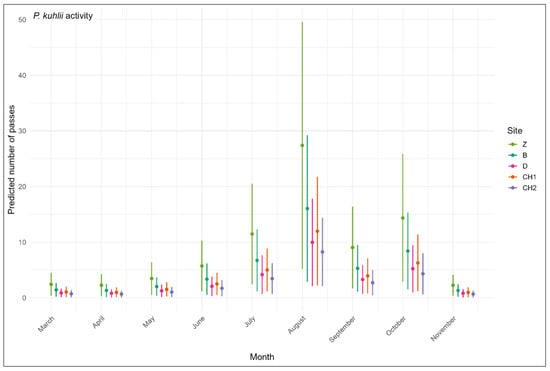
Figure 3.
Monthly variations in Pipistrellus kuhlii activity across five forests in northeast Algeria according to the best models (Table 4) using the number of passes as a measure of activity level. The period was included as a random effect in the predictions.
For E. isabellinus, the second most active bat species in our study, the site and the month (with no interaction) are also key predictors of bat activity (AICcWt = 0.82, Table 5). Flight activity varied significantly across sites (Figure 4). Bordj Zemoura (p < 0.001) and Djaafra (p < 0.001) exhibited higher levels of bat activity compared to Boumergad. In contrast, the activity in Chettaba holm oak forest (p = 0.189) and Aleppo pine forest (p = 0.128) were not significantly different from the reference site. Bat activity peaked significantly in August (p = 0.009), while a significant decline can be observed in November (p = 0.005). Activity in October tended to decrease, though this result was marginally significant (p = 0.071). For the months of April (p = 0.747), May (p = 0.543), June (p = 0.635), July (p = 0.264), and September (p = 0.213), bat activity was not significantly different from activity measured in March.

Table 5.
Candidate generalized linear models to explain Eptesicus isabellinus activity (month, region, site and temperature) in five forests in northeast Algeria from March to November 2023. Model classification is based on the corrected Akaike’s Information Criterion (ΔAICc) and Akaike weights (AICcWt). K refers to the number of parameters. The retained model is in bold.
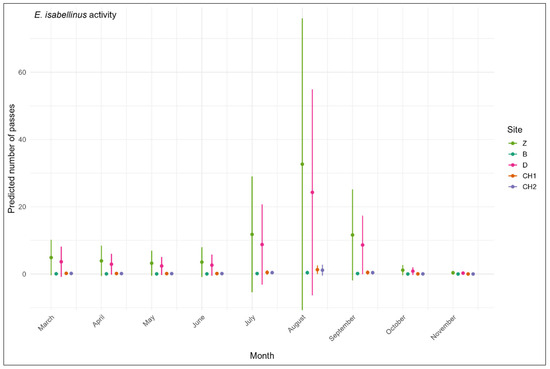
Figure 4.
Monthly variations in Eptesicus isabellinus activity across five forests in northeast Algeria according to the best models (Table 5) using the number of passes as a measure of activity level. The period was included as a random effect in the predictions.
4. Discussion
The aim of our study was to explore the seasonal variations in bat activity in South Mediterranean forests. This was quite challenging as the authors in [29] reported that forests were the least visited sites by bats among the habitat types they sampled in a nearby area. A similar result was published for Spain [49]. The lack of potential prey, an explanation for low bat activity that was suggested for cereal fields [50], is very unlikely to apply to forests. These may be less attractive to bats that forage in open space and may mainly be visited by species exhibiting high maneuverability, which typically emit very weak echolocation calls [37], and are therefore difficult to detect: rhinolophids, Plecotus, and most Myotis species [51,52].
Our results show that bat activity is mainly shaped by seasonality, including month and site. There was a critical challenge in interpreting our findings because of the limited baseline data in the area. Furthermore, the monitoring design (active recording by one observer) and the use of two different detectors were methodological limitations. Active acoustic monitoring induces short recording duration, which significantly lowers the probability of detection for some species [53,54]. However, mobile acoustic transects could detect more bat activity than fixed-point surveys as it was reported for open landscapes [54,55]. The use of one device per site and night is another limitation as it can often miss some bat species, mainly in woody vegetation [56,57,58]. An optimal field monitoring would include several passive detectors set for several nights on the ground, in the canopy, and in forest clearings [59]. Lastly, the site effect must be carefully considered due to the pitfalls of comparing datasets collected with two different bat detector brands. However, both detectors were time expansion detectors, and were used in the same field design.
4.1. Bat Assemblage Composition
Despite those possible pitfalls, bat assemblages were quite similar among the five forests, except the noticeable lower species richness in the suburban forest of Boumergad. Such result was reported in central Spain where species richness was lowered by urbanization despite the presence of synanthropic species [60]. This sensitivity of bats to urbanization was reviewed in the last decade [61]. In our dataset, except one Myotis species, the assemblage of this suburban forest is composed of the most widely distributed species in Northwestern Africa [62,63,64,65], particularly in the cities [29].
These species were also the most acoustically detected, with P. kuhlii being the main one, particularly in Boumergad, due to its synurbic adaptations [66], but it is also often the most active species in the whole Mediterranean area [22,25,67,68,69,70,71,72], even in arid zones [39,73]. The second most active species was E. isabellinus at Bordj Zemoura and Djaafra, and T. teniotis in Boumergad and Chettaba forests. As the calls of the latter are easy to detect and identify, the use of two different bat detectors was probably not a bias. Similarly, T. teniotis was also the second most active species in the city of Constantine, nearby Chettaba forests [29], whereas E. isabellinus was the second most active species in Tunisian studies [25,39,50,74,75], in coastal forests of Morocco [27], and over small ponds in dry Mediterranean forests of Spain [76]. H. savii was the third most acoustically detected in Djaffra, probably because of its mountainous nature [77]. It was also particularly active in two previously sampled western Algerian regions [21] and outside the city of Constantine, but not in the forests [29]. P. pipistrellus and M. schreibersii were mainly recorded in the Chettaba Aleppo pine forest. Both species were previously recorded in the Constantine area, being less active than the previous ones [29]. On the contrary, P. pipistrellus was the most active bat species in coastal Morocco [27] and in various surveys in Spain [76,78]. M. schreibersii can be considered “locally frequent” as in Jbel Mghilla National Park (Tunisia) [23]. The activity of the other species, which are more difficult to detect (see above), cannot be compared among sampled sites and with other bat studies in Northwestern Africa.
4.2. Spatial and Temporal Variation in Bat Activity
The highest bat activity was recorded in Bordj Zemoura forest, which is an open forest suitable for a large number of species, including short-range, mid-range, and long-range echolocators foraging from the ground to over the canopy [79]. In a nearby area, Djaafra forest, which is higher and denser, with taller trees, exhibited the second highest bat activity. Chettaba forests, 300 km apart, are similarly dense, and mainly differ from each other by the tree composition (Aleppo pine vs. holm oak). In conifer-dominated landscapes, bat activity was more closely related to forest structural complexity and prey availability than to tree species composition [80]. Similar findings were reported in several different forest types [24,81,82]. On the contrary, an increasing richness with the abundance of conifers and no noted effects of structure were found in a French pine forest [83]. At last, in these four Algerian forests, no relation could be inferred between tree height and bat activity, as it was previously reported [26]. Only the tree density seemed to slightly influence bat activity. A similar result was recorded in southeast Spain [49]. In temperate pine forests, higher bat activity was recorded in young stands and open areas of pine forests [84,85], due to higher prey availability [86,87]. However, some studies have shown higher insect availability inside or at the edges of stands [87,88,89,90]. Other stand parameters may be more important than the availability of insects [87,91,92].
Located in an urban environment, Boumergad forest is exposed to multiple anthropogenic pressures that significantly influence bat activity. This finding aligns with broader research about the negative impact of urbanization on bats [61,93]. Artificial light modifies nocturnal insect behavior and abundance [94], while noise pollution deteriorates the echolocation efficiency of bats [95].
Bat activity was significantly higher during summer, particularly in August, and early autumn among all sites, while in arid areas of Tunisia and Algeria, a lower bat activity was reported in August than in the previous and next months [39,73]. This low summer activity was related to shallow torpor during the hottest days, whereas nightly temperature is never so high in the surveyed Mediterranean forests, which grow to heights between 900 and 1200 m a.s.l. In southeast Spain, Lison and Calvo [76] reported an increasing bat activity in forest from June to September, and a parallel decreasing activity at ponds and emergence of insect prey [96,97], suggesting a displacement or widening of the hunting areas [76]. Contrary to our hypothesis, the energy requirements of pregnancy do not imply higher spring activity of bats which give birth from March to June in North Africa [98,99]. Lactation in July could increase the energy demand but it is more likely that forests offer more insect prey as summer drought increases in open areas [100,101,102].
4.3. Spatial and Temporal Activity of P. kuhlii and E. isabellinus
Most of the overall bat activity in the five studied forests is allotted to P. kuhlii, with a lower value in Djaafra forest, where H. savii and E. isabellinus were particularly active, and almost in Boumergad forest. Kuhl’s pipistrelle is a generalist open air aerial insectivore that may utilize cluttered environments as well, as long as clutter density is not very high [103]. The high level of P. kuhlii activity in this suburban forest highlights the synanthropic behavior of this species [61,66,104]. The lower activity in Djaafra forest could be related to interspecific resource competition as the three species (P. kuhlii, H. savii and E. isabellinus) share the same guild [105,106], and forage on flying insects, mainly Lepidoptera [107].
The observed seasonal activity pattern of P. kuhlii among sites aligns with its reproductive biology and energy requirements in Mediterranean climates [108], with low activity in early spring, followed by an increase as the season progresses. The peak of activity in August, also recorded in Tiaret [21], can be related to young gaining independent flight [108] after parturition, usually occurring from mid-May to late June [100,109,110] or sometimes earlier in Lebanon, Syria, Iran, Libya, and Saudi Arabia [111,112,113,114]. August is also the molting season for this species, an energetically stressful period requiring more active foraging [115]. The second lower peak of activity in October is probably related to the mating season, and to the storing of reserves [100].
Eptesicus isabellinus was mainly active in Bordj Zemoura and Djaafra forests, and rarely detected in Chettaba forests, and mostly in the suburban Boumergad forest. Even if this species can roost in buildings [116], its foraging areas are located far from human settlements [117]. The low levels of activity in Chettaba forests are more difficult to explain. Neither altitude nor tree density or composition can be related to the difference from Bordj Zemoura and Djaafra forests. In fact, E. isabellinus was poorly recorded in Constantine area [29] and in Tiaret region (western Algeria) [21], contrary to most areas surveyed in Tunisia [20,25,50,74]. Interestingly, Djaafra forest is located at an altitude of 1200 m, over the 300–600 m optimal range for the species in Spain [117].
The temporal distribution of E. isabellinus activity is marked by a single, very sharp peak in August, mostly in Bordj Zemoura and Djaafra forests. A similar pattern was reported from Andalucía, where it was correlated with temperature and insect abundance [118]. Moreover, a different diet between the pregnancy and the lactation—post-lactation period was recorded [119], supporting a displacement of foraging areas from agricultural zones to an Aleppo pine forest. In southeast Spain, the activity of E. isabellinus in forest increased from June to September, when it decreased at ponds [76]. So, even if E. isabellinus occurs in Mediterranean areas that consist mainly of forests and scrublands, it prefers to forage close to watercourses and ponds [117] or also in pastures with trees [118] and agricultural fields [49]. In the arid Bou Hedma National Park (Tunisia), a different seasonal activity pattern was found with two peaks in late spring—early summer and early autumn, which exclude the hottest summer months, and is more related to the biological cycle of the bat [39,120]. In this park, lactation occurs in May and June, as it was observed in other parts of Tunisia [121], and in Libya [111].
5. Conclusions
Despite limitations, this study is the first to explore the spatial and seasonal activity of bats in South Mediterranean forests, a poorly documented topic, highlighting the importance of forests as habitats for several species, at least during some periods of the year. Our results suggest that forests should deserve special attention in bat conservation efforts throughout the region. Nevertheless, more studies are needed to improve our knowledge on the relation between forests and roosting/foraging bats, including the impact of management [10] and fires [122,123], and develop conservation strategies in order to limit the consequences of the recent environmental changes.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/d17110750/s1. Table S1: Summary of multicollinearity checking for key predictor variables in models for overall bat activity, Pipistrellus kuhlii and Eptesicus isabellinus activity. Figure S1: DHARMa residual diagnostics—QQ plot and residuals vs. predicted values for the best model of overall bat activity. No significant deviations were detected. Figure S2: DHARMa residual diagnostics—QQ plot and residuals vs. predicted values for the best model of Pipistrellus kuhlii activity. No significant deviations were detected. Figure S3: DHARMa residual diagnostics—QQ plot and residuals vs. predicted values for the best model of Eptesicus isabellinus activity. No significant deviations were detected.
Author Contributions
Conceptualization and methodology, A.B., M.A.-A., F.Z.B., F.B. and S.A.; investigation, A.B. and M.A.-A.; data curation, A.B., M.A.-A., I.K. and M.B.; writing—review and editing, A.B., M.A.-A., F.Z.B., F.B., I.K. and S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Raw data can be obtained on request to the first author.
Acknowledgments
The authors are grateful to Ferhat Boukhari and Abdelnour Tabi for their help during field trips, as well as Ridha Dalhoumi. The authors would also like to acknowledge all the members of the Forest Conservation of Bordj Bou Arreridj for their invaluable assistance and protection. Smail and Hamza Abbaz hosted the second author in the Chettaba forest.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- FAO and Plan Bleu. State of Mediterranean Forests 2018; Food and Agriculture Organization of the United Nations: Roma, Italy; Plan Bleu: Marseille, France, 2018. [Google Scholar]
- Martín-Ortega, P.; Picard, N.; García-Montero, L.G.; Del Rio, S.; Penas, A.; Marchetti, M.; Lasserre, B.; Özdemir, E.; García-Robredo, F.; Pascual, C. Importance of Mediterranean forests. In State of Mediterranean Forests; Bourlion, N., Garavaglia, V., Picard, N., Eds.; Food and Agriculture Organization of the United Nations: Roma, Italy; Plan Bleu: Marseille, France, 2018; pp. 31–50. [Google Scholar]
- FAO. State of Mediterranean Forests 2013; Food and Agriculture Organization of the United Nations: Roma, Italy; Plan Bleu: Marseille, France, 2013. [Google Scholar]
- Bentouati, A.; Oudjehih, B.; Alatou, D. Croissance en hauteur dominante et classes de fertilité du pin d’Alep (Pinus halepensis Mill.) dans le massif de Ouled-Yakoub et des Benioudjana (Khenchela–Aures). Sci. Technol. C 2005, 23, 57–62. [Google Scholar]
- Kadik, B. Contribution à l’étude du pin d’Alep (Pinus halepensis mill.) en Algérie: Écologie, dendrométrie, morphologie. Ph.D. Thesis, Université Paul Cézanne, Aix-Marseille, France, 1983. [Google Scholar]
- Dahmani-Megrerouche, M. Typologie et dynamique des chênaies vertes en Algérie. Forêt Méditerranéenne 2002, 23, 117–132. [Google Scholar]
- Simmons, N.B.; Cirranello, A.L. Bats of the World. A Taxonomic and Geographic Database. Version 1.9. 2025. Available online: https://batnames.org/ (accessed on 10 March 2025).
- Kehal, L.; Zouidi, M.; Keddari, D.; Hadjout, S.; Borsali, A.H. Comparative analysis of forest soil properties from sub-humid and semi-arid areas of Djebel El Ouahch biological reserve in Algeria. S. Asian J. Exp. Biol. 2022, 12, 349–356. [Google Scholar] [CrossRef]
- Rodríguez-San Pedro, A.; Simonetti, J.A. The relative influence of forest loss and fragmentation on insectivorous bats: Does the type of matrix matter? Landsc. Ecol. 2015, 30, 1561–1572. [Google Scholar] [CrossRef]
- Russo, D.; Billington, G.; Bontadina, F.; Dekker, J.; Dietz, M.; Gazaryan, S.; Jones, G.; Meschede, A.; Rebelo, H.; Reiter, G. Identifying key research objectives to make European forests greener for bats. Front. Ecol. Evol. 2016, 4, 87. [Google Scholar] [CrossRef]
- Langridge, J.; Pisanu, B.; Laguet, S.; Archaux, F.; Tillon, L. The role of complex vegetation structures in determining hawking bat activity in temperate forests. For. Ecol. Manag. 2019, 448, 559–571. [Google Scholar] [CrossRef]
- Brigham, R.M. Bats in forests: What we know and what we need to learn. In Bats in Forests. Conservation and Management; Lacki, M.J., Hayes, J.P., Kurta, A., Eds.; The Johns Hopkins University Press: Baltimore, MD, USA, 2007; pp. 1–15. [Google Scholar]
- Kunz, T.H.; Braun de Torrez, E.; Bauer, D.; Lobova, T.; Fleming, T.H. Ecosystem services provided by bats. Ann. N. Y. Acad. Sci. 2011, 1223, 1–38. [Google Scholar] [CrossRef]
- Lacki, M.J.; Johnson, J.S.; Dodd, L.E.; Baker, M.D. Prey consumption of insectivorous bats in coniferous forests of North-Central Idaho. Northwest Sci. 2007, 81, 199–205. [Google Scholar] [CrossRef]
- Böhm, S.M.; Wells, K.; Kalko, E.K.V. Top-down control of herbivory by birds and bats in the canopy of temperate broad-leaved oaks (Quercus robur). PLoS ONE 2011, 6, e17857. [Google Scholar] [CrossRef]
- Aulagnier, S.; Haffner, P.; Mitchell-Jones, A.J.; Moutou, F.; Zima, J. Mammifères d’Europe, d’Afrique du Nord et du Moyen-Orient, 6th ed.; Delachaux et Niestlé: Paris, France, 2024. [Google Scholar]
- Ahmim, M. Écologie et biologie de la conservation des Chiroptères de la région de la Kabylie des Babors (Algérie). Ph.D. Thesis, Université Abderrahmane Mira, Bejaia, Algeria, 2014. [Google Scholar]
- Dalhoumi, R. Les Chiroptères du Parc National de Bou Hedma: Cycle annuel et utilisation de l’habitat. Ph.D. Thesis, Université de Carthage, Bizerte, Tunisia, 2016. [Google Scholar]
- Loumassine, H.-E. Écologie des Chiroptères dans quelques biotopes en Algérie Occidentale. Ph.D. Thesis, Université Ibn Khaldoun, Tiaret, Algeria, 2018. [Google Scholar]
- Russo, D.; Jones, G. Use of foraging habitats by bats in a Mediterranean area determined by acoustic surveys: Conservation implications. Ecography 2003, 26, 197–209. [Google Scholar] [CrossRef]
- Rainho, A. Summer foraging habitats of bats in a Mediterranean Region of the Iberian Peninsula. Acta Chiropterol. 2007, 9, 171–181. [Google Scholar] [CrossRef]
- Novella-Fernandez, R.; Juste, J.; Ibañez, C.; Nogueras, J.; Osborne, P.E.; Razgour, O. The role of forest structure and composition in driving the distribution of bats in Mediterranean Regions. Sci. Rep. 2022, 12, 3224. [Google Scholar] [CrossRef] [PubMed]
- Dalhoumi, R.; Hedfi, A.; Aissa, P.; Aulagnier, S. Bats of Jebel Mghilla National Park (Central Tunisia): First survey and habitat-related activity. Trop. Zool. 2014, 27, 53–62. [Google Scholar] [CrossRef]
- Allegrini, C.; Korine, C.; Krasnov, B.R. Insectivorous bats in Eastern Mediterranean Planted pine forests—Effects of forest structure on foraging activity, diversity, and implications for management practices. Forests 2022, 13, 1411. [Google Scholar] [CrossRef]
- Dbiba, Y.; Dakki, M.; Mansouri, I.; El Mouden, E.H. Bats of the Safi-Essaouira Provinces (Morocco): New Inputs to the knowledge of bat populations of the Atlantic littoral. Int. J. Zool. 2023, 2023, 6624548. [Google Scholar] [CrossRef]
- Kaci, K.; Ahmim, M.; Dalhoumi, R. Winter habitat use and nocturnal activity of the Kuhl’s pipistrelle (Pipistrellus kuhlii) and the European free-tailed bat (Tadarida teniotis) in a Mediterranean region (Algeria). Zool. Ecol. 2024, 34, 169–179. [Google Scholar] [CrossRef]
- Ait Abdesselam, M.; Dalhoumi, R.; Ouabed, A.; Farid, B.; Aulagnier, S. Bat Species richness and activity in a Mediterranean area of Northwest Africa: Urban–rural shifts. Anim. Taxon. Ecol. 2025, 71, 36–50. [Google Scholar] [CrossRef]
- Zamora-Gutierrez, V.; MacSwiney, G.M.C.; Martínez Balvanera, S.; Robredo Esquivelzeta, E. The evolution of acoustic methods for the study of bats. In 50 Years of Bat Research: Foundations and New Frontiers; Springer: Berlin/Heidelberg, Germany, 2021; pp. 43–59. [Google Scholar]
- Neuweiler, G. The Biology of Bats; Oxford University Press: New York, NJ, USA, 2000. [Google Scholar]
- Ziouche, S.; Baali, F.; Moutassem, D.; Djazouli, Z. Stratégies de choix de l’emplacement des nids d’hiver de Thaumetopoea pityocampa (Denis & Schiffermüller, 775) au niveau de trois pinèdes dans la région de Bordj Bou Arreridj (Algérie). Agrobiologia 2017, 7, 412–426. [Google Scholar]
- Bouttaba, C.; Nouibat, B.; Benmechiche, M. Peri-urban forests: An exploratory study of users’ recreational activities: The case of the El Meridj-Est recreational forest in Constantine, Algeria. J. Degrad. Min. Lands Manag. 2024, 11, 5695–5706. [Google Scholar] [CrossRef]
- Bourenane, H.; Bouhadad, Y. Impact of land use changes on landslides occurrence in urban area: The case of the Constantine City (NE Algeria). Geotech. Geol. Eng. 2021, 39, 1–21. [Google Scholar] [CrossRef]
- Lemouissi, S. Approche phytosociologique de la végétation dans le massif forestier de Chettabah (Constantine). Master’s Thesis, Université Frères Mentouri, Constantine, Algeria, 2014. [Google Scholar]
- Kanouni, M.R.; Hani, I.; Bousba, R.; Beldjazia, A.; Khamar, H. Structural variability of Aleppo pine stands in two forests in Northeastern Algeria. Biodiversitas J. Biol. Divers. 2020, 21, 2848–2853. [Google Scholar] [CrossRef]
- Russ, J. Bat Calls of Britain and Europe; Pelagic Publishing: Exeter, UK, 1999. [Google Scholar]
- Barataud, M. Écologie Acoustique des Chiroptères d’Europe; Biotope: Mèze, France; Muséum National d’Histoire Naturelle: Paris, France, 2012. [Google Scholar]
- Disca, T.; Allegrini, B.; Prié, V. Caractéristiques acoustiques des cris d’écholocation de 16 espèces de Chiroptères (Mammalia, Chiroptera) du Maroc. Vespère 2014, 3, 209–229. [Google Scholar]
- Dalhoumi, R.; Aïssa, P.; Aulagnier, S. Bat species richness and activity in Bou Hedma National Park (Central Tunisia). Turk. J. Zool. 2016, 40, 864–875. [Google Scholar] [CrossRef]
- Ahmim, M.; Dalhoumi, R.; Măntoiu, D.S. First data on the acoustic characteristics of some chiropteran species from Northern Algeria. Bioacoustics 2020, 29, 499–517. [Google Scholar] [CrossRef]
- The R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: http://www.R-project.org/ (accessed on 18 November 2024).
- Brooks, M.E.; Kristensen, K.; Van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Mächler, M.; Bolker, B.M. glmm TMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Sugiura, N. Further analysis of the data by Akaike’s information criterion and the finite corrections: Further analysis of the data by Akaike’s. Commun. Stat.-Theory Methods 1978, 7, 13–26. [Google Scholar] [CrossRef]
- Hurvich, C.M.; Tsai, C.-L. Regression and time series model selection in small samples. Biometrika 1989, 76, 297–307. [Google Scholar] [CrossRef]
- Mazerolle, M.J.; Mazerolle, M.M.J. Package ‘AICcmodavg’. 2020. Available online: https://cran.uib.no/web/packages/AICcmodavg/AICcmodavg.pdf (accessed on 14 January 2025).
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. Available online: https://cran.r-project.org/web/packages/DHARMa/vignettes/DHARMa.html (accessed on 14 January 2025).
- Lüdecke, D.; Ben-Shachar, M.S.; Patil, I.; Waggoner, P.; Makowski, D. Performance: An R package for assessment, comparison and testing of statistical models. J. Open Source Softw. 2021, 6, 3139. [Google Scholar] [CrossRef]
- Gori, M.; Betti, A.; Melacci, S. Machine Learning. A Constraint-Based Approach, 2nd ed.; Morgan Kaufmann Publishers: Cambridge, MA, USA, 2024. [Google Scholar]
- Lisón, F.; Calvo, J.F. The significance of water infrastructures for the conservation of bats in a semiarid Mediterranean landscape. Anim. Conserv. 2011, 14, 533–541. [Google Scholar] [CrossRef]
- Dalhoumi, R.; Nefla, A.; Bedoui, W.; Ouni, R.; Aissa, P.; Aulagnier, S. Preliminary study of habitat-related bat fauna of Mastouta-Bishshouk region (Northwest Tunisia). Zoodiversity 2019, 53, 23–30. [Google Scholar] [CrossRef]
- Tillon, L.; Barataud, M.; Giosa, S.; Aulagnier, S. Acoustic detection of radiotracked foraging bats in temperate lowland forests. Mamm. Biol. 2019, 95, 155–159. [Google Scholar] [CrossRef]
- Dekker, J.; Steen, W.; Bouman, H.B.; van der Vliet, R.E. Differences in acoustic detectability of bat species hamper environmental impact assessment studies. Eur. J. Wildl. Res. 2022, 68, 14. [Google Scholar] [CrossRef]
- Teets, K.D.; Loeb, S.C.; Jachowski, D.S. Detection probability of bats using active versus passive monitoring. Acta Chiropterol. 2019, 21, 205–213. [Google Scholar] [CrossRef]
- Perks, S.J.; Goodenough, A.E. Comparing acoustic survey data for European bats: Do walked transects or automated fixed-point surveys provide more robust data? Wildl. Res. 2021, 49, 314–323. [Google Scholar] [CrossRef]
- Fisher-Phelps, M.; Schwilk, D.; Kingston, T. Mobile acoustic transects detect more bat activity than stationary acoustic point counts in a semi-arid and agricultural landscape. J. Arid. Environ. 2017, 136, 38–44. [Google Scholar] [CrossRef]
- Fischer, J.; Stott, J.; Law, B.S.; Adams, M.D.; Forrester, R.I. Designing effective habitat studies: Quantifying multiple sources of variability in bat activity. Acta Chiropterol. 2009, 11, 127–137. [Google Scholar] [CrossRef]
- Skalak, S.L.; Sherwin, R.E.; Brigham, R.M. Sampling period, size and duration influence measures of bat species richness from acoustic surveys. Methods Ecol. Evol. 2012, 3, 490–502. [Google Scholar] [CrossRef]
- Kubista, C.E.; Bruckner, A. Within-site variability of field recordings from stationary, passively working detectors. Acta Chiropterol. 2017, 19, 189–197. [Google Scholar] [CrossRef]
- Froidevaux, J.S.; Zellweger, F.; Bollmann, K.; Obrist, M.K. Optimizing passive acoustic sampling of bats in forests. Ecol. Evol. 2014, 4, 4690–4700. [Google Scholar] [CrossRef]
- Tena, E.; Tellería, J. Modelling the distribution of bat activity areas for conservation in a Mediterranean mountain range. Anim. Conserv. 2022, 25, 65–76. [Google Scholar] [CrossRef]
- Russo, D.; Ancillotto, L. Sensitivity of bats to urbanization: A review. Mamm. Biol. 2015, 80, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Aulagnier, S.; Cuzin, F.; Thévenot, M. Mammifères Sauvages du Maroc. Peuplement, Répartition, Écologie; Société Française pour l’Étude et la Protection des Mammifères: Paris, France, 2017. [Google Scholar]
- Dalhoumi, R.; Aissa, P.; Aulagnier, S. Taxonomie et répartition des Chiroptères de Tunisie. Rev. Suisse Zool. 2011, 118, 265–292. [Google Scholar] [CrossRef]
- Puechmaille, S.J.; Hizem, W.M.; Allegrini, B.; Abiadh, A. Bat fauna of Tunisia: Review of records and new records, morphometrics and echolocation data. Vespertilio 2012, 16, 211–239. [Google Scholar]
- Ahmim, M.; Oubaziz, B. New data on the distribution of bats (Mammalia: Chiroptera) in Algeria. Acta Soc. Zool. Bohemicae 2017, 81, 17–30. [Google Scholar]
- Ancillotto, L.; Tomassini, A.; Russo, D. The fancy city life: Kuhl’s pipistrelle, Pipistrellus kuhlii, benefits from urbanisation. Wildl. Res. 2015, 42, 598–606. [Google Scholar] [CrossRef]
- Davy, C.M.; Russo, D.; Fenton, M.B. Use of native woodlands and traditional olive groves by foraging bats on a Mediterranean Island: Consequences for conservation. J. Zool. 2007, 273, 397–405. [Google Scholar] [CrossRef]
- Di Salvo, I.; Russo, D.; Sarà, M. Habitat preferences of bats in a rural area of Sicily determined by acoustic surveys. Hystrix 2009, 20, 137–146. [Google Scholar]
- Georgiakakis, P.; Vasilakopoulos, P.; Mylonas, M.; Russo, D. Bat species richness and activity over an elevation gradient in Mediterranean shrublands of Crete. Hystrix 2010, 21, 43–56. [Google Scholar]
- Herrera, J.M.; Costa, P.; Medinas, D.; Marques, J.T.; Mira, A. Community composition and activity of insectivorous bats in Mediterranean olive farms. Anim. Conserv. 2015, 18, 557–566. [Google Scholar] [CrossRef]
- Ancillotto, L.; Ariano, A.; Nardone, V.; Budinski, I.; Rydell, J.; Russo, D. Effects of free-ranging cattle and landscape complexity on bat foraging: Implications for bat conservation and livestock management. Agric. Ecosyst. Environ. 2017, 241, 54–61. [Google Scholar] [CrossRef]
- Tzortzakaki, O.; Papadatou, E.; Kati, V.; Giokas, S. Winners and losers in an urban bat community: A case study from Southeastern Europe. Hystrix 2019, 30, 134–140. [Google Scholar]
- Loumassine, H.E.; Bonnot, N.; Allegrini, B.; Bendjeddou, M.L.; Bounaceur, F.; Aulagnier, S. How arid environments affect spatial and temporal activity of bats. J. Arid. Environ. 2020, 180, 104206. [Google Scholar] [CrossRef]
- Dalhoumi, R.; Aïssa, P.; Hamouda, B.; Aulagnier, S. Bat species richness and activity in Dghoumes National Park (Southwest Tunisia): A preliminary survey. Arx. Miscel-Lània Zool. 2020, 18, 89–100. [Google Scholar] [CrossRef]
- Dalhoumi, R.; El Mokni, R.; Ouni, R.; Hamouda, B.; Aulagnier, S. Bats of the Tunisian desert: Preliminary data using acoustic identification and first record of Taphozous nudiventris in the country. Diversity 2023, 15, 1108. [Google Scholar] [CrossRef]
- Lisón, F.; Calvo, J. Bat activity over small ponds in dry Mediterranean forests: Implications for conservation. Acta Chiropterol. 2014, 16, 95–101. [Google Scholar] [CrossRef]
- Kipson, M.; Gazaryan, S.; Horáček, I. Savi’s pipistrelle Hypsugo savii (Bonaparte, 1837). In Handbook of the Mammals of Europe; Russo, D., Ed.; Springer Nature: Cham, Switzerland, 2025; pp. 95–112. [Google Scholar]
- Lisón, F.; Calvo, J.F. Ecological niche modelling of three pipistrelle bat species in semiarid Mediterranean Landscapes. Acta Oecol. 2013, 47, 68–73. [Google Scholar] [CrossRef]
- Froidevaux, J.; Zellweger, F.; Bollmann, K.; Jones, G.; Obrist, M.K. From field surveys to LiDAR: Shining a light on how bats respond to forest structure. Remote Sens. Environ. 2016, 175, 242–250. [Google Scholar] [CrossRef]
- Froidevaux, J.S.P.; Barbaro, L.; Vinet, O.; Larrieu, L.; Bas, Y.; Molina, J.; Calatayud, F.; Brin, A. Bat responses to changes in forest composition and prey abundance depend on landscape matrix and stand structure. Sci. Rep. 2021, 11, 10586. [Google Scholar] [CrossRef]
- Bender, M.J.; Perea, S.; Castleberry, S.B.; Miller, D.A.; Wigley, T.B. Influence of Insect abundance and vegetation structure on site-occupancy of bats in managed pine forests. For. Ecol. Manag. 2021, 482, 118839. [Google Scholar] [CrossRef]
- Perea, S.; Vicente-Santos, A.; Larsen-Gray, A.L.; Gandhi, K.J.; Greene, D.U.; Barnes, B.F.; Castleberry, S.B. Disentangling winter relationships: Bat responses to forest stand structure, environmental conditions, and prey composition. For. Ecol. Manag. 2025, 578, 122484. [Google Scholar] [CrossRef]
- Charbonnier, Y.M.; Barbaro, L.; Barnagaud, J.-Y.; Ampoorter, E.; Nezan, J.; Verheyen, K.; Jactel, H. Bat and bird diversity along independent gradients of latitude and tree composition in European forests. Oecologia 2016, 182, 529–537. [Google Scholar] [CrossRef]
- Tibbels, A.E.; Kurta, A. Bat activity is low in thinned and unthinned stands of red pine. Can. J. For. Res. 2003, 33, 2436–2442. [Google Scholar] [CrossRef]
- Węgiel, A.; Grzywiński, W.; Ciechanowski, M.; Jaros, R.; Kalcounis-Rüppell, M.; Kmiecik, A.; Kmiecik, P.; Węgiel, J. The foraging activity of bats in managed pine forests of different ages. Eur. J. For. Res. 2019, 138, 383–396. [Google Scholar] [CrossRef]
- Charbonnier, Y.; Barbaro, L.; Theillout, A.; Jactel, H. Numerical and functional responses of forest bats to a major insect pest in pine plantations. PLoS ONE 2014, 9, e109488. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.D.; Miller, D.A.; Kalcounis-Rueppell, M.C. Use of forest edges by bats in a managed pine forest landscape. J. Wildl. Manag. 2010, 74, 26–34. [Google Scholar] [CrossRef]
- Kalcounis, M.C.; Brigham, R.M. Intraspecific variation in wing loading affects habitat use by little brown bats (Myotis lucifugus). Can. J. Zool. 1995, 73, 89–95. [Google Scholar] [CrossRef]
- Menzel, J.M.; Menzel, M.A., Jr.; Kilgo, J.C.; Ford, W.M.; Edwards, J.W.; McCracken, G.F. Effect of habitat and foraging height on bat activity in the coastal plain of South Carolina. J. Wildl. Manag. 2005, 69, 235–245. [Google Scholar] [CrossRef]
- Müller, J.; Mehr, M.; Bässler, C.; Fenton, M.B.; Hothorn, T.; Pretzsch, H.; Klemmt, H.-J.; Brandl, R. Aggregative response in bats: Prey abundance versus habitat. Oecologia 2012, 169, 673–684. [Google Scholar] [CrossRef]
- Dodd, L.E.; Lacki, M.J.; Britzke, E.R.; Buehler, D.A.; Keyser, P.D.; Larkin, J.L.; Rodewald, A.D.; Wigley, T.B.; Wood, P.B.; Rieske, L.K. Forest structure affects trophic linkages: How silvicultural disturbance impacts bats and their insect prey. For. Ecol. Manag. 2012, 267, 262–270. [Google Scholar] [CrossRef]
- Titchenell, M.A.; Williams, R.A.; Gehrt, S.D. Bat response to shelterwood harvests and forest structure in oak-hickory forests. For. Ecol. Manag. 2011, 262, 980–988. [Google Scholar] [CrossRef]
- Stone, E.L.; Harris, S.; Jones, G. Impacts of artificial lighting on bats: A review of challenges and solutions. Mamm. Biol. 2015, 80, 213–219. [Google Scholar] [CrossRef]
- Gaston, K.J.; Bennie, J.; Davies, T.W.; Hopkins, J. The ecological impacts of nighttime light pollution: A mechanistic appraisal. Biol. Rev. 2013, 88, 912–927. [Google Scholar] [CrossRef] [PubMed]
- Schaub, A.; Ostwald, J.; Siemers, B.M. Foraging bats avoid noise. J. Exp. Biol. 2008, 211, 3174–3180. [Google Scholar] [CrossRef]
- Florencio, M.; Serrano, L.; Gómez-Rodríguez, C.; Millán, A.; Díaz-Paniagua, C. Inter-and intra-annual variations of macroinvertebrate assemblages are related to the hydroperiod in Mediterranean temporary ponds. Hydrobiologia 2009, 634, 167–183. [Google Scholar] [CrossRef]
- Florencio, M.; Díaz-Paniagua, C.; Serrano, L.; Bilton, D.T. Spatio-temporal nested patterns in macroinvertebrate assemblages across a pond network with a wide hydroperiod range. Oecologia 2011, 166, 469–483. [Google Scholar] [CrossRef]
- Gaisler, J.; Madkour, G.; Pelikán, J. On the bats (Chiroptera) of Egypt. Československá akademie věd v Brně. Přírodovědné práce Ústavů Československé akademie vĕd v Brnĕ Acta scientiarum naturalium Academiae scientiarum Bohemoslovacae Brno. Ser. Nova 1972, 6, 1–40. [Google Scholar]
- Dalhoumi, R.; Morellet, N.; Aissa, P.; Aulagnier, S. Seasonal activity pattern and habitat use by the Kuhl’s pipistrelle (Pipistrellus kuhlii) in an arid environment. Eur. J. Wildl. Res. 2018, 64, 36. [Google Scholar] [CrossRef]
- Yekwayo, I.; Pryke, J.S.; Roets, F.; Samways, M.J. Surrounding vegetation matters for arthropods of small, natural patches of indigenous forest. Insect Conserv. Divers. 2016, 9, 224–235. [Google Scholar] [CrossRef]
- Uhler, J.; Redlich, S.; Zhang, J.; Hothorn, T.; Tobisch, C.; Ewald, J.; Thorn, S.; Seibold, S.; Mitesser, O.; Morinière, J. Relationship of insect biomass and richness with land use along a climate gradient. Nat. Commun. 2021, 12, 5946. [Google Scholar] [CrossRef]
- van Deventer, H.D.; Gaigher, R.; Pryke, J.S. Grassland arthropod assemblages differ between a variety of neighbouring biotopes and small features in a timber production landscape. Biodivers. Conserv. 2025, 34, 2939–2955. [Google Scholar] [CrossRef]
- Kalko, E.K.; Schnitzler, H.-U. Plasticity in echolocation signals of European pipistrelle bats in search flight: Implications for habitat use and prey detection. Behav. Ecol. Sociobiol. 1993, 33, 415–428. [Google Scholar] [CrossRef]
- Ancillotto, L.; Santini, L.; Ranc, N.; Maiorano, L.; Russo, D. Extraordinary range expansion in a common bat: The potential roles of climate change and urbanisation. Sci. Nat. 2016, 103, 15. [Google Scholar] [CrossRef]
- Korine, C.; Pinshow, B. Guild structure, foraging space use, and distribution in a community of insectivorous bats in the Negev Desert. J. Zool. 2004, 262, 187–196. [Google Scholar] [CrossRef]
- Razgour, O.; Korine, C.; Saltz, D. Does interspecific competition drive patterns of habitat use in desert bat communities? Oecologia 2011, 167, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Feldman, R.; Whitaker, J.O.; Yom-Tov, Y. Dietary composition and habitat use in a desert insectivorous bat community in Israel. Acta Chiropterol. 2000, 2, 15–22. [Google Scholar]
- Speakman, J.R.; Thomas, D.W. Physiological ecology and energetics of bats. In Bat Ecology; Kunz, T.H., Fenton, M.B., Eds.; The University of Chicago Press: Chicago, MI, USA, 2003; pp. 430–490. [Google Scholar]
- Sharifi, M.; Vaissi, S.; Javanbakht, H.; Akmali, V. Postnatal growth and wing development in Kuhl’s pipistrelle Pipistrellus kuhlii (Chiroptera: Vespertilionidae) in captivity. Zool. Stud. 2012, 51, 1235–1247. [Google Scholar]
- Benda, P.; Lucan, R.K.; Obuch, J.; Reiter, A.; Andreas, M.; Backor, P.; Bohnenstengel, T.; Eid, E.K.; Sevcik, M.; Vallo, P. Bats (Mammalia: Chiroptera) of the Eastern Mediterranean and Middle East. Part 8. Bats of Jordan: Fauna, ecology, echolocation, ectoparasites. Acta Soc. Zool. Bohemicae 2010, 74, 185–353. [Google Scholar]
- Benda, P.; Spitzenberger, F.; Hanák, V.; Andreas, M.; Reiter, A.; Ševčík, M.; Šmíd, J.; Uhrin, M. Bats (Mammalia: Chiroptera) of the Eastern Mediterranean and Middle East. Part 11. On the bat fauna of Libya II. Acta Soc. Zool. Bohemicae 2014, 78, 1–162. [Google Scholar]
- Benda, P.; Andreas, M.; Kock, D.; Lucan, R.K.; Munclinger, P.; Nova, P.; Obuch, J.; Ochman, K.; Reiter, A.; Uhrin, M. Bats (Mammalia: Chiroptera) of the Eastern Mediterranean. Part 4. Bat Fauna of Syria: Distribution, systematics, ecology. Acta Soc. Zool. Bohemicae 2006, 70, 1–329. [Google Scholar]
- Lewis, R.E.; Harrison, D.L. Notes on bats from the Republic of Lebanon. Proc. Zool. Soc. Lond. 1962, 138, 473–486. [Google Scholar] [CrossRef]
- Alagaili, A. Biological, Ecological, and Conservational Study of Kuhl’s Bat (Pipistrellus kuhlii) from Unizah Province, Saudi Arabia. Ph.D. Thesis, University of Arkansas, Fayetteville, NC, USA, 2008. [Google Scholar]
- Alagaili, A.N.; James, D.A.; Mohammed, O.B. Timing and pattern of molt in Kuhl’s bat, Pipistrellus kuhlii, in Saudi Arabia. Acta Chiropterol. 2011, 13, 465–470. [Google Scholar] [CrossRef]
- Kelm, D.H.; Popa-Lisseanu, A.G.; Dehnhard, M.; Ibáñez, C. Non-invasive monitoring of stress hormones in the bat Eptesicus isabellinus–Do fecal glucocorticoid metabolite concentrations correlate with survival? Gen. Comp. Endocrinol. 2016, 226, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Lisón, F.; Haz, Á.; Calvo, J.F. Preferencia de hábitat del murciélago hortelano meridional Eptesicus isabellinus (Temminck, 1840) en ambientes mediterráneos semiáridos. Anim. Biodivers. Conserv. 2014, 37, 59–67. [Google Scholar] [CrossRef]
- Pérez Jordá, J.L. Ecología del murciélago hortelano, Eptesicus serotinus, en Andalucía. Ph.D. Thesis, Universidad de Sevilla, Sevilla, Spain, 1994. [Google Scholar]
- Lisón, F.; Espinosa, J.A.L.; Calvo, J.F.; Jones, G. Diet of the meridional serotine Eptesicus isabellinus in an urban semiarid Mediterranean landscape. Acta Chiropterol. 2015, 17, 371–378. [Google Scholar] [CrossRef]
- Dalhoumi, R.; Morellet, N.; Aissa, P.; Aulagnier, S. Seasonal activity pattern and habitat use by the isabelline serotine bat (Eptesicus isabellinus) in an arid environment of Tunisia. Acta Chiropterol. 2017, 19, 141–153. [Google Scholar] [CrossRef]
- Baker, R.J.; Davis, B.L.; Jordan, R.G.; Binous, A. Karyotypic and morphometric studies of Tunisian mammals: Bats. Mammalia 1974, 38, 695–705. [Google Scholar] [CrossRef]
- Loeb, S.C.; Waldrop, T.A. Bat activity in relation to fire and fire surrogate treatments in southern pine stands. For. Ecol. Manag. 2008, 255, 3185–3192. [Google Scholar] [CrossRef]
- López-Baucells, A.; Flaquer, C.; Mas, M.; Puig-Montserrat, X. Recurring fires in Mediterranean habitats and their impact on bats. Biodivers. Conserv. 2021, 30, 385–402. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).