Implications of Ecological Drivers on Roan Antelope Populations in Mokala National Park, South Africa
Abstract
1. Introduction
2. Methods
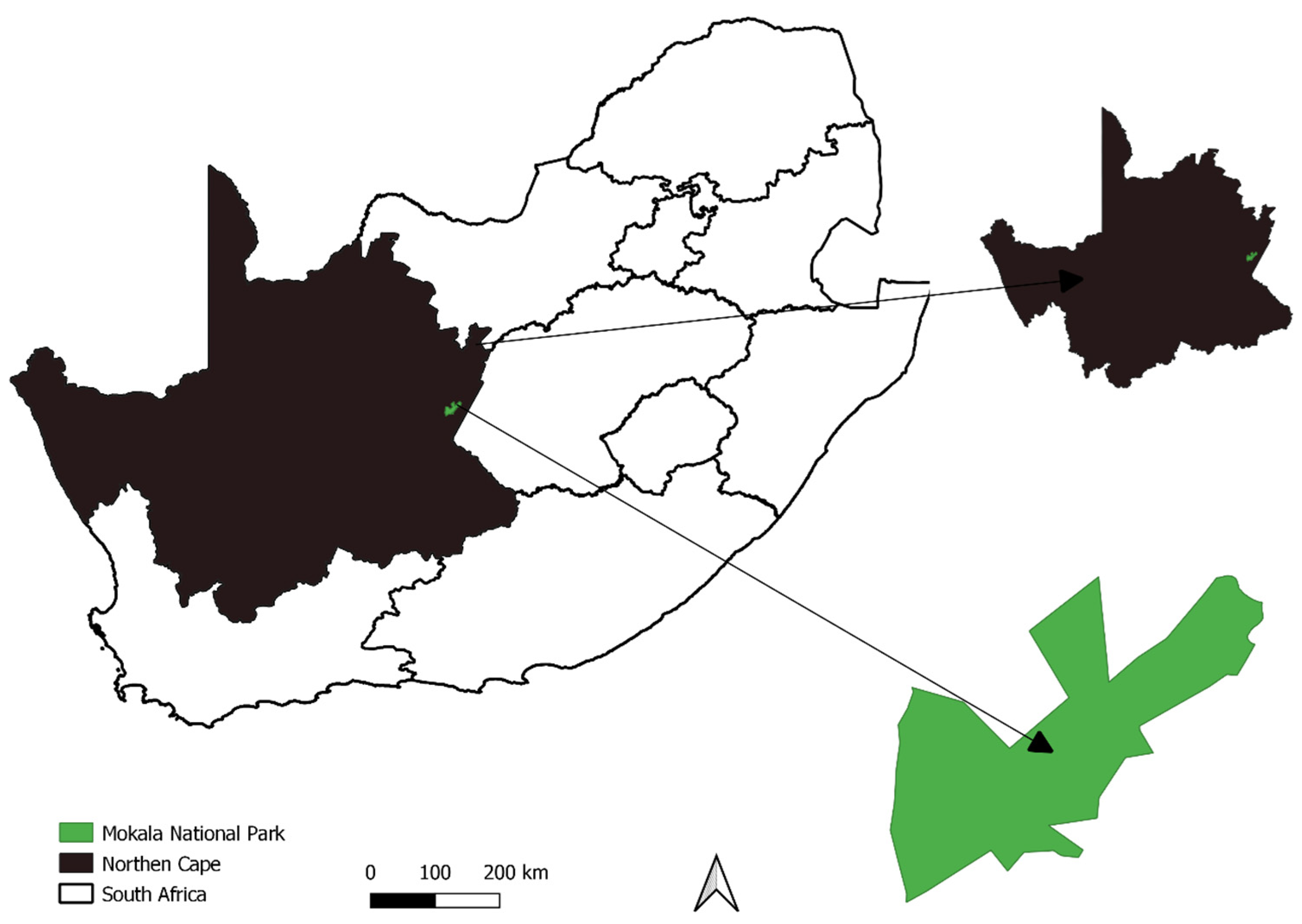
2.1. Study Area
2.2. Data Collection and Modelling
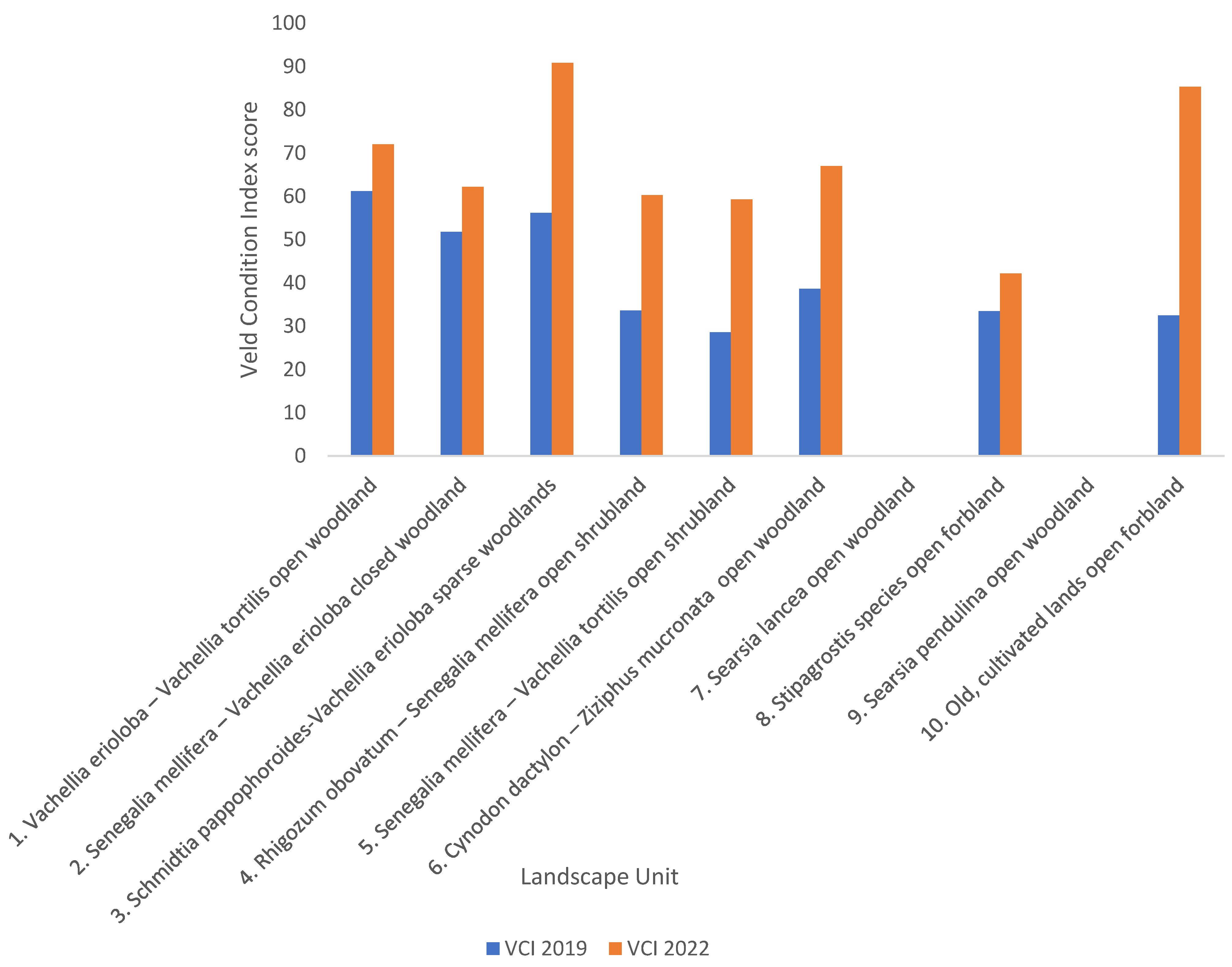
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chape, S.; Harrison, J.; Spalding, M.; Lysenko, I. Measuring the extent and effectiveness of protected areas as an indicator for meeting global biodiversity targets. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 443–455. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Anderson, R.P.; Dudik, M.; Ferriera, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Kruger, J.; Parrini, F.; Koen, J.; Collins, K.; Nel, E.J.; Child, M.F. A conservation assessment of Hippotragus equinus. In The Red List of Mammals of South Africa, Swaziland and Lesotho; Child, M.F., Roxburgh, L., Do Linh San, E., Raimondo, D., Davies-Mostert, H.T., Eds.; South African National Biodiversity Institute and Endangered Wildlife Trust: Midrand, South Africa, 2016. [Google Scholar]
- Tidmarsh, C.E.M.; Havenga, C.M. The wheel-point method of survey and measurement of semi-open grasslands and Karoo vegetation in South Africa. Mem. Bot. Surv. S. Afr. 1955, 29, 1–49. [Google Scholar]
- van Vliet, A.J.H.; de Groot, R.S. Phenological events as indicators of climate change. In RSPB/WWF Workshop on Effects of Climate Change on Flora and Fauna; University of East Anglia: Norwich, UK, 1999; pp. 14–15. [Google Scholar]
- O’Donnell, M.S.; Ignizio, D.A. Bioclimatic Predictions for Supporting Ecological Applications in the Conterminous United States. US Geol. Surv. Data Ser. 2012, 691, 10. [Google Scholar]
- Havemann, C.P.; Retief, T.A.; Collins, K.; Fynn, R.W.S.; Tosh, C.A.; de Bruyn, P.J.N. Home range and habitat use of roan antelope Hippotragus equinus in Northern Botswana. J. Arid Environ. 2022, 196, 104648. [Google Scholar] [CrossRef]
- Kass, J.M.; Vilela, B.; Aiello-Lammens, M.E.; Muscarella, R.; Merow, C.; Anderson, R.P. Wallace: A flexible platform for reproducible modeling of species niches and distributions built for community expansion. Methods Ecol. Evol. 2018, 9, 1151–1156. [Google Scholar] [CrossRef]
- Muller, K.; O’connor, T.G.; Henschel, J.R. Impact of a severe frost event in 2014 on woody vegetation within the Nama-Karoo and semi-arid savanna biomes of South Africa. J. Arid. Environ. 2016, 133, 112–121. [Google Scholar] [CrossRef]
- Holt, R.D.; Gaines, M.S. Analysis of adaptation in heterogeneous landscape: Implications for the evolution of fundamental niches. Evol. Ecol. 1992, 6, 433–447. [Google Scholar] [CrossRef]
- EWT Red Data List 2022. Available online: https://ewt.org.za/red-list/ (accessed on 23 November 2022).
- Blanchet, F.G.; Cazelles, K.; Gravel, D. Co-occurrence is not evidence of ecological interactions. Ecol. Lett. 2020, 23, 1050–1063. [Google Scholar] [CrossRef]
- Tyowua, B.T.; Orsar, J.T.; Agbelusi, E.A. Habitat preference of Roan Antelope (Hippotragus equinus, Desmarest, 1840) In Kainji Lake National Park, Nigeria. J. Res. For. Wildl. Environ. 2012, 4, 13–21. [Google Scholar]
- Sannier, C.A.D.; Taylor, J.C.; Du Plessis, W.; Campbell, K. Real-time vegetation monitoring with NOAA-AVHRR in Southern Africa for wildlife management and food security assessment. Int. J. Remote Sens. 1998, 19, 621–639. [Google Scholar] [CrossRef]
- Knoop, M.-C.; Owen-Smith, N. Foraging ecology of roan antelope: Key resources during critical periods. Afr. J. Ecol. 2006, 44, 228–236. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1 km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Strimbeck, G.R.; Schaberg, P.G.; Fossdal, C.G.; Schröder, W.P.; Kjellsen, T.D. Extreme low temperature tolerance in woody plants. Front. Plant Sci. 2015, 6, 884. [Google Scholar] [CrossRef]
- AgroClimatology Staff. ARC-ISCW AgroClimatology Daily Data Report (DDR Format, Limited Quality Control). In ARC-ISCW Climate Information System; ARC-Institute for Soil, Climate and Water: Pretoria, South Africa, 2023. [Google Scholar]
- Elsen, P.R.; Monahan, W.B.; Dougherty, E.R.; Merenlender, A.M. Keeping pace with climate change in global terrestrial protected areas. Sci. Adv. 2020, 6, eaay0814. [Google Scholar] [CrossRef]
- Moeslund, J.E.; Arge, L.; Bøcher, P.K.; Dalgaard, T.; Odgaard, M.V.; Nygaard, B.; Svenning, J.-C. Topographically controlled soil moisture is the primary driver of local vegetation patterns across a lowland region. Ecosphere 2013, 4, 91. [Google Scholar] [CrossRef]
- Owen-Smith, N. Functional heterogeneity in resources within landscapes and herbivore population dynamics. Landsc. Ecol. 2004, 19, 761–771. [Google Scholar] [CrossRef]
- Lesica, P.; Kittelson, P. Precipitation and temperature are associated with advanced flowering phenology in a semi-arid grassland. J. Arid. Environ. 2010, 74, 1013–1017. [Google Scholar] [CrossRef]
- Bezuidenhout, H.; Bradshaw, P.L.; Bradshaw, M.; Zietsman, P.C. Landscape units of Mokala National Park, Northern Cape Province, South Africa. Navorsinge Van Die Nas. Mus. Bloemfontein 2015, 31, 1–27. [Google Scholar]
- Climate Source, Inc. Downscaled OSU PRISM Climate Data: Corvallis, Oreg., The Climate Source. 2011. Available online: http://www.climatesource.com/ (accessed on 23 October 2023).
- Bothma J du, P.; van Rooyen, N.; van Rooyen, M.W. Using diet and plant resources to set wildlife stocking densities in African savannas. Wildl. Soc. Bull. 2004, 32, 840–851. [Google Scholar] [CrossRef]
- Meroni, M.; Fasbender, D.; Rembold, F.; Atzberger, C.; Klisch, A. Near real-time vegetation anomaly detection with MODIS NDVI: Timeliness vs. accuracy and effect of anomaly computation options. Remote Sens. Environ. 2018, 221, 508–521. [Google Scholar] [CrossRef]
- Mzileni, N.; Ferreira, S.; Bezuidenhout, H.; Smit, I.; de Klerk, J. Mokala National Park Herbivore Off-Take Recommendations 2022: An Integrated Approach Combining Local Knowledge with Data Derived from Animal Census, Herbivore Models, and Vegetation Monitoring and Satellite Imagery; Internal Report 35/2022, Scientific Services; South African National Parks: Kimberley, South Africa, 2022. [Google Scholar]
- Edwards, D. A broad-scale structural classification of vegetation for practical purposes. Bothalia 1983, 14, 705–812. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Climatologies at high resolution for the earth’s land surface areas. Sci. Data 2017, 4, 170122. [Google Scholar] [CrossRef]
- IUCN SSC Antelope Specialist Group. Hippotragus equinus. 2017. Available online: https://doi.org/10.2305/IUCN.UK.2017-2.RLTS.T10167A50188287.en (accessed on 16 November 2022).
- Bezuidenhout, H.; Botha, J.; Ramaswiela, T.; O’Connor, T. Key determinants of long-term compositional variation of the herbaceous layer in a semi-arid African savanna: Rainfall, soil type, and plant species functional types. Suid-Afr. Tydskr. Vir Natuurwetenskap En Tegnol. 2018, 37, 14. [Google Scholar]
- Klein, D.R. Variation in quality of caribou and reindeer forage plants associated with season, plant part, and phenology. Rangifer 1990, 10, 123–130. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extrem. 2015, 10 Pt A, 4–10. [Google Scholar] [CrossRef]
- Mawdsley, J.R.; O’Mallay, R.O.; Ojima, D.S. A review of, climate-change adaptation strategy for wildlife management and biodiversity conservation. Conserv. Biol. 2009, 23, 1080–1089. [Google Scholar] [CrossRef]
- Roux, D.J.; Foxcroft, L.C. The development and application of strategic adaptive management within South African National Parks. Koedoe 2011, 53, 1049. [Google Scholar] [CrossRef]
- CaraDonna, P.J.; Bain, J.A. Frost sensitivity of leaves and flowers of subalpine plants is related to tissue type and phenology. J. Ecol. 2016, 104, 55–64. [Google Scholar] [CrossRef]
- Ghorbanian, A.; Mohammadzadeh, A.; Jamali, S. Linear and Non-Linear Vegetation Trend Analysis throughout Iran Using Two Decades of MODIS NDVI Imagery. Remote Sens. 2022, 14, 3683. [Google Scholar] [CrossRef]
- Gonḉalves, M.; Siegismund, H.R.; van Vuuren, B.J.; Ferrand, N.; Godinho, R. Evolutionary history of the roan antelope across its African range. J. Biogeogr. 2020, 48, 2812–2827. [Google Scholar] [CrossRef]
- Brown, C.L.; Coe, P.K.; Clark, D.A.; Wisdom, M.J.; Rowland, M.M.; Averett, J.P.; Johnson, B.K. Climate change effects on understory plant phenology: Implications for large herbivore forage availability. Environ. Res. Ecol. 2022, 1, 011002. [Google Scholar] [CrossRef]
- Kimanzi, J.K.; Sanderson, R.A.; Rushton, S.P. Habitat suitability modelling and implications for management of roan antelopes in Kenya. Afr. J. Ecol. 2013, 52, 111–121. [Google Scholar] [CrossRef]
- Chen, F.; Weber, K.T. Assessing the impact of seasonal precipitation and temperature on vegetation in a grass-dominated rangeland. Rangel. J. 2014, 36, 185–190. [Google Scholar] [CrossRef]
- Kogan, F. Application of vegetation index and brightness temperature for drought detection. Adv. Space Res. 1995, 15, 91–100. [Google Scholar] [CrossRef]
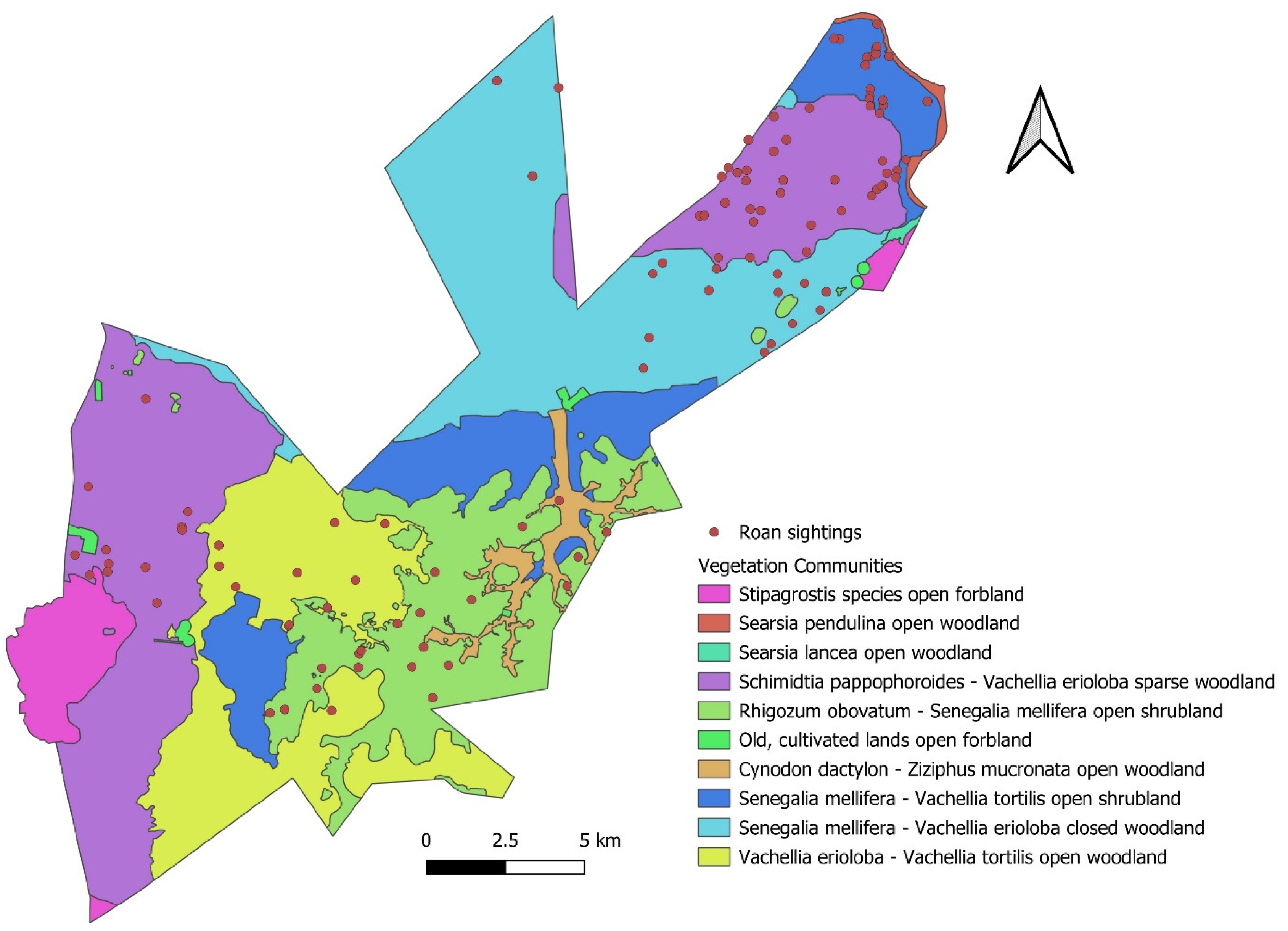

| Habitat Description | ||||
|---|---|---|---|---|
| Landscape Unit | Landscape | Geology and Soil | Roan | |
| 1 | Vachellia erioloba–Vachellia tortilis open woodland | Undulating plains, open woodland | Aeolian sand covering the Dwyka Formation with deep sandy soil | Present |
| 2 | Senegalia mellifera–Vachellia erioloba closed woodland | Flat plains, open woodland | Aeolian sand covering the Dwyka Formation with deep sandy soil | Present |
| 3 | Schmidtia pappophoroides–Vachellia erioloba sparse woodlands | Flat plains, sparse woodland | Aeolian sand covering the Dwyka Formation with deep sandy soil | Present |
| 4 | Rhigozum obovatum–Senegalia mellifera open shrubland | Rolling hills, open shrubland | Andesitic lava and dolerite with rocky shallow soil | Present |
| 5 | Senegalia mellifera–Vachellia tortilis open shrubland | Slightly undulating foot slopes | Andesitic lava, dolerite, shale, and rocky outcrops with shallow soil | Present |
| 6 | Cynodon dactylon–Ziziphus mucronata e open woodland | Slightly undulating clayey drainage line | Alluvium | Absent |
| 7 | Searsia lancea open woodland | Slightly undulating rocky drainage line | Calcrete | Absent |
| 8 | Stipagrostis species open woodland | Slightly undulating valley bottomlands | Calcrete | Absent |
| 9 | Searsia pendulina open woodland | Flat Riet River | Alluvium | Absent |
| 10 | Old, cultivated lands open woodland | Flat cultivated land | Aeolian sand covering the Dwyka Formation | Absent |
| Bioclimatic Predictor | Unit | Definition | Interpretation |
|---|---|---|---|
| Bio 1—Annual Mean Temperature | Degrees Celsius | The annual mean temperature | The annual mean temperature. |
| Bio 2—Annual Mean Diurnal Range | Degrees Celsius | The mean of the monthly temperature ranges (monthly maximum minus monthly minimum). | Indicates the relevance of temperature fluctuation for different species. |
| Bio—3 Isothermy | Percentage | Quantifies how large the day-to-night temperatures oscillate relative to the summer-to-winter (annual) oscillations. | Species distribution may be influenced by large or small temperature fluctuations within a month relative to the year. |
| Bio 4—Temperature Seasonality (standard deviation) | Degrees Celsius | The amount of temperature variation over a given year based on standard deviation of monthly temperature averages. | Temperature seasonality is a measure of temperature change over the course of a year. The larger the standard deviation the greater the variability of temperature. |
| Bio 5—Max Temperature of Warmest Month | Degrees Celsius | The maximum monthly temperature occurrence over a given year (time series) or averaged set of years (normal) | Used to determine whether species distributions are affected by warm temperature anomalies throughout the year. |
| Bio 6—Minimum Temperature of Coldest Month | Degrees Celsius | The minimum monthly temperature occurrence over a given year or averaged specified years. | This determines whether species distributions are affected by cold temperature anomalies throughout the year. |
| Bio 7—Annual Temperature Range | Degrees Celsius | Measure of temperature variation over a given period of time | Used to determine whether species distributions are affected by ranges of extreme temperature conditions. |
| Bio 8—Mean Temperature of Wettest Quarter | Degrees Celsius | Approximates the mean temperatures that prevail during the wettest season. | This index approximates mean temperature during the wettest three months of the year, which may influence species’ seasonal distribution. |
| Bio 9—Mean Temperature of Driest Month | Degrees Celsius | Approximates the mean temperatures that prevail during the driest season. | This index approximates mean temperature during the driest three months of the year, which may influence species’ seasonal distribution. |
| Bio 10—Mean Temperature of Warmest Quarter | Degrees Celsius | Approximates the mean temperatures that prevail during the warmest quarter | The mean temperature during the warmest three months indicates the influence on species seasonal distribution. |
| Bio 11—Mean Temperature of Coldest Quarter | Degree Celsius | Approximates the mean temperatures that prevail during the coldest quarter | The mean temperature during the coldest three months indicates the influence on species seasonal distribution. |
| Bio 12—Annual Precipitation | mm | The sum of all total monthly precipitation values | Important for determining the importance of water availability to species distribution. |
| Bio 13—Precipitation of Wettest Month | mm | Identifies the total precipitation that prevails during the wettest month. | The wettest month is useful if extreme precipitation conditions during the year influence a species’ potential range. |
| Bio 14 –Precipitation of Driest Month | mm | Identifies the total precipitation that prevails during the driest month. | The driest month is useful if extreme precipitation conditions during the year influence a species’ potential range. |
| Bio 15—Precipitation Seasonality (CV) | mm | The measure of the variation in monthly precipitation totals over the course of the year. | Species distributions can be strongly influenced by variability in precipitation. |
| Bio 16—Precipitation of Wettest Quarter | mm | Approximates total precipitation that prevails during the wettest quarter. | Provides total precipitation during the wettest three months of the year, which may affect species’ seasonal distributions. |
| Bio 17—Precipitation of Driest Quarter | mm | Approximates total precipitation that prevails during the driest quarter. | Provides total precipitation during the driest three months of the year, which may affect species’ seasonal distributions. |
| Bio 18—Precipitation of the Warmest Quarter | mm | Approximates total precipitation during the warmest quarter | Provides total precipitation during the warmest three months of the year, which may influence species’ distribution. |
| Bio 19—Precipitation of Coldest Quarter | mm | Approximates total precipitation during the coldest quarter | Provides total precipitation during the coldest three months of the year, which may influence species’ distribution. |
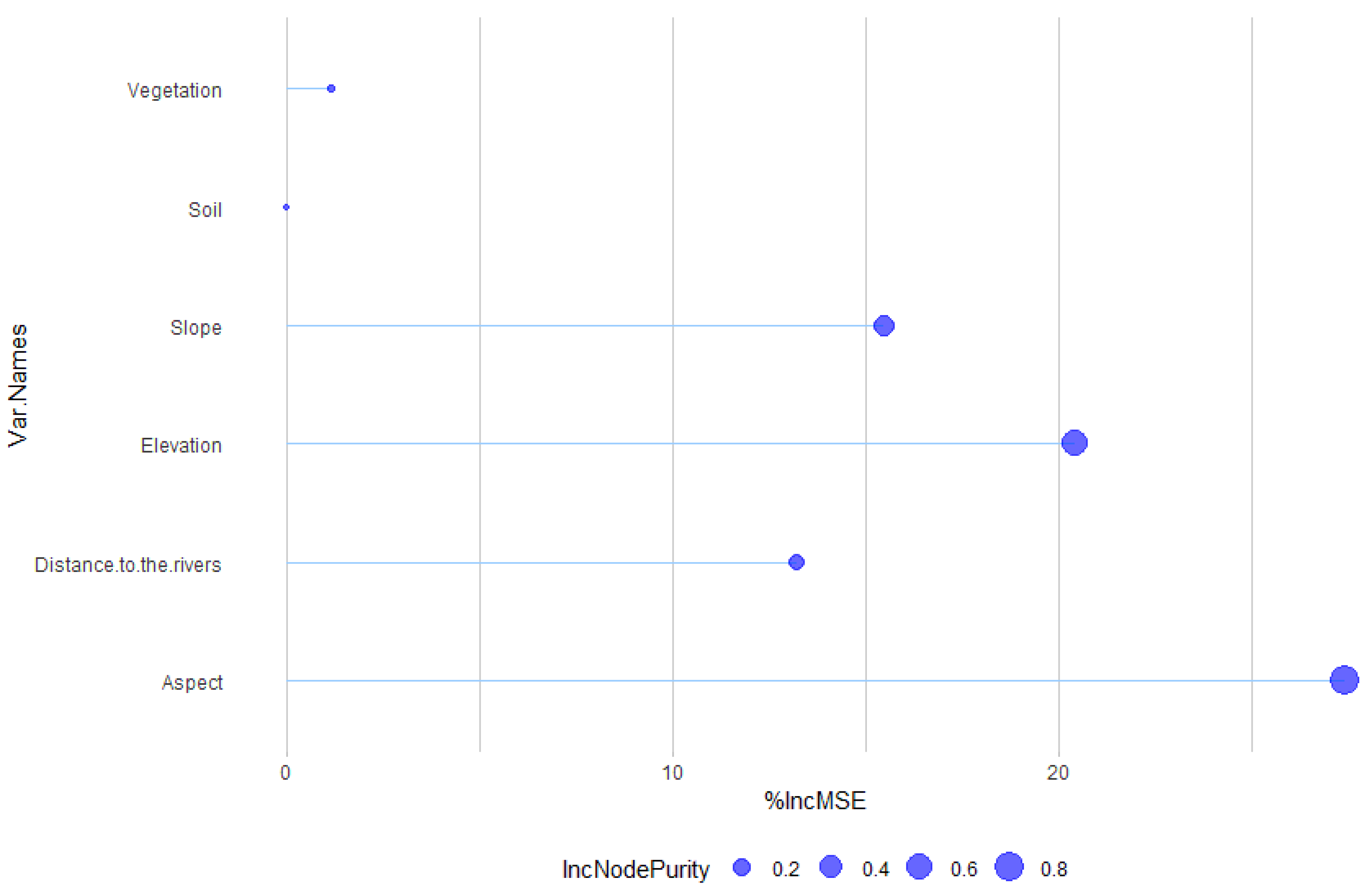
| Environmental Variable | %IncMSE | IncNodePurity |
|---|---|---|
| Aspect | 27.40 | 0.84 |
| Elevation | 20.42 | 0.59 |
| Slope | 15.44 | 0.32 |
| Distance to the river | 13.22 | 0.16 |
| Vegetation | 1.13 | 0.01 |
| Soil | 0.00 | 0.00 |
| MODEL | FEATURE | AUCTRAIN | AUCDIFF | AUCTEST | AICC | ∆AICC | W.AIC | PARA |
|---|---|---|---|---|---|---|---|---|
| 1 | rm.1_fc.L | 0.97 | 0.06 | 0.87 | 289.38 | 49.60 | 0.00 | 8 |
| 2 | rm.2_fc.L | 0.94 | 0.04 | 0.87 | 262.53 | 22.75 | 0.00 | 6 |
| 3 | rm.3_fc.L | 0.91 | 0.03 | 0.87 | 258.57 | 18.78 | 0.00 | 5 |
| 4 | rm.1_fc.LQ | 0.99 | 0.03 | 0.91 | 249.59 | 9.80 | 0.01 | 7 |
| 5 | rm.2_fc.LQ | 0.98 | 0.02 | 0.92 | 244.97 | 5.18 | 0.07 | 6 |
| 6 | rm.3_fc.LQ | 0.97 | 0.02 | 0.92 | 239.78 | 0.00 | 0.87 | 5 |
| 7 | rm.1_fc.H | 1.00 | 0.01 | 0.98 | 264.38 | 24.60 | 0.00 | 8 |
| 8 | rm.2_fc.H | 1.00 | 0.00 | 0.95 | 257.85 | 18.06 | 0.00 | 7 |
| 9 | rm.3_fc.H | 0.99 | 0.01 | 0.94 | 248.13 | 8.34 | 0.01 | 5 |
| 10 | rm.1_fc.LQH | 1.00 | 0.02 | 0.94 | NA | NA | NA | 11 |
| 11 | rm.2_fc.LQH | 0.99 | 0.02 | 0.92 | 271.17 | 31.39 | 0.00 | 8 |
| 12 | rm.3_fc.LQH | 0.98 | 0.02 | 0.92 | 246.35 | 6.56 | 0.03 | 6 |
| 13 | rm.1_fc.LQHP | 1.00 | 0.01 | 0.96 | 260.21 | 20.43 | 0.00 | 8 |
| 14 | rm.2_fc.LQHP | 0.99 | 0.03 | 0.94 | 274.28 | 34.50 | 0.00 | 8 |
| 15 | rm.3_fc.LQHP | 0.99 | 0.04 | 0.93 | 249.34 | 9.55 | 0.01 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maruping-Mzileni, N.T.; Bezuidenhout, H.; Ferreira, S.; Ramoelo, A.; Mapuru, M.; Munyai, L.; Erusan, R. Implications of Ecological Drivers on Roan Antelope Populations in Mokala National Park, South Africa. Diversity 2024, 16, 355. https://doi.org/10.3390/d16060355
Maruping-Mzileni NT, Bezuidenhout H, Ferreira S, Ramoelo A, Mapuru M, Munyai L, Erusan R. Implications of Ecological Drivers on Roan Antelope Populations in Mokala National Park, South Africa. Diversity. 2024; 16(6):355. https://doi.org/10.3390/d16060355
Chicago/Turabian StyleMaruping-Mzileni, Nkabeng Thato, Hugo Bezuidenhout, Sam Ferreira, Abel Ramoelo, Morena Mapuru, Lufuno Munyai, and Roxanne Erusan. 2024. "Implications of Ecological Drivers on Roan Antelope Populations in Mokala National Park, South Africa" Diversity 16, no. 6: 355. https://doi.org/10.3390/d16060355
APA StyleMaruping-Mzileni, N. T., Bezuidenhout, H., Ferreira, S., Ramoelo, A., Mapuru, M., Munyai, L., & Erusan, R. (2024). Implications of Ecological Drivers on Roan Antelope Populations in Mokala National Park, South Africa. Diversity, 16(6), 355. https://doi.org/10.3390/d16060355







