Abstract
After the end-Triassic extinction, parvipelvian ichthyosaurs diversified and became dominant elements of marine ecosystems worldwide. By the Early Jurassic, they achieved a thunniform body plan that persisted for the last 100 m.y.a of their evolution. Diversification and extinctions of thunniform ichthyosaurs, and their swimming performance, have been studied from different perspectives. The transformation of limbs into hydrofoil-like structures for better control and stability during swimming predates thunniform locomotion. Despite their importance as control surfaces, fin evolution among thunnosaurs remains poorly understood. We explore ichthyosaur fin diversity using anatomical networks. Our results indicate that, under a common hydrofoil controller fin, the bone arrangement diversity of the ichthyosaur fin was greater than traditionally assumed. Changes in the connectivity pattern occurred stepwise throughout the Mesozoic. Coupled with other lines of evidence, such as the presence of a ball-and-socket joint at the leading edge of some derived Platypterygiinae, we hypothesize that fin network disparity also mirrored functional disparity likely associated with different capabilities of refined maneuvering. The ball-and-socket articulation indicates that this local point could be acting like a multiaxial intrafin joint changing the angle of attack and thus affecting the maneuverability, similar to the alula of flying birds. Further studies on large samples and quantitative experimental approaches would be worthy to test this hypothesis.
1. Introduction
Ichthyosauromorphs diversified in the aftermath of the Permo-Triassic mass extinction [1,2]. The macromorphological evolutionary changes in their body plan provide canonical examples of convergence among tetrapods secondarily adapted to the marine environment (SECAD from hereon) [3]. As early as the Anisian (Middle Triassic), some ichthyosauromorphs evolved fusiform bodies with dorsal and well-developed caudal fins [4]. Since then, and throughout the Jurassic and much of the Cretaceous, Ichthyosauria Ichthyosauriomorphs (ichthyosaurs from hereon) have been dominant elements in marine ecosystems worldwide. Within this clade, thunnosaurian ichthyosaurs are easily recognizable by their streamlined body deepest at the pectoral region and tapering posteriorly to the peduncle of the lunate caudal fin [5,6] (Figure 1). Alongside Neoceti cetaceans, ichthyosaurs were the only tetrapods to evolve a thunniform body plan suitable for long-distance cruising [7,8,9] and the first vertebrates to achieve thunniform bodies [10].
As required, throughout the wide arc of SECAD lineages, the shift from continental to marine lifestyle was coupled with the transformation of the columnar and weight-bearing limbs of continental forms into paddles or fins, both for propulsion and/or steering during swimming [11,12,13]. Both functional categories of modified limbs (paddle-shaped limb or hydrofoil-shaped) imply the enclosing of limb bones into soft-tissue envelopes and the lengthening of the distal region by the addition of bones [14]. As a result, all SECAD have better-integrated limbs in comparison with their terrestrial ancestors. However, among them, the evolutionary strategy and adaptation path followed by ichthyosaurs were unique. Network analysis by Fernández et al. [15] showed that the most widespread evolutionary strategy among SECAD was the enclosing of limb bones in soft-tissue “envelopes” (like “baby mittens”), without drastically impacting the underlying connectivity pattern of the bones. In contrast, the strategy depicted by ichthyosaurs involved “zipping up” their fingers so that digital bones (transformed into carpal-like elements) were connected not only proximodistally with the surrounding bones but also laterally. This strategy resulted in highly integrated and homogeneous forefins in ichthyosaurs, allowing them to explore new regions of the morphospace [15].
In the last decades, the knowledge of the speed and mode of ichthyosaur evolution and extinction increased significantly. Integrative analyses of disparity and evolutionary rates indicate that the evolution of the lineage was characterized by a Triassic early burst followed by an evolutionary bottleneck leading to a long-term reduction of the evolutionary rates and disparity throughout the Jurassic and Cretaceous [2]. On the other hand, disparity and diversity data of Cretaceous forms show that the extinction of ichthyosaurs was characterized by a two-phase pathway: an early Cenomanian extinction that radically reduced their ecological diversity, and a final extinction event at the end of the Cenomanian [16]. However, within this general framework, two key episodes of ichthyosaur evolution are particularly significant due to their impact on the diversity and morphological innovation of the group, and both had ophthalmosaurian parvipelvians as their main protagonists: the Early/Middle Jurassic and the Jurassic/Cretaceous transitions. This clade of parvipelvians accounts for more than half of the entire evolutionary history of ichthyosaurs and is known for drastic transformations in their forefins, including the emergence of pre-radial and post-ulnar zeugopodial elements and numerous accessory digits. The Early/Middle Jurassic transition, although poorly documented [17,18,19], witnessed the emergence of the ophthalmosaurians. In contrast, the Jurassic/Cretaceous transition marks a profound drop in the diversity (and probably disparity) of the clade [16,20].
Understanding the evolutionary transformation of ichthyosaur fins is crucial for taking the first steps in comprehending the role of forefins during swimming in these marine reptiles, particularly as they evolved into efficient thunnosaurian cruisers. Here we analyze the morphological disparity of ichthyosaurs by exploring how the underlying connectivity pattern of fins transformed during ichthyosaurs’ evolutionary history. We increased the taxon sample of anatomical networks of fins from 3 [15] to 16 including forefins of Mixosaurus cornalianus and 14 parvipelvians. Finally, framed against the phylogeny, we track the changes in the connectivity pattern of ichthyosaur forefins over 147 million years (from the Annisian up to the Albian) comprising most of the evolutionary history of the ichthyosauromorphs.
The results of analyses of the fin networks highlighted that, within a clear trend towards better integrated and modular forefins, ichthyosaurs depicted a broad array of connectivity patterns. The overall similarity of the fin morphology (i.e., hydrofoil design) hides a striking underlying disparity of bone arrangements. We also found that major evolutionary changes in fin networks occur stepwise. Given the significance of forefins as control surfaces during swimming we proposed that the forefin disparity mirrored functional disparity as well, likely associated with disparity of the refined maneuverability principally among derived thunniform swimmers.
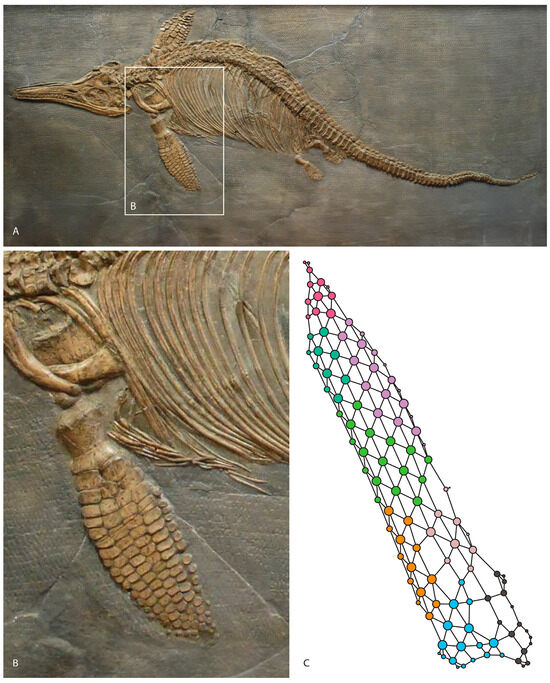
Figure 1.
Ichthyosaurus somersetensis holotype from the Hettangian of England modified from [21] (A). Left forefin on dorsal view (B). Anatomical network model of the forefin (C).
2. Materials and Methods
We built undirected and unweighted anatomical network models of the forefin for a total of 16 Ichthyosaurian taxa (Supplementary Material, Table S1), in addition to the SECAD dataset of [15]. For the selection of taxa and specimens, we chose complete fins in their anatomical position without any deformation. In cases where this was not possible, we reconstructed the missing parts using all available information, ensuring that at least the minimum number of fin elements were positioned in their most conservative configuration. Anatomical network analysis seeks to describe and analyze the underlying connectivity pattern of the bone elements and their connections, being sutures, contacts, and articulations. This kind of analysis adapts concepts of network analysis to anatomy, where network metrics are interpreted as metrics of anatomical complexity, integration, heterogeneity, and modularity (following [22] and references therein). Each element of the forefin is represented as a node, and contacts among them are depicted as links connecting the nodes. Osteological information is based on personal examination (MF, LC, AM) and published specimens. Network models were created in the open-source software Gephi v.0.10.0 [23], which was implemented for calculation of the network’s descriptors, including those descriptors developed specifically for anatomical networks (heterogeneity and parcellation based on [22]). These metrics are anatomically interpreted as measures of the complexity of connections (density, number of connections divided by the maximum possible number of connections), anatomical integration both locally (average clustering coefficient, number of connections between the neighbors of a node divided by the maximum possible number of connections in the neighborhood, on average) as well as along the entire length of the structure (average path length, average of the path length between any pair of nodes), the variability of connections (heterogeneity, standard deviation of connections divided by the mean number of connections), and anatomical modularity (parcellation, based on the number of modules and the number of nodes in each module). For a detailed description of the network metrics and how they are calculated see [22] and references therein. Data from ichthyosaur limbs was subjected to two PCA analyses: one with the complete SECAD dataset from [15] adding new network models obtained herein (Figure 2) and a second considering solely the ichthyosaur information to gain detailed observations (Figure 3). A major change compared to the [15] analysis, is that we now include the average path length metric as well, under normalized variance–covariance correlation because the av. path length is measured in different units compared to the other metrics. Finally, based on the phylogenetic hypothesis presented in [24], a reconstruction of the ancestral states was made in TNT v. 1.6 [25] by mapping the network metrics as continuous characters using the built-in optimization.
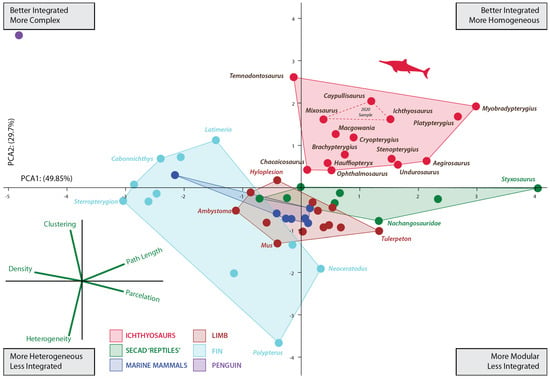
Figure 2.
Principal component analysis (PCA) scatter diagram showing morphospace occupation defined by the first two PCAs explaining 77.288% of the variation. Red dashed lines represent the convex hull morphospace occupied by the three ichthyosaurs previously analyzed [15]. See Table S2 for details on network properties of analyzed taxa.
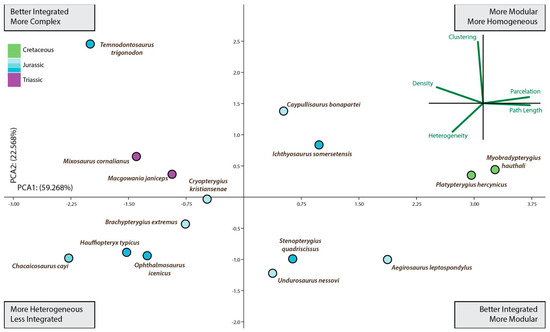
Figure 3.
Ichthyosauria forefin morphospace plotted separately to aid comparison of morphospace occupancy through time. This is a second PCA using only data from ichthyosaurs. If we focus on the Ichthyosauria forefin morphospace occupancy over time derived from the second PCA (Figure 3), from Late Triassic represented by Mixosaurus up to the Albian (Late Cretaceous) represented by Platypterygius hercynicus, there were no major shifts in the fin morphospace occupation but an overall trend toward better integrated and more modular fins. Thus, this long-term tendency spanned for approximately 137 million years comprising most of the evolutionary history of the Ichthyosauriomorpha.
3. Results
3.1. Morphospace Analyses
The increased taxon sampling and the inclusion of another descriptor in the analyses (average path length) complemented previous results. As in the former analysis including only three ichthyosaurs, the increased sample shows that the limb-to-fin transition of ichthyosaurs followed a unique strategy among SECAD. After the initial shift between the pattern of the basal ichthyosauromorh (e.g., Nanchangosaurus and Hupehsuchus) and ichthyosaur fins, ichthyosaurs explore new regions of the morphospace. As depicted in Figure 2, the morphospace occupied by ichthyosaurs does not overlap with that of any other SECAD, a difference that is also confirmed statistically with a PERMANOVA analysis (Supplementary Material File S1). Nonetheless, this finding should be interpreted with caution until more taxa from other lineages of marine reptiles can be incorporated into the study. Within a general path to homogeneous reintegration (sensu [15]), the pattern of connectivity changes depicted by their networks indicates that the disparity among ichthyosaur fins was greater than previously assumed. Thus, the morphospace is expanded in all directions.
After the Triassic/Jurassic crisis, ichthyosaurs occupied a large morphospace (in blue color on Figure 3) spreading alongside positive values on PCA1 and PCA2 except for the outlying Temnodontosaurus with low negative values on PC1. Within the common path to complex reintegration of their fore appendages, Jurassic forms spread across the empty morphospace. Some of them, like Chacaicosaurus, Hauffiopteryx, and Ophthalmosaurus, have the proximal elements better connected than phalanges resulting in relatively more heterogeneous networks. On the other hand, the connections of Ichthyosaurus and Caypullisaurus fins are distributed almost evenly across the networks resulting in a relatively more homogeneous fin. Temnodontosaurus trigonodon is the only parvipelvian with a diverging pattern (less homogeneous connectivity across the fin). The disparate location of this taxon is not surprising as this taxon reduced the number of primary digits to three. Cretaceous Myobradypterygius hauthali and Platypterygius hercynicus are clustered together and separated from the Jurassic thunniforms; this is due to their distinctive fin morphology characterized by the increased number of tightly packed phalanges resulting in extremely homogeneous and better-integrated fins. However, the low sampling of Late Cretaceous taxa could underestimate the morphospace occupation during the last episodes of the evolutionary history of the lineage.
3.2. Connectivity Changes in the Forefins across Phylogeny
The analysis of the anatomical networks of SECAD fins [15] indicated that as early as the Middle Triassic, the evolutionary strategy in ichthyosaurs of “zipping-up” their fingers was established and that, through the Jurassic, thunniform ichthyosaurs followed an adaptation path to homogeneous reintegration of their forefins. The analysis of an expanded sample (Table S2), mapped across phylogeny under maximum parsimony, indicates four points where major changes happen and that these evolutionary changes occurred stepwise (Figure 4 and Figure S1). The first step is noted at the Ichthyosauria node, denoted the early and drastic changes in the underlying connectivity pattern of limb elements, promoted by the “re-integration” of the fingers, that clearly impacted the network parameters. The whole fin integration increases but without losing much of its modularity. While nodes, edges, and average clustering coefficient increase, heterogeneity and parcellations decrease. At the Parvipelvian node, no major changes occurred except for the ongoing trends toward more homogeneous fins expressed by a decrease in heterogeneity (H) values. The second step likely occurred in the Early Jurassic. At this point, the fins became larger, but slightly less integrated and modular. After relatively long stability, two successive steps took place during the Middle and Late Jurassic. The last step is the one that registers the most abrupt change in the values of the network descriptors, marking a notable increase toward even more integrated and homogeneous networks.
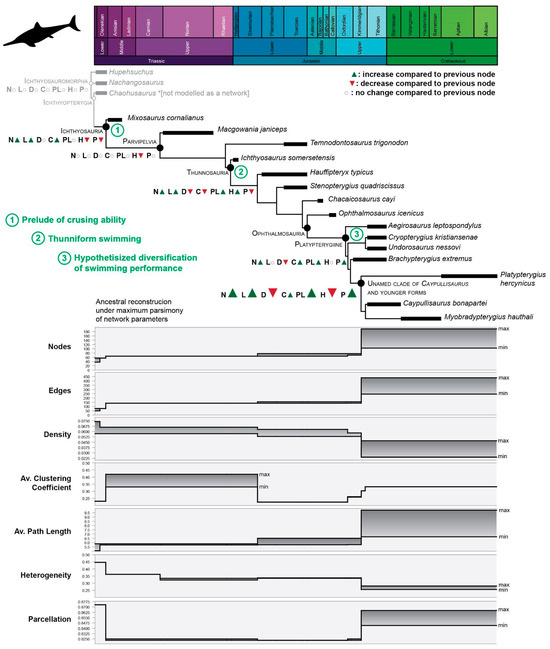
Figure 4.
Fin evolution of Ichthyosauria. Changes in the connectivity pattern through phylogeny. On cladogram, in gray, non-Ichthyosauria ichthyosauromorphs were added for comparison; in green, 1–3, major evolutionary events related to swimming. Bottom: stepwise pattern of connectivity changes, each network property is illustrated separately to aid visualization. Abbreviations of network properties as for Table 1.
4. Discussion
4.1. Morphospace Occupation
Analyses of ichthyosaur disparity, based on phylogenetic data sets [2,26], identified clear differences in morphospace occupation between Triassic and post-Triassic forms. These contributions proposed that ichthyosaurs passed through an evolutionary bottleneck close to the Triassic–Jurassic boundary and that after this key period, ichthyosaur evolution showed a long-term reduction in evolutionary rates and disparity. Other approaches integrating ecomorphological metrics and functional disparity for ecospace modeling [27,28,29] agreed with these general results. Particularly, [28] found that, after the Triassic–Jurassic crisis, ichthyosaurs again achieved relatively high diversity in the Early Jurassic but throughout the Middle and Late Jurassic, the proportional disparity of ichthyosaurs becomes increasingly diminished. However, these general outcomes do not match with the disparity of the connectivity pattern of the forefins found here (Figure 3). The analysis of the forefin networks showed no evidence of disparity retraction after the Early Jurassic as depicted by the morphospace occupation of the Middle Jurassic and younger thunnosaurs. Noteworthy, within a general tendency towards more integrated and modular fins, the thunnosauria morphospace is expanded in all directions. Similar results have been obtained through the analysis of humerus and zeugopodium morphology among ophthalmosaurids [20]. Other lines of evidence, like those provided by bone microanatomy, e.g., [30,31], also suggest that thunnosaurs, and particularly ophthalmosaurids, were ecologically diverse throughout the Jurassic.
4.2. Fin Connectivity and Functional Disparity
The exploration of functional disparity focuses on morphological diversity (and its innovations) with a recognized impact on the way of life of animals [28,32]. In the particular case of the Mesozoic SECAD, since the pioneering contributions of Massare [5,33], most of the ecomorphological approaches have been focused on the feeding apparatus [34,35,36] and paleohistology [37,38]. However, swimming performance is a key factor for the SECAD not only for dispersal during steady swimming but also for foraging. Thus, the skeletal thunniform body plan has been linked to the ecological abilities for the capture of fast pelagic prey such as fast swimming belemnite cephalopods [10]. The evolution of the thunniform body plan of ichthyosaurus has also been explored in terms of energetic performance. Assuming that all post-Triassic ichthyosaurs were thunniform swimmers, it has been proposed that body size was a key factor in the evolution of swimming [39]. These contributions deal mainly with the steady locomotion of ichthyosaurs; however, different maneuverability performances are crucial for surviving escaping from predators and/or capturing elusive prey. Although belemnites were important items of thunnosaur diets, the gut content of Cretaceous Platypterygiinae, as well as tooth and skull morphology [16,40], indicate that they probably fed on a wide range of prey, including other vertebrates.
The role of the pectoral appendages of vertebrate swimmers as control surfaces is well known. Changes in the orientation of the control surfaces with respect to the body axis, as well as small changes in orientation at the leading and trailing edges, have an impact on stability and maneuverability. This is true for flexible pectoral fins of fishes [41] but also, although to a lesser extent, in relatively stiff flippers like those of sharks and odontocetes. Among odontocetes, the lack of maneuverability is compensated by changing small turn radii of flexible forms for higher turning rates and they depict different turning performances [42]. In sharks, the majority of the pectoral fin area is internally supported by collagenous ceratotrichia, which cannot be actively moved [43]. Most of the stabilization relies on changes in the angle of attack or asynchronous pectoral fin movement [44,45]. Despite ichthyosauromorphs being axial swimmers through their evolution and having paired fins that must have acted on stability and maneuverability, the disparity of hydrofoils across thunnosaur clades has not been explored other than as an eventual source of phylogenetic or taxonomic information [24,46]. Given the functional relevance of fins as control surfaces, features such as density, clustering, or path length of their bone arrangements could be considered not only as expressions of morphological disparity but also as functional disparity among thunnosaurs and, thus, suggests different ecological niches.
In addition to the observed disparity of connectivity patterns of the forefin of ichthyosaurs, an eloquent feature that still remains undescribed must be addressed. This is the presence of a ball-and-socket joint between the distal end of the humerus and extra-zeugopial accessory elements on the leading edge of the forefin of some Late Jurassic-Cretaceous ichthyosaurs. Thus in Platypterygius australis QM F3348 [47] and in the Late Jurassic Platypterygiine MLP 85-I-15-1 [48] (Figure S2) the proximal surface of the extra zeugopodial element anterior to radius is short (antero-posteriorly) and notably convex and articulates with a strongly concave and small distal articular surface of the humerus. A similar condition occurred in the forefin of the Late Jurassic Platypterygiine Sumpalla [20] although in this taxon the articular facet on the distal humerus is not so well demarcated. This peculiar ball-and-socket joint between the humerus and the pre-radial accessory fin elements indicates that this local point could be acting like a multiaxial joint. If so, then subtle, intrafin movements at this point would indicate considerable changes on the leading edge. That is as a vortex generator that increases the lift force and enhances maneuverability during locomotion analogous to the function of the alula in flying birds [49]. Noteworthy, the forefin of Platypterygius americanus (UW 2421, Figure S2) [50], shows another very interesting condition: a ball-and-socket joint occurs on the trailing edge between the humerus and a pisiform. This condition suggests that the diversity of maneuvering abilities among derived ichthyosaurus may have been even greater. Quantitative experimental approaches would be worthy to test this hypothesis.
Unfortunately, the fins of QM F3348, MLP 85-I-15-1, and UW 2421, which are eloquent examples of ball-and-socket joints, could not be modeled for this study because they are very incomplete. It is expected that the exploration of deposits such as those of the Cretaceous Zapata Formation in Southern Chile [51,52] may provide more complete specimens in the near future.
4.3. Stepwise Evolution of Ichthyosaur Hydrofoils
Along the phylogeny is a clear trend, expressed across the succession of major steps of connectivity changes, towards better integrated, more modular, and more homogeneous fins in ichthyosaurs (Figure 4). These major changes could be interpreted as steps of a stepwise evolutionary pattern of limb-to-controller hydrofoil transition within ichthyosaurs. It is known that, on very broad scales, morphological iteration (and convergence) occurs frequently [53,54]. Whether this stepwise pattern denotes, at the lowest scale, a morphological iteration in the evolution of more efficient controller flipper-hydrofoils is a worthy question to be empirically tested in the future.
The results of network analysis framed against phylogeny show that the underlying connectivity patterns changed as ichthyosaurs evolved thunniform body plan very early in phylogeny. Thus, the first step of the connectivity changes coincides with the emergence of Ichthyosauria soon after the emergence of Ichthyosauromorhs at the Olenekian [1]. Some noteworthy modifications on the forefin, as the lack of centralia [55] pre-dated these changes. The ongoing fin evolution throughout the Early Jurassic indicates that morphological changes that accompanied the emergence of parvipelvians and thunnosaurs, such as mesopodialization and the development of the thunniform body plan, respectively, predate the next steps of important changes in bone connectivity. The paucity of Aalenian–Bathonian records [56] obscures understanding of the fin transition between the Early and Middle Jurassic and the sudden appearance of ophthalmosaurid ichthyosaurs. Unfortunately, the most complete specimens of early ophthalmosaurids (i.e., Mollesaurus and Argovisaurus) lack their fins [19,57]. However, the comparison between Chacaicosaurus and Ophthalmosaurus icenicus, as well as the ancestral reconstruction using parsimony analysis of the network parameters (Figure 4), suggest that the complexity of the propodeal–epipodial joint (as was the morphological innovation of the appearance of the pae) did not produce drastic changes in the connectivity pattern. In the same way, the rise of Platypterygiine by the Late Jurassic is not mirrored by changes in the fin networks. It is likely that along evolution, the morphological innovations of the forefins (associated with the emergency of major clades) provided the structural framework that allowed the subsequent diversification of the bone connectivity that ultimately triggered an ecological diversity (e.g., diversity of refined maneuverability among thunniform swimming).
Noteworthy, as also indicated by other ecological and diversity parameters [16,20], the Jurassic–Cretaceous transition seems to reduce the disparity of the forefin. The only survival lineage shows the most extreme pattern of homogeneous integration but also a restricted occupation of the morphospace.
5. Conclusions and Future Directions
The generalized hydrofoil design of ichthyosaur fins hides a great diversity of bone arrangements. The occupation of the morphospace through time shows a clear evolutionary trend towards better integrated and modular forefins. Within this common path, the disparity of thunnosaurs (as mirrored by the large occupation of morphospace areas) persisted throughout the Jurassic. A key period occurred at the Jurassic–Cretaceous boundary. Late Cretaceous-derived Platypterygiine explores a vacant restricted new area of the available space.
The connectivity pattern diversity (i.e., variations of density, clustering, path length, and nodes and edge values) may also represent functional diversity. Based on the role of the forefin as the control surface of swimming, we argue that the morphospace occupation can be interpreted in ecological–functional terms. The controller hydrofoils of ichthyosaurs are assumed to be relatively stiff and with restricted mobility [37]. However, the number of nodes, density, clustering, and path length of their bony arrangement indicate that not all fins should have had the same performance in terms of partial surface deformation and/or in terms of relative stiffness. Noteworthily, some derived Platypterygiines had a ball-and-socket joint point on the leading edge of their fins that could have facilities for localized bending of the leading edge substantially affecting the angle of attack during swimming. Based on the integration of the outcomes of network analysis and gross anatomy of the leading edge we propose diverse maneuverability capacities among members of the large clade Platypterygiine. Further studies on large samples and quantitative experimental approaches would be worthy to test this hypothesis. The mapping of the bone arrangements of the forefin on phylogeny shows that evolutionary changes occurred stepwise along the Mesozoic.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d16060349/s1, Figure S1: Mapped network properties of analyzed taxa; Figure S2: Forefins showing ball-and-socket joints; File S1: Results of PERMANOVA; Table S1: List of the specimens used for the construction of the anatomical networks of the forefin; Table S2: Network properties of analyzed taxa.
Author Contributions
Conceptualization, M.S.F., E.V. and L.C.; methodology, E.V., L.C. and A.M.; investigation, M.S.F., L.C., A.M. and E.V.; writing—original draft preparation, M.S.F.; writing—review and editing, L.C. and A.M. All authors contributed to drafting the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the Universidad Nacional de La Plata, programa de incentivos docentes UNLP-N981 (M.S.F.) and the Agencia Nacional de Investigaciones científicas de Argentina PICT 2020-2067 (M.S.F.), PICT 2022-0963 (L.C.) and PICT 2019-0327 (M.S.F., E.V.).
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary Materials.
Acknowledgments
Yanina Herrera (MLP), Martín Ezcurra (MACN), Alberto Garrido, and Belén Bollini (MOZ) are thanked for allowing access to the specimens under their care. The authors also wish to extend their thanks to the reviewers for their comments, which substantially improved the quality of the manuscript. Finally, we want to thank Nathalie Bardet, the academic editor, for her invitation to contribute to this special volume dedicated to Mesozoic marine reptile faunas.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Motani, R.; Jiang, D.; Tintori, A.; Ji, C.; Huang, J. Pre- versus Post-Mass Extinction Divergence of Mesozoic Marine Reptiles Dictated by Time-Scale Dependence of Evolutionary Rates. Proc. R. Soc. B Biol. Sci. 2017, 284, 20170241. [Google Scholar] [CrossRef] [PubMed]
- Moon, B.C.; Stubbs, T.L. Early High Rates and Disparity in the Evolution of Ichthyosaurs. Commun. Biol. 2020, 3, 68. [Google Scholar] [CrossRef]
- Kelley, N.P.; Pyenson, N.D. Evolutionary Innovation and Ecology in Marine Tetrapods from the Triassic to the Anthropocene. Science 2015, 348, aaa3716. [Google Scholar] [CrossRef] [PubMed]
- Renesto, S.; Dal Sasso, C.; Fogliazza, F.; Ragni, C. New Findings Reveal That the Middle Triassic Ichthyosaur Mixosaurus cornalianus Is the Oldest Amniote with a Dorsal Fin. Acta Palaeontol. Pol. 2020, 65, 511–522. [Google Scholar] [CrossRef]
- Massare, J.A. Swimming Capabilities of Mesozoic Marine Reptiles: Implications for Method of Predation. Paleobiology 1988, 14, 187–205. [Google Scholar] [CrossRef]
- Massare, J.A. Faunas, Behavior, and Evolution. In Ancient Marine Reptiles, 1st ed.; Callaway, J.M., Nicholls, E.L., Eds.; Elsevier: Amsterdam, The Netherlands, 1997; pp. 401–421. ISBN 978-0-12-155210-7. [Google Scholar] [CrossRef]
- Webb, P.W. Body Form, Locomotion and Foraging in Aquatic Vertebrates. Am. Zool. 1984, 24, 107–120. [Google Scholar] [CrossRef]
- Webb, P.W. Simple Physical Principles and Vertebrate Aquatic Locomotion. Am. Zool. 1988, 28, 709–725. [Google Scholar] [CrossRef]
- Motani, R. Scaling Effects in Caudal Fin Propulsion and the Speed of Ichthyosaurs. Nature 2002, 415, 309–312. [Google Scholar] [CrossRef]
- Motani, R.; Shimada, K. Skeletal Convergence in Thunniform Sharks, Ichthyosaurs, Whales, and Tunas, and Its Possible Ecological Links through the Marine Ecosystem Evolution. Sci. Rep. 2023, 13, 16664. [Google Scholar] [CrossRef]
- Caldwell, M.W. Modified Perichondral Ossification and the Evolution of Paddle-Like Limbs in Ichthyosaurs and Plesiosaurs. J. Vertebr. Paleontol. 1997, 17, 534–547. [Google Scholar] [CrossRef]
- Caldwell, M.W. From Fins to Limbs to Fins: Limb Evolution in Fossil Marine Reptiles. Am. J. Med. Genet. 2002, 112, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, E.E. Unraveling the Influences of Soft-Tissue Flipper Development on Skeletal Variation Using an Extinct Taxon. J. Exp. Zoolog. B Mol. Dev. Evol. 2012, 318, 545–554. [Google Scholar] [CrossRef]
- DeBlois, M.C.; Motani, R. Flipper Bone Distribution Reveals Flexible Trailing Edge in Underwater Flying Marine Tetrapods. J. Morphol. 2019, 280, 908–924. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.S.; Vlachos, E.; Buono, M.R.; Alzugaray, L.; Campos, L.; Sterli, J.; Herrera, Y.; Paolucci, F. Fingers Zipped up or Baby Mittens? Two Main Tetrapod Strategies to Return to the Sea. Biol. Lett. 2020, 16, 20200281. [Google Scholar] [CrossRef]
- Fischer, V.; Bardet, N.; Benson, R.B.J.; Arkhangelsky, M.S.; Friedman, M. Extinction of Fish-Shaped Marine Reptiles Associated with Reduced Evolutionary Rates and Global Environmental Volatility. Nat. Commun. 2016, 7, 10825. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, Z. A New Pliosaur from the Bajocian of the Neuquen Basin, Argentina. Palaeontology 1997, 40, 135–147. [Google Scholar]
- Fernández, M.S.; Talevi, M. Ophthalmosaurian (Ichthyosauria) Records from the Aalenian–Bajocian of Patagonia (Argentina): An Overview. Geol. Mag. 2014, 151, 49–59. [Google Scholar] [CrossRef]
- Miedema, F.; Bastiaans, D.; Scheyer, T.M.; Klug, C.; Maxwell, E.E. A Large New Middle Jurassic Ichthyosaur Shows the Importance of Body Size Evolution in the Origin of the Ophthalmosauria. BMC Ecol. Evol. 2024, 24, 34. [Google Scholar] [CrossRef]
- Campos, L.; Fernández, M.S.; Herrera, Y.; Garrido, A. Morphological Disparity in the Evolution of the Ophthalmosaurid Forefin: New Clues from the Upper Jurassic of Argentina. Pap. Palaeontol. 2021, 7, 1995–2020. [Google Scholar] [CrossRef]
- Lomax, D.R.; Massare, J.A. Two New Species of Ichthyosaurus from the Lowermost Jurassic (Hettangian) of Somerset, England. Pap. Palaeontol. 2017, 3, 1–20. [Google Scholar] [CrossRef]
- Esteve-Altava, B.; Pierce, S.E.; Molnar, J.L.; Johnston, P.; Diogo, R.; Hutchinson, J.R. Evolutionary Parallelisms of Pectoral and Pelvic Network-Anatomy from Fins to Limbs. Sci. Adv. 2019, 5, eaau7459. [Google Scholar] [CrossRef] [PubMed]
- Bastian, M.; Heymann, S.; Jacomy, M. Gephi: An Open Source Software for Exploring and Manipulating Networks. Proc. Int. AAAI Conf. Web Soc. Media 2009, 3, 361–362. [Google Scholar] [CrossRef]
- Campos, L.; Fernández, M.S.; Bosio, V.; Herrera, Y.; Manzo, A. Revalidation of Myobradypterygius hauthali Huene, 1927 and the Phylogenetic Signal within the Ophthalmosaurid (Ichthyosauria) Forefins. Cretac. Res. 2024, 157, 105818. [Google Scholar] [CrossRef]
- Goloboff, P.A.; Morales, M.E. TNT version 1.6, with a graphical interface for MacOS and Linux, including new routines in parallel. Cladistics 2023, 39, 144–153. [Google Scholar] [CrossRef]
- Thorne, P.M.; Ruta, M.; Benton, M.J. Resetting the Evolution of Marine Reptiles at the Triassic-Jurassic Boundary. Proc. Natl. Acad. Sci. USA 2011, 108, 8339–8344. [Google Scholar] [CrossRef] [PubMed]
- Dick, D.G.; Maxwell, E.E. The Evolution and Extinction of the Ichthyosaurs from the Perspective of Quantitative Ecospace Modelling. Biol. Lett. 2015, 11, 20150339. [Google Scholar] [CrossRef]
- Stubbs, T.L.; Benton, M.J. Ecomorphological Diversifications of Mesozoic Marine Reptiles: The Roles of Ecological Opportunity and Extinction. Paleobiology 2016, 42, 547–573. [Google Scholar] [CrossRef]
- Reeves, J.C.; Moon, B.C.; Benton, M.J.; Stubbs, T.L. Evolution of Ecospace Occupancy by Mesozoic Marine Tetrapods. Palaeontology 2021, 64, 31–49. [Google Scholar] [CrossRef]
- Houssaye, A.; Martin Sander, P.; Klein, N. Adaptive Patterns in Aquatic Amniote Bone Microanatomy—More Complex than Previously Thought. Integr. Comp. Biol. 2016, 56, 1349–1369. [Google Scholar] [CrossRef]
- Talevi, M.; Fernández, M.S. Unexpected Skeletal Histology of an Ichthyosaur from the Middle Jurassic of Patagonia: Implications for Evolution of Bone Microstructure among Secondary Aquatic Tetrapods. Naturwissenschaften 2012, 99, 241–244. [Google Scholar] [CrossRef]
- Anderson, P.S.L.; Friedman, M.; Brazeau, M.D.; Rayfield, E.J. Initial Radiation of Jaws Demonstrated Stability despite Faunal and Environmental Change. Nature 2011, 476, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Massare, J.A. Tooth Morphology and Prey Preference of Mesozoic Marine Reptiles. J. Vertebr. Paleontol. 1987, 7, 121–137. [Google Scholar] [CrossRef]
- Kelley, N.P.; Motani, R. Trophic Convergence Drives Morphological Convergence in Marine Tetrapods. Biol. Lett. 2015, 11, 20140709. [Google Scholar] [CrossRef] [PubMed]
- Foffa, D.; Young, M.T.; Stubbs, T.L.; Dexter, K.G.; Brusatte, S.L. The Long-Term Ecology and Evolution of Marine Reptiles in a Jurassic Seaway. Nat. Ecol. Evol. 2018, 2, 1548–1555. [Google Scholar] [CrossRef]
- Delsett, L.L.; Pyenson, N.; Miedema, F.; Hammer, Ø. Is the Hyoid a Constraint on Innovation? A Study in Convergence Driving Feeding in Fish-Shaped Marine Tetrapods. Paleobiology 2023, 49, 684–699. [Google Scholar] [CrossRef]
- Houssaye, A.; De Buffrénil, V. Bone Histology and the Adaptation to Aquatic Life in Tetrapods. In Vertebrate Skeletal Histology and Paleohistology; CRC Press: Boca Raton, FL, USA, 2021; pp. 744–756. ISBN 978-1-351-18959-0. [Google Scholar]
- Houssaye, A.; Fish, F.E. Functional (Secondary) Adaptation to an Aquatic Life in Vertebrates: An Introduction to the Symposium. Integr. Comp. Biol. 2016, 56, 1266–1270. [Google Scholar] [CrossRef] [PubMed]
- Gutarra, S.; Moon, B.C.; Rahman, I.A.; Palmer, C.; Lautenschlager, S.; Brimacombe, A.J.; Benton, M.J. Effects of Body Plan Evolution on the Hydrodynamic Drag and Energy Requirements of Swimming in Ichthyosaurs. Proc. R. Soc. B Biol. Sci. 2019, 286, 20182786. [Google Scholar] [CrossRef] [PubMed]
- Kear, B.P.; Boles, W.E.; Smith, E.T. Unusual Gut Contents in a Cretaceous Ichthyosaur. Proc. R. Soc. Lond. B Biol. Sci. 2003, 270, S206–S208. [Google Scholar] [CrossRef]
- Gerstner, C.L. Maneuverability of Four Species of Coral-Reef Fish That Differ in Body and Pectoral-Fin Morphology. Can. J. Zool. 1999, 77, 1102–1110. [Google Scholar] [CrossRef]
- Fish, F.E.; Lauder, G.V. Control Surfaces of Aquatic Vertebrates: Active and Passive Design and Function. J. Exp. Biol. 2017, 220, 4351–4363. [Google Scholar] [CrossRef]
- Fish, F.E. Aquatic locomotion: Environmental constraints that drive convergent evolution. In Convergent Evolution: Animal Form and Function; Springer International Publishing: Cham, Switzerland, 2023; pp. 477–522. [Google Scholar]
- Fish, F.E.; Shannahan, L.D. The role of the pectoral fins in body trim of sharks. J. Fish Biol. 2000, 56, 1062–1073. [Google Scholar] [CrossRef]
- Hoffmann, S.L.; Porter, M.E. Body and pectoral fin kinematics during routine yaw turning in bonnethead sharks (Sphyrna tiburo). Integr. Org. Biol. 2019, 1, obz014. [Google Scholar] [CrossRef] [PubMed]
- Motani, R. Phylogeny of the Ichthyopterygia. J. Vertebr. Paleontol. 1999, 19, 473–496. [Google Scholar] [CrossRef]
- Wade, M. Platypterygius australis, an Australian Cretaceous Ichthyosaur. Lethaia 1984, 17, 99–113. [Google Scholar] [CrossRef]
- Fernández, M.S. Nuevo Material de Caypullisaurus bonapartei Fernández (Reptilia: Ichthyosauridae) Del Jurásico Superior de La Cuenca Neuquina, Argentina. Ameghiniana 1998, 35, 21–24. [Google Scholar]
- Lee, S.; Kim, J.; Park, H.; Jabłoński, P.G.; Choi, H. The Function of the Alula in Avian Flight. Sci. Rep. 2015, 5, 9914. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, E.E.; Kear, B.P. Postcranial Anatomy of Platypterygius americanus (Reptilia: Ichthyosauria) from the Cretaceous of Wyoming. J. Vertebr. Paleontol. 2010, 30, 1059–1068. [Google Scholar] [CrossRef]
- Pardo-Pérez, J.; Frey, E.; Stinnesbeck, W.; Fernández, M.S.; Rivas, L.; Salazar, C.; Leppe, M. An Ichthyosaurian Forefin from the Lower Cretaceous Zapata Formation of Southern Chile: Implications for Morphological Variability within Platypterygius. Palaeobiodivers. Palaeoenviron. 2012, 92, 287–294. [Google Scholar] [CrossRef]
- Stinnesbeck, W.; Frey, E.; Rivas, L.; Perez, J.P.; Cartes, M.L.; Soto, C.S.; Lobos, P.Z. A Lower Cretaceous Ichthyosaur Graveyard in Deep Marine Slope Channel Deposits at Torres Del Paine National Park, Southern Chile. Geol. Soc. Am. Bull. 2014, 126, 1317–1339. [Google Scholar] [CrossRef]
- Vermeij, G.J. Historical Contingency and the Purported Uniqueness of Evolutionary Innovations. Proc. Natl. Acad. Sci. USA 2006, 103, 1804–1809. [Google Scholar] [CrossRef]
- Jablonski, D. Scale and Hierarchy in Macroevolution. Palaeontology 2007, 50, 87–109. [Google Scholar] [CrossRef]
- Motani, R.; Jiang, D.-Y.; Tintori, A.; Rieppel, O.; Chen, G.-B.; You, H. First Evidence of Centralia in Ichthyopterygia Reiterating Bias from Paedomorphic Characters on Marine Reptile Phylogenetic Reconstruction. J. Vertebr. Paleontol. 2015, 35, e948547. [Google Scholar] [CrossRef]
- Fischer, V.; Weis, R.; Thuy, B. Refining the Marine Reptile Turnover at the Early–Middle Jurassic Transition. PeerJ 2021, 9, e10647. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.S. A New Ichthyosaur from the Los Molles Formation (Early Bajocian), Neuquen Basin, Argentina. J. Paleontol. 1999, 73, 677–681. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
