Seasonal Phenotypic Variation in the Aeolian Wall Lizard, Podarcis raffonei, of the Capo Grosso (Vulcano) Population
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Hue
3.2. Saturation
3.3. Brightness
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Repeated-Measures ANOVAs | |||||
| 2017/18 | |||||
| Source | Sum of Squares | df | Mean sq. | F | Sig. |
| Intercept | 176,069.1 | 1 | 176,069.1 | 4751.502 | <0.001 |
| Sex | 89.6 | 1 | 89.6 | 2.418 | 0.129 |
| Error | 1296.9 | 35 | 37.1 | ||
| Month | 1949.6 | 2 | 974.8 | 68.012 | <0.001 |
| Month × Sex | 309.3 | 2 | 154.6 | 10.789 | <0.001 |
| Error | 1003.3 | 70 | 14.3 | ||
| 2017/18–2022 | |||||
| Source | Sum of Squares | df | Mean sq. | F | Sig. |
| Intercept | 43,571.68 | 1 | 43,571.68 | 3964.010 | <0.001 |
| Season | 338.35 | 2 | 169.18 | 15.391 | <0.001 |
| Error | 197.85 | 18 | 11.99 | ||
| Year | 2519.98 | 1 | 2519.98 | 255.474 | <0.001 |
| Year × Season | 278.57 | 2 | 139.28 | 14.120 | <0.001 |
| Error | 177.55 | 18 | 9.86 | ||
| Spring 2017-2021-2022 | |||||
| Source | Sum of Squares | df | Mean sq. | F | Sig. |
| Intercept | 20,331.86 | 1 | 20,331.86 | 921.495 | <0.001 |
| Sex | 1205.30 | 1 | 1205.30 | 54.627 | <0.001 |
| Error | 132.38 | 6 | 22.06 | ||
| Year | 481.71 | 2 | 240.86 | 20.783 | <0.001 |
| Year × Sex | 90.33 | 2 | 45.17 | 3.897 | 0.050 |
| Error | 139.07 | 12 | 11.59 | ||
| GLMs | |||||
| Source | Sum of Squares | df | Mean sq. | F | Sig. |
| Intercept | 0.11 | 1 | 0.11 | 0.001 | 0.973 |
| Sex | 1257.07 | 1 | 1257.07 | 13.739 | <0.001 |
| Status | 14.33 | 1 | 14.33 | 0.157 | 0.695 |
| Year | 351.52 | 1 | 351.52 | 3.842 | 0.059 |
| Size | 120.95 | 1 | 120.95 | 1.322 | 0.259 |
| Error | 2927.84 | 32 | 91.50 | ||
| Repeated-Measures ANOVAs | |||||
| 2017/18 | |||||
| Source | Sum of Squares | df | Mean sq. | F | Sig. |
| Intercept | 131,187.4 | 1 | 131,187.4 | 11,863.48 | <0.001 |
| Sex | 15.0 | 1 | 15.0 | 1.36 | 0.252 |
| Error | 387.0 | 35 | 11.1 | ||
| Month | 7645.0 | 2 | 3822.5 | 326.00 | <0.001 |
| Month × Sex | 45.1 | 2 | 22.6 | 1.92 | 0.154 |
| Error | 820.8 | 70 | 11.7 | ||
| 2017/18–2022 | |||||
| Source | Sum of Squares | df | Mean sq. | F | Sig. |
| Intercept | 52012.90 | 1 | 52012.90 | 4439.017 | <0.001 |
| Season | 448.54 | 2 | 224.27 | 19.140 | <0.001 |
| Error | 210.91 | 18 | 11.72 | ||
| Year | 124.30 | 1 | 124.30 | 17.386 | <0.001 |
| Year × Season | 667.59 | 2 | 333.79 | 46.686 | <0.001 |
| Error | 128.70 | 18 | 7.15 | ||
| Spring 2017–2022 | |||||
| Source | Sum of Squares | df | Mean sq. | F | Sig. |
| Intercept | 14,422.90 | 1 | 131,187.4 | 1483.638 | <0.001 |
| Sex | 14.42 | 1 | 14.42 | 1.484 | 0.269 |
| Error | 58.33 | 6 | 9.72 | ||
| Year | 1682.51 | 2 | 841.26 | 212.850 | <0.001 |
| Year × Sex | 59.17 | 2 | 29.59 | 7.486 | <0.001 |
| Error | 47.43 | 12 | 3.95 | ||
| GLMs | |||||
| Source | Sum of Squares | df | Mean sq. | F | Sig. |
| Intercept | 379.47 | 1 | 379.47 | 7.785 | 0.009 |
| Sex | 3.24 | 1 | 3.24 | 0.067 | 0.798 |
| Status | 0.77 | 1 | 0.77 | 0.016 | 0.901 |
| Year | 1971.83 | 1 | 1971.83 | 40.453 | <0.001 |
| Size | 57.73 | 1 | 57.73 | 1.184 | 0.285 |
| Repeated-Measures ANOVAs | |||||
| 2017/18 | |||||
| Source | Sum of Squares | df | Mean sq. | F | Sig. |
| Intercept | 111,221.6 | 1 | 111,221.6 | 6964.543 | <0.001 |
| Sex | 0.3 | 1 | 0.3 | 0.018 | 0.893 |
| Error | 558.9 | 35 | 16.0 | ||
| Month | 489.7 | 2 | 244.8 | 40.466 | <0.001 |
| Month × Sex | 273.1 | 2 | 136.5 | 22.568 | <0.001 |
| Error | 423.5 | 70 | 6.1 | ||
| 2017/18–2022 | |||||
| Source | Sum of Squares | df | Mean sq. | F | Sig. |
| Intercept | 41,981.66 | 1 | 41,981.66 | 6556.818 | <0.001 |
| Season | 65.46 | 2 | 32.73 | 5.112 | 0.017 |
| Error | 115.25 | 18 | 6.40 | ||
| Year | 1.16 | 1 | 1.16 | 0.416 | 0.527 |
| Year × Season | 466.46 | 2 | 233.23 | 83.452 | <0.001 |
| Error | 50.31 | 18 | 2.79 | ||
| Spring 2017–2022 | |||||
| Source | Sum of Squares | df | Mean sq. | F | Sig. |
| Intercept | 8994.485 | 1 | 8994.48 | 916.091 | <0.001 |
| Sex | 2.583 | 1 | 2.58 | 0.263 | 0.626 |
| Error | 58.910 | 6 | 9.82 | ||
| Year | 241.144 | 2 | 120.57 | 20.136 | <0.001 |
| Year×Sex | 2.569 | 2 | 1.28 | 0.215 | 0.810 |
| Error | 71.856 | 12 | 5.99 | ||
| GLMs | |||||
| Source | Sum of Squares | df | Mean sq. | F | Sig. |
| Intercept | 129.11 | 1 | 129.11 | 6.486 | 0.016 |
| Sex | 13.26 | 1 | 13.26 | 0.666 | 0.421 |
| Status | 75.40 | 1 | 75.40 | 3.787 | 0.060 |
| Year | 827.87 | 1 | 827.87 | 41.587 | <0.001 |
| Year × Status | 410.65 | 1 | 410.65 | 20.629 | <0.001 |
| Size | 28.52 | 1 | 28.52 | 1.433 | 0.240 |
References
- Cuthill, I.C.W.; Allen, L.; Arbuckle, K.; Caspers, B.; Chaplin, G.; Hauber, M.E.; Hill, G.E.; Jablonski, N.G.; Jiggins, C.D.; Kelber, A.; et al. The biology of color. Science 2017, 357, eaan0221. [Google Scholar] [CrossRef] [PubMed]
- Stokes, D.L. Things we like: Human preferences among similar organisms and implications for conservation. Hum. Ecol. 2007, 35, 361–369. [Google Scholar] [CrossRef]
- Prokop, P.; Fančovičová, J. Does colour matter? The influence of animal warning coloration on human emotions and willingness to protect them. Anim. Conserv. 2013, 16, 458–466. [Google Scholar] [CrossRef]
- Lifshitz, N.; St. Clair, C. Coloured ornamental traits could be effective and non-invasive indicators of pollution exposure for wildlife. Conserv. Physiol. 2016, 4, cow028. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Whiting, M.J.; Du, W.; Wu, Z.; Luo, S.; Yue, B.; Fu, J.; Qi, Y. Colour Variation in the Crocodile Lizard (Shinisaurus crocodilurus) and its Relationship to Individual Quality. Biology 2022, 11, 1314. [Google Scholar] [CrossRef] [PubMed]
- Mynott, S.; Daniels, C.; Widdicombe, S.; Stevens, M. Using camouflage for conservation: Colour change in juvenile European lobster. bioRxiv 2018. [Google Scholar] [CrossRef]
- Sherpa, S.; Salvi, D.; Silva-Rocha, I.; Capblancq, T.; Paris, J.R.; Carretero, M.A.; Ficetola, G.F. Reconstructing the complex colonisation histories of lizards across Mediterranean archipelagos. J. Biogeogr. 2024, 51, 157–172. [Google Scholar] [CrossRef]
- Capula, M. Genetic variation and differentiation in the lacertid lizard, Podarcis wagleriana (Reptilia: Lacertidae). Biol. J. Linn. Soc. 1994, 52, 177–196. [Google Scholar] [CrossRef]
- D’Amico, M.; Bastianelli, G.; Faraone, F.P.; Lo Valvo, M. The spreading of the invasive Italian wall lizard on Vulcano, the last island inhabited by the critically endangered Aeolia wall lizard. Herp. Con. Bio. 2018, 13, 146–157. [Google Scholar]
- Damas-Moreira, I.; Riley, J.L.; Harris, D.J.; Whiting, M.J. Can behaviour explain invasion success? A comparison between sympatric invasive and native lizards. Anim. Behav. 2019, 151, 195–202. [Google Scholar] [CrossRef]
- Downes, S.; Bauwens, D. An experimental demonstration of direct behavioural interference in two Mediterranean lacertid lizard species. Anim. Behav. 2002, 63, 1037–1046. [Google Scholar] [CrossRef]
- Herrel, A.; Huyghe, K.; Vanhooydonck, B.; Backeljau, T.; Breugelmans, K.; Grbac, I.; Van Damme, R.; Irschick, D.J. Rapid large-scale evolutionary divergence in morphology and performance associated with exploitation of a different dietary resource. Proc. Natl. Acad. Sci. USA 2008, 105, 4792–4795. [Google Scholar] [CrossRef] [PubMed]
- Rocha, I. Podarcis sicula. In Invasive Species Compendium; CAB International: Wallingford, UK, 2016. [Google Scholar] [CrossRef]
- Nevo, E.; Gorman, G.C.; Soulé, M.; Yang, E.J.; Clover, R.; Jovasnovic, V. Competitive exclusion between Insular Lacerta species (Sauria, Lacertidae). Oecologia 1972, 10, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, B.; Josic, P.; Buric, D.; Tkalec, M.; Lisicic, D.; Blazevic, S.A.; Hranilovic, D. Coexisting lacertid lizard species Podarcis siculus and Podarcis melisellensis differ in dopamine brain concerntrations. J. Comp. Physiol. 2019, 205, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Capula, M. Competitive exclusion between Podarcis lizards from Tyrrhenian islands: Inference from comparative specie distribution. In Proceedings of the IV Ordinary General Meeting of the Societas Europea Herpetologica, Budapest, Hungary, 19–23 August 1991; pp. 89–93. [Google Scholar]
- Caruso, Y.; Macale, D.; Luiselli, L.; Vignoli, L. Thermoregulation comparisons between a threatened native and an invasive lizard species. Herpetol. J. 2021, 31, 70–76. [Google Scholar] [CrossRef]
- Capula, M.; Lo Cascio, P. Podarcis raffonei (Mertens, 1952). In Fauna d’Italia, Reptilia, Edizioni Calderini de; Corti, C., Capula, M., Luiselli, L., Razzetti, E., Sindaco, R., Eds.; Il Sole 24 ORE: Bologna, Italy, 2011; pp. 401–407. [Google Scholar]
- Capula, M. Natural hybridization in Podarcis sícula and P. wagleriana (Reptilia: Lacertidae). Biochem. Syst. Ecol. 1993, 21, 373–380. [Google Scholar] [CrossRef]
- Storniolo, F.; Zuffi, M.A.L.; Coladonato, A.J.; Di Vozzo, L.; Giglio, G.; Gini, A.E.; Leonetti, F.L.; Luccini, S.; Mangiacotti, M.; Scali, S.; et al. Patterns of variations in dorsal colouration of the Italian wall lizard Podarcis siculus. Biol. Open 2021, 10, bio058793. [Google Scholar] [CrossRef] [PubMed]
- Pellitteri-Rosa, D.; Gazzola, A.; Todisco, S.; Mastropasqua, F.; Liuzzi, C. Lizard colour plasticity tracks background seasonal changes. Biol. Open 2020, 9, bio052415. [Google Scholar] [CrossRef] [PubMed]
- Ortega, J.; Marti’N, J.; Crochet, P.-A.; LóPez, P.; Clobert, J. Seasonal and interpopulational phenotypic variation in morphology and sexual signals of Podarcis liolepis lizards. PLoS ONE 2019, 14, e0211686. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Silva-Rocha, I.; Carretero, M.A.; Vignoli, L.; Sacchi, R.; Melotto, A.; Scali, S.; Salvi, D. Status of the largest extant population of the critically endangered Aeolian lizard Podarcis raffonei (Capo Grosso, Vulcano island). PLoS ONE 2021, 16, e0253631. [Google Scholar] [CrossRef]
- Paris, J.R.; Ficetola, G.F.; Ferrer Obiol, J.; Silva-Rocha, I.; Carretero, M.A.; Salvi, D. Does hybridisation with an invasive species threaten Europe’s most endangered reptile? Genomic assessment of Aeolian lizards on Vulcano island. bioRxiv 2024. [Google Scholar] [CrossRef]
- Corti, C.; Lo Cascio, P. I lacertidi italiani. In Mediterraneo, Guide Naturalistiche; L’Epos: Palermo, Italy, 1999; Volume 10. [Google Scholar]
- Stevens, M.; Párraga, C.A.; Cuthill, I.C.; Partridge, J.C.; Troscianko, T.S. Using digital photography to study animal coloration. Biol. J. Linn. Soc. 2007, 90, 211–237. [Google Scholar] [CrossRef]
- Smith, K.R.; Cadena, V.; Endler, J.A.; Kearney, M.R.; Porter, W.P.; Stuart-Fox, D. Color change for thermoregulation versus camouflage in free ranging lizards. Am. Nat. 2016, 188, 668–678. [Google Scholar] [CrossRef]
- Guo, K.; Zhong, J.; Zhu, L.; Xie, F.; Du, Y.; Ji, X. The thermal dependence and molecular basis of physiological color change in Takydromus septentrionalis (Lacertidae). Biol. Open 2021, 10, bio058503. [Google Scholar] [CrossRef]
- Datacolor. SpyderCHECKR Camera Color Correction for Photo and Video. Version 1.6. 2022. Available online: https://www.datacolor.com/spyder/ (accessed on 16 July 2024).
- Rasband, W.S. ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, 1997–2018. Available online: https://imagej.net/ij/ (accessed on 16 July 2024).
- Chernov, V.; Alander, J.; Bochko, V. Integer-based accurate conversion between RGB and HSV color spaces. Comput. Electr. Eng. 2015, 46, 328–337. [Google Scholar] [CrossRef]
- Burger, W.; Burge, M.J. Principles of Digital Image Processing—Fundamental Techniques; Springer: London, UK, 2009; pp. 202–207. [Google Scholar]
- StatSoft, Inc. STATISTICA (Data Analysis Software System), Version 12. 2014. Available online: www.statsoft.com (accessed on 16 July 2024).
- Faraone, F.P.; Lo Valvo, M. Seasonal variation in colour of the Sicilian wall lizard Podarcis wagleriana. In Proceedings of the Riassunti del 6° Congresso Nazionale della Societas Herpetologica Italica (SHI), Rome, Italy, 27 September–1 October 2006; p. 25. [Google Scholar]
- Galán, P. Cambios estacionales de coloración y comportamiento agonístico, de cortejo y de apareamiento en el lacértido Podarcis bocagei. Rev. Esp. Herp. 1995, 9, 57–75. [Google Scholar]
- Sacchi, R.; Scali, S.; Mangiacotti, M.; Ruffo, D. Colour variation of the Maltese wall lizards (Podarcis filfolensis) at population and individual levels in the Linosa island. Rend. Lincei Sci. Fis. Nat. 2021, 32, 565–575. [Google Scholar] [CrossRef]
- Galán, P. Ontogenetic and sexual variation in the coloration of the lacertid lizards Iberolacerta monticola and Podarcis bocagei. Do the females prefer the greener males? Anim. Biol. 2008, 58, 173–198. [Google Scholar] [CrossRef]
- Merilaita, S.; Stevens, M. Crypsis through Background Matching. In Animal Camouflage: Mechanisms and Function; Stevens, M., Merilaita, S., Eds.; Cambridge University Press: Cambridge, UK, 2017; pp. 17–33. [Google Scholar]
- Olsson, M.; Stuart-Fox, D.; Ballen, C. Genetics and evolution of colour patterns in reptiles. Semin. Cell Dev. Biol. 2013, 24, 529–541. [Google Scholar]
- Lo Cascio, P.; Sciberras, A. “Cold-blooded” travellers around Sicily and Recent herpetofauna of circum-Sicilian and Maltese Islands. In Life on Islands. 1 Biodiversity in Sicily and Surrounding Islands; La Mantia, T., Badalamenti, E., Carapezza, A., Lo Cascio, P., Troia, A., Eds.; Edizioni Danaus: Palermo, Italy, 2020; pp. 368–369. [Google Scholar]

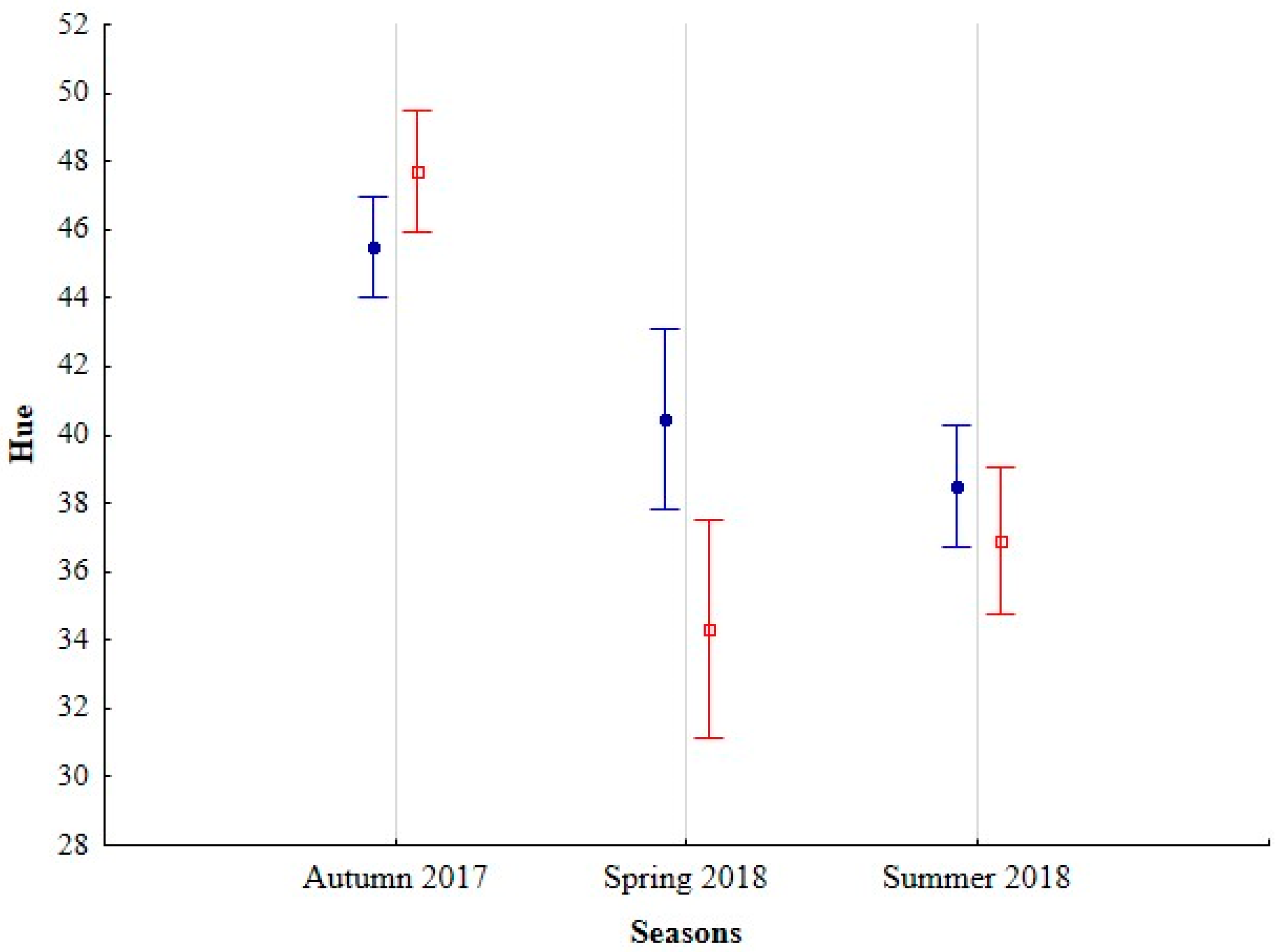
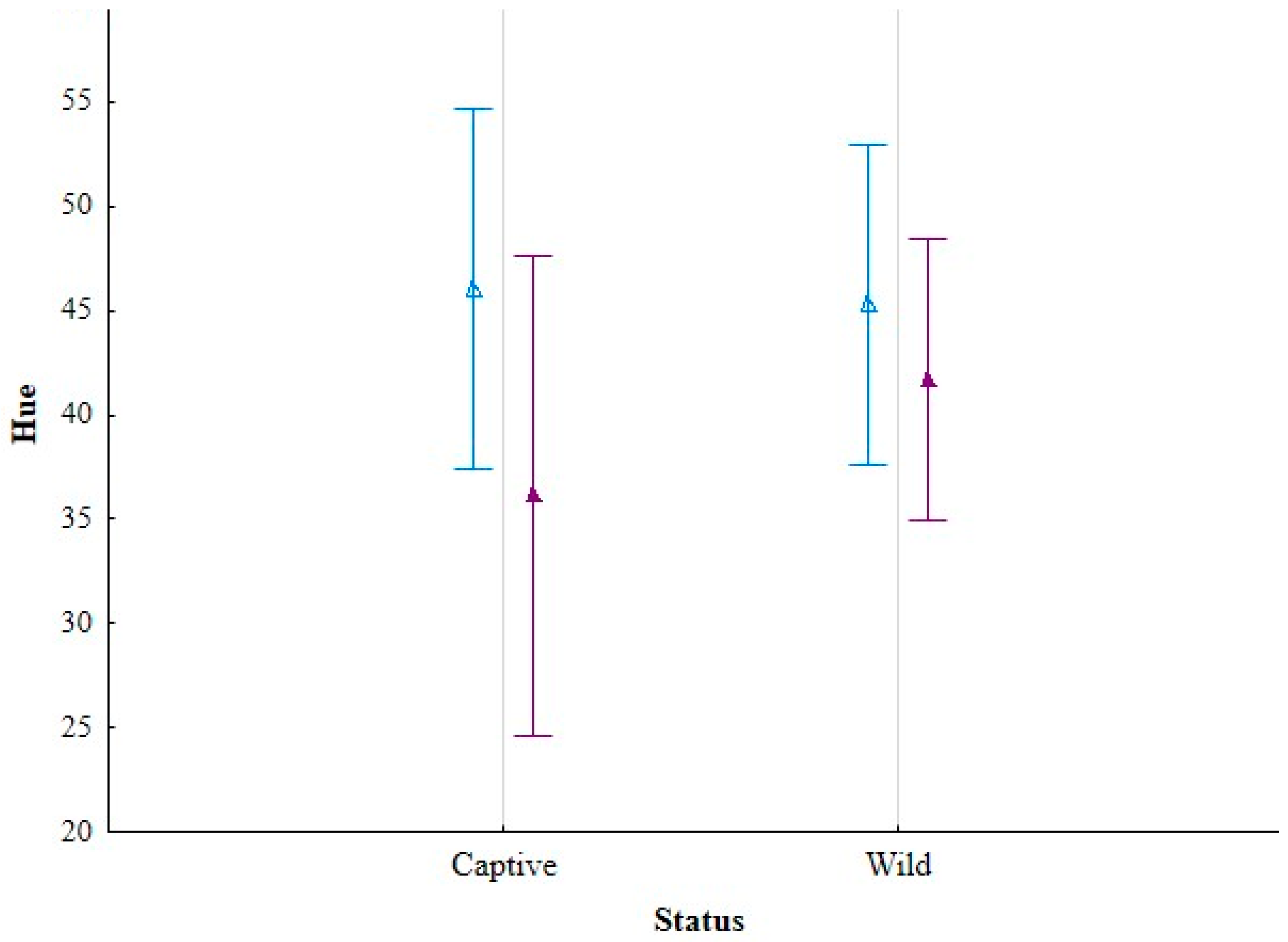


| Hue | Saturation | Brightness | |
|---|---|---|---|
| r-mANOVA 2017/18 | Season | Season | Season |
| Season × Sex | Season × Sex | ||
| r-mANOVA 2017/18–2022 | Year | Year | Season |
| Season | Season | ||
| Year × Season | Year × Season | Year × Season | |
| r-mANOVA Spring 2017–2021–2022 | Year | Year | Year |
| Sex | |||
| GLM Spring 2021–2022 Captive and Wild | Sex | Year | Year |
| Year × Status |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gambioli, B.; Macale, D.; Vignoli, L. Seasonal Phenotypic Variation in the Aeolian Wall Lizard, Podarcis raffonei, of the Capo Grosso (Vulcano) Population. Diversity 2024, 16, 485. https://doi.org/10.3390/d16080485
Gambioli B, Macale D, Vignoli L. Seasonal Phenotypic Variation in the Aeolian Wall Lizard, Podarcis raffonei, of the Capo Grosso (Vulcano) Population. Diversity. 2024; 16(8):485. https://doi.org/10.3390/d16080485
Chicago/Turabian StyleGambioli, Benedetta, Daniele Macale, and Leonardo Vignoli. 2024. "Seasonal Phenotypic Variation in the Aeolian Wall Lizard, Podarcis raffonei, of the Capo Grosso (Vulcano) Population" Diversity 16, no. 8: 485. https://doi.org/10.3390/d16080485
APA StyleGambioli, B., Macale, D., & Vignoli, L. (2024). Seasonal Phenotypic Variation in the Aeolian Wall Lizard, Podarcis raffonei, of the Capo Grosso (Vulcano) Population. Diversity, 16(8), 485. https://doi.org/10.3390/d16080485






