Prioritising Ex Situ Conservation for Malagasy Mammal Species in Line with IUCN’s ‘One Plan Approach to Conservation’
Abstract
1. Introduction
2. Materials and Methods
2.1. Species List and Threat Status
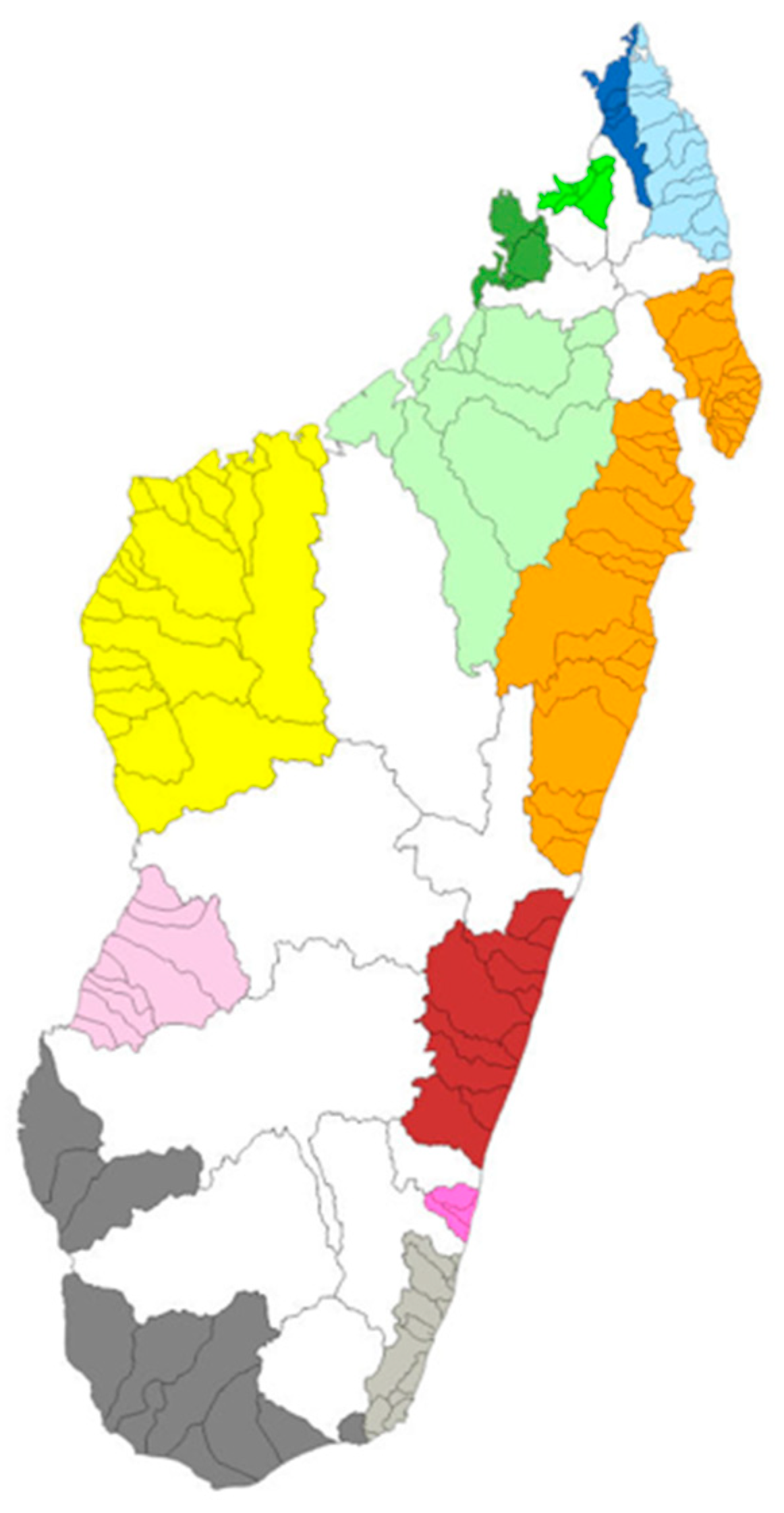
2.2. Distribution
2.3. ZIMS and “Zootierliste”
2.4. CITES
2.5. EDGE Score
2.6. Prioritization
2.7. Protected Area and Key Biodiversity Area Coverage
3. Results
3.1. Species List and Threat Status
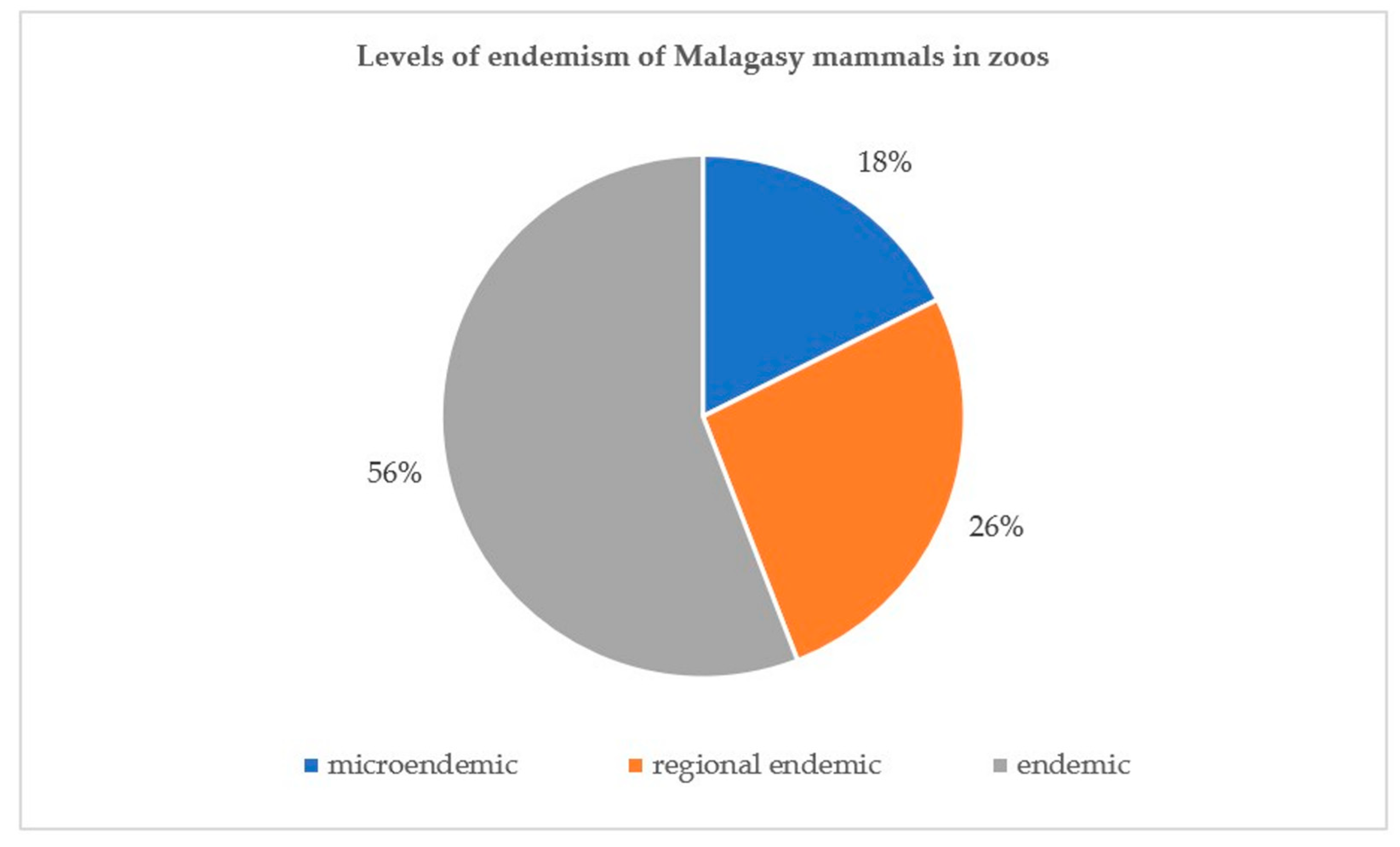
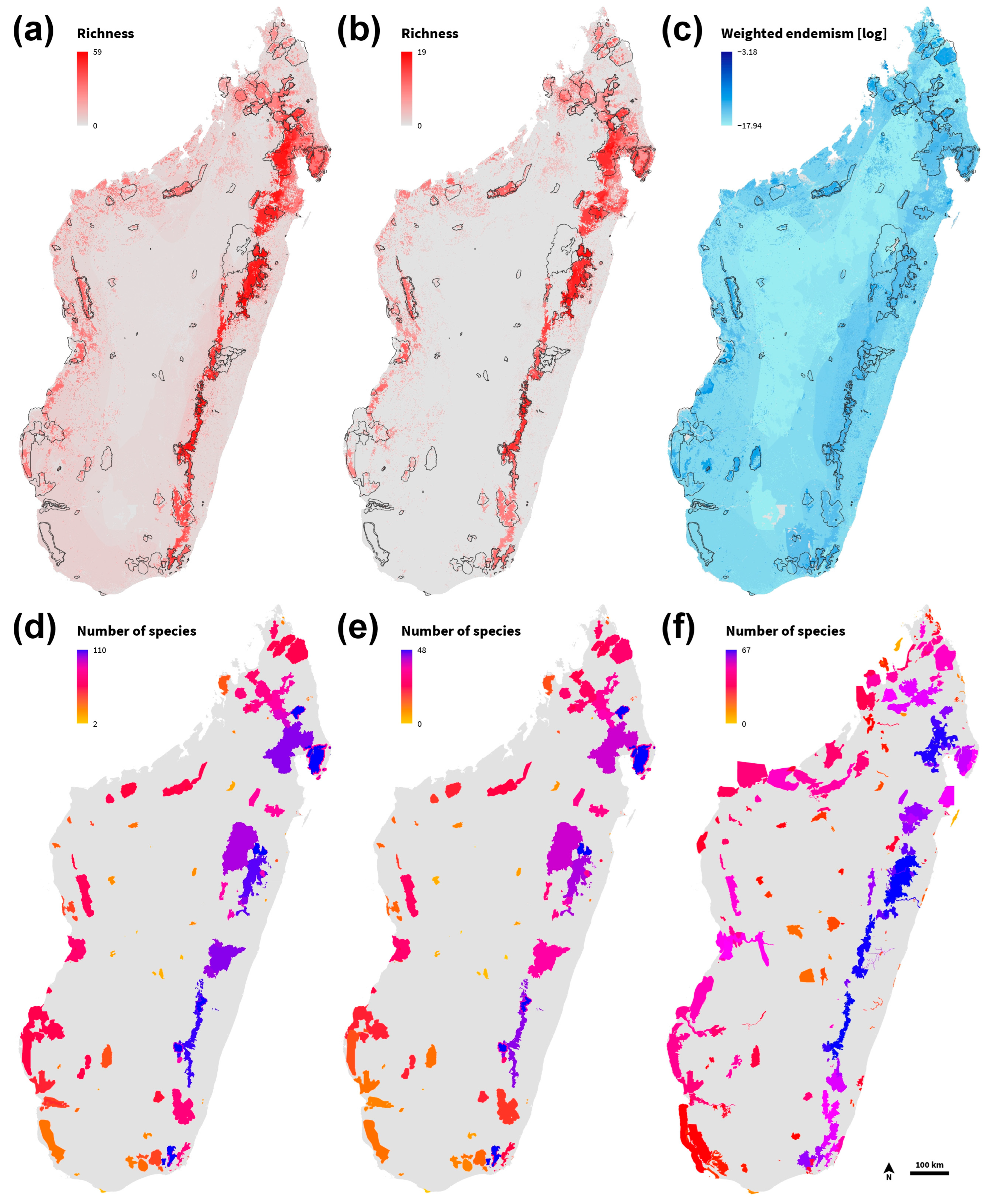
3.2. Distribution
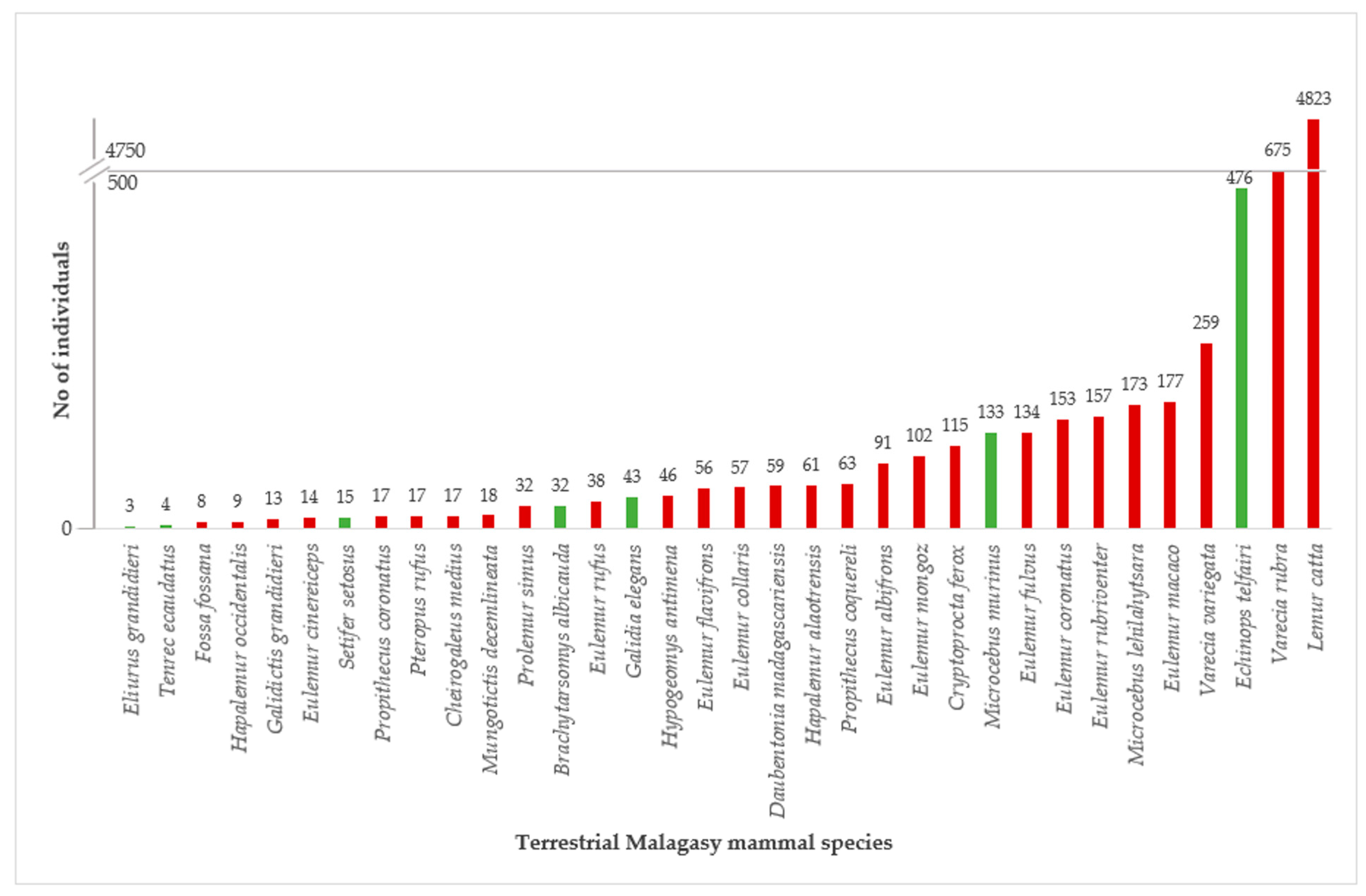
3.3. ZIMS and “Zootierliste”
3.4. CITES
3.5. EDGE of Existence Programme
3.6. Prioritization
3.7. Protected Area and Key Biodiversity Area Coverage
4. Discussion
5. Recommendations
- Include overlooked threatened taxa into breeding programs, such as small-bodied and nocturnal species, e.g., Nesomyidae, Cheirogaleus spp., Lepilemur spp. This is easiest for threatened species where historic expertise exists (e.g., Mirza coquereli, Phaner furcifer) or where similar, often closely related, less threatened species are kept in zoos but could be exchanged with their threatened counterparts (e.g., Echinops telfairi with threatened members of Tenrecidae).
- Aim to increase the number of individuals for threatened species that are prioritized for breeding but currently kept in very small populations. Examples are Prolemur simus, Eulemur mongoz, and many other lemur species.
- Reduce the number of non-threatened species or the number of individuals of commonly kept threatened species. Examples of the latter are Lemur catta and Varecia rubra.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Family Species | Threat Status | Population Trend | Tot. Ind. | Tot.M | Tot.F | Tot. O | Tot. Inst. | No. of Births | Tot. Breeding Inst. | EEP/ESB/SSP | Species Kept | From (year) | Until (year) | ZIMS/ZTL | EDGE | EDGE Rank | CITES |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cheirogaleoidae | |||||||||||||||||
| Allocebus trichotis | EN | ↓ | past | 1991 | 2002 | ZIMS | 4.31 | 307 | I | ||||||||
| Cheirogaleus crossleyi | VU | ↓ | past | 1967 | 1971 | ZIMS | I | ||||||||||
| Cheirogaleus major | VU | ↓ | past | 1978 | 1993 | ZIMS | I | ||||||||||
| Cheirogaleus medius | VU | ↓ | 17 | 5 | 2 | 10 | 1 | 3 | 1 | today | 1965 | ZIMS | I | ||||
| Microcebus lehilahytsara | NT | ↓ | 173 | 89 | 83 | 1 | 7 | 28 | 2 | EEP | today | 2005 | ZIMS | 3.75 | 452 | I | |
| Microcebus murinus | LC | ↓ | 133 | 65 | 65 | 3 | 28 | 21 | 4 | EEP/SSP | today | 1967 | ZIMS | I | |||
| Microcebus myoxinus | VU | ↓ | past | 1910 | 1911 | ZIMS | 3.7 | 471 | I | ||||||||
| Microcebus rufus | VU | ↓ | past | 1973 | 2005 | ZIMS | 3.79 | 441 | I | ||||||||
| Mirza coquereli | EN | ↓ | past | 1986 | 2012 | ZIMS | 5.11 | 101 | I | ||||||||
| Mirza zaza | VU | ↓ | past | 1982 | 2012 | ZIMS | I | ||||||||||
| Phaner furcifer | EN | ↓ | past | 1986 | 1996 | ZIMS | 4.01 | 394 | I | ||||||||
| Daubentoniidae | I | ||||||||||||||||
| Daubentonia madagascariensis | EN | ↓ | 59 | 28 | 29 | 2 | 15 | 4 | 4 | EEP/SSP | today | 1862 | ZIMS | 20.13 | 2 | I | |
| Eupleridae | |||||||||||||||||
| Cryptoprocta ferox | VU | ↓ | 115 | 64 | 51 | 0 | 55 | 13 | 4 | EEP/SSP | today | 1954 | ZIMS | 4.93 | 143 | II | |
| Fossa fossana | VU | ↓ | 8 | 6 | 2 | 0 | 3 | 2 | 2 | today | 1966 | ZIMS | 4.61 | 198 | II | ||
| Galidia elegans | LC | ↓ | 43 | 20 | 20 | 3 | 13 | 8 | 4 | ESB | today | 1966 | ZIMS | ||||
| Galidictis fasciata | VU | ↓ | past | 1905 | 1963 | ZTL | 4.2 | 366 | |||||||||
| Galidictis grandidieri | EN | ↓ | 13 | 8 | 5 | 0 | 4 | 0 | 0 | today | 2017 | ZIMS | 4.89 | 149 | |||
| Mungotictis decemlineata | EN | ↓ | 18 | 7 | 11 | 0 | 9 | 1 | 1 | ESB | today | 1997 | ZIMS | 4.89 | 151 | ||
| Salanoia concolor | VU | ↓ | past | 1902 | 1913 | ZTL | 4.22 | 364 | |||||||||
| Indriidae | |||||||||||||||||
| Indri indri | CR | ↓ | past | 1965 | 1965 | ZIMS | 10.44 | 16 | I | ||||||||
| Propithecus coquereli | CR | ↓ | 63 | 29 | 33 | 1 | 15 | 12 | 4 | EEP/SSP | today | 1962 | ZIMS | 4.52 | 218 | I | |
| Propithecus coronatus | CR | ↓ | 17 | 12 | 5 | 0 | 6 | 3 | 3 | EEP | today | 1987 | ZIMS | 4.56 | 208 | I | |
| Propithecus diadema | CR | ↓ | past | 1993 | 2012 | ZIMS | 5.23 | 72 | I | ||||||||
| Propithecus tattersalli | CR | ↓ | past | 1987 | 2008 | ZIMS | 5.31 | 58 | I | ||||||||
| Propithecus verreauxi | CR | ↓ | past | 1984 | 2002 | ZIMS | 4.50 | 225 | I | ||||||||
| Lemuridae | |||||||||||||||||
| Eulemur albifrons | VU | ↓ | 91 | 48 | 39 | 4 | 31 | 5 | 2 | SSP | today | 1969 | ZIMS | 4.23 | 354 | I | |
| Eulemur cinereiceps | CR | ↓ | 14 | 7 | 7 | 0 | 5 | 0 | 0 | today | 2002 | ZIMS | I | ||||
| Eulemur collaris | EN | ↓ | 57 | 30 | 26 | 1 | 21 | 3 | 3 | SSP | today | 1962 | ZIMS | 4.23 | 355 | I | |
| Eulemur coronatus | EN | ↓ | 153 | 82 | 69 | 2 | 50 | 10 | 7 | EEP/SSP | today | 1955 | ZIMS | 4.42 | 265 | I | |
| Eulemur flavifrons | CR | ↓ | 56 | 28 | 28 | 0 | 20 | 7 | 4 | EEP/SSP | today | 1985 | ZIMS | 5.00 | 124 | I | |
| Eulemur fulvus | VU | ↓ | 134 | 71 | 60 | 3 | 45 | 3 | 3 | SSP | today | 1972 | ZIMS | I | |||
| Eulemur macaco | EN | ↓ | 177 | 89 | 87 | 1 | 67 | 16 | 13 | EEP | today | 1904 | ZIMS | 3.70 | 469 | I | |
| Eulemur mongoz | CR | ↓ | 102 | 60 | 39 | 3 | 33 | 12 | 9 | EEP/SSP | today | 1898 | ZIMS | 5.07 | 110 | I | |
| Eulemur rubriventer | VU | ↓ | 157 | 85 | 70 | 2 | 53 | 4 | 4 | EEP | today | 1925 | ZIMS | 3.68 | 478 | I | |
| Eulemur rufus | VU | ↓ | 62 | 29 | 31 | 2 | 28 | 5 | 3 | SSP | today | 1963 | ZIMS | 3.55 | 551 | I | |
| Eulemur sanfordi | EN | ↓ | past | 1969 | 2015 | ZIMS | 4.25 | 346 | I | ||||||||
| Hapalemur alaotrensis | CR | ↓ | 61 | 34 | 24 | 3 | 16 | 6 | 3 | EEP | today | 1985 | ZIMS | 5.38 | 48 | I | |
| Hapalemur aureus | CR | ↓ | past | 1988 | 1995 | ZIMS | 5.41 | 47 | I | ||||||||
| Hapalemur griseus | VU | ↓ | past | 1962 | 2022 | ZIMS | 3.99 | 399 | I | ||||||||
| Hapalemur occidentalis | VU | ↓ | 9 | 6 | 3 | 0 | 4 | 1 | 1 | today | 1991 | ZIMS | 3.99 | 401 | I | ||
| Lemur catta | EN | ↓ | 4823 | 2415 | 2023 | 385 | 555 | 369 | 113 | EEP/SSP | today | 1961 | ZIMS | 4.76 | 167 | I | |
| Prolemur simus | CR | ↓ | 32 | 10 | 20 | 2 | 10 | 3 | 2 | EEP | today | 1987 | ZIMS | 5.33 | 54 | I | |
| Varecia rubra | CR | ↓ | 675 | 358 | 307 | 10 | 191 | 32 | 15 | EEP/SSP | today | 1983 | ZIMS | 11.31 | 13 | I | |
| Varecia variegata | CR | ↓ | 259 | 133 | 95 | 14 | 82 | 11 | 5 | EEP/SSP | today | 1989 | ZIMS | 11.29 | 14 | I | |
| Lepilemuridae | |||||||||||||||||
| Lepilemur mustelinus | VU | ↓ | past | 1969 | 1973 | ZIMS | I | ||||||||||
| Lepilemur ruficaudatus | CR | ↓ | past | 1986 | 1993 | ZIMS | I | ||||||||||
| Nesomyidae | |||||||||||||||||
| Brachytarsomys albicauda | LC | ? | 32 | 9 | 13 | 10 | 6 | 17 | 3 | today | 2018 | ZIMS | |||||
| Eliurus grandidieri | LC | ? | 3 | 1 | 2 | 0 | 1 | 0 | 0 | today | 2008 | ZIMS | |||||
| Eliurus myoxinus | LC | ? | past | 1967 | 1967 | ZIMS | |||||||||||
| Hypogeomys antimena | CR | ? | 46 | 21 | 23 | 2 | 15 | 8 | 5 | today | 1990 | ZIMS | 4.94 | 139 | |||
| Pteropodidae | |||||||||||||||||
| Pteropus rufus | VU | ↓ | 17 | 5 | 2 | 10 | 1 | 3 | 1 | today | 2020 | ZIMS | II | ||||
| Tenrecidae | |||||||||||||||||
| Echinops telfairi | LC | – | 476 | 198 | 187 | 91 | 143 | 42 | 9 | SSP | today | 1975 | ZIMS | ||||
| Hemicentetes nigriceps | LC | ? | past | 1966 | 2022 | ZIMS | |||||||||||
| Hemicentetes semispinosus | LC | ? | past | 1965 | 2003 | ZIMS | |||||||||||
| Microgale dobsoni | LC | ↓ | past | 1966 | 1970 | ZIMS | |||||||||||
| Microgale talazaci | LC | ↓ | past | 1966 | 1978 | ZIMS | |||||||||||
| Microgale thomasi | LC | ↓ | past | 1966 | 1969 | ZIMS | |||||||||||
| Setifer setosus | LC | – | 15 | 6 | 4 | 5 | 8 | 0 | 0 | today | 1966 | ZIMS | |||||
| Tenrec ecaudatus | LC | – | 4 | 4 | 0 | 0 | 4 | 0 | 0 | today | 1900 | ZIMS |
Appendix B
| Family Species | Species Kept | Endemism | IUCN | EDGE Score |
|---|---|---|---|---|
| Cheirogaleidae | ||||
| Microcebus gerpi | no | microendemic | CR | 5.14 |
| Microcebus berthae | no | microendemic | CR | 4.45 |
| Microcebus manitatra | no | microendemic | CR | 0.00 |
| Microcebus mamiratra | no | microendemic | EN | 5.14 |
| Phaner parienti | no | microendemic | EN | 4.73 |
| Microcebus bongolavensis | no | microendemic | EN | 4.45 |
| Microcebus jollyae | no | microendemic | EN | 4.45 |
| Microcebus margotmarshae | no | microendemic | EN | 4.45 |
| Microcebus sambiranensis | no | microendemic | EN | 4.45 |
| Microcebus simmonsi | no | microendemic | EN | 4.45 |
| Cheirogaleus lavasoensis | no | microendemic | EN | 0.00 |
| Cheirogaleus thomasi | no | microendemic | EN | 0.00 |
| Microcebus ganzhorni | no | microendemic | EN | 0.00 |
| Microcebus jonahi | no | microendemic | EN | 0.00 |
| Microcebus tanosi | no | microendemic | EN | 0.00 |
| Cheirogaleus andysabini | no | microendemic | EN | 0.00 |
| Microcebus danfossi | no | microendemic | VU | 4.45 |
| Microcebus ravelobensis | no | microendemic | VU | 4.45 |
| Microcebus rufus | no | microendemic | VU | 3.79 |
| Microcebus myoxinus | no | microendemic | VU | 3.70 |
| Microcebus griseorufus | no | microendemic | LC | 0.00 |
| Cheirogaleus grovesi | no | microendemic | DD | 0.00 |
| Microcebus boraha | no | microendemic | DD | 0.00 |
| Cheirogaleus sibreei | no | regional endemic | CR | 5.68 |
| Phaner electromontis | no | regional endemic | EN | 4.70 |
| Allocebus trichotis | no | regional endemic | EN | 4.31 |
| Phaner furcifer | no | regional endemic | EN | 4.01 |
| Cheirogaleus shethi | no | regional endemic | EN | 0.00 |
| Microcebus tavaratra | no | regional endemic | VU | 3.75 |
| Cheirogaleus crossleyi | no | regional endemic | VU | 0.00 |
| Cheirogaleus major | no | regional endemic | VU | 0.00 |
| Microcebus marohita | no | endemic | CR | 5.14 |
| Mirza coquereli | no | endemic | EN | 5.11 |
| Phaner pallescens | no | endemic | EN | 4.72 |
| Microcebus macarthurii | no | endemic | EN | 4.45 |
| Microcebus arnholdi | no | endemic | VU | 4.45 |
| Mirza zaza | no | endemic | VU | 0.00 |
| Microcebus lehilahytsara | yes | regional endemic | NT | 3.75 |
| Cheirogaleus medius | yes | endemic | VU | 0.00 |
| Microcebus murinus | yes | endemic | LC | 0.00 |
| Daubentoniidae | ||||
| Daubentonia madagascariensis | yes | endemic | EN | 20.13 |
| Emballonuridae | ||||
| Paremballonura atrata | no | endemic | LC | 0.00 |
| Paremballonura tiavato | no | endemic | LC | 0.00 |
| Coleura kibomalandy | no | endemic | DD | 0.00 |
| Eupleridae | ||||
| Salanoia concolor | no | microendemic | VU | 4.22 |
| Eupleres major | no | endemic | EN | 5.30 |
| Eupleres goudotii | no | endemic | VU | 4.61 |
| Galidictis fasciata | no | endemic | VU | 4.20 |
| Galidictis grandidieri | yes | microendemic | EN | 4.89 |
| Mungotictis decemlineata | yes | regional endemic | EN | 4.89 |
| Cryptoprocta ferox | yes | endemic | VU | 4.93 |
| Fossa fossana | yes | endemic | VU | 4.61 |
| Galidia elegans | yes | endemic | LC | 0.00 |
| Hipposideridae | ||||
| Paratriaenops auritus | no | regional endemic | VU | 4.08 |
| Macronycteris commersoni | no | endemic | NT | 0.00 |
| Paratriaenops furcula | no | widespread | LC | 0.00 |
| Indriidae | ||||
| Propithecus tattersalli | no | microendemic | CR | 5.31 |
| Propithecus candidus | no | microendemic | CR | 5.25 |
| Propithecus perrieri | no | microendemic | CR | 5.21 |
| Avahi cleesei | no | microendemic | CR | 4.63 |
| Avahi unicolor | no | microendemic | CR | 4.63 |
| Propithecus deckenii | no | microendemic | CR | 4.50 |
| Avahi betsileo | no | microendemic | EN | 4.63 |
| Avahi mooreorum | no | microendemic | EN | 4.63 |
| Propithecus edwardsi | no | microendemic | EN | 4.54 |
| Avahi occidentalis | no | microendemic | VU | 4.60 |
| Avahi peyrierasi | no | microendemic | VU | 3.94 |
| Indri Indri | no | regional endemic | CR | 10.44 |
| Propithecus diadema | no | regional endemic | CR | 5.23 |
| Propithecus verreauxi | no | regional endemic | CR | 4.50 |
| Avahi meridionalis | no | regional endemic | EN | 4.63 |
| Avahi ramanantsoavanai | no | regional endemic | VU | 3.94 |
| Avahi laniger | no | endemic | VU | 3.94 |
| Propithecus coronatus | yes | microendemic | CR | 4.56 |
| Propithecus coquereli | yes | microendemic | CR | 4.52 |
| Lemuridae | ||||
| Hapalemur aureus | no | microendemic | CR | 5.41 |
| Eulemur sanfordi | no | regional endemic | EN | 4.25 |
| Hapalemur meridionalis | no | endemic | VU | 4.01 |
| Hapalemur griseus | no | endemic | VU | 3.99 |
| Eulemur rufifrons | no | endemic | VU | 0.00 |
| Hapalemur alaotrensis | yes | microendemic | CR | 5.38 |
| Eulemur flavifrons | yes | microendemic | CR | 5.00 |
| Varecia rubra | yes | regional endemic | CR | 11.31 |
| Prolemur simus | yes | regional endemic | CR | 5.33 |
| Eulemur mongoz | yes | regional endemic | CR | 5.07 |
| Eulemur coronatus | yes | regional endemic | EN | 4.42 |
| Eulemur collaris | yes | regional endemic | EN | 4.23 |
| Eulemur macaco | yes | regional endemic | EN | 3.70 |
| Eulemur rufus | yes | regional endemic | VU | 3.55 |
| Varecia variegata | yes | endemic | CR | 11.29 |
| Eulemur cinereiceps | yes | endemic | CR | 0.00 |
| Lemur catta | yes | endemic | EN | 4.76 |
| Eulemur albifrons | yes | endemic | VU | 4.23 |
| Hapalemur occidentalis | yes | endemic | VU | 3.99 |
| Eulemur rubriventer | yes | endemic | VU | 3.68 |
| Eulemur fulvus | yes | endemic | VU | 0.00 |
| Lepilemuridae | ||||
| Lepilemur septentrionalis | no | microendemic | CR | 4.96 |
| Lepilemur ahmansoni | no | microendemic | CR | 0.00 |
| Lepilemur grewcockorum | no | microendemic | CR | 0.00 |
| Lepilemur hollandorum | no | microendemic | CR | 0.00 |
| Lepilemur jamesorum | no | microendemic | CR | 0.00 |
| Lepilemur ruficaudatus | no | microendemic | CR | 0.00 |
| Lepilemur sahamalaza | no | microendemic | CR | 0.00 |
| Lepilemur tymerlachsoni | no | microendemic | CR | 0.00 |
| Lepilemur aeeclis | no | microendemic | EN | 0.00 |
| Lepilemur betsileo | no | microendemic | EN | 0.00 |
| Lepilemur dorsalis | no | microendemic | EN | 0.00 |
| Lepilemur edwardsi | no | microendemic | EN | 0.00 |
| Lepilemur fleuretae | no | microendemic | EN | 0.00 |
| Lepilemur hubbardorum | no | microendemic | EN | 0.00 |
| Lepilemur leucopus | no | microendemic | EN | 0.00 |
| Lepilemur microdon | no | microendemic | EN | 0.00 |
| Lepilemur otto | no | microendemic | EN | 0.00 |
| Lepilemur petteri | no | microendemic | EN | 0.00 |
| Lepilemur randrianasoloi | no | microendemic | EN | 0.00 |
| Lepilemur scottorum | no | microendemic | EN | 0.00 |
| Lepilemur wrightae | no | microendemic | EN | 0.00 |
| Lepilemur mustelinus | no | microendemic | VU | 0.00 |
| Lepilemur ankaranensis | no | regional endemic | EN | 4.25 |
| Lepilemur milanoii | no | regional endemic | EN | 0.00 |
| Lepilemur seali | no | regional endemic | VU | 0.00 |
| Miniopteridae | ||||
| Miniopterus ambohitrensis | no | microendemic | LC | 0.00 |
| Miniopterus griffithsi | no | microendemic | DD | 0.00 |
| Miniopterus egeri | no | regional endemic | LC | 0.00 |
| Miniopterus mahafaliensis | no | regional endemic | LC | 0.00 |
| Miniopterus petersoni | no | regional endemic | DD | 0.00 |
| Miniopterus brachytragos | no | endemic | LC | 0.00 |
| Miniopterus gleni | no | endemic | LC | 0.00 |
| Miniopterus majori | no | endemic | LC | 0.00 |
| Miniopterus sororculus | no | endemic | LC | 0.00 |
| Miniopterus aelleni | no | widespread | LC | 0.00 |
| Miniopterus manavi | no | widespread | LC | 0.00 |
| Molossidae | ||||
| Mops atsinanana | no | endemic | LC | 0.00 |
| Mops jobimena | no | endemic | LC | 0.00 |
| Mops leucostigma | no | endemic | LC | 0.00 |
| Mormopterus jugularis | no | endemic | LC | 0.00 |
| Otomops madagascariensis | no | endemic | LC | 0.00 |
| Mops leucogaster | no | widespread | LC | 0.00 |
| Myzopodidae | ||||
| Myzopoda schliemanni | no | regional endemic | LC | 0.00 |
| Myzopoda aurita | no | endemic | LC | 0.00 |
| Nesomyidae | ||||
| Macrotarsomys ingens | no | microendemic | EN | 4.76 |
| Voalavo antsahabensis | no | microendemic | EN | 4.69 |
| Nesomys lambertoni | no | microendemic | EN | 4.66 |
| Eliurus penicillatus | no | microendemic | EN | 0.00 |
| Eliurus petteri | no | microendemic | EN | 0.00 |
| Eliurus danieli | no | microendemic | LC | 0.00 |
| Eliurus antsingy | no | microendemic | DD | 0.00 |
| Eliurus ellermani | no | microendemic | DD | 0.00 |
| Macrotarsomys petteri | no | microendemic | DD | 0.00 |
| Eliurus carletoni | no | regional endemic | LC | 0.00 |
| Brachytarsomys villosa | no | endemic | VU | 4.01 |
| Brachyuromys betsileoensis | no | endemic | LC | 0.00 |
| Brachyuromys ramirohitra | no | endemic | LC | 0.00 |
| Eliurus majori | no | endemic | LC | 0.00 |
| Eliurus minor | no | endemic | LC | 0.00 |
| Eliurus myoxinus | no | endemic | LC | 0.00 |
| Eliurus tanala | no | endemic | LC | 0.00 |
| Eliurus webbi | no | endemic | LC | 0.00 |
| Gymnuromys roberti | no | endemic | LC | 0.00 |
| Macrotarsomys bastardi | no | endemic | LC | 0.00 |
| Monticolomys koopmani | no | endemic | LC | 0.00 |
| Nesomys audeberti | no | endemic | LC | 0.00 |
| Nesomys rufus | no | endemic | LC | 0.00 |
| Voalavo gymnocaudus | no | endemic | LC | 0.00 |
| Hypogeomys antimena | yes | microendemic | CR | 4.94 |
| Brachytarsomys albicauda | yes | endemic | LC | 0.00 |
| Eliurus grandidieri | yes | endemic | LC | 0.00 |
| Nycteridae | ||||
| Nycteris madagascariensis | no | endemic | DD | 0.00 |
| Pteropodidae | ||||
| Eidolon dupreanum | no | endemic | VU | 0.00 |
| Rousettus madagascariensis | no | endemic | VU | 0.00 |
| Pteropus rufus | yes | endemic | VU | 0.00 |
| Rhinonycteridae | ||||
| Triaenops menamena | no | endemic | LC | 0.00 |
| Tenrecidae | ||||
| Microgale jenkinsae | no | microendemic | EN | 4.46 |
| Microgale jobihely | no | microendemic | EN | 4.46 |
| Microgale monticola | no | microendemic | VU | 3.77 |
| Oryzorictes tetradactylus | no | microendemic | DD | 0.00 |
| Limnogale mergulus | no | regional endemic | VU | 4.47 |
| Microgale nasoloi | no | regional endemic | VU | 3.77 |
| Microgale dryas | no | regional endemic | VU | 3.66 |
| Hemicentetes nigriceps | no | regional endemic | LC | 0.00 |
| Microgale longicaudata | no | regional endemic | LC | 0.00 |
| Geogale aurita | no | endemic | LC | 0.00 |
| Hemicentetes semispinosus | no | endemic | LC | 0.00 |
| Microgale brevicaudata | no | endemic | LC | 0.00 |
| Microgale cowani | no | endemic | LC | 0.00 |
| Microgale dobsoni | no | endemic | LC | 0.00 |
| Microgale drouhardi | no | endemic | LC | 0.00 |
| Microgale fotsifotsy | no | endemic | LC | 0.00 |
| Microgale gracilis | no | endemic | LC | 0.00 |
| Microgale grandidieri | no | endemic | LC | 0.00 |
| Microgale gymnorhyncha | no | endemic | LC | 0.00 |
| Microgale majori | no | endemic | LC | 0.00 |
| Microgale parvula | no | endemic | LC | 0.00 |
| Microgale principula | no | endemic | LC | 0.00 |
| Microgale pusilla | no | endemic | LC | 0.00 |
| Microgale soricoides | no | endemic | LC | 0.00 |
| Microgale taiva | no | endemic | LC | 0.00 |
| Microgale talazaci | no | endemic | LC | 0.00 |
| Microgale thomasi | no | endemic | LC | 0.00 |
| Oryzorictes hova | no | endemic | LC | 0.00 |
| Echinops telfairi | yes | endemic | LC | 0.00 |
| Setifer setosus | yes | endemic | LC | 0.00 |
| Tenrec ecaudatus | yes | endemic | LC | 0.00 |
| Vespertilionidae | ||||
| Neoromicia malagasyensis | no | microendemic | VU | 4.23 |
| Hypsugo bemainty | no | microendemic | LC | 0.00 |
| Neoromicia robertsi | no | microendemic | DD | 0.00 |
| Neoromicia matroka | no | regional endemic | LC | 0.00 |
| Myotis goudoti | no | endemic | LC | 0.00 |
| Scotophilus marovaza | no | endemic | LC | 0.00 |
| Scotophilus robustus | no | endemic | LC | 0.00 |
| Pipistrellus raceyi | no | endemic | DD | 0.00 |
| Scotophilus tandrefana | no | endemic | DD | 0.00 |
References
- Richardson, K.; Steffen, W.; Lucht, W.; Bendtsen, J.; Cornell, S.E.; Donges, J.F.; Drüke, M.; Fetzer, I.; Bala, G.; von Bloh, W. Earth beyond six of nine planetary boundaries. Sci. Adv. 2023, 9, eadh2458. [Google Scholar] [CrossRef] [PubMed]
- De Vos, J.M.; Joppa, L.N.; Gittleman, J.L.; Stephens, P.R.; Pimm, S.L. Estimating the normal background rate of species extinction. Conserv. Biol. 2015, 29, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Myers, N. Threatened biotas: “hot spots” in tropical forests. Environmentalist 1988, 8, 187–208. [Google Scholar] [CrossRef] [PubMed]
- Byers, O.; Lees, C.; Wilcken, J.; Schwitzer, C. The One Plan Approach: The philosophy and implementation of CBSG’s approach to integrated species conservation planning. WAZA Mag. 2013, 14, 2–5. [Google Scholar]
- Traylor-Holzer, K.; Leus, K.; Bauman, K. Integrated Collection Assessment and Planning (ICAP) workshop: Helping zoos move toward the One Plan Approach. Zoo Biol. 2019, 38, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Conde, D.A.; Flesness, N.; Colchero, F.; Jones, O.R.; Scheuerlein, A. An emerging role of zoos to conserve biodiversity. Science 2011, 331, 1390–1391. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, D.J.; Fa, J.E.; Oldfield, S.; Harrop, S.R. Bring the captive closer to the wild: Redefining the role of ex situ conservation. Oryx 2012, 46, 18–23. [Google Scholar] [CrossRef]
- Collins, A.S.; Ali, J.R.; Razakamanana, T. Introduction to the Geology of Madagascar. In The New History of Madagascar; Goodman, S.M., Ed.; Princeton University Press: Princeton, NJ, USA, 2022. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Ganzhorn, J.U.; Lowry, P.P.; Schatz, G.E.; Sommer, S. The biodiversity of Madagascar: One of the world’s hottest hotspots on its way out. Oryx 2001, 35, 346–348. [Google Scholar] [CrossRef]
- Antonelli, A.; Smith, R.J.; Perrigo, A.L.; Crottini, A.; Hackel, J.; Testo, W.; Farooq, H.; Torres Jiménez, M.F.; Andela, N.; Andermann, T.; et al. Madagascar’s extraordinary biodiversity: Evolution, distribution, and use. Science 2022, 378, eabf0869. [Google Scholar] [CrossRef]
- Goodman, S.M. Updated estimates of biotic diversity and endemism for Madagascar—Revisited after 20 years. Oryx 2023, 57, 561–565. [Google Scholar] [CrossRef]
- Ceballos, G.; Ehrlich, P.R. Discoveries of new mammal species and their implications for conservation and ecosystem services. Proc. Natl. Acad. Sci. USA 2009, 106, 3841–3846. [Google Scholar] [CrossRef] [PubMed]
- Moura, M.R.; Jetz, W. Shortfalls and opportunities in terrestrial vertebrate species discovery. Nat. Ecol. Evol. 2021, 5, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, G.; Zimmermann, E.; Randrianambinina, B.; Rasoloharijaona, S.; Rakotondravony, D.; Guschanski, K.; Radespiel, U. The ever-increasing diversity in mouse lemurs: Three new species in north and northwestern Madagascar. Mol. Phylogenet. Evol. 2007, 43, 309–327. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.M.; Soarimalala, V. Introduction to Mammals. In The New History of Madagascar; Goodman, S.M., Ed.; Princeton University Press: Princeton, NJ, USA, 2022; pp. 1737–1769. [Google Scholar]
- Schüßler, D.; Blanco, M.B.; Salmona, J.; Poelstra, J.; Andriambeloson, J.B.; Miller, A.; Randrianambinina, B.; Rasolofoson, D.W.; Mantilla-Contreras, J.; Chikhi, L.; et al. Ecology and morphology of mouse lemurs (Microcebus spp.) in a hotspot of microendemism in northeastern Madagascar, with the description of a new species. Am. J. Primatol. 2020, 82, e23180. [Google Scholar] [CrossRef] [PubMed]
- Ceballos, G.; Ehrlich, P.R.; Dirzo, R. Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines. Proc. Natl. Acad. Sci. USA 2017, 114, E6089–E6096. [Google Scholar] [CrossRef] [PubMed]
- IUCN. The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/ (accessed on 31 December 2023).
- Davidson, A.D.; Shoemaker, K.T.; Weinstein, B.; Costa, G.C.; Brooks, T.M.; Ceballos, G.; Radeloff, V.C.; Rondinini, C.; Graham, C.H. Geography of current and future global mammal extinction risk. PLoS ONE 2017, 12, e0186934. [Google Scholar] [CrossRef] [PubMed]
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.; Underwood, E.C.; D’amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C. Terrestrial Ecoregions of the World: A New Map of Life on Earth: A new global map of terrestrial ecoregions provides an innovative tool for conserving biodiversity. BioScience 2001, 51, 933–938. [Google Scholar] [CrossRef]
- Rafanoharana, S.C.; Andrianambinina, F.O.D.; Rasamuel, H.A.; Waeber, P.O.; Wilmé, L.; Ganzhorn, J.U. Projecting forest cover in Madagascar’s protected areas to 2050 and its implications for lemur conservation. Oryx 2024, 58, 155–163. [Google Scholar] [CrossRef]
- Ralimanana, H.; Perrigo, A.L.; Smith, R.J.; Borrell, J.S.; Faurby, S.; Rajaonah, M.T.; Randriamboavonjy, T.; Vorontsova, M.S.; Cooke, R.S.C.; Phelps, L.N.; et al. Madagascar’s extraordinary biodiversity: Threats and opportunities. Science 2022, 378, eadf1466. [Google Scholar] [CrossRef]
- Suzzi-Simmons, A. Status of deforestation of Madagascar. Glob. Ecol. Conserv. 2023, 42, e02389. [Google Scholar] [CrossRef]
- Dufils, J. Remaining forest cover. In The Natural History of Madagascar; Goodman, S.M., Benstead, J.P., Eds.; University of Chicago Press: Chicago, IL, USA, 2003; pp. 88–96. [Google Scholar]
- Harper, G.J.; Steininger, M.K.; Tucker, C.J.; Juhn, D.; Hawkins, F. Fifty years of deforestation and forest fragmentation in Madagascar. Environ. Conserv. 2008, 34, 325–333. [Google Scholar] [CrossRef]
- Gade, D.W. Deforestation and its effects in highland Madagascar. Mt. Res. Dev. 1996, 16, 101–116. [Google Scholar] [CrossRef]
- Brooks, T.M.; Mittermeier, R.A.; Da Fonseca, G.A.; Gerlach, J.; Hoffmann, M.; Lamoreux, J.F.; Mittermeier, C.G.; Pilgrim, J.D.; Rodrigues, A.S. Global biodiversity conservation priorities. Science 2006, 313, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Hrdina, A.; Romportl, D. Evaluating global biodiversity hotspots–Very rich and even more endangered. J. Landsc. Ecol. 2017, 10, 108–115. [Google Scholar] [CrossRef]
- Nori, J.; Loyola, R.; Villalobos, F. Priority areas for conservation of and research focused on terrestrial vertebrates. Conserv. Biol. 2020, 34, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Slik, F.; Zheng, S.; Lindenmayer, D.B. Undescribed species have higher extinction risk than known species. Conserv. Lett. 2022, 15, e12876. [Google Scholar] [CrossRef]
- WWF. Terrestrial Ecoregions of the World; World Wildlife Fund: Gland, Switzerland, 2012; Available online: https://www.worldwildlife.org/publications/terrestrial-ecoregions-of-the-world (accessed on 16 July 2022).
- QGIS Association. QGIS Geographic Information System; QGIS Association: Berne, Switzerland, 2024. [Google Scholar]
- Wilmé, L.; Goodman, S.M.; Ganzhorn, U., Jr. Biogeographic evolution of Madagascar’s microendemic biota. Science 2006, 312, 1063–1065. [Google Scholar] [CrossRef]
- ArcGIS Pro; Version 3.0; Esri Inc.: New York, NY, USA, 2022; Available online: https://www.esri.com/en-us/arcgis/products/arcgis-pro/overview (accessed on 30 August 2022).
- ZIMS. Species360 Zoological Information Management System; ZIMS: Minneapolis, MN, USA, 2024; Available online: https://zims.species360.org (accessed on 16 February 2024).
- Graf, R.; Pfleiderer, J.; Fritsche, M.; Schmidt, J.; Mantei, R.; Peter, S.; Spangenberg, F. Zootierliste [ZTL]. 2021. Available online: https://www.zootierliste.de (accessed on 17 February 2024).
- CITES. The CITES Appendices; CITES: Geneva, Switzerland, 2023; Available online: https://cites.org/eng/app/appendices.php (accessed on 19 February 2024).
- EDGE. Edge of Existence Programme; Zoological Society of London: London, UK, 2021. [Google Scholar]
- Chamberlain, S. Rredlist: ‘IUCN’ Red List Client, R package version 0.7.1; R Core Team: Vienna, Austria, 2022; Available online: https://cran.R-project.Org/package=rredlist (accessed on 16 February 2024).
- Jung, M.; Dahal, P.R.; Butchart, S.H.M.; Donald, P.F.; De Lamo, X.; Lesiv, M.; Kapos, V.; Rondinini, C.; Visconti, P. A global map of terrestrial habitat types. Sci. Data 2020, 7, 256. [Google Scholar] [CrossRef]
- BirdLife International. World Database of Key Biodiversity Areas. Developed by the KBA Partnership: BirdLife International, International Union for the Conservation of Nature, American Bird Conservancy, Amphibian Survival Alliance, Conservation International, Critical Ecosystem Partnership Fund, Global Environment Facility, Re: Wild, NatureServe, Rainforest Trust, Royal Society for the Protection of Birds, Wildlife Conservation Society and World Wildlife Fund. June 2024 Version, 2024. Available online: http://keybiodiversityareas.org/kba-data/request (accessed on 16 February 2024).
- Eken, G.; Bennun, L.; Brooks, T.M.; Darwall, W.; Fishpool, L.D.C.; Foster, M.; Knox, D.; Langhammer, P.; Matiku, P.; Radford, E.; et al. Key Biodiversity Areas as Site Conservation Targets. BioScience 2004, 54, 1110–1118. [Google Scholar] [CrossRef]
- Crisp, M.D.; Laffan, S.; Linder, H.P.; Monro, A. Endemism in the Australian flora. J. Biogeogr. 2001, 28, 183–198. [Google Scholar] [CrossRef]
- Ganzhorn, J.; Goodman, S.; Ramanamanjato, J.; Ralison, J.; Rakotondravony, D.; Rakotosamimanana, B. Effects of fragmentation and assessing minimum viable populations of lemurs in Madagascar. Bonn. Zool. Monogr. 2000, 46, 265–272. [Google Scholar]
- Ceballos, G.; Ehrlich, P.R. Global mammal distributions, biodiversity hotspots, and conservation. Proc. Natl. Acad. Sci. USA 2006, 103, 19374–19379. [Google Scholar] [CrossRef] [PubMed]
- Fattorini, S. Endemism in historical biogeography and conservation biology: Concepts and implications. Biogeographia 2017, 32, 47–75. [Google Scholar] [CrossRef]
- Rakotoarivelo, A.R.; Willows-Munro, S.; Schoeman, M.C.; Lamb, J.M.; Goodman, S.M. Cryptic diversity in Hipposideros commersoni sensu stricto (Chiroptera: Hipposideridae) in the western portion of Madagascar. BMC Evol. Biol. 2015, 15, 235. [Google Scholar] [CrossRef]
- Goodman, S.M.; Ramasindrazana, B.; Naughton, K.M.; Appleton, B. Description of a new species of the Miniopterus aelleni group (Chiroptera: Miniopteridae) from upland areas of central and northern Madagascar. Zootaxa 2015, 3936, 538–558. [Google Scholar] [CrossRef] [PubMed]
- Merson, S.D.; Dollar, L.J.; Johnson, P.J.; Macdonald, D.W. Poverty not taste drives the consumption of protected species in Madagascar. Biodivers. Conserv. 2019, 28, 3669–3689. [Google Scholar] [CrossRef]
- Reuter, K.E.; Gilles, H.; Wills, A.R.; Sewall, B.J. Live capture and ownership of lemurs in Madagascar: Extent and conservation implications. Oryx 2016, 50, 344–354. [Google Scholar] [CrossRef]
- Borgelt, J.; Dorber, M.; Høiberg, M.A.; Verones, F. More than half of data deficient species predicted to be threatened by extinction. Commun. Biol. 2022, 5, 679. [Google Scholar] [CrossRef]
- Waeber, P.O.; Wilmé, L.; Mercier, J.-R.; Camara, C.; Lowry, P.P. How effective have thirty years of internationally driven conservation and development efforts been in Madagascar? PLoS ONE 2016, 11, e0161115. [Google Scholar] [CrossRef]
- Reimes, T.; Nijssen, T.; Valente, L. Captive populations of lemurs in European zoos: Mismatch between current species representation and ex-situ conservation needs. Lemur News 2021, 23, 60–66. [Google Scholar]
- Samonds, K.E.; Goodman, S.M.; Alumbaugh, J.L.; Simmons, N.B. Fossil and subfossil bats. In The New Natural History of Madagascar; Goodman, S.M., Ed.; Princeton University Press: Princeton, NJ, USA, 2022; pp. 1859–1862. [Google Scholar]
- Bottrill, M.C.; Joseph, L.N.; Carwardine, J.; Bode, M.; Cook, C.; Game, E.T.; Grantham, H.; Kark, S.; Linke, S.; McDonald-Madden, E. Is conservation triage just smart decision making? Trends Ecol. Evol. 2008, 23, 649–654. [Google Scholar] [CrossRef]
- Garbutt, N. Handbook of Mammals of Madagascar; Bloomsbury Publishing: London, UK, 2023. [Google Scholar]
- Purvis, A.; Gittleman, J.L.; Cowlishaw, G.; Mace, G.M. Predicting extinction risk in declining species. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2000, 267, 1947–1952. [Google Scholar] [CrossRef]
- Kappeler, P.; Radespiel, U.; Rasoloarison, R.; Salmona, J.; Yoder, A. Cheirogalidae: Microcebus, mouse lemurs, tsidy, tsy-tsy. In The New History of Madagascar; Goodman, S.M., Soarimalala, V., Eds.; Princeton University Press: Princeton, NJ, USA, 2022; pp. 1927–1932. [Google Scholar]
- IUCN/SSC. Guidelines on the Use of Ex Situ Management for Species Conservation; Version 2.0; IUCN Species Survival Commission: Gland, Switzerland, 2014. [Google Scholar]
- Roullet, D. The European captive population of crowned sifaka: 25 years of management. Primate Conserv. 2014, 2014, 99–107. [Google Scholar] [CrossRef]
- Zehr, S.M.; Roach, R.G.; Haring, D.; Taylor, J.; Cameron, F.H.; Yoder, A.D. Life history profiles for 27 strepsirrhine primate taxa generated using captive data from the Duke Lemur Center. Sci. Data 2014, 1, 140019. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Akcakaya, H.R.; Andelman, S.J.; Bakarr, M.I.; Boitani, L.; Brooks, T.M.; Chanson, J.S.; Fishpool, L.D.; Da Fonseca, G.A.; Gaston, K.J. Global gap analysis: Priority regions for expanding the global protected-area network. BioScience 2004, 54, 1092–1100. [Google Scholar] [CrossRef]
- Brooks, T.M.; Bakarr, M.I.; Boucher, T.; Da Fonseca, G.A.; Hilton-Taylor, C.; Hoekstra, J.M.; Moritz, T.; Olivieri, S.; Parrish, J.; Pressey, R.L. Coverage provided by the global protected-area system: Is it enough? BioScience 2004, 54, 1081–1091. [Google Scholar] [CrossRef]
- Schwitzer, C.; King, T.; Robsomanitrandrasana, E.; Chamberlan, C.; Rasolofoharivelo, T. Integrating Ex situ and In situ Conservation of Lemurs. In Lemurs of Madagascar: A Strategy for Their Conservation 2013–2016; IUCN SSC Primate Specialist Group, Bristol Conservation and Science Foundation, and Conservation International: Bristol, UK, 2013; pp. 146–152. [Google Scholar]
- Hung, C.-M.; Shaner, P.-J.L.; Zink, R.M.; Liu, W.-C.; Chu, T.-C.; Huang, W.-S.; Li, S.-H. Drastic population fluctuations explain the rapid extinction of the passenger pigeon. Proc. Natl. Acad. Sci. USA 2014, 111, 10636–10641. [Google Scholar] [CrossRef]
- Gamalo, L.E.; Ilham, K.; Jones-Engel, L.; Gill, M.; Sweet, R.; Aldrich, B.; Phiapalath, P.; Van Bang, T.; Ahmed, T.; Kite, S. Removal from the wild endangers the once widespread long-tailed macaque. Am. J. Primatol. 2024, 86, e23547. [Google Scholar] [CrossRef]



| Species | Family | Endemism Level | IUCN Status | EDGE Score |
|---|---|---|---|---|
| Hapalemur aureus | Lemuridae | microendemic | CR | 5.41 |
| Propithecus tattersalli | Indriidae | microendemic | CR | 5.31 |
| Propithecus candidus | Indriidae | microendemic | CR | 5.25 |
| Propithecus perrieri | Indriidae | microendemic | CR | 5.21 |
| Microcebus gerpi | Cheirogaleidae | microendemic | CR | 5.14 |
| Lepilemur septentrionalis | Lepilemuridae | microendemic | CR | 4.96 |
| Avahi cleesei | Indriidae | microendemic | CR | 4.63 |
| Avahi unicolor | Indriidae | microendemic | CR | 4.63 |
| Propithecus deckenii | Indriidae | microendemic | CR | 4.50 |
| Microcebus berthae | Cheirogaleidae | microendemic | CR | 4.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rose, A.; Tuchtfeldt, M.; Lammers, R.; Rode-White, J.; Markolf, M.; Pagel, T.; Rödder, D.; Ziegler, T. Prioritising Ex Situ Conservation for Malagasy Mammal Species in Line with IUCN’s ‘One Plan Approach to Conservation’. Diversity 2024, 16, 456. https://doi.org/10.3390/d16080456
Rose A, Tuchtfeldt M, Lammers R, Rode-White J, Markolf M, Pagel T, Rödder D, Ziegler T. Prioritising Ex Situ Conservation for Malagasy Mammal Species in Line with IUCN’s ‘One Plan Approach to Conservation’. Diversity. 2024; 16(8):456. https://doi.org/10.3390/d16080456
Chicago/Turabian StyleRose, Anna, Marie Tuchtfeldt, Robin Lammers, Johanna Rode-White, Matthias Markolf, Theo Pagel, Dennis Rödder, and Thomas Ziegler. 2024. "Prioritising Ex Situ Conservation for Malagasy Mammal Species in Line with IUCN’s ‘One Plan Approach to Conservation’" Diversity 16, no. 8: 456. https://doi.org/10.3390/d16080456
APA StyleRose, A., Tuchtfeldt, M., Lammers, R., Rode-White, J., Markolf, M., Pagel, T., Rödder, D., & Ziegler, T. (2024). Prioritising Ex Situ Conservation for Malagasy Mammal Species in Line with IUCN’s ‘One Plan Approach to Conservation’. Diversity, 16(8), 456. https://doi.org/10.3390/d16080456






