Diversity Begets Diversity: Structural Heterogeneity Determines Fine-Scale Epiphyte Community Structure in a Temperate Rainforest
Abstract
1. Introduction
2. Materials and Methods
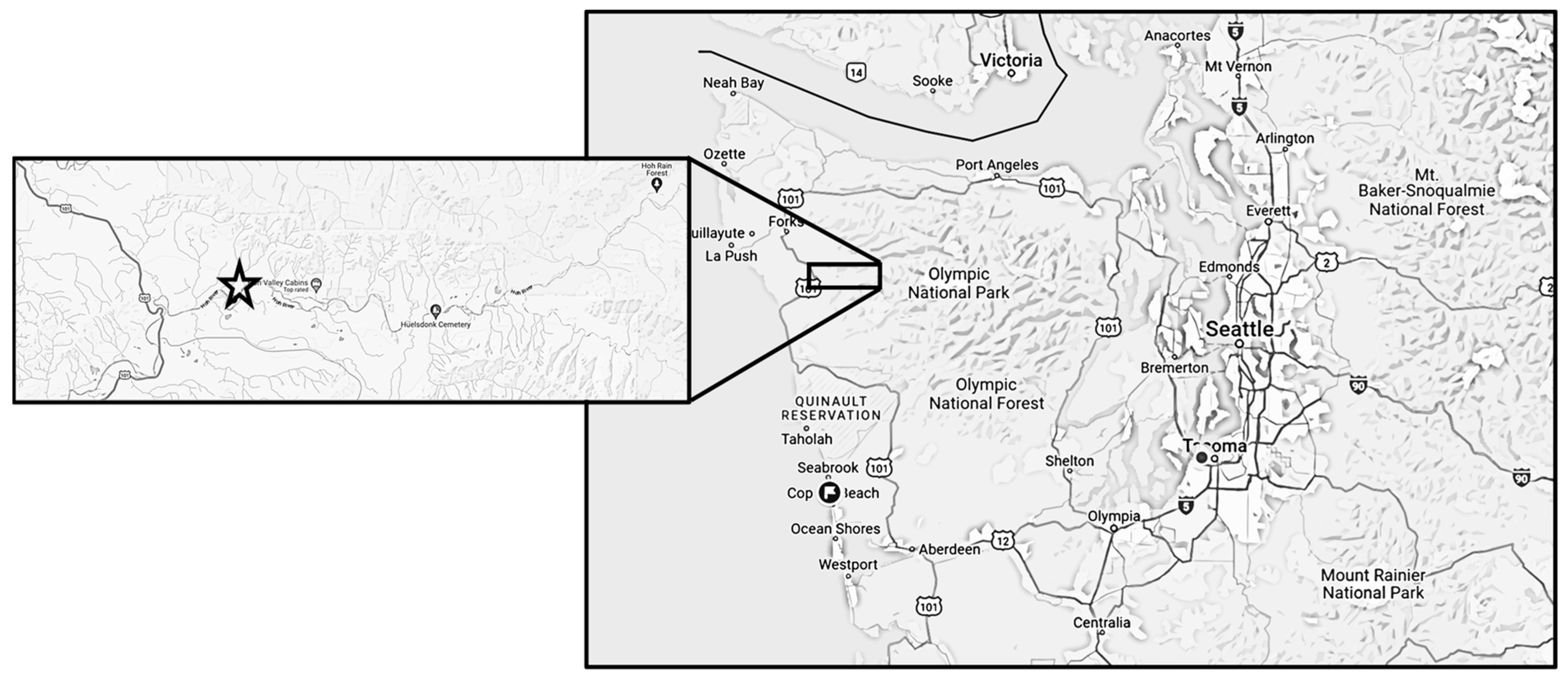
2.1. Study Area
2.2. Study Design and Data Collection
2.3. Statistical Analyses
3. Results
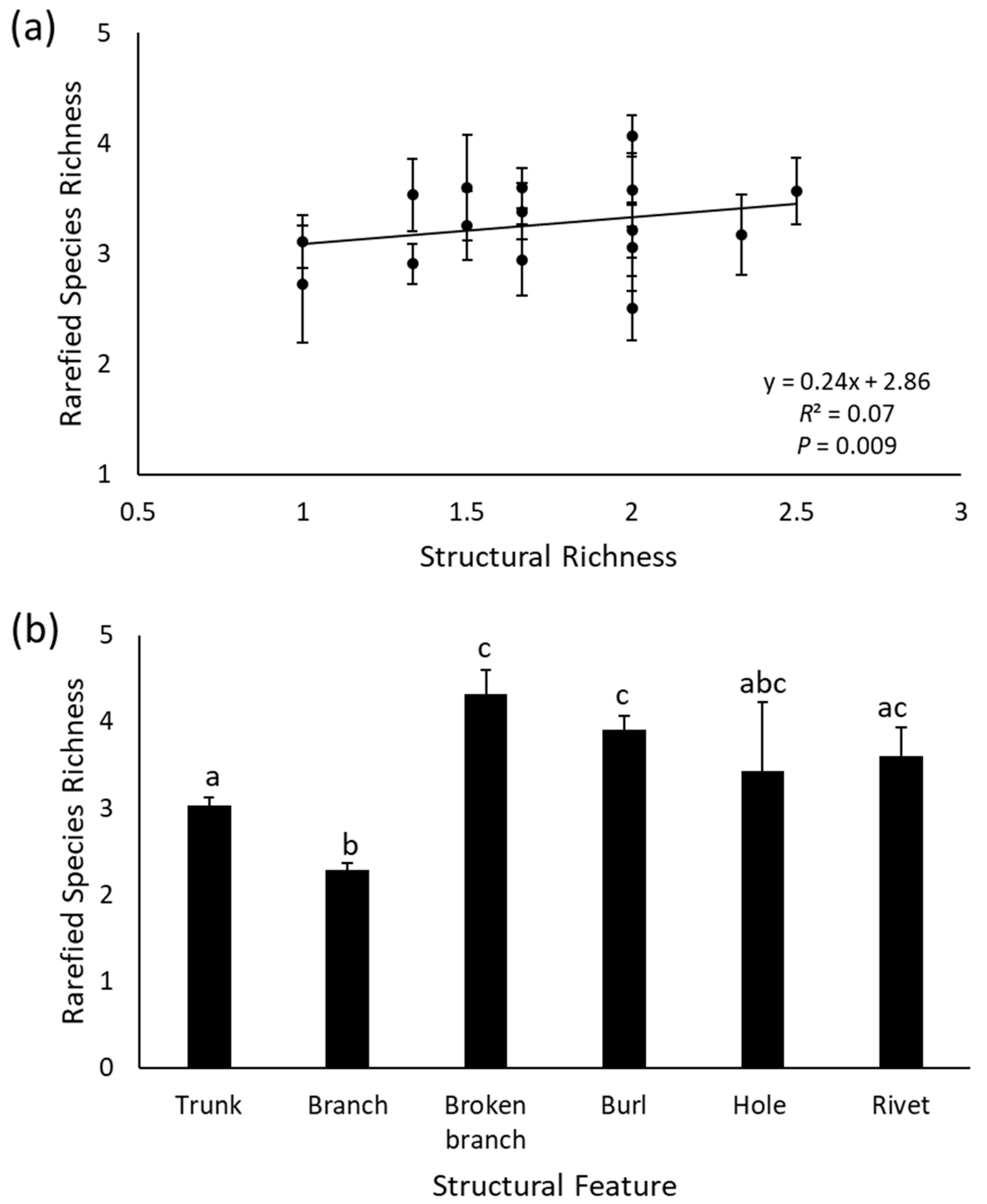
3.1. Effect of Structure
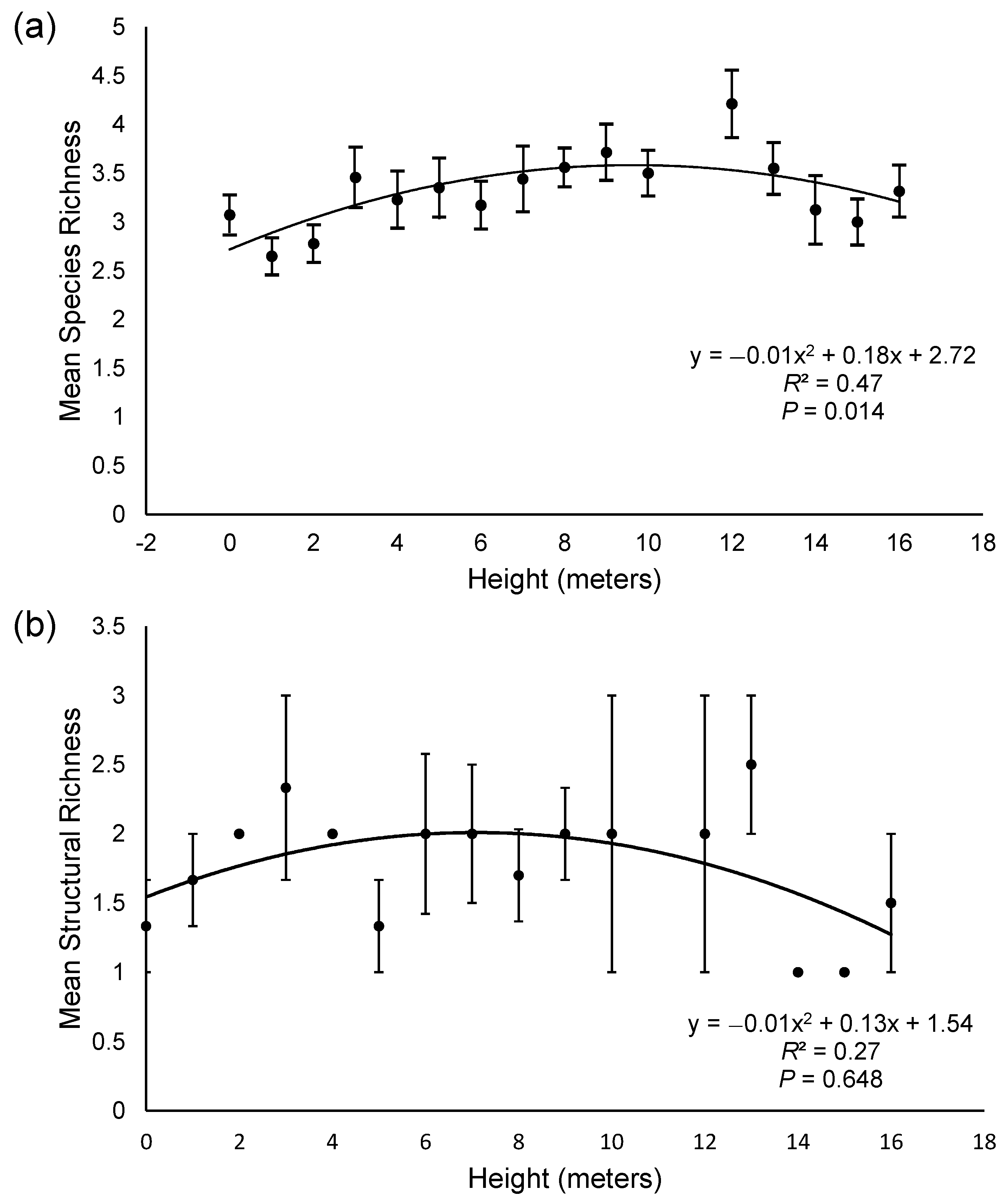
3.2. Effect of Height
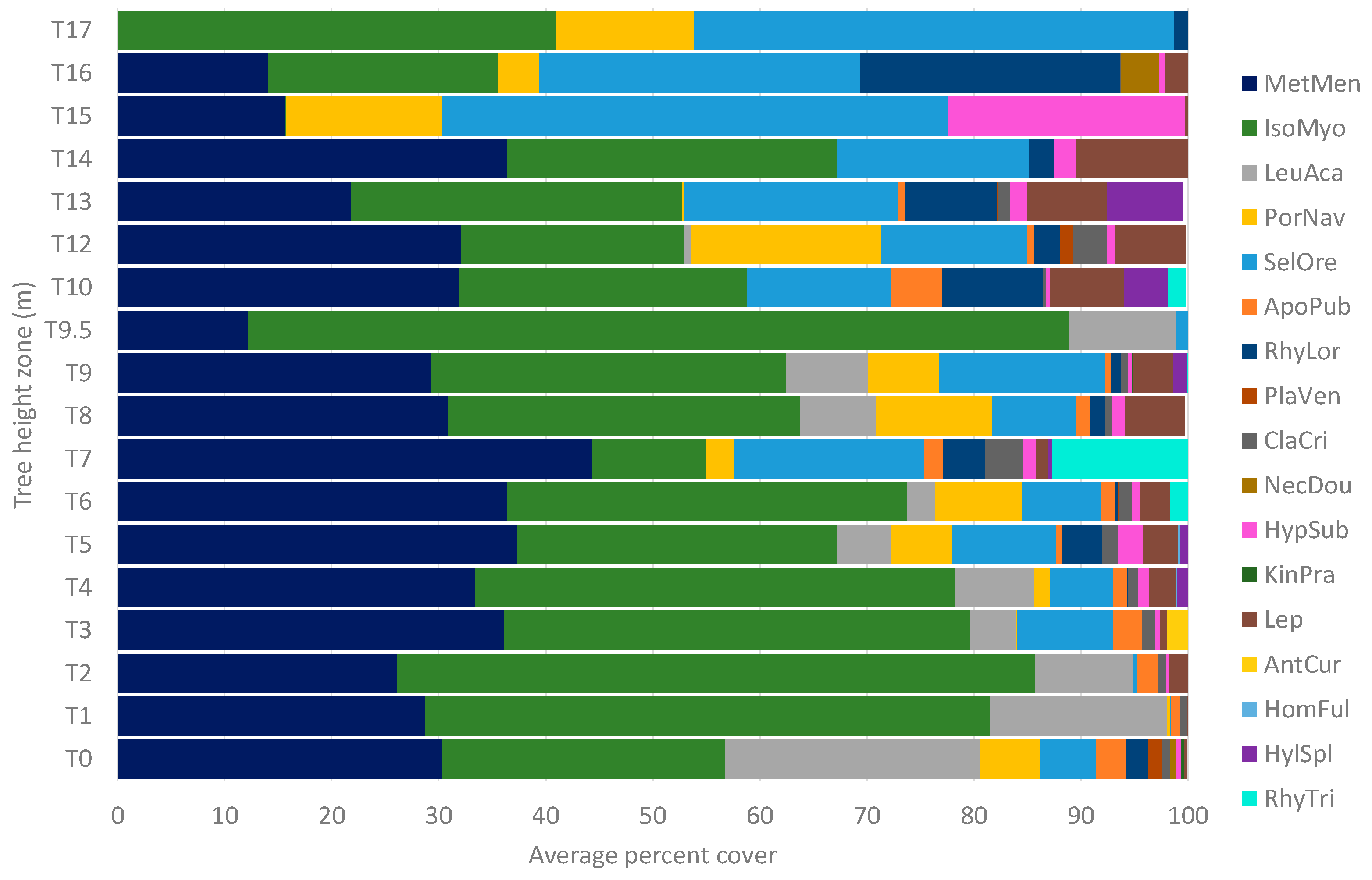
3.3. Species Distributions
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hutchinson, G.E. Homage to Santa Rosalina or Why Are There So Many Kinds of Animals? Am. Nat. 1959, 93, 145–159. [Google Scholar] [CrossRef]
- MacArthur, R.H.; MacArthur, J.W. On Bird Species Diversity. Ecology 1961, 42, 594–598. [Google Scholar] [CrossRef]
- Rosenzweig, M.L. Species Diversity in Space and Time; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Stein, A.; Gerstner, K.; Kreft, H. Environmental Heterogeneity as a Universal Driver of Species Richness across Taxa, Biomes and Spatial Scales. Ecol. Lett. 2014, 17, 866–880. [Google Scholar] [CrossRef] [PubMed]
- Graham, N.A.J.; Nash, K.L. The Importance of Structural Complexity in Coral Reef Ecosystems. Coral Reefs 2013, 32, 315–326. [Google Scholar] [CrossRef]
- Messmer, V.; Jones, G.P.; Munday, P.L.; Holbrook, S.J.; Schmitt, R.J.; Brooks, A.J. Habitat Biodiversity as a Determinant of Fish Community Structure on Coral Reefs. Ecology 2011, 92, 2285–2298. [Google Scholar] [CrossRef] [PubMed]
- Firth, L.B.; Crowe, T.P. Competition and Habitat Suitability: Small-Scale Segregation Underpins Large-Scale Coexistence of Key Species on Temperate Rocky Shores. Oecologia 2010, 162, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Franklin, J.F.; Van Pelt, R. Spatial Aspects of Structural Complexity in Old-Growth Forests. J. For. 2004, 102, 22–28. [Google Scholar] [CrossRef]
- Stagoll, K.; Lindenmayer, D.B.; Knight, E.; Fischer, J.; Manning, A.D. Large Trees Are Keystone Structures in Urban Parks. Conserv. Lett. 2012, 5, 115–122. [Google Scholar] [CrossRef]
- Yamaura, Y.; Unno, A.; Royle, J.A. Sharing Land via Keystone Structure: Retaining Naturally Regenerated Trees May Efficiently Benefit Birds in Plantations. Ecol. Appl. 2023, 33, e2802. [Google Scholar] [CrossRef]
- Manning, A.D.; Fischer, J.; Lindenmayer, D.B. Scattered Trees Are Keystone Structures—Implications for Conservation. Biol. Conserv. 2006, 132, 311–321. [Google Scholar] [CrossRef]
- Woods, C.L.; Cardelús, C.L.; DeWalt, S.J. Microhabitat Associations of Vascular Epiphytes in a Wet Tropical Forest Canopy. J. Ecol. 2015, 103, 421–430. [Google Scholar] [CrossRef]
- Victoriano-Romero, E.; Valencia-Díaz, S.; García-Franco, J.G.; Mehltreter, K.; Toledo-Hernández, V.H.; Flores-Palacios, A. Interactions between Epiphytes during Canopy Soil Formation: An Experiment in a Lower Montane Cloud Forest of Southeast Mexico. Plant Biol. 2023, 25, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Lindenmayer, D.B.; Laurance, W.F.; Franklin, J.F.; Likens, G.E.; Banks, S.C.; Blanchard, W.; Gibbons, P.; Ikin, K.; Blair, D.; McBurney, L.; et al. New Policies for Old Trees: Averting a Global Crisis in a Keystone Ecological Structure. Conserv. Lett. 2014, 7, 61–69. [Google Scholar] [CrossRef]
- Azuma, W.A.; Komada, N.; Ogawa, Y.; Ishii, H.; Nakanishi, A.; Noguchi, Y.; Kanzaki, M. One Large Tree Crown Can Be Defined as a Local Hotspot for Plant Species Diversity in a Forest Ecosystem: A Case Study in Temperate Old-Growth Forest. Plant Ecol. 2022, 223, 99–112. [Google Scholar] [CrossRef]
- Emmons, L.H. Ecology and Resource Partitioning among Nine Species of African Rain Forest Squirrels. Ecol. Monogr. 1980, 50, 31–54. [Google Scholar] [CrossRef]
- Sushma, H.S.; Singh, M. Resource Partitioning and Interspecific Interactions among Sympatric Rain Forest Arboreal Mammals of the Western Ghats, India. Behav. Ecol. 2006, 17, 479–490. [Google Scholar] [CrossRef]
- Woods, C.L.; Nevins, L.M.; Didier, E.J. Structural Heterogeneity of Trees Influences Epiphyte Distributions in a Northern Temperate Rainforest. J. Veg. Sci. 2019, 30, 1134–1142. [Google Scholar] [CrossRef]
- Johansson, D. Ecology of Vascular Epiphytes in West African Rain Forest. Acta Phytogeogr. Suec. 1974, 59, 1–136. [Google Scholar]
- Hietz, P.; Hietz-Seifert, U. Structure and Ecology of Epiphyte Communities of a Cloud Forest in Central Veracruz, Mexico. J. Veg. Sci. 1995, 6, 719–728. [Google Scholar] [CrossRef]
- Mellado-Mansilla, D.; León, C.A.; Ortega-Solís, G.; Godoy-Güinao, J.; Moreno, R.; Díaz, I.A. Vertical Patterns of Epiphytic Bryophyte Diversity in a Montane Nothofagus Forest in the Chilean Andes. N. Z. J. Bot. 2017, 55, 514–529. [Google Scholar] [CrossRef]
- Kenkel, N.C.; Bradfield, G.E. Epiphytic Vegetation on Acer macrophyllum: A Multivariate Study of Species-Habitat Relationships. Vegetatio 1986, 68, 43–53. [Google Scholar] [CrossRef]
- Bartels, S.F.; Chen, H.Y.H. Mechanisms Regulating Epiphytic Plant Diversity. Crit. Rev. Plant Sci. 2012, 31, 391–400. [Google Scholar] [CrossRef]
- Zimmerman, J.K.; Olmsted, I.C. Host Tree Utilization by Vascular Epiphytes in a Seasonally Inundated Forest (Tintal) in Mexico. Biotropica 1992, 24, 402. [Google Scholar] [CrossRef]
- Elias, J.P.C.; Mortara, S.R.; Nunes-Freitas, A.F.; Van den Berg, E.; Ramos, F.N. Host Tree Traits in Pasture Areas Affect Forest and Pasture Specialist Epiphyte Species Differently. Am. J. Bot. 2021, 108, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Callaway, R.M.; Reinhart, K.O.; Moore, G.W.; Moore, D.J.; Pennings, S.C. Epiphyte Host Preferences and Host Traits: Mechanisms for Species-Specific Interactions. Oecologia 2002, 132, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Wyse, S.V.; Burns, B.R. Short Communication: Do Host Bark Traits Influence Trunk Epiphyte Communities? N. Z. J. Ecol. 2011, 35, 296–301. [Google Scholar]
- Izuddin, M.; Webb, E.L. The Influence of Tree Architecture, Forest Remnants, and Dispersal Syndrome on Roadside Epiphyte Diversity in a Highly Urbanized Tropical Environment. Biodivers. Conserv. 2015, 24, 2063–2077. [Google Scholar] [CrossRef]
- Zotz, G.; Vollrath, B. The Epiphyte Vegetation of the Palm Socratea exorrhiza-Correlations with Tree Size, Tree Age and Bryophyte Cover. J. Trop. Ecol. 2003, 19, 81–90. [Google Scholar] [CrossRef][Green Version]
- Adhikari, Y.P.; Hoffmann, S.; Kunwar, R.M.; Bobrowski, M.; Jentsch, A.; Beierkuhnlein, C. Vascular Epiphyte Diversity and Host Tree Architecture in Two Forest Management Types in the Himalaya. Glob. Ecol. Conserv. 2021, 27, e01544. [Google Scholar] [CrossRef]
- Zhao, M.; Geekiyanage, N.; Xu, J.; Khin, M.M.; Nurdiana, D.R.; Paudel, E.; Harrison, R.D. Structure of the Epiphyte Community in a Tropical Montane Forest in SW China. PLoS ONE 2015, 10, e0122210. [Google Scholar] [CrossRef]
- Wagner, K.; Mendieta-Leiva, G.; Zotz, G. Host Specificity in Vascular Epiphytes: A Review of Methodology, Empirical Evidence and Potential Mechanisms. AoB Plants 2015, 7, plu092. [Google Scholar] [CrossRef] [PubMed]
- Spicer, M.E.; Woods, C.L. A Case for Studying Biotic Interactions in Epiphyte Ecology and Evolution. Perspect. Plant Ecol. Evol. Syst. 2022, 54, 125658. [Google Scholar] [CrossRef]
- Lyons, B.; Nadkarni, N.M.; North, M.P. Spatial Distribution and Succession of Epiphytes on Tsuga heterophylla (Western Hemlock) in an Old-Growth Douglas-Fir Forest. Can. J. Bot. 2000, 78, 957–968. [Google Scholar]
- Király, I.; Nascimbene, J.; Tinya, F.; Ódor, P. Factors Influencing Epiphytic Bryophyte and Lichen Species Richness at Different Spatial Scales in Managed Temperate Forests. Biodivers. Conserv. 2013, 22, 209–223. [Google Scholar] [CrossRef]
- Dickinson, K.J.M.; Mark, A.F.; Dawkins, B. Ecology of Lianoid/Epiphytic Communities in Coastal Podocarp Rain Forest, Haast Ecological District, New Zealand. J. Biogeogr. 1993, 20, 687. [Google Scholar] [CrossRef]
- Hofstede, R.G.M.; Dickinson, K.J.M.; Mark, A.F. Distribution, Abundance and Biomass of Epiphyte-Lianoid Communities in a New Zealand Lowland Nothofagus-Podocarp Temperate Rain Forest: Tropical Comparisons. J. Biogeogr. 2002, 28, 1033–1049. [Google Scholar] [CrossRef]
- Clement, J.P.; Shaw, D.C. Crown Structure and the Distribution of Epiphyte Functional Group Biomass in Old-Growth Pseudotsuga menziesii Trees. Ecoscience 1999, 6, 243–254. [Google Scholar] [CrossRef]
- Harmon, M.E.; Franklin, J.F. Tree Seedlings on Logs in Picea-Tsuga Forests of Oregon and Washington. Ecology 1989, 70, 48–59. [Google Scholar] [CrossRef]
- East, A.E.; Jenkins, K.J.; Happe, P.J.; Bountry, J.A.; Beechie, T.J.; Mastin, M.C.; Sankey, J.B.; Randle, T.J. Channel-Planform Evolution in Four Rivers of Olympic National Park, Washington, USA: The Roles of Physical Drivers and Trophic Cascades. Earth Surf. Process. Landf. 2017, 42, 1011–1032. [Google Scholar] [CrossRef]
- Nadkarni, N.M. Biomass and Mineral Capital of Epiphytes in an Acer macrophyllum Community of a Temperate Moist Coniferous Forest, Olympic Peninsula, Washington State. Can. J. Bot. 1984, 62, 2223–2228. [Google Scholar] [CrossRef]
- Perry, D.R. A Method of Access into the Crowns of Emergent and Canopy Trees. Biotropica 1978, 10, 155–157. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.B.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Wagner, H.; Barbour, M.; Bedward, M.; Bolker, B.; Borcard, D.; et al. Vegan, Community Ecology PackageR Package version 2.6-7. 2024. Available online: https://github.com/vegandevs/vegan (accessed on 20 July 2024).
- McCune, B.; Grace, J.B. Analysis of Ecological Communities; MjM Software Design: Gleneden Beach, OR, USA, 2002; ISBN 0972129006. [Google Scholar]
- Martinez Arbizu, P. pairwiseAdonis, Version 0.4.1; Pairwise Multilevel Comparison Using Adonis; R Package. 2020. Available online: https://github.com/pmartinezarbizu/pairwiseAdonis (accessed on 20 July 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Schneider, K.N.; Winemiller, K.O. Structural Complexity of Woody Debris Patches Influences Fish and Macroinvertebrate Species Richness in a Temperate Floodplain-River System. Hydrobiologia 2008, 610, 235–244. [Google Scholar] [CrossRef]
- Freiberg, M. Spatial Distribution of Vascular Epiphytes on Three Emergent Canopy Trees in French Guiana. Biotropica 1996, 28, 345–355. [Google Scholar] [CrossRef]
- Hoffman, G.R.; Kazmierski, R.G. An Ecologic Study of Epiphytic Bryophytes and Lichens on Pseudotsuga menziesii on the Olympic Peninsula, Washington I. A Description of the Vegetation. Bryologist 1969, 72, 149–169. [Google Scholar] [CrossRef]
- Ingram, S.W.; Nadkarni, N.M. Composition and Distribution of Epiphytic Organic Matter in a Neotropical Cloud Forest, Costa Rica. Biotropica 1993, 25, 370. [Google Scholar] [CrossRef]
- Woods, C.L.; Maleta, K.; Ortmann, K. Plant–Plant Interactions Change during Succession on Nurse Logs in a Northern Temperate Rainforest. Ecol. Evol. 2021, 11, 9631–9641. [Google Scholar] [CrossRef]
- Fukasawa, Y.; Ando, Y. Species Effects of Bryophyte Colonies on Tree Seedling Regeneration on Coarse Woody Debris. Ecol. Res. 2018, 33, 191–197. [Google Scholar] [CrossRef]
- Zotz, G.; Schultz, S. The Vascular Epiphytes of a Lowland Forest in Panama—Species Composition and Spatial Structure. Plant Ecol. 2008, 195, 131–141. [Google Scholar] [CrossRef]
- Wang, X.; Long, W.; Schamp, B.S.; Yang, X.; Kang, Y.; Xie, Z.; Xiong, M. Vascular Epiphyte Diversity Differs with Host Crown Zone and Diameter, but Not Orientation in a Tropical Cloud Forest. PLoS ONE 2016, 11, e0158548. [Google Scholar] [CrossRef]
- Cardelús, C.L. Vascular Epiphyte Communities in the Inner-Crown of Hyeronima alchorneoides and Lecythis ampla at La Selva Biological Station, Costa Rica. Biotropica 2007, 39, 171–176. [Google Scholar] [CrossRef]
- Naranjo, C.; Iriondo, J.M.; Riofrio, M.L.; Lara-Romero, C. Evaluating the Structure of Commensalistic Epiphyte–Phorophyte Networks: A Comparative Perspective of Biotic Interactions. AoB Plants 2019, 11, plz011. [Google Scholar] [CrossRef] [PubMed]
- Burns, K.C. Meta-Community Structure of Vascular Epiphytes in a Temperate Rainforest. Botany 2008, 86, 1252–1259. [Google Scholar] [CrossRef]
- Taylor, A.; Burns, K. Epiphyte Community Development throughout Tree Ontogeny: An Island Ontogeny Framework. J. Veg. Sci. 2015, 26, 902–910. [Google Scholar] [CrossRef]
- Burns, K.C.; Zotz, G. A Hierarchical Framework for Investigating Epiphyte Assemblages: Networks, Meta-Communities, and Scale. Ecology 2010, 91, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Laube, S.; Zotz, G. Neither Host-Specific nor Random: Vascular Epiphytes on Three Tree Species in a Panamanian Lowland Forest. Ann. Bot. 2006, 97, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Wang, S.; Li, Y.; Chen, Y.; Zhao, H.; Wang, J.; Liu, F.; Yuan, Z. Importance of Bark Physicochemical Properties in an Epiphytic Bryophyte Community within a Temperate Deciduous Broadleaf Forest. Diversity 2023, 15, 688. [Google Scholar] [CrossRef]
- Tatsumi, S.; Ohgue, T.; Azuma, W.A.; Nishizawa, K. Bark Traits Affect Epiphytic Bryophyte Community Assembly in a Temperate Forest. Plant Ecol. 2023, 224, 1089–1095. [Google Scholar] [CrossRef]
- Gentry, A.H.; Dodson, C.H. Diversity and Biogeography of Neotropical Vascular Epiphytes. Ann. Mo. Bot. Gard. 1987, 74, 205–233. [Google Scholar] [CrossRef]

| Average % Cover on Trunk Structures | Average % Cover on Branches | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Code | Family | Life Form | Broken Branch | Burl | Hole | Rivet | Trunk | Top | Bottom | Side | |
| Antitrichia curtipendula | AntCur | Leucodontaceae | Moss | 0.00 | 25.45 | 0.00 | 0.00 | 7.88 | 0.00 | 0.00 | 33.33 | |
| Apometzgeria pubescens | ApoPub | Haplomitriaceae | Liverwort | 3.86 | 0.00 | 0.88 | 0.00 | 28.60 | 0.00 | 0.00 | 0.00 | |
| Claopodium crispifolium | ClaCri | Leskeaceae | Moss | 0.00 | 7.65 | 0.77 | 0.76 | 90.83 | 30.88 | 22.73 | 15.28 | |
| Dicranum fuscescens | DicFus | Dicranaceae | Moss | 8.33 | 0.00 | 0.00 | 0.00 | 58.33 | 0.00 | 0.00 | 81.22 | |
| Homalothecium fulgesens | HomFul | Brachytheciaceae | Moss | 0.00 | 8.33 | 0.00 | 0.00 | 25.00 | 0.00 | 0.00 | 0.00 | |
| Hylocomium slpendens | HylSlp | Hylocomiaceae | Moss | 27.83 | 13.04 | 1.38 | 0.00 | 24.42 | 0.00 | 0.00 | 0.00 | |
| Hypnum subimponens | HypSub | Hypnaceae | Moss | 3.27 | 7.74 | 0.00 | 0.00 | 88.99 | 31.25 | 0.00 | 18.75 | |
| Isothecium myosuroides | IsoMyo | Lembophyllaceae | Moss | 1.58 | 5.35 | 1.63 | 2.46 | 88.90 | 18.75 | 31.54 | 45.40 | |
| Kindbergia oregana | KinOre | Brachytheciaceae | Moss | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 33.33 | |
| Kindbergia praelonga | KinPra | Brachytheciaceae | Moss | 33.33 | 0.00 | 0.00 | 0.00 | 33.33 | 0.00 | 0.00 | 20.98 | |
| Lepraria spp. | Lep | Stereocaulaceae | Lichen | 1.24 | 3.25 | 0.24 | 0.00 | 95.27 | 0.00 | 0.00 | 33.33 | |
| Leucolepis acanthroneura | LeuAca | Mniaceae | Moss | 10.06 | 14.32 | 1.38 | 1.12 | 73.12 | 44.95 | 0.00 | 10.61 | |
| Metaneckera menziesii | MetMen | Neckeraceae | Moss | 0.05 | 3.40 | 0.28 | 1.47 | 94.80 | 11.29 | 25.75 | 59.76 | |
| Mnium spinulosum | MniSpi | Mniaceae | Moss | 0.00 | 0.00 | 0.00 | 0.00 | 33.33 | 0.00 | 0.00 | 0.00 | |
| Neckera douglasii | NecDou | Neckeraceae | Moss | 0.00 | 9.96 | 0.00 | 0.00 | 56.71 | 0.00 | 0.00 | 80.98 | |
| Plagiomnium venustrum | PlaVen | Mniaceae | Moss | 0.00 | 20.99 | 0.00 | 0.00 | 45.68 | 33.33 | 0.00 | 0.00 | |
| Polypodium glycyrrhiza | PolGly | Pteridaceae | Fern | 0.00 | 0.00 | 0.00 | 0.00 | 66.67 | 0.00 | 0.00 | 0.00 | |
| Porella navicularis | PorNav | Porellaceae | Liverwort | 5.46 | 16.81 | 2.47 | 0.13 | 75.13 | 30.73 | 11.08 | 39.29 | |
| Rhytidiadelphus loreus | RhyLor | Hylocomiaceae | Moss | 10.96 | 52.39 | 0.00 | 0.00 | 36.65 | 45.83 | 4.80 | 56.26 | |
| Rhytidiadelphus triquetrus | RhyTri | Hylocomiaceae | Moss | 0.00 | 0.00 | 0.00 | 0.00 | 33.33 | 0.00 | 0.00 | 12.01 | |
| Selaginella oregana | SelOre | Selaginellaceae | Lycophyte | 3.68 | 17.46 | 1.47 | 0.72 | 76.66 | 13.33 | 42.48 | 31.25 | |
| Scapania bolanderi | ScaBol | Scapaniaceae | Liverwort | 0.00 | 33.33 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamilton, K.M.; Woods, C.L. Diversity Begets Diversity: Structural Heterogeneity Determines Fine-Scale Epiphyte Community Structure in a Temperate Rainforest. Diversity 2024, 16, 484. https://doi.org/10.3390/d16080484
Hamilton KM, Woods CL. Diversity Begets Diversity: Structural Heterogeneity Determines Fine-Scale Epiphyte Community Structure in a Temperate Rainforest. Diversity. 2024; 16(8):484. https://doi.org/10.3390/d16080484
Chicago/Turabian StyleHamilton, Kaela M., and Carrie L. Woods. 2024. "Diversity Begets Diversity: Structural Heterogeneity Determines Fine-Scale Epiphyte Community Structure in a Temperate Rainforest" Diversity 16, no. 8: 484. https://doi.org/10.3390/d16080484
APA StyleHamilton, K. M., & Woods, C. L. (2024). Diversity Begets Diversity: Structural Heterogeneity Determines Fine-Scale Epiphyte Community Structure in a Temperate Rainforest. Diversity, 16(8), 484. https://doi.org/10.3390/d16080484






