Abstract
The main objective of the study was to evaluate the population characteristics of Betula nana under different anthropogenic influences. The study was conducted in the vicinity of the exploited Šepeta peatland (northeastern Lithuania). The population status of B. nana was determined by comparing the ramet density and morphology (height, branching, and leaf size), the age structure, the number of generative ramets, and their flowering characteristics in four study areas at different distances from the exploited peatlands and in different habitats. Around 20 environmental factors were included in the analysis, covering water levels, peat, and vegetation characteristics. Shading, drainage and increased amounts of nitrogen in the habitats are the main factors contributing to the differences and structure of B. nana cenopopulations. Although taller ramets with larger leaves are observed under the changed conditions as an adaptation to shading, the negative anthropogenic effects in the most affected habitats are reflected in a reduction in the number of flowering ramets, lower vegetative regeneration, and an increase in the number of dead twigs on mature ramets.
Keywords:
catkins; cenopopulation; drainage; dwarf birch; raised bog; ramets; morphometric parameters 1. Introduction
Dwarf birch (Betula nana L.) is a widespread circumpolar Arctic–montane species. Plants distributed in the Eurasian part of the range are classified as B. nana subsp. nana [1,2]. In Europe, the subspecies is common in the northern regions (Greenland, Iceland, Scandinavia and Finland) [1]. It is also widely distributed in Northern Estonia; meanwhile, further south, its occurrence decreases [3]. In Latvia, Lithuania, and in Poland, B. nana is a climatic glacial relict outside its continuous distribution area with scattered locations reaching the southwestern border in France [3,4].
The species occurring on the boundary of their range are more sensitive to environmental changes, especially in the habitats which undergo modification processes due to anthropogenic activity [5].
Most studies of B. nana have been carried out in countries covering its continuous range. Publications include studies on distribution and synecology [6], hybridisation with other birch species [6,7,8] conservation genetics [9], seed banks [10], climate changes impact [11], etc.
Some detailed surveys on relict populations of this species at the southern edge of its range have been carried out mainly in Poland [12,13,14,15,16,17,18], sometimes including populations from other countries such as Russia, Belarus, and Finland [4]. Betula nana is recognised as endangered in Latvia [19], Lithuania [20], and Poland [21]. In Lithuania, there are available data mainly on B. nana distribution and decline trends, but detailed studies of populations have not been carried out [5,20].
Our study is focused on anthropogenically impacted fragments of B. nana population that survived after drainage of a large raised bog and occurring in different habitats.
Our main objective was to assess the possibility of survival of B. nana population under human pressure. The main questions we aimed to answer were: (1) How do B. nana habitats change under anthropogenic pressure? (2) How does anthropogenic pressure change the abundance of B. nana ramets and their morphology? (3) How does this affect the flowering characteristics of B. nana ramets? (4) Are there differences in the age structure of B. nana populations between habitats affected by different anthropogenic influences? (5) Which habitat changes have a decisive influence on the structure of B. nana populations?
2. Materials and Methods
Study site. Lithuania is between central latitude oceanic and continental climates with an average annual precipitation of 675 mm, a hydrothermal coefficient (HTC) of 1.3–1.9, and the yearly mean air temperature is 6.2 °C [22]. The country’s climate is favourable to the formation of peatlands; they cover about 7.8% of the territory [23]. Figure 1 shows a climate diagram based on 30 years (1991–2020) of data from the Lithuanian Hydrometeorological Service at the Panevėžys station [24], which is closest to the Šepeta peatland.
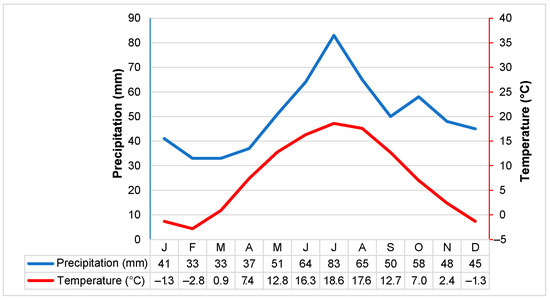
Figure 1.
Climate diagram based on data from the Lithuanian Hydrometeorological Service 1991–2020 at the Panevėžys station. The monthly average air temperature and the monthly total precipitation are shown in red and blue, respectively.
Šepeta peatland (1650 ha) [25] is in the northeastern part of Lithuania (55.787492, 25.014157) (Figure 2). It is a former natural raised bog that has been gradually drained and exploited since the early 20th century. The exploitation of the bog accelerated, particularly in the 1960s, and today, 90% of its territory is either in industrial use or is exploited. Only the edges of the bog, which are inconvenient for use, have remained. Although not as drastically as the main part, they are still affected by drainage. According to the Šepeta survey carried out more than 80 years ago [26], B. nana was distributed throughout the bog. It was one of the first discoveries of this glacial relict in Lithuania [27].
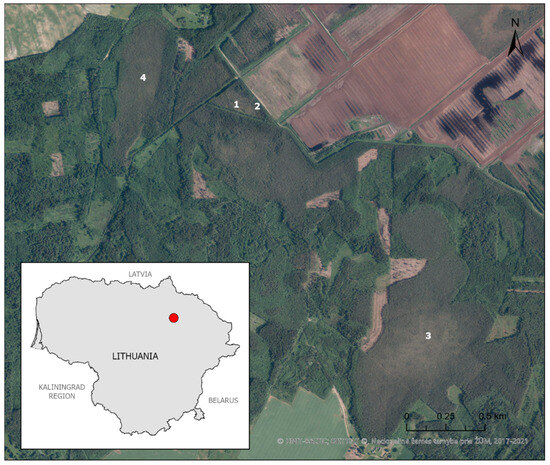
Figure 2.
Location of study areas (1–4) in Šepeta peatland (northeastern Lithuania).
Study areas. The research was conducted in 2022 and 2023. During the pilot study in 2022, four study areas (cenopopulations) with different habitat characteristics and anthropogenic pressure in the preserved fragments of the raised bog situated to the south and west of the exploited peatland were selected (Figure 2). These bog fragments were of different sizes and at different distances from the exploited peatland, so they were differently anthropogenically affected.
The 1st and the 2nd study areas were situated in the same fragment of the bog, located closest to the exploited peatland, bordered by drainage ditches on all sides. The 2nd study area located along the main drainage ditch was treated as the most affected by peatland drainage. The 1st study area was situated in the central part of this fragment and seemed less affected by drainage. The 3rd study area was located more than 1 km from the exploited peatland area in the largest and relatively most natural raised bog fragment. The 4th study area was located about 500 m to the west of the exploited peatland border.
The habitats of the 1st and the 3rd areas are characterised as semi-open areas of a raised bog with solitary trees (mainly dwarf pines Pinus sylvestris), a richly developed shrub layer of B. nana, and a moss layer with equally dominant green and Sphagnum mosses. The 2nd area is in the transitional stage between semi-open raised bog habitat to bog pine forest, whereas the 4th can be classified as a true forest habitat. The vegetation of both latter areas was characterised by taller and denser Pinus tree cover. Since each study area is a plant community that differs in structure and specific species composition, we have treated each study area as if it contained different Betula nana cenopopopulations sensu Rabotnov [28].
Study object. Betula nana L. is a shrub up to 1 m tall that forms prostrate to ascending, abundantly branched shoots. The twigs are stiff and persistently pubescent, sometimes with resin glands. The leaves are with short (1–4 mm long) petiola and with orbicular to broadly obovatelamina (5–20 mm long). The male catkins are erect to patent and 5–13 mm long. The female catkins are erect, broadly cylindrical, and 7–14 mm long [2,29].
Betula nana is a clonal plant that reproduces mainly by vegetative means [2]. The main object of our study was the ramet, the clonally formed offspring of the genet [30,31]. We did not investigate whether individual ramets in the study plots were derived from one or more genets, as the method is destructive. Genetic markers are usually used for the detection of genets and the assignment of ramets to the corresponding genets [32]. We did not perform such studies. However, in our study, we tested whether the young shoots were of vegetative or generative origin.
Field studies of cenopopulations. In 2023, more detailed research of B. nana cenopopulations was conducted. Three study plots (3 m × 3 m) were selected in each of the four study areas (12 plots in total). When selecting the study plots, care was taken to ensure that they were evenly distributed across the study area and representative of its vegetation.
For each study plot, the following parameters were recorded:
- (1)
- Cover and abundance of each plant species following the Braun–Blanquet scale [33].
- (2)
- Percentage cover of tree, shrub/subshrub, herb, and bryophyte layers and B. nana.
- (3)
- Physical and chemical parameters of peat (conductivity; pH; concentrations total nitrogen, NH4+, NO3−, P, K, Mg, Cl, organic material and organic carbon, peat decomposition degree, and peat moisture). Peat samples were selected from 10 randomly chosen sites, and a pooled sample (approximately 500 g of peat) was prepared. The analysis of peat samples was performed in the Agrochemical Research Laboratory of the Lithuanian Research Centre for Agriculture and Forestry. In the laboratory, the pH was measured using the potenciometrical method according to LSTEN 13037 [34]. Spectrophotometrical methods were used for the measurements of P, atomic emission spectral method for K, atomic absorption emission spectrometrical method for Ca and Mg, colourimetric method with FIA star for N-NO3−, N-NH4, and Cl was measured with the argentometrical method LSTEN 13652 [35]. The peat decomposition degree was ascertained according to LST 1957:2022 [36], organic material and peat moisture according to [37] LST EN 13040, and organic carbon according to ISO10694:19995 [38].
- (4)
- Water table. Free groundwater table before the ground surface was measured using 20 mm diameter PVC pipes perforated with 50 mm holes. The pipes were hammered down to a depth of 1 m from the surface of the peat at the centre of each study plot. After half an hour, the water table depth from the peat surface was measured with a stable measuring tape. If water did not appear at 1 m, its depth was estimated as >1 m.
Additionally, to assess the shading of the study plots, the percentage cover of trees within 5 m radius of the study plot was estimated.
In five subplots (1 m × 1 m) arranged in corners and the centre of each study plot (60 subplots in total), the following B. nana population parameters were evaluated:
- (1)
- The total number of ramets (sensu [39]) and the number of ramets in each of the five stages of development. The first four stages followed [15]: stage 1—one-year-old unbranched, stage 2—two-year-old unbranched, stage 3—two-year-old branched, stage 4—branched ramets that were three or more years old. Additionally, we distinguished the 5th stage for the ramets with signs of ageing and degradation (shoots with a grey-coloured main stem and long, thick, and stiff branches and with occasional thin leafy twigs).
- (2)
- The number of flowering ramets, followed by the number of male and female catkins on each.
Morphometric measurements. In July, nine ramets were accidentally cut in each study plot. Five leaves from the central part of the ramet were selected for their morphometrical measurements. In the laboratory, the length of the main shoot was measured. Ramet branching was estimated as the number of twig apices (total, dead and live).
Leaf width was measured at the widest part of the leaf and the length from the point of attachment of the petiole to the apex. The area of the leaf was ascertained using the software Digimizer 6.3.0. Each sampling plot was photographed with the camera (Canon EOS 550D, EFS 18–55 mm lens, Tokyo, Japan) mounted on a tripod, maintaining a uniform distance (0.3 m) from the lens to the surface (MedCalc Software Ltd., Ostend, Belgium).
Preparations for the ramet age determination were made according to the basic principles outlined in [40], with slight modifications. Firstly, segments of about 50 mm from the lower part of the ramets were cut and sanded with 400–600 grit sandpaper. The sanded segments were soaked in water for about 10 min to make them softer and easier to cut into thin (1–2 mm) strips. The cut strips were soaked in methylene blue dye for about 10 min to obtain a better view of the annual rings. The stained preparations were rinsed with water and air-dried. The age of each ramet was ascertained by counting annual rings using a microscope Nikon SMZ 800 and imaging analysis NIS-Elements D (Ver 4.00) software (Nikon, Tokyo, Japan).
Statistical analysis. The normality of the data distribution was evaluated using the Kolmogorov–Smirnov test. For comparison of the parameters that showed non-normal distribution (the peat physical–chemical parameters, cover of B. nana, density of ramets, number of flowering ramets and catkins) between study areas, the nonparametric Kruskal–Wallis, and Mann–Whitney tests were used. The parametric one-way ANOVA was used to identify significant differences in ramet age and stem and leaf morphometric parameters. Tukey’s HSD post hoc multiple comparison test was used to examine significant differences.
Pearson correlation was used to determine correlations between ramet age and their height and diameter.
Calculations were performed using SPSS software (version 16) [41]. All p-values of less than 0.05 were considered statistically significant.
Principle component analysis (PCA) was performed for twenty environmental variables across the study plots to determine the main variables that identify the most responsive parameters in the data set. All environmental variables were ln-transformed before ordination analyses. Variables with loadings above 0.30 (considered significant) [42] were used to interpret the retained PC axes. PCA analysis was processed using the PAST version 4.17 [43].
3. Results
3.1. Characteristics of Betula nana Habitats
The differences in environmental parameters were ascertained for all study areas (Table A1). The 2nd study area had the highest levels of many chemical parameters, especially by the total nitrogen, ammonium and K amounts that were significantly different compared to other study areas (Figure 3). This area was characterised by the higher conductivity, the highest degree of peat decomposition, the lowest amounts of organic material, organic carbon as well as the lowest water level and degree of peat moisture. Conversely, the 4th study area was characterised by the lowest levels and quantities of many parameters (N, ammonium, nitrate, K, Ca, Mg, Cl, conductivity). The 1st and 3rd study areas were similar regarding the most physical and chemical parameters (N, ammonium, nitrates, Mg, electric conductivity, organic carbon, peat moisture, and water level). Although significant differences were ascertained between study areas in pH (Kruskal–Wallis test), they varied within a very small range (from 3.8 to 4.00) (Table A1).
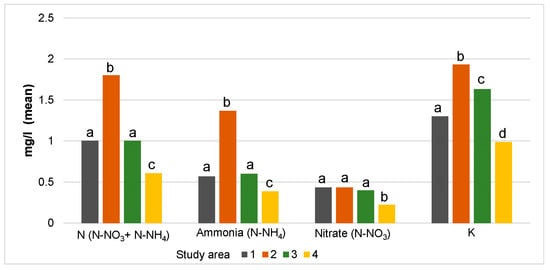
Figure 3.
The mean amount of nitrogen and potassium nutrients between study areas (1–4). Different letters indicate significant differences (p < 0.05) between the parameters of different study areas (1–4) according to the Mann–Whitney U test analysis.
The vegetation characteristics showed significant differences in the study plots of different areas. The most distinctive study plots were those of the third study area. They were characterised by the lowest tree cover and the highest cover of the bryophyte layer. A more detailed description of the vegetation in B. nana study plots is provided in Table A2.
The first two PCA axes accounted for 77.2% of the total variance in the environmental data (Table 1). The environmental variables on the first axis of PCA were preliminarily classified as vegetation (biotic)-related factors, while the second axis was more associated with physical–chemical (abiotic) environmental parameters. The first PCA explained 43.4% of the total variance and chiefly indicated the gradients of tree cover, surrounding study plot, and tree and dwarf shrub cover within the study plot. The second PCA axis explained 33.8% of the variance and was associated with the amount of nutrients, including total ammonia, nitrate ions, and water level.

Table 1.
Summary of principal component analysis (PCA) results for environmental variables recorded in the study plots. Values in bold indicate variables (≥0.3) that most influence each PCs.
3.2. Characteristics Betula nana Population in Šepeta Peatland
3.2.1. Cover, Density, and Flowering of Betula nana
One-way ANOVA revealed significant differences between the abundance (cover) of B. nana in subplots of the study areas. The lowest was in the 1st study area, and the highest was in the 4th study area (Figure 4A). The same tendency was ascertained in ramet density between studied areas (Figure 4B).
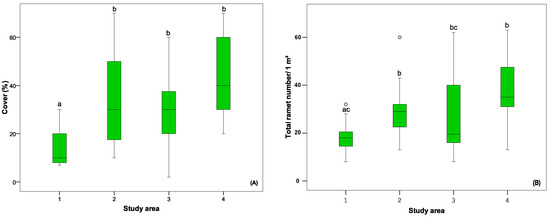
Figure 4.
The comparison of B. nana population parameters between study areas: (A) cover, (B) density of ramets. Different letters indicate significant differences (p < 0.05) between the parameters of different study areas (1–4) according to the Mann–Whitney U test analysis.
Across all study areas, the proportion of ramets of different stages was similar. The young shoots were all of vegetative origin, growing from a rhizome or stem, i.e., they were all ramets and not genets. The ramets of the 4th stage were notable for their abundance, and their density differences in the studied areas were similar to those of the total ramet density. However, the density of the ramets of other stages was incredibly low, and the differences were not significant between study areas (Figure 5).
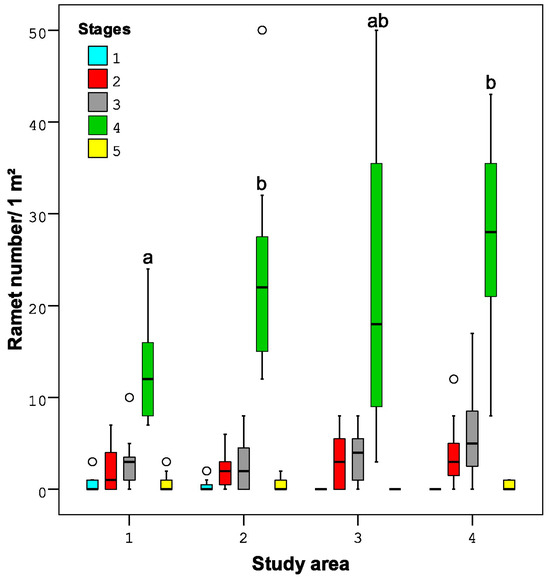
Figure 5.
The proportion of different stages of B. nana ramets between study areas. Stage 1—one-year-old unbranched ramets, stage 2—two-year-old unbranched ramets, stage 3—two-year-old branched ramets, stage 4—branched ramets that were three or more years old, and stage 5—ramets with signs of ageing and degradation. Different letters indicate significant differences (p < 0.05)) between the parameters of different study areas (1–4) according to the Mann–Whitney U test analysis.
The number of flowering ramets was significantly different in the study areas, and made 12.2% in the 1st study area, 0.45% in the 2nd, 22.4% in the 3rd and 4.5% in the 4th. By the total number the highest number of flowering ramets were ascertained in the subplots of the 3rd study area and the lowest in the 2nd (Figure 6).
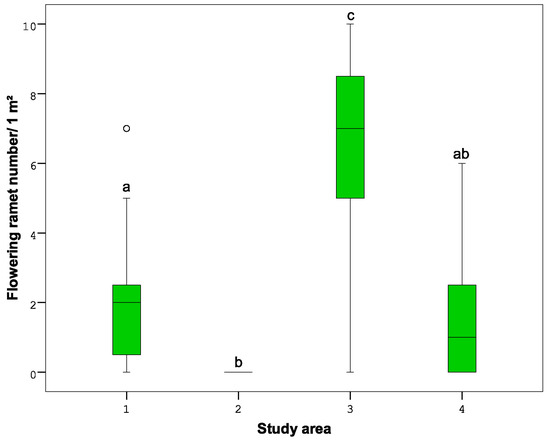
Figure 6.
Number of flowering ramets in different study areas (1–4). Different letters indicate significant differences (p < 0.05) between the parameters of different study areas (1–4) according to the Mann–Whitney U test analysis.
The proportion of male and female catkins was similar between study plots; however, their number per flowering ramet differed significantly in the study areas: the highest in the 3rd study area and the lowest in the 2nd one (Table 2).

Table 2.
Number of female (♀) and male (♂) catkins per flowering ramet in different cenopopulations. Different lowercase letters in the column “Study Area” indicate that there are significant differences (p > 0.05) between the parameters of varying study areas (1–4) according to the Mann–Whitney U test analysis.
3.2.2. Age and Morphometric Parameters of the Ramets
The age of ramets studied in the laboratory ranged from 5 to 16 years. The one-way ANOVA revealed significant differences between the age of the ramets from the 3rd and 4th study areas (Figure 7A). Significant differences between these study areas were demonstrated by comparing the height of the ramets. However, the tendency was the opposite (Figure 7B). No significant differences were shown between the stem’s diameter in different study areas (Figure 7C).
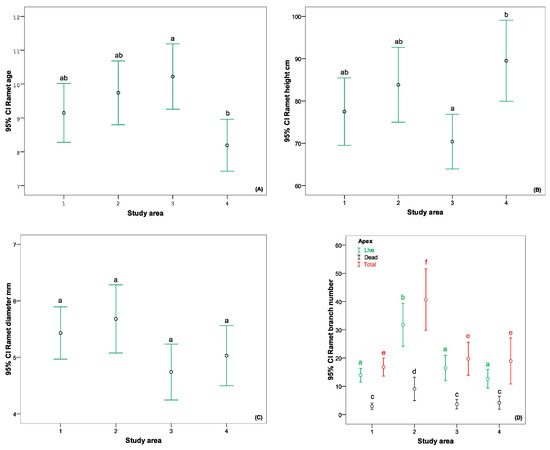
Figure 7.
The comparison of B. nana age and ramet morphometric parameters: (A) age, (B) ramet hight, (C) ramet diameter, (D) branching. Different lowercase letters indicate that there are significant differences (p < 0.05) according to Tukey’s HSD post hoc multiple comparison test.
Ramet diameter was in quite strong correlation with its age and height (Table 3).

Table 3.
Pearson correlations between age, height, and diameter of ramets. Significant correlations (p < 0.05) are in green, darker shades indicates stronger correlations).
While analysing the branching of the ramets, it was ascertained that the ramets of the 2nd area were significantly different from those of other study areas in various terms (total number of twigs, also number of live and dead twigs) (Figure 7D). Number of twigs showed the strongest correlation with stem diameter, and it was not in correlation with ramet age (Table 3).
We also ascertained significant differences in leaf parameters. The biggest difference in leaf length was between B. nana leaves growing in the 3rd and the 4th study areas, with significant differences not only between them but also between B. nana growing in the other two study plots. Concerning leaf width, only the leaves of B. nana growing in the 3rd study plot were significantly different (Figure 8A). The same trend was found for the leaf area (Figure 8B).
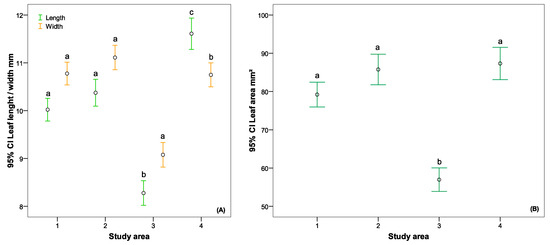
Figure 8.
The comparison of B. nana leaf parameters: (A) leaf length and width, (B) leaf area. Different lowercase letters indicate that there are significant differences (p < 0.05) according to Tukey’s HSD post hoc multiple comparison test.
4. Discussion
4.1. Environmental Differences in Betula nana Cenopopulations
Despite many differences in peat composition, the main environmental variables determining differences in our study areas and plots seemed to be the water level, which determines the dryness of the habitat, the amount of nitrogen (of various forms) in the peat, showing its fertility, and the tree cover in the study areas and their surroundings, which determines the light conditions of the habitat. All of them are related to anthropogenic pressures, especially drainage, of the peatlands. Many studies show that the degree of peat decomposition, and hence the nutrient content, increases with the drainage [44,45,46,47,48]. This is reflected in our study area, which is closest to exploited peatland and the main ditch surrounding it. The analysis of environmental factors confirms our decision to treat them as separate isolated study areas (the 1st and the 2nd) albeit close to each other within the same fragment. We expected higher peat decomposition and mineral content in the 4th study area, which, like the 2nd one, is overgrown by pine trees. Both natural and human-caused factors can cause pine establishment in the mires [49,50,51]. Though low peat decomposition degree, relatively high water level, low amount of nutrients, and dense cover of not-very-tall pine trees suggest quite natural succession from open bog to bog woodland in the 4th study area, it can not be assumed that initially it may have been human-induced. The enhanced growth of trees in peatlands influenced by drainage can be observed up to 400 m from the cutoff ditch [52].
4.2. Cover, Density, and Flowering of Betula nana in Different Cenopopulations
All cenopopulations of B. nana that we studied in the environs of Šepeta peatland are characterised by low ramet density as compared to those provided for Svalbard and Norway [10] and for reference sites in Poland [14,15,16]. Ramet density recorded by us, in the most open habitat, is like the habitats affected by shading of shrubs of Polish populations [15]. In terms of shrub and subshrub cover, the habitats of all the cenopopulations we studied are like those in Poland. Differences in shading in our study areas are already assessed not only by the development of shrubs or subshrubs but also by the development of tree canopy. Alsos et al. [10] associate the lower ramet density with lower seed germinations. We observe significant differences between the 1st and the 4th study areas concerning ramet density. The emergence of new ramets may be limited by the cover of dense shrubs (Rhododendron tomentosum and Vaccinium uliginosum) [16]. The lowest cover and density of B. nana in our study can be attributed to the abundance of Calluna vulgaris and Empetrum nigrum. As in the Polish populations [16], the number of juvenile (1–3 stage) ramets in our study was very low and exclusively derived from vegetative growth. The lowest percentage was in the study area closest to the ditch (16%), while the others had almost twice as many (30%). This shows that the most drained part of the peatland has the most reduced vegetative regeneration.
Our results are also in accordance with those obtained by Ejankowski et al. [16], that sexual reproduction is negatively affected by shade. This was confirmed by a significantly higher number of flowering ramets and by the abundance of male and female catkins in the most open study area.
Ejankowski [15] found that the highest number of flowering ramets is recorded at sites with optimal water levels and decreases under waterlogged conditions. Our research confirms the same trend in the opposite direction as the water regime changes—the number of flowering ramets and catkins decreases with decreasing water level. This is primarily due to facilitating the conditions for tree growth while changing the lighting conditions.
The abundance of catkins, even when plants are growing under optimal conditions, does not suggest successful generative reproduction [4]. The flowers can often be damaged by insects [4,16]. As mentioned above, seedlings derived from seeds were not used during our study. Sexual reproduction of B. nana is rare both at the southern edge of the range [4,15] and under severe Arctic climatic conditions [10]. The sexual reproduction of B. nana may not be so successful in relict populations compared to central ones within the continuous distribution range [4]. In general, it is considered that vegetative propagation is of greater importance than generative ones in B. nana, as in many shrubs occurring in open areas [53]. Whatever the subsequent reproductive success of seed propagation, by the abundance of flowering, we can conclude that cenopopulations in the open habitat similar to that in the species’ main distribution area indicate better conditions for B. nana than those in the habitats overgrown by trees.
4.3. Age and Morphometric Parameters of the Ramets in Different Cenopopulations
The ramet age determined in our study was similar to that of the Polish populations [15]. Similar to this study, no close correlation was found between ramet age and their height. The same tendency has been assessed for the trees [54]. However, we found a correlation between the of age dwarf birch ramet and its diameter. As shrub species age, there may be a decrease in the width of radial growth, or a constant/increasing growth with greater biomass as the shrub individual is able to access greater resources [55].
Our studies have shown that a critical factor changing the growth of B. nana vegetative ramets is changes in vegetation. Tree cover influences environmental conditions, e.g., light availability and quality, soil temperature and moisture [56,57]. Our research supports the statements that the increasing density of trees in peat bogs can reduce the abundance of dwarf birch [14,16]. Still, we did not notice any reduction in one of the indicators of vegetative growth—ramet height. In contrast, in shady habitats, the ramets, despite their age and anthropogenic impact on the habitat, are taller than in open ones. Several studies have shown the stem elongation and decrease in stem diameter of various plants caused by shade [41,58,59,60,61,62,63]. However, our investigations confirmed a different trend—stem diameter in most cases increases with stem elongation. According to Schmitt et al. 1995, plants adjust their growth strategies to outgrow competitors in shady habitats, among which elongation of stem-like structures and elevation of leaves.
Our results partly agree with Wein and Bliss [64] that low growth of B. nana is characteristic of the habitats with high water levels—the ramets are lowest where the habitats are not only the least covered by trees, but also where the water levels are highest.
We expected that the 2nd study area would have the highest plants because it contains the most mineral substances, e.g., nitrogen compounds and shade. Instead, in the 2nd area, we found the most branched plants. Some surveys show that more branching is the adaptation of plants to shady habitats to minimise shading within the crown of an individual plant [65]. Some studies have revealed that an increase of B. nana in growth can also be achieved by plasticity in the production of branched shoots [66].
It is relevant to point out that in the study area closest to the exploited peatland, a higher number of twigs is accompanied by a higher number of dead branches. This probably reflects the unfavourable status of the population caused by significant habitat desiccation due to its drainage. Other changes in vegetation, such as a greater cover of brown mosses rather than Sphagnum mosses, and taller trees, such as those typical of forest habitats rather than bog ones, are also evidence that this area is more strongly affected by drainage [39]. The plants of drier hummocks initially even benefit from drainage. Later, their opportunities to thrive will be limited.
4.4. Variations in Leaf Sizes
Apart from stem height, plants exhibit other morphological and physiological changes such as expanded leaf size and area increasing the efficiency of light capture [67]. Our research fully supports this. We found quite a wide variation of leaf sizes (width, length and area) within studied cenopopulations significantly differing according to the degree of shading conditions in the habitat. Since a wide range of leaf sizes were found, it was essential for us to find out whether they fell within the range of leaf sizes reported in the literature.
The leaves of B. nana are described as small and short-petiolate. The leaf lamina is dark green, glabrous, orbicular to broadly obovate, or reniform, with a deeply crenate margin [2,29,68]. The leaf lamina length of B. nana found in our studied cenopopulations falls within the 5–20 mm range, as de Groot et al. [2] have reported. Even the maximal leaf lamina length found in the raised pine forest cenopopulation did not exceed the specified upper limit. The regional identification key indicates [69] that the width of the leaf sheet of B. nana is often greater than its length. This is confirmed by leaf length and width ranges from neighbouring countries Latvia (5–15/10–22 mm) [19], Poland (4–15/5–15 mm) [70], and Nordic countries (6–17/6.5–15(20) mm) [29]. Leaf length was also lower than leaf width in the cenopopulations we studied, apart from the raised bog pine forest cenopopulation mentioned above.
5. Conclusions
Due to anthropogenic activity, essential changes in the wet, nutrient-poor, and open habitats to drier, more fertile, and shady influence the status of B. nana cenopopulations. Adaptation to shady habitats leads to taller ramets with wider diameters and larger leaves. Although changes in environmental conditions seem favourable for the vegetative ramet growth, the deterioration of the cenopopulation, additionally affected by human-induced drainage and fragmentation, is evidenced in various levels: by a marked reduction in the number of flowering ramets, reduced vegetative regeneration, and an increase in the number of dead twigs on mature ramets.
Author Contributions
Conceptualization, I.J. and Z.S.; methodology, I.J., A.B., and Z.S.; data curation, A.B.; formal analysis, I.J. and M.K., investigation, I.J., A.B., and Z.S.; writing—original draft preparation, I.J., Z.S., and A.R.; visualization, A.B. and M.K.; supervision, I.J.; writing—review and editing, I.J., A.B., A.R., M.K., and Z.S.; funding acquisition, I.J.; project administration, I.J. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the Research Council of Lithuania (LMTLT), agreement No. S-LIP-22-63.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data is not publicly available due to privacy concerns.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Environmental parameters of varying study areas (1–4). Different lowercase letters in the column “Study Area” indicate that there are significant differences (p > 0.05) between the parameters of varying study areas (1–4) according to the Mann–Whitney U test analysis.
Table A1.
Environmental parameters of varying study areas (1–4). Different lowercase letters in the column “Study Area” indicate that there are significant differences (p > 0.05) between the parameters of varying study areas (1–4) according to the Mann–Whitney U test analysis.
| Parameter | Study Area | Minimum | Maximum | Mean | Std. Deviation |
|---|---|---|---|---|---|
| pH 1:5 (H2O) | 1a | 4.0 | 4.0 | 4.000 | 0.000 |
| 2b | 3.9 | 4.0 | 3.967 | 0.0488 | |
| 3b | 3.8 | 4.0 | 3.933 | 0.0976 | |
| 4c | 3.8 | 3.9 | 3.829 | 0.0469 | |
| N (N-NO3+ N-NH4) mg/L | 1a | 0.6 | 1.5 | 1.000 | 0.3873 |
| 2b | 1.2 | 2.5 | 1.800 | 0.5542 | |
| 3a | 0.8 | 1.3 | 1.000 | 0.2236 | |
| 4c | 0.3 | 1.0 | 0.607 | 0.2895 | |
| Ammonia (N-NH4) mg/L | 1a | 0.4 | 0.8 | 0.567 | 0.1759 |
| 2b | 0.9 | 2.1 | 1.367 | 0.5434 | |
| 3a | 0.4 | 1.0 | 0.600 | 0.2928 | |
| 4c | 0.2 | 0.6 | 0.386 | 0.1657 | |
| Nitrate (N-NO3) mg/L | 1a | 0.2 | 0.7 | 0.433 | 0.2127 |
| 2a | 0.3 | 0.6 | 0.433 | 0.1291 | |
| 3a | 0.3 | 0.5 | 0.400 | 0.0845 | |
| 4b | 0.1 | 0.4 | 0.221 | 0.1251 | |
| P mg/L | 1a | 0.3 | 0.4 | 0.367 | 0.488 |
| 2a | 0.3 | 0.4 | 0.367 | 0.488 | |
| 3b | 0.2 | 0.3 | 0.233 | 0.488 | |
| 4b | 0.2 | 0.3 | 0.264 | 0.497 | |
| K mg/L | 1a | 1.0 | 1.7 | 1.300 | 0.3047 |
| 2b | 1.2 | 2.5 | 1.933 | 0.5627 | |
| 3c | 1.5 | 1.9 | 1.633 | 0.1952 | |
| 4d | 0.8 | 1.2 | 0.986 | 0.1657 | |
| Ca mg/L | 1ab | 13 | 19 | 16.00 | 2.535 |
| 2a | 16 | 19 | 17.67 | 1.291 | |
| 3a | 17 | 18 | 17.33 | 0.488 | |
| 4b | 15 | 16 | 15.29 | 0.469 | |
| Mg mg/L | 1a | 6 | 8 | 7.33 | 0.976 |
| 2a | 7 | 9 | 8.00 | 0.845 | |
| 3a | 7 | 8 | 7.67 | 0.488 | |
| 4b | 6 | 7 | 6.29 | 0.469 | |
| Cl mg/L | 1a | 7 | 11 | 9.67 | 1.952 |
| 2b | 7 | 9 | 8.33 | 0.976 | |
| 3b | 7 | 9 | 8.33 | 0.976 | |
| 4c | 7 | 8 | 7.36 | 0.497 | |
| Electrical conductivity mS/m | 1ac | 2.08 | 2.86 | 2.5567 | 0.35318 |
| 2b | 2.58 | 3.14 | 2.9433 | 0.26623 | |
| 3a | 2.44 | 2.75 | 2.5533 | 0.14450 | |
| 4c | 2.39 | 2.58 | 2.4686 | 0.08708 | |
| Organic material % | 1a | 93.77 | 95.66 | 94.7533 | 0.80064 |
| 2b | 93.07 | 95.22 | 94.0700 | 0.91515 | |
| 3c | 92.62 | 94.03 | 93.5067 | 0.65249 | |
| 4b | 93.22 | 94.78 | 94.0857 | 0.69841 | |
| Organic carbon (C) % | 1a | 50.69 | 56.89 | 54.5600 | 2.85215 |
| 2b | 47.99 | 54.24 | 51.9767 | 2.92685 | |
| 3a | 52.76 | 57.84 | 54.9633 | 2.20254 | |
| 4a | 51.26 | 57.78 | 54.0429 | 2.96446 | |
| Peat decomposition % | 1a | 27. 46 | 29.88 | 28.3833 | 1.10539 |
| 2b | 29.50 | 32.04 | 31.1800 | 1.22975 | |
| 3c | 29.04 | 31.21 | 30.1733 | 0.91972 | |
| 4b | 30.61 | 32.43 | 31.3000 | 0.87596 | |
| Moisture % | 1a | 89.14 | 90.29 | 89.8067 | 0.50415 |
| 2b | 87.77 | 88.48 | 88.0933 | 0.30359 | |
| 3ac | 88.25 | 90.68 | 89.6233 | 1.05269 | |
| 4c | 88.28 | 89.86 | 89.2136 | 0.72834 | |
| Water level depth m | 1a | 37 | 45 | 42.33 | 3.904 |
| 2b | >1 | >1 | >1 | 0.000 | |
| 3a | 24 | 54 | 38.33 | 12.715 | |
| 4a | 44 | 49 | 45.43 | 2.344 | |
| Herb cover % | 1a | 0 | 60 | 15.00 | 16.583 |
| 2b | 0 | 40 | 4.40 | 10.253 | |
| 3a | 3 | 60 | 21.40 | 16.612 | |
| 4a | 0 | 35 | 10.36 | 12.475 | |
| Tree cover % | 1a | 20 | 60 | 43.33 | 17.593 |
| 2a | 30 | 60 | 43.33 | 12.910 | |
| 3b | 5 | 30 | 18.33 | 10.635 | |
| 4a | 0 | 40 | 37.33 | 10.328 | |
| Shrub and subshrub cover % (Betula nana excluded) | 1a | 0.00 | 70.00 | 30.3571 | 27.62832 |
| 2a | 2.00 | 40.00 | 20.8000 | 10.73845 | |
| 3b | 3.00 | 80.00 | 51.8667 | 23.03992 | |
| 4ac | 0.00 | 40.00 | 19.0000 | 11.5824 | |
| Moss cover % | 1a | 70 | 80 | 76.67 | 4.880 |
| 2b | 40 | 80 | 61.67 | 17.078 | |
| 3c | 80 | 90 | 83.33 | 4.880 | |
| 4ac | 70 | 85 | 78.21 | 6.682 |

Table A2.
Characteristics of vegetation in Betula nana study plots. Species names follow [71,72].
Table A2.
Characteristics of vegetation in Betula nana study plots. Species names follow [71,72].
| Study Area | 1 | 2 | 3 | 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of study plot | 1.1 | 1.2 | 1.3 | 2.1 | 2.2 | 2.3 | 3.1 | 3.2 | 3.3 | 4.1 | 4.2 | 4.3 |
| Cover of tree layer [%]: | 50 | 20 | 60 | 40 | 60 | 30 | 20 | 5 | 30 | 40 | 40 | 40 |
| Cover of shrub and subshrub layers [%] | 80 | 60 | 80 | 85 | 60 | 60 | 80 | 80 | 80 | 80 | 40 | 70 |
| Cover of herb layer [%] | 2 | 50 | 5 | 8 | 5 | 2 | 1 | 50 | 60 | 5 | 10 | 10 |
| Cover of moss layer [%] | 80 | 80 | 70 | 40 | 65 | 80 | 80 | 95 | 80 | 80 | 70 | 85 |
| Trees | ||||||||||||
| Pinus sylvestris | 3 | 2 | 3 | 3 | 3 | 2 | 2 | 1 | 2 | 3 | 2 | 3 |
| Betula pubescens | + | 2 | + | |||||||||
| Betula pendula | 1 | 1 | ||||||||||
| B. pubescens × pendula | + | + | 1 | |||||||||
| Shrubs and subshrubs | ||||||||||||
| Betula nana | 2 | 3 | 3 | 4 | 4 | 3 | 3 | 3 | 3 | 3 | 3 | 4 |
| Andromeda polifolia | 2 | 1 | 2 | 1 | 1 | 2 | 1 | 2 | 1 | 1 | 2 | |
| Vaccinium oxycoccos | 2 | 3 | 1 | 3 | 2 | 2 | 2 | 2 | 1 | 1 | 2 | |
| Empetrum nigrum | 3 | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | ||
| Calluna vulgaris | 3 | 3 | 2 | 2 | 1 | 2 | 3 | 3 | 3 | |||
| Rhododendron tomentosum | 2 | 1 | 1 | 3 | 3 | 1 | 3 | 1 | ||||
| Vaccinium uliginosum | + | |||||||||||
| Herbs | ||||||||||||
| Eriophorum vaginatum | 3 | 1 | 1 | 1 | 3 | 4 | 1 | 2 | 2 | |||
| Melampyrum pratense | 1 | 1 | + | 1 | 1 | |||||||
| Betula pubescens juv. | + | 1 | 1 | |||||||||
| Pinus sylvestris juv. | + | + | + | |||||||||
| Rubus chamaemorus | + | + | ||||||||||
| Carex rostrata | + | + | ||||||||||
| Carex sp. | + | |||||||||||
| Phragmites australis | + | |||||||||||
| Mosses | ||||||||||||
| Sphagnum divinum | 2 | 1 | 2 | 1 | 2 | 3 | 3 | 2 | 3 | 2 | 3 | 3 |
| Polytrichum strictum | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 1 | + | 1 | 2 | |
| Pleurozium schreberi | 2 | 2 | 3 | 2 | 1 | 2 | 3 | 2 | 3 | 1 | 3 | |
| Sphagnum cuspidatum | + | 3 | 2 | 1 | 1 | 1 | 1 | 1 | ||||
| Sphagnum fallax | 3 | 1 | + | 1 | 1 | 1 | 3 | 1 | ||||
| Dicranum polysetum | 2 | 1 | 2 | 1 | + | 1 | ||||||
| Sphagnum angustifolium | + | 1 | 1 | 1 | 1 | 1 | ||||||
| Sphagnum flexuosum | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Sphagnum fuscum | 1 | 1 | 1 | |||||||||
| Pohlia sphagnicola | + | |||||||||||
| Calliergon stramineum | + | |||||||||||
| Sphagnum capillifolium | 1 | |||||||||||
| Sphagnum balticum | + | |||||||||||
| Sphagnum rubellum | + |
References
- Jalas, J.; Suominen, J. Atlas Florae Europaeae; Salicaceae to Balanophoracea; Committee for Mapping the Flora of Europe and Societas Biologica Fennica Vanamo: Helsinki, Finland, 1976; Volume 3, p. 57. [Google Scholar]
- de Groot, W.J.; Thomas, P.A.; Wein, R.W. Betula nana L. and Betula glandulosa Michx. J. Ecol 1997, 85, 241–264. [Google Scholar] [CrossRef]
- Drzymulska, D. Postglacial occurrence and decline of Betula nana L. (dwarf birch) in northeastern Poland. Est. J. Earth Sci. 2014, 63, 76–87. [Google Scholar] [CrossRef]
- Jadwiszczak, K.A.; Kłosowski, S.; Zalewska, I.; Banaszek, A.; Chrzanowska, A. Genetic diversity and sexual reproduction in relict populations of Betula nana. Silva Fenn. 2017, 51, id5643. [Google Scholar] [CrossRef]
- Gostyńska-Jakuszewska, M.; Lekavičius, A. Selected Boreal and Subboreal species in the flora of Poland and the Lithuanian SSR. Part I. Fragm. Flor. Geobot. 1989, 34, 299–314. [Google Scholar]
- Fredskild, B. The genus Betula in Greenland-Holocene history, present distribution and synecology. Nord. J. Bot. 2008, 11, 393–412. [Google Scholar] [CrossRef]
- Anamthawat-Jónsson, K.; Thór Thórsson, A. Natural hybridisation in birch: Triploid hybrids between Betula nana and B. pubescens. Plant Cell Tissue Organ Cult. 2003, 75, 99–107. [Google Scholar] [CrossRef]
- Anamthawat-Jónsson, K.; Karlsdóttir, L.; Thórsson, T.; Jóhannsson, M.H. Naturally occurring triploid birch hybrids from woodlands in Iceland are partially fertile. New For. 2021, 52, 659–678. [Google Scholar] [CrossRef]
- Alsos, I.G.; Engelskjøn, T. Conservation genetics and population history of Betula nana, Vaccinium uliginosum, and Campanula rotundifolia in the Arctic Archipelago of Svalbard. Arct. Antarct. Alp. Res. 2002, 34, 408–418. [Google Scholar] [CrossRef]
- Alsos, I.G.; Spjelkavik, S.; Engelskjøn, T. Seed bank size and composition of Betula nana, Vaccinium ulginosum, and Campanula rotundifolia habitats in Svalbard and Northern Norway. Can. J. Bot. 2003, 81, 220–231. [Google Scholar] [CrossRef]
- Hollesen, J.; Buchwal, A.; Rachlewicz, G.; Hansen, B.; Hansen, M.O.; Stecher, O.; Elberling, B. Winter warming as an important co-driver for Betula nana growth in western Greenland during the past century. Global Chang. Biol. 2015, 21, 2410–2423. [Google Scholar] [CrossRef]
- Dąbrowska, G.; Dzialuk, A.; Burnicka, O.; Ejankowski, W.; Gugnacka-Fiedor, W.; Goc, A. Genetic diversity of postglacial relict shrub Betula nana revealed by RAPD analysis. Dendrobiology 2006, 55, 19–23. [Google Scholar]
- Dąbrowska, G.B.; Henryk, P.; Dąbrowski, H.B.; Szyp-Borowska, I. Genetic diversity of Betula nana in Sweden and conservation implications for protection of relict Polish populations. Folia For. Pol. Ser. A-For. 2021, 63, 225–231. [Google Scholar] [CrossRef]
- Ejankowski, W. The influence of ground water level on the demography and population structure of the dwarf birch Betula nana L. Ecol. Quest. 2004, 6, 63–68. [Google Scholar]
- Ejankowski, W. Effect of waterlogging on regeneration in the dwarf birch (Betula nana). Biologia 2008, 3, 670–676. [Google Scholar] [CrossRef]
- Ejankowski, W. Demographic variation of dwarf birch (Betula nana) in communities dominated by Ledum palustre and Vaccinium uliginosum. Biologia 2010, 65, 248–253. [Google Scholar] [CrossRef]
- Ejankowski, W.; Kunz, M. Reconstructing of vegetation dynamics in “Linje” Peat-bog (N Poland) using remote sensing method. Biodiv. Res. Conserv. 2006, 1–2, 111–113. [Google Scholar]
- Jadwiszczak, K.A.; Drzymulska, D.; Banaszek, A.; Jadwiszczak, P. Population history, genetic variation and conservation status of the endangered birch species Betula nana L. in Poland. Silva Fenn. 2012, 46, 465–477. [Google Scholar] [CrossRef]
- Cinovskis, R. Betula nana L. In Latvijas Sarkanā grāmata. Vaskulārie aug; Andrušaitis, G., Ed.; Latvijas Universitātes Bioloģijas institūts: Riga, Latvia, 2003; Volume 3, p. 298. [Google Scholar]
- Patalauskaitė, D. Beržas keružis. Betula nana L. In Red Data Book of Lithuania. Animals, Plants, Fungi; Rašomavičius, V., Ed.; Aplinkos Ministerija: Vilnius, Lithuania, 2021; p. 466. [Google Scholar]
- Kruszelnicki, J.; Fabiszewski, J. Betula nana L. Brzoza karłowata [Betula nana L. Dwarf birch]. In Polska Czerwona Księga Roślin [Polish Red Data Book of Plants]; Kaźmierczakowa, R., Zarzycki, K., Eds.; Instytut Botaniki im. W. Szafera PAN, Instytut Ochrony Przyrody PAN: Krakow, Poland, 2001; pp. 82–83. [Google Scholar]
- Bukantis, A.; Gulbinas, Z.; Kazakevičius, S.; Kilkus, K.; Mikelinskienė, A.; Morkūnaitė, R.; Rimkus, E.; Samuila, M.; Stankūnavičius, G.; Valiuškevičius, G.; et al. Klimato Svyravimų Poveikis Fiziniams Geografiniams Procesams Lietuvoje; Geografijos Institutas, Vilniaus Universitetas: Vilnius, Lithuania, 2001; pp. 1–280. [Google Scholar]
- Povilaitis, A.; Taminskas, J.; Gulbinas, Z.; Linkevičienė, R.; Pileckas, M. Lietuvos Šlapynės ir jų Vandensauginė Reikšmė; Apyaušris: Vilnius, Lithuania, 2011; p. 52. [Google Scholar]
- LHT. Lietuvos Hidrometeorologijos Tarnyba. Available online: https://www.meteo.lt/en/ (accessed on 1 August 2024).
- Liužinas, R. Lietuvos Durpynų Kadastras I, 1st ed.; Lietuvos Respublikos Aplinkos Apsaugos Ministerija: Vilnius, Lithuania, 1995; p. 302. [Google Scholar]
- Brundza, K. Šepeta. Aukštapelkio monografija. Žemės Akad. Metraštis 1940, 13, 1–208. [Google Scholar]
- Ivanauskas, T. Gamtos paminklai ir jų klausimas Lietuvoje. Švietimo Darbas 1921, 1–2, 13–33. [Google Scholar]
- Rabotnov, T.A. Dynamics of Plant Coenotic Populations. In The Population Structure of Vegetation. Handbook of Vegetation Science; White, J., Ed.; Springer: Dordrecht, The Netherlands, 1985; Volume 3, pp. 121–142. [Google Scholar] [CrossRef]
- Jonsell, B. Betula L. In Flora Nordica; Lycopodiaceae–Polygonaceae; Jonsel, B., Karlssson, T., Eds.; Flora Nordica: Stockholm, Sweden, 2000; Volume 1, pp. 197–203. [Google Scholar]
- Falińska, K. Osobnik, Populacja, Fitocenoza; PWN: Warszawa, Poland, 1990; pp. 1–309. [Google Scholar]
- Scrosati, R. An updated definition of genet applicable to clonal seaweeds, bryophytes, and vascular plants. Basic Appl. Ecol. 2002, 3, 97–99. [Google Scholar] [CrossRef]
- Tsujimoto, M.; Araki, K.S.; Honjo, M.N.; Yasugi, M.; Nagano, A.J.; Akama, S.; Hatakeyama, M.; Shimizu-Inatsugi, R.; Sese, J.; Shimizu, K.K.; et al. Genet assignment and population structure analysis in a clonal forest-floor herb, Cardamine leucantha, using RAD-seq. AoB Plants 2019, 11, plz080. [Google Scholar] [CrossRef] [PubMed]
- Braun-Blanquet, J. Pflanzensoziologie, Grundzüge der Vegetationskunde, 3rd ed.; Springer: Wien, Austria; New York, NY, USA, 1964; p. 866. [Google Scholar]
- LST EN 13037; Dirvožemio Gerinimo Medžiagos ir Auginimo Terpės. pH Nustatymas. Lietuvos Standartizacijos Departamentas: Vilnius, Lithuania, 2012; pp. 1–8.
- LST EN 13652:2006; Dirvožemio Gerinimo Medžiagos ir Auginimo Terpės. Vandenyje Tirpių Maisto Medžiagų ir Elementų Eekstrahavimas. Lietuvos Standartizacijos Departamentas: Vilnius, Lithuania, 2006; pp. 1–15.
- LST 1957:2022; Durpės, Skirtos Daržininkystei, Sodininkystei ir Dekoratyvinei Sodininkystei. Savybės, Tyrimo Metodai, Techninės Tiekimo Sąlygos. Lietuvos Standartizacijos Departamentas: Vilnius, Lithuania, 2022; pp. 1–26.
- LST EN 13040:2008; Dirvožemio Gerinimo Medžiagos ir Auginimo Terpės. Mėginių Paruošimas Cheminiams ir Fizikiniams Tyrimams, Sausųjų Medžiagų Kiekio, Drėgnio ir Laboratorijoje Tankinto Piltinio Tankio Nustatymas. Lietuvos Standartizacijos departamentas: Vilnius, Lithuania, 2009; pp. 1–17.
- ISO 10694:1995; Soil Quality Determination of Organic and Total Carbon after Dry Combustion (Elementary Analysis). International Organization for Standardization: Geneva, Switzerland, 1995; pp. 1–18.
- Harper, J.L. Population Biology of Plants; Academic Press: London, UK, 1977; pp. 1–892. [Google Scholar]
- Gärtner, H.; Schweingruber, F.H. Microscopic Preparation Techniques for Plant Stem Analysis; Verlag Dr. Kessel: Remagen, Germany, 2013; pp. 19–29. [Google Scholar]
- SPSS Inc. SPSS for Windows, Version 16.0; SPSS Inc.: Chicago, IL, USA, 2007; pp. 1–165. [Google Scholar]
- McGarigal, K.; Stafford, S.; Cushman, S. Ordination: Principal components analysis. In Multivariate Statistics for Wildlife and Ecology Research; McGarigal, K., Stafford, S., Cushman, S., Eds.; Springer: New York, NY, USA, 2000; pp. 19–80. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Szajdak, L.W.; Jezierski, A.; Wegner, K.; Meysner, T.; Szczepański, M. Influence of Drainage on Peat Organic Matter: Implications for Development, Stability, and Transformation. Molecules 2020, 25, 2587. [Google Scholar] [CrossRef]
- Koeselman, W.; Verhoeven, J.T.A. Eutrophication of fen ecosystems: External and internal nutrient sources and restoration strategies. In Restoration of Temperate Wetlands; Wheeler, B.D., Shaw, S.C., Fojt, W.J., Robertson, R.A., Eds.; John Wiley & Sons: Chichester, UK, 1995; pp. 91–112. [Google Scholar]
- Laine, J.; Vanha-Majamaa, I. Vegetation ecology along a trophic gradient on drained pine mires in southern Finland. Ann. Bot. Fenn 1992, 29, 213–233. [Google Scholar]
- Laine, J.; Vasander, H.; Laiho, R. Long-term effects of water level drawdown on the vegetation of drained pine mires in southern Finland. J. Appl. Ecol 1995, 32, 785–802. [Google Scholar] [CrossRef]
- Harris, L.I.; Moore, T.R.; Roulet, N.T.; Pinsonneault, A.J. Limited effect of drainage on peat properties, porewater chemistry, and peat decomposition proxies in a boreal peatland. Biogeochemistry 2020, 151, 43–62. [Google Scholar] [CrossRef]
- Edvardsson, J.; Šimanauskienė, R.; Taminskas, J.; Baužienė, I.; Stoffel, M. Increased tree establishment in Lithuanian peat bogs detected using a combination of field and remotely sensed approaches. Sci. Total Environ. 2015, 505, 113–120. [Google Scholar] [CrossRef]
- Dudová, L.; Hájek, M.; Hájková, P. The origin and vegetation development of the Rejvíz pine bog and the history of the surrounding landscape during the holocene. Preslia 2010, 82, 223–246. [Google Scholar]
- Felèchoux, F.; Buttler, A.; Gillet, F. Dynamics of bog-pine-dominated mires in the Jura Mountains, Switzerland: A tentative scheme based on synusial phytosociology. Folia Geobot 2000, 35, 273–288. [Google Scholar] [CrossRef]
- Paal, J.; Jürjendal, I.; Suija, A.; Kull, A. Impact of drainage on vegetation of transitional mires in Estonia. Mires Peat 2016, 18, 1–19. [Google Scholar]
- Ebert, T.A.; Ebert, C.A. A method for studying vegetation dynamics when there are no obvious individuals: Virtual-population analysis applied to the tundra shrub Betula nana L. Vegetatio 1989, 85, 33–44. [Google Scholar] [CrossRef]
- Nowakowska, J.; Gazda, A.; Tomski, A.; Szwagrzyk, J. Drainage ditches enhance forest succession in a raised bog but do not affect the spatial pattern of tree encroachment. PLoS ONE 2021, 16, e0247760. [Google Scholar] [CrossRef]
- Myers-Smith, I.H.; Hallinger, M.; Blok, D.; Sass-Klaassen, U.; Rayback, S.A.; Weijers, S.; Trant, A.J.; Tape, K.D.; Naito, A.T.; Wipf, S.; et al. Methods for measuring arctic and alpine shrub growth: A review. Earth-Sci. Rev. 2015, 140, 1–13. [Google Scholar] [CrossRef]
- Breshears, D.D.; Rich, P.M.; Barnes, F.J.; Campbell, K. Overstory-imposed heterogeneity in solar radiation and soil moisture in a semiarid woodland. Ecol. Appl. 1997, 7, 1201–1215. [Google Scholar] [CrossRef]
- Breshears, D.D.; Nyhan, J.W.; Heil, C.E.; Wilcox, B.P. Effects of woody plants on microclimate in a semiarid woodland: Soil temperature and evaporation in canopy and intercanopy patches. Int. J. Plant Sci. 1998, 159, 1010–1017. [Google Scholar] [CrossRef]
- Wen, B.; Zhang, Y.; Hussain, S.; Wang, S.; Zhang, X.; Yang, J.; Xu, M.; Qin, S.; Yang, W.; Liu, W. Slight Shading Stress at Seedling Stage Does not Reduce Lignin Biosynthesis or Affect Lodging Resistance of Soybean Stems. Agronomy 2020, 10, 544. [Google Scholar] [CrossRef]
- Franklin, K.A. Shade avoidance. New Phytol. 2008, 179, 930–944. [Google Scholar] [CrossRef]
- Casal, J.J. Shade avoidance. Arab. Book Am. Soc. Plant Biol. 2012, 10, e0157. [Google Scholar] [CrossRef]
- Casal, J.J. Photoreceptor signaling networks in plant responses to shade. Annu. Rev. Plant Biol 2013, 64, 403–427. [Google Scholar] [CrossRef]
- Hutchinson, T.C. Comparative studies of the ability of species to withstand prolonged periods of darkness. J. Ecol. 1967, 55, 291–299. [Google Scholar] [CrossRef]
- Schmitt, J.; McCormac, A.C.; Smith, H. A test of the adaptive plasticity hypothesis using transgenic and mutant plants disabled inphytochrome-mediated elongation responses to neighbors. Am. Nat. 1995, 146, 937–953. [Google Scholar] [CrossRef]
- Wein, R.W.; Bliss, L.C. Primary production in arctic cottongrass tussock tundra communities. Arct. Alp. Res. 1974, 6, 261–274. [Google Scholar] [CrossRef]
- Pickett, S.T.A.; Kempf, J.S. Branching patterns in forest shrubs and understorey trees in relation to habitat. New Phytol. 1980, 86, 219–228. [Google Scholar] [CrossRef]
- Bret-Harte, M.S.; Shaver, G.R.; Zoerner, J.P.; Johnstone, J.F.; Wagner, J.L.; Chavez, A.S.; Gunkelman, R.F.; Lippert, S.C.; Laundre, J.A. Developmental plasticity allows Betula nana to dominate tundra subjected to an alternative environment. Ecology 2001, 82, 18–32. [Google Scholar] [CrossRef]
- Gommers, C.M.; Visser, E.J.; St Onge, K.R.; Voesenek, L.A.; Pierik, R. Shade tolerance: When growing tall is not an option. Trends Plant Sci. 2013, 18, 65–71. [Google Scholar] [CrossRef]
- Furlow, J.J. Betulaceae Gray. Betula L. In Flora of North America North of Mexico; Flora of North America Editorial Committee: New York, NY, USA; Oxford, UK, 1997; Volume 3, Available online: http://www.efloras.org/florataxon.aspx?flora_id=1&taxon_id=10101 (accessed on 27 May 2024).
- Lekavičius, A. Vadovas Augalams Pažinti; Mokslas: Vilnius, Lithuania, 1989; pp. 54–56. [Google Scholar]
- Gostyńska-Jakuszewska, M. Betulaceae, Brzozowate. In Flora Polski. Rośliny Naczyniowe, 3. Dwuliścienne Wolnopłatkowe-Jednookwiatowe, 2nd ed.; Jasiewicz, W.A., Ed.; Instytut Botaniki im. W. Szafera, Polska Akademia Nauk: Kraków, Poland, 1992; pp. 7–18. [Google Scholar]
- Hodgetts, N.G.; Söderström, L.; Blockeel, T.L.; Caspari, S.; Ignatov, M.S.; Konstantinova, N.A.; Lockhart, N.; Papp, B.; Schröck, C.; Sim-Sim, M.; et al. An annotated checklist of bryophytes of Europe, Macaronesia and Cyprus. J. Bryol. 2020, 42, 1–116. [Google Scholar] [CrossRef]
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Available online: http://www.plantsoftheworldonline.org/ (accessed on 8 May 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).