Abstract
The research was carried out in calcareous fen habitats which share coverage with Natura 2000 sites designated under the EU Habitats and the Birds Directive. A total of 27 taxa of molluscs were recorded: 23 gastropod and 4 bivalve species. Anisus vorticulus, one of the species of Community interest whose conservation requires designation of special conservation areas within the Habitats Directive Natura 2000, was subrecedent and accedent in mollusc communities. Calcareous fen habitats offer the aquatic organisms harsh environmental conditions including a relatively high temperature of the water up to 33.29 °C (undrained fens), oxygen deficits in the water, high pH of up to 11.08 (fen pools) and conductivity above 3000 μS cm−1 (fen ditches). Therefore molluscs have to face extreme environmental conditions. Temperature of the water, pH, dissolved oxygen and conductivity were the parameters most associated with the distribution of mollusc species in the calcareous fen habitats. The abundance of submerged and floating macrophytes, the degree of habitat persistence and the fish predation pressure on molluscs also exerted a significant effect on their distribution. The calcareous fen habitats that are listed in Annex I of the European Union Habitats Directive create a unique valuable ecosystem that contributes to the natural diversity of aquatic organisms.
1. Introduction
Freshwater ecosystems which cover less than 1% of the Earth’s surface including inland wetlands are inhabited by at least 10% of the Earth’s species. According to [1,2] and the Living Planet Index (LPI), freshwater populations have declined more than other organisms by an average of 83% since 1970. Anthropogenic stressors, including fragmentation, habitat loss and water quality degradation, account for around half the threats to these populations. The rate of biodiversity loss in freshwater is twice the rate in terrestrial and marine ecosystems. What is more, wetlands globally have declined three times faster than forests. Calcareous fens are a unique type of wetlands which have a substrate of non-acidic peat and require a steady supply of oxygen-poor groundwater rich in calcium and magnesium bicarbonate. Calcareous fens are especially vulnerable to surface and sub-surface disturbances affecting their groundwater regime [3]. The fen water balance is controlled by precipitation, evapotranspiration, surface and groundwater inflows and outflows. Calcareous fens accumulate carbon-rich deposits through carbonate precipitation and slow organic matter decomposition which can be affected by a lowering water table. Ongoing climate change is altering the carbon balance in fens and threatening their flora and fauna [4]. Calcareous fens support an incredibly rich and diverse range of plants and animals, including rare and endangered species and have a high conservation value on a world scale [5,6,7]. Therefore the calcareous fens with Cladium mariscus and species of the Caricion davallianae are protected under the EU Habitats Directive [8] (code of the Habitats Directive Annex I: 7210). Despite the protection of Annex I of the EU Habitats Directive [8], fens remain seriously threatened habitats which are disappearing quickly from the landscape [9]. According to [10], the threat level for freshwater gastropods is the highest in Europe; in contrast, the threat level for freshwater bivalves is the highest in North America. Considering the EU 27 level, 667 freshwater mollusc species are included in the IUCN Red List. Some gastropod species are considered to be priority wetland species globally, including Anisus vorticulus (Troschel, 1834), Segmentina nitida (O.F. Müller, 1774) and Valvata macrostoma Mörch, 1864 and listed in the Biodiversity Action Plan [11].
Due to the problems highlighted above, the objectives of our survey were the following: (1) to analyse the structure of mollusc communities in calcareous fen habitats, (2) to determine the most predictive environmental factors structuring the mollusc communities, (3) to assess the role of calcareous fens as unique habitats for rare and threaten species.
2. Materials and Methods
2.1. Study Area
The research was carried out in calcareous fen habitats located within two macroregions: the Western Polesie and the Wołyń Polesie, Poland (Figure 1). The research area shares coverage with Natura 2000 sites, i.e., Torfowiska Chełmskie (PLH060023, area of 2090.2 ha) designated under the EU Habitats Directive and Chełmskie Torfowiska Węglanowe (PLB060002, area of 4309.42 ha) designated under the EU Birds Directive [12]. The area of PLH60023 is much smaller than the area of PLB060002 (Figure 1). Natura 2000 site Torfowiska Chełmskie (PLH060023), which was established in 2004, protects four unique habitats: alkaline fens (code of the Habitats Directive Annex I: 7230), calcareous fens with Cladium mariscus and species of the Caricion davallianae (code of the Habitats Directive Annex I: 7210), Molinia meadows on calcareous, peaty or clayey-silt-laden soils (Molinion caeruleae) (code of the Habitats Directive Annex I: 6410) and semi-natural dry grasslands and scrubland facies on calcareous substrates (Festuco-Brometalia) (code of the Habitats Directive Annex I: 6210). The research area also included three nature reserves: the Bagno Serebryskie, the Brzeźno and the Roskosz. Calcareous fen habitats were created as a result of the accumulation of organic and mineral material in the depressions in the ground, which are the result of karst processes. Calcareous fens are fed solely by rainfall and water flowing from the surrounding hills. The largest participation in processes of the fen-forming plant here was the Cladium mariscus, which covers approximately 50% of the fens area. Most of the fens are cut by canal drainage related to the network of ponds. The study area of calcareous fen habitats included the following: UF—undrained fens (five sampling sites UF1-UF5), DF—drained fens (six sampling sites DF1-DF6), FD—fen ditches (four sampling sites FD1-FD4) and FP—fen pools (six sampling sites FP1-FP6) (Figure 1). In total, 21 sampling sites were selected within calcareous fen habitats (Supplementary Materials Tables S1 and S2).
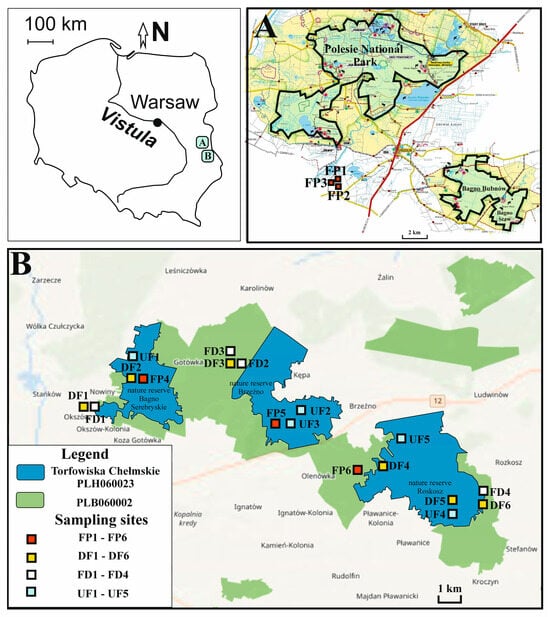
Figure 1.
Location of the study area and sampling sites within two macroregions: the Western Polesie (A) and the Wołyń Polesie (B), Poland. Abbreviations: UF—undrained fens, DF—drained fens, FD—fen ditches, FP—fen pools.
2.2. Field and Laboratory Methods
The material was collected once a month from April to October in 2016 and 2017. The mollusc samples were collected according to quantitative methods from each of the calcareous fen habitats: undrained fens (N = 39), drained fens (N = 28), fen ditches (N = 39), fen pools (N = 58). Samples of molluscs were taken using a square-framed pond net with dimensions of 25 cm × 25 cm, dragging it 10 times over a length of 1 m, which amounted to a research area of 2.5 m2. A total of 164 samples of Mollusca were collected. The collected material was transported to the laboratory in plastic containers. The samples were washed using a 0.5 mm mesh sieve and then preserved in 80% ethanol. Molluscs were identified at the species level based on their morphological and anatomical features according to [13]. Empty shells were not taken into account. Immediately before mollusc sampling, the physical and chemical parameters of the water were measured in situ using a portable HI 9828 m (Hanna Instruments, Smithfield, Rhode Island, USA). The measurement included the temperature of the water, pH, oxidation-reduction potential (ORP), dissolved oxygen (DO), electrical conductivity (EC), specific resistance (SR), total dissolved solids (TDS) and salinity.
The following environmental characteristics of each sampling site were evaluated:
- Permanence/astatism (expressed as the number of months during which water was present at a site).
- Fish predation pressure on molluscs.
- The overall coverage (percentage) of a site by vegetation.
- The coverage of helophytes.
- The coverage of floating macrophytes.
- The coverage of submersed macrophytes.
- Diversity of riparian vegetation.
- Moving the meadows.
These data were scored from 0 (absence/undeveloped) to 4 (complete cover/strongly developed) for the statistical analyses.
2.3. Numerical and Statistical Analyses
The structure of the mollusc communities was analysed using the following indices [14,15]:
- Dominance index D% divided into five classes (eudominants > 10.0% of a sample, dominants 5.1–10.0% of a sample, subdominants 2.1–5.0% of a sample, recedents 1.1–2.0% of a sample and subrecedents ≤ 1.0% of a sample).
- Frequency index F% divided into four classes (euconstants 75.1–100.0% of samples, constants 50.1–75.0% of samples, accessory species 25.1–50.0% of samples, and accedents ≤ 25.0% of samples).
- The Shannon–Wiener index H’.
- The density of Mollusca is estimated as the number of individuals per square metre.
The values of the biological and environmental data did not reveal a normal distribution according to the Lilliefors test of normality. Therefore non-parametric tests have been used. The significance of the differences in the median values of the physical and chemical parameters of the water, the number of mollusc species and the density between the calcareous fen habitats was calculated using the Kruskal–Wallis test and the multiple comparison post hoc tests. Cluster analysis was applied to group calcareous fen habitats according to the similarity of species composition of mollusc communities. The statistical analyses were performed using Statistica version 13.3. Canonical ordination analyses for relating the taxonomic composition of Mollusca to the environmental data (the physical and chemical parameters of the water, environmental characteristics) were performed using CANOCO for Windows version 4.5 [16]. The appropriate type of analysis was selected to analyse the biological data using detrended correspondence analysis (DCA) and the length of the gradient. The gradient length (4.026) exceeded 3 SD (the standard deviation), therefore an unimodal direct ordination, i.e., canonical correspondence analysis (CCA) with a forward selection was used to reduce the large set of environmental variables. Species that occurred at less than 10% of the sampling sites were excluded from the statistical analyses following a preliminary exploration of their influence in the initial DCA analysis.
3. Results
3.1. Environmental Variables
The temperature of the water ranged from 6.39 °C (the lowest value was measured in fen pools) to 33.29 °C (the highest value was measured in undrained fens) (Table 1). A very high pH value of up to 11.08 was recorded for the fen pools. The value of the conductivity ranged from 168 µS cm−1 (the lowest value was measured in undrained fens) to 3232 µS cm−1 (the highest value was measured in fen ditches). The maximum value of salinity up to 1.60 PSU was recorded in the fen ditches. Redox potential ranged from −455.7 mV (the lowest was measured in undrained fens) to 249.5 mV (the highest value was measured in fen pools). The Kruskal–Wallis test and multiple comparison post hoc tests revealed statistically significant differences in the values of the conductivity (EC), specific resistance (SR), total dissolved solids (TDS) and salinity between the calcareous fen habitats (Table 1).

Table 1.
The physical and chemical parameters of the water and the results of the Kruskal–Wallis test and multiple comparison post hoc tests (superscript denotes significant differences between the calcareous fen habitats). Abbreviations: UF—undrained fens, DF—drained fens, FD—fen ditches, FP—fen pools, N—number of samples.
3.2. The Structure of Mollusc Communities
A total of 27 taxa of Mollusca were recorded in the calcareous fen habitats: 23 gastropod species and 4 bivalve species (Table 2). Planorbis planorbis (Linnaeus, 1758) was eudominant and constant in the mollusc communities in all calcareous fen habitats. Three constant species: Bithynia tentaculata (Linnaeus, 1758), Bathyomphalus contortus (Linnaeus, 1758) and P. planorbis were found in all types of calcareous fen habitats (Table 2). The rare gastropod species Bithynia leachii (Sheppard, 1823) was subrecedent in the fen ditches whereas A. vorticulus was subrecedent in the fen pools. Invasive alien species (IAS), i.e., Physa acuta (Draparnaud, 1805) was recorded in the fen ditches (subrecedent and accedent). Only a few pea clam species (Sphaeriidae) including Musculium lacustre (O.F. Müller, 1774) (accedent) and Sphaerium corneum (Linnaeus, 1758) (accessory species) occurred in the mollusc communities. In contrast, not any unionid mussels were found in the calcareous fen habitats. The maximum value up to 3.04 of the Shannon-Wiener index H’ was recorded for the mollusc community in the fen ditches (Table 2).

Table 2.
The values of the dominance (D%), frequency (F%) and theShannon–Wiener (H’) indices calculated for the mollusc communities in the calcareous fen habitats. Abbreviations: UF—undrained fens, DF—drained fens, FD—fen ditches, FP—fen pools, N—number of samples.
The maximum number of mollusc species (12 species) and maximum mollusc density of 142 individuals m−2 were recorded in the fen pools. The median number of mollusc species and density amount to 6 and 26.5 individuals m−2, respectively. The Kruskal–Wallis test and multiple comparison post hoc tests revealed statistically significant differences in the median number of mollusc species and density between the calcareous fen habitats (Figure 2 and Figure 3).
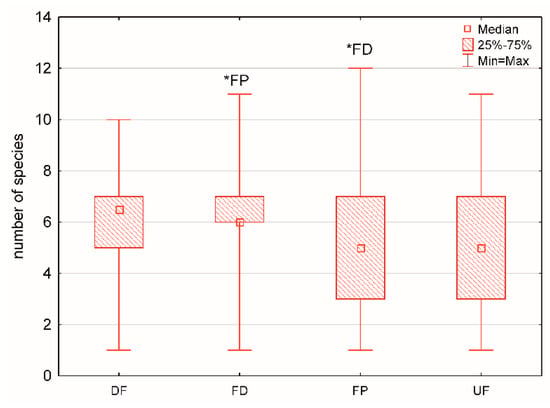
Figure 2.
Box-and-whisker plot showing the number of mollusc species in the calcareous fen habitats (* significant differences between the habitats, the Kruskal–Wallis test and multiple comparison post hoc tests, H = 8.81, p = 0.0319). Abbreviations: UF—undrained fens, DF—drained fens, FD—fen ditches, FP—fen pools.
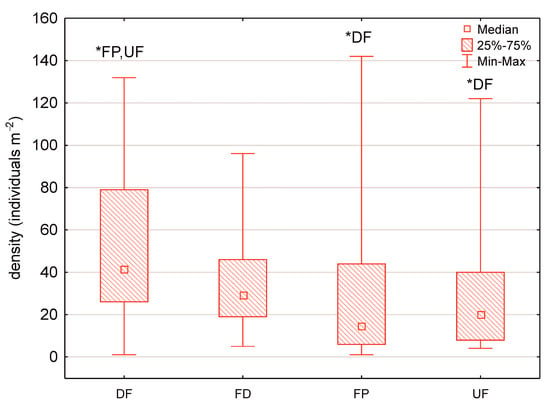
Figure 3.
Box-and-whisker plot showing the density of Mollusca in the calcareous fen habitats (* significant differences between the habitats, the Kruskal–Wallis test and multiple comparison post hoc tests, H = 17.83, p = 0.0005). Abbreviations: UF—undrained fens, DF—drained fens, FD—fen ditches, FP—fen pools.
3.3. Mollusc Communities in Relation to Environmental Data
Canonical correspondence analysis (CCA) based on the species data and the physical and chemical parameters of the water showed that temperature of the water, pH, dissolved oxygen and conductivity were the only parameters most associated with the distribution of mollusc species in the calcareous fen habitats. The relationship between the species composition of Mollusca and these physical and chemical parameters of the water was significant (The Monte Carlo test of significance of the first canonical axis: F-ratio = 2.903, p-value = 0.0302; test of significance of all canonical axes F-ratio = 1.583, p-value = 0.0080) (Figure 4). Some patterns of mollusc distribution were identified. Planorbarius corneus (Linnaeus, 1758), P. planorbis, Anisus leucostoma (Millet, 1813) and B. tentaculata were associated with the higher pH and lower conductivity. Radix balthica (Linnaeus, 1758), Viviparus contectus (Millet, 1813), M. lacustre, S. corneum, Lymnaea stagnalis (Linnaeus, 1758) and Hippeutis complanatus (Linnaeus, 1758) were positively influenced by higher concentration of dissolved oxygen and negatively influenced by conductivity (Figure 4).
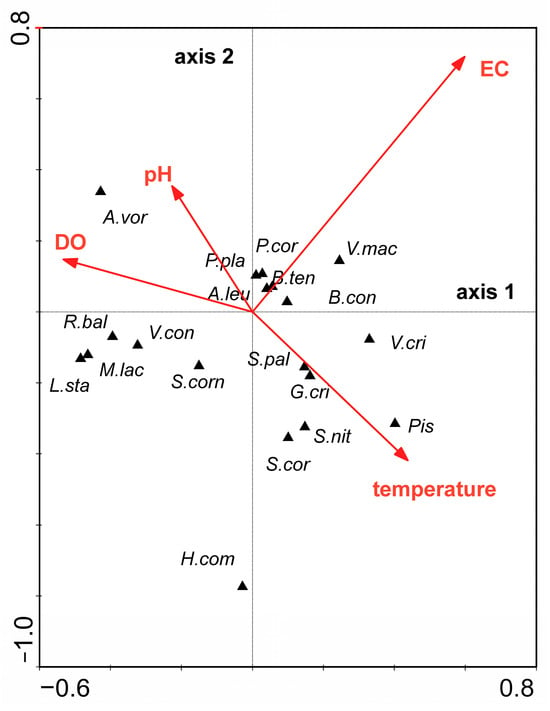
Figure 4.
Ordination diagram (biplot) based on canonical correspondence analysis (CCA) of the Mollusca data and the physical and chemical parameters of the water (arrows represent statistically significant parameters of the water). Abbreviations: EC—electrical conductivity; DO—dissolved oxygen; temperature—temperature of the water. A.vor—Anisus vortex; A.leu—Anisus leucostoma; B.con—Bathyomphalus contortus; B.ten—Bithynia tentaculata; G.cri—Gyraulus crista; H.com—Hippeutis complanatus; L.sta—Lymnaea stagnalis; M.lac—Musculium lacustre; P.cor—Planorbarius corneus; P.pla—Planorbis planorbis; Pis—Pisidium sp.; S.nit—Segmentina nitida; R.bal—Radix balthica; S.corn—Sphaerium corneum; S.cor—Stagnicola corvus; S.pal—Stagnicola palustris; V.cri—Valvata cristata; V.mac—Valvata macrostoma; V.con—Viviparus contectus.
Among environmental characteristics, the abundance of submerged and floating macrophytes, degree of habitat persistence and the fish predation pressure on molluscs also exerted a significant effect on the distribution of the mollusc species (The Monte Carlo test of significance of the first canonical axis: F-ratio = 9.146, p-value = 0.0020; test of significance of all canonical axes F-ratio = 4.138, p-value = 0.0020) (Figure 5). Viviparus contectus, H. complanatus, S. corneum, R. balthica and L. stagnalis were the species most associated with the abundance of floating and submerged macrophytes. The degree of habitat persistence exerted a significant effect on the distribution pattern of Gyraulus crista (Linnaeus, 1758), V. macrostoma, A. leucostoma, Pisidium sp., B. contortus and S. nitida (Figure 5).
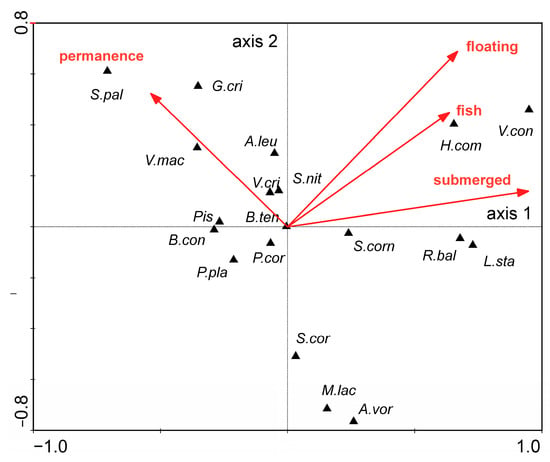
Figure 5.
Ordination diagram (biplot) based on canonical correspondence analysis (CCA) of the Mollusca data and the selected environmental data (arrows represent statistically significant environmental characteristics). Abbreviations: permanence—habitat permanence; floating—floating macrophytes; submerged—submerged macrophytes; fish—fish predation pressure. Abbreviations for mollusc species see Figure 4.
3.4. The Similarity of the Species Composition of Molluscs between the Types of Calcareous Fen Habitats
The cluster analysis showed the smallest distance between the fen pools (FP) and the undrained fens (UF), i.e., the species composition of mollusc communities in FP is most similar to the species composition of mollusc communities in UF. The FD joins the cluster FP-UF, i.e., the species composition of molluscs is more similar to the species composition in FP-UF than in the drained fens (DF) (Figure 6).
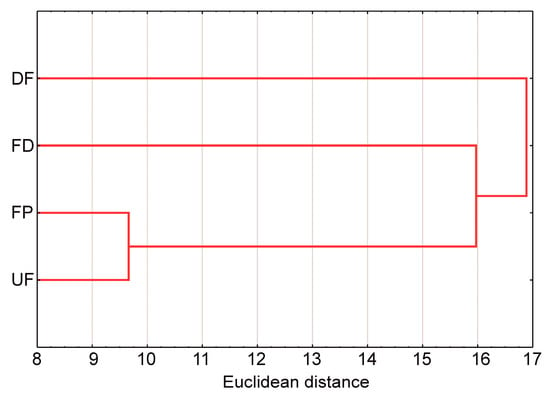
Figure 6.
Dendrogram for a nearest neighbour cluster analysis based on Euclidean distances for the mollusc communities of the calcareous fen habitats. Abbreviations: UF—undrained fens, DF—drained fens, FD—fen ditches, FP—fen pools.
4. Discussion
4.1. Diversity of Mollusc Communities in Calcareous Fen Habitats
Our survey showed that unique calcareous fen habitats offer the aquatic organisms harsh environmental conditions: relatively high temperature of the water up to 33.29 °C (undrained fens), oxygen deficits in the water, high pH up to 11.08 (fen pools) and conductivity above 3000 μS cm−1 (fen ditches). Therefore, they have to face extreme environmental conditions. Our survey of the mollusc communities revealed the occurrence of 27 species, including 4 bivalve species in unique calcareous fen habitats. In comparison, 28 mollusc species including 9 bivalve species were recorded in the Wicken Fen National Nature Reserve, UK [17], 10 mollusc species in the Central European fens [9,18] and 11 mollusc species in Scandinavian fens [19]. Our results showed that unique calcareous fen environments are inhabited by unique mollusc species. Due to their adaptive biology and ecology, they can survive oxygen deficits in the water, high pH and high water temperatures, changes in salinity and drying of aquatic environments. However, only one species, typical of swamps, peat bog reservoirs, drainage ditches and periodic water bodies, P. planorbis was eudominant and euconstant in the calcareous fen habitats. Both P. planorbis and P. corneus (euconstant in the calcareous fen habitats) inhabit water bodies rich in calcium and higher pH with conductivity of about 1300 µS cm−1 and increasing salinity [20]. Planorbarius corneus and P. planorbis, tolerant to dissolved oxygen deficits, can survive a few months without water. Both species retract deep into shells and form mucous membranes (epiphragm) between the aperture and the body which prevent them from desiccation [13]. Our results confirmed the finding of [21] that planorbid species dominate in mollusc communities of temporary (periodic) water bodies. Due to the specific biology and the drought resistance, P. corneus, P. planorbis, A. leucostoma and A. spirorbis can inhabit calcareous fen habitats. Bathyomphalus contortus, S. nitida and A. leucostoma prefer permanent water bodies; however, they can survive in periodic environments of calcareous fen habitats producing epiphragm. On the other hand, some planorbid species, i.e., A. vortex and H. complanatus prefer permanent water bodies because they are less drought-resistant. Therefore these species are accedents in mollusc communities in the calcareous fen habitats. Two prosobranch species typical for periodic water bodies, i.e., V. cristata and V. macrostoma were accessory species in mollusc communities of calcareous fen habitats. What is more, V. cristata tolerates pH up to 9.6 and salinity up to 5 PSU, whereas V. macrostoma tolerates conductivity of up to 720 µS cm−1 [13,22]. Some prosobranch and planorbid species, i.e., V. cristata, V. macrostoma, B. contortus and S. nitida which were recorded in calcareous fen habitats, are considered to be associated usually with floating and submerged macrophytes [13]. The abundance of macrophytes as one of the predictive environmental factors that influence the mollusc diversity in European fen habitats was highlighted by [19].
Our results showed that the species composition of mollusc communities in the undrained fens (UF) was most similar to the species composition of mollusc communities in the fen pools (FP) as well as to the fen ditches (FD). This phenomenon can be explained by the relatively high frequency and dominance indices of planorbid species, especially P. corneus, P. planorbis and B. contortus in the UF and FP (eudominant and dominant). Planorbid species, especially P. corneus and P. planorbis can be considered typical of the extreme environmental conditions including those in the calcareous fen habitats. It should be also emphasised that the fen pools are inhabited by diverse molluscs including rare and near-threatened species on a European scale, i.e., A. vorticulus. The mollusc communities in fen pools (FP) are very similar to those in untransformed fens. This phenomenon can be used in the conservation and protection of these valuable mollusc communities. The fen pools are easy to shape by interfering with the spatial structure and the composition and structure of vegetation. They can also be easily created de novo in the case of a decline in the water table in fens, which is likely as the climate warms and droughts increase. The potential of these water bodies in the context of protecting dragonflies (Odonata) was already pointed out by [23].
Species diversity of fen molluscs increases from acid bogs towards calcareous fens. Even a very small habitat fragment of a calcareous fen hosts more species than a large, calcium-rich Sphagnum fen [9,18]. According to [9,18,24], the extremely mineral-rich European low-altitude fens are clear diversity hotspots for mollusc fauna, especially large and well-preserved fragments. Therefore the calacareous fen habitats located within two macroregions: the Western Polesie and the Wołyń Polesie, Poland should be considered as unique.
4.2. Molluscs in Relation to the Environmental Variables in Unique Calcareous Fen Habitats
Our research showed that pH, conductivity, the abundance of submerged and floating macrophytes or the fish predation pressure on molluscs exerted a significant effect on the distribution of the mollusc species. Freshwater molluscs are especially sensitive to low pH. Gastropods are more sensitive to low pH than fish and they can give an early warning signal of acidification of freshwater ecosystems. In the case of a pH decrease, gastropods will disappear from freshwater habitats before fish [25]. Calcium is necessary for the development of gastropod embryos and the biomineralisation of shells. At low pH, calcium uptake by gastropods can be impaired. Low pH changes shell properties, lowers adult growth rate and reduces fecundity [26]. In contrast, pH values ranged from 6.88 to 11.08 in investigated calcareous fen habitats. Therefore, the positive correlation between the distribution of mollusc species and pH can be explained by their physiological make up. In comparison, the limiting calcium concentration lies between 2.7 and 4 mg dm−3, and pH 4.8 and 5.5 for mollusc species in Sphagnum-fens. One of the exceptions, the fingernail clam Pisidium casertanum (Poli, 1791) can inhabit such water conditions [27]. Our results showed the correlation between the diversity of mollusc species and lower values of conductivity, whereas M. lacustre was recorded in fen ditches with relatively high conductivity. These results confirmed the survey of [20] who found that most of the mollusc species occur at sampling sites with median values of conductivity from 400 to 600 μS cm−1, whereas M. lacustre is more tolerant to the higher values of conductivity than other species. Our results also support the survey of [9,28] that pH and conductivity are important parameters structuring the mollusc communities in calcareous fen habitats in Europe. These results are consistent with [17], who highlighted the importance of dissolved oxygen, the temperature of the water and conductivity for aquatic organisms including mollusca in fen habitats.
Oxidation-reduction potential is rarely used in studies on aquatic ecology. However, some authors recommend it as the best predictor of species diversity for many taxa groups in fens including the calcareous fen habitats because potential redox reflects changes in water chemistry along a mineral richness gradient [9]. Redox potentials determine the potential energy requirements or yields during biotic or abiotic processes that transform chemical compounds in water habitats. The concentration of dissolved oxygen in the water is one of the primary determinants of redox. If dissolved oxygen is present in the water, the value of redox amounts to 200 mV [29]. Our results showed wide ranges of the dissolved oxygen concentration in the water and redox potential above 200 mV. Thus, the dissolved oxygen in the water was one of the predictors of mollusc diversity in calcareous fen habitats. The abundance of submerged and floating macrophytes and the fish predation pressure on molluscs exerted a significant effect on the distribution of the mollusc species in calcareous fen habitats. Gastropods can graze on submerged macrophytes removing periphyton and decaying tissues. A larger population of macrophytes increases the surface area of periphyton, sites for shelter and oviposition. Gastropods reduce the density of the algal and bacterial periphyton, which are potentially deleterious to macrophytes. Such interactions between submerged macrophytes, gastropods, periphyton algae and bacteria can be regarded as mutualistic [30]. Pulmonate gastropods can achieve a net uptake of the dissolved organic carbon (DOC) produced by macrophytes and metabolise it [31]. The important role of submerged macrophytes as a rich food source for gastropods and safe shelter from predators in shallow waterbodies was also confirmed by [32]. The increase in water temperature can result in higher periphyton biomass and in turn, higher biomass and density of gastropods in fish-absent ecosystems. With the decrease in gastropod populations, the fish population can decrease over time and the growth of periphyton can increase [33].
4.3. Calcareous Fens as Unique Habitats for Rare and Threatened Species
Natura 2000 is the largest coordinated network of protected areas in the world and it preserves specific types of natural habitats and species that are considered valuable and endangered throughout Europe. Natura 2000, designated under the EU Habitats and Birds Directive includes the area of our surveys, i.e., calcareous fen habitats Torfowiska Chełmskie (PLH060023) and Chełmskie Torfowiska Węglanowe (PLB060002). Calcareous fens are listed in Annex I of the European Union (EU) Habitats Directive ([8], habitat code 7230, 7210 and 7140) as habitats requiring special conservation measures.
According to the European Red List of Non-marine Molluscs [34], 75 species (8.8% of European mollusc species) are classified as Near Threatened (NT), including A. vorticulus. In Poland, in addition to the protection provided by law, A. vorticulus is regarded as rare and threatened with extinction and therefore is included in the Red List of Threatened Animals as Near Threatened (NT) [35]. The dramatic decrease in the population density has led to a consideration of A. vorticulus within European law regulations. Therefore, under Annexes II and IV to the Habitats Directive [8] of EU, and its consolidation version of 01.01.2007, which includes the latest version of the annexes, A. vorticulus is one of the species of Community interest whose conservation requires designation of special conservation areas within the Habitats Directive Natura 2000. Anisus vorticulus was subrecedent and accident in mollusc communities of calcareous fen habitats (fen pools). This species prefers oligo and mesotrophic, well-vegetated and calcium-rich water bodies including marshes, floodplains, pools, oxbow lakes or slow-flowing rivers [36,37]. Channelisation, dredging, acidification, habitat fragmentation, and nutrient enrichment are the major threats to A. vorticulus [38]. The protected site PLH60023 as the Special Area of Conservation (SAC), which includes three separate, fragmented enclaves protects habitats and some species of flora and fauna. However, the list does not include A. vorticulus, which occurs in this area in the results of our surveys [39].
Mollusc species which occur in the calcareous fen habitats are included in the European Red List of Non-marine Molluscs and the Red List of Threatened Animals in Poland. According to the Red List of Threatened Animals in Poland, B. leachii is listed as a Near Threatened (NT), V. macrostoma and M. lacustre are classified as Vulnerable (VU). In contrast, Stagnicola palustris (O.F. Müller, 1774), Stagnicola corvus (Gmelin, 1778), A. spirorbis and H. complanatus are classified as Data Deficient (DD) species [34,35,40]. These species are rare in European countries [41,42]. In total, all mollusc species which were recorded in the calcareous fen habitats are included in the European Red List of Non-marine Molluscs excluding P. acuta.
Gastropod species B. leachii (subrecedent and accedent in the calcareous fen habitats), extremely resistant to desiccation, inhabits shallow, well-vegetated ditches, wetlands, ponds and oxbow lake shallow areas of slowly running waters [13]. Bithynia leachii is classified as Critically Endangered (CR) in some European countries [41]. Valvata macrostoma (accessory species in calcareous fen habitats), one of the most threatened species associated with the regulation of river valleys and wetland drainage, inhabits ditches, flooded meadows, peat bog reservoirs and ponds. Valvata macrostoma prefers well-vegetated water bodies of conductivity up to 720 µS cm−1 [13,22].
According to [43], M. lacustre (recedent and accedent in the calcareous fen habitats) is the most common species in swamps, ponds, marsh drains as well as in the well-vegetated margins of rivers and canals.
The area of PLH60023 is much smaller and fragmentary and does not overlap with the area of PLB060002. However, waterbirds play an unquestionable role in the widespread distribution of aquatic organisms including molluscs among wetlands isolated by land [44]. Despite limited mobility, molluscs can be transferred by many local and long-distance vectors, such as birds or insects. Wildfowl can transfer molluscs internally (if molluscs survive passage through their digestive system) or externally (if molluscs adhere to beaks, feathers and legs) [45].
Freshwater molluscs, which are hololimnic organisms, have limited mobility. Therefore, they reflect the abiotic or biotic state of aquatic ecosystems, which represents the impact of environmental change on the habitat, community and ecosystem, especially in the context of climatic and environmental emergencies. In Europe, molluscs are affected by a series of threats that combine to lead to declining populations [34]. The main threat concerns the decline in water quality as a result of anthropogenic pollution including eutrophication, changes to flow regimes, and over-frequent dredging. The drainage of wetlands and riverside meadows, agricultural drainage and over-frequent dredging, habitat fragmentation and climate change also pose a threat to freshwater molluscs [46].
5. Conclusions
The calcareous fen habitats that are listed in Annex I of the European Union (EU) Habitats Directive create a unique valuable ecosystem that contributes to the natural diversity of aquatic organisms. The present results showed the occurrence of 27 taxa, including 4 bivalve species. The calcareous fen habitats provide habitats for rare and threatened mollusc species that are classified into different categories of threat under the European Red List of Non-marine Molluscs and the Red List of Threatened Animals.
The unique calcareous fen habitats offer freshwater molluscs harsh environmental conditions: relatively high temperature of the water, oxygen deficits in the water, and conductivity. Therefore, molluscs of calcareous fen habitats have to face extreme environmental conditions. Climate change will further intensify harsh environmental conditions. An increase in air temperature affects the drying of aquatic environments, especially isolated and shallow ones. As the water temperature increases, the concentration of dissolved oxygen in the water decreases, the concentration of total dissolved solids increases, and conductivity and salinity increase.
Anisus vorticulus, one of the species of Community interest whose conservation requires designation of special conservation areas within the Habitats Directive Natura 2000, was subrecedent and accedent in mollusc communities of calcareous fen habitats (fen pools). Due to the research area sharing coverage with Natura 2000 sites, i.e., Torfowiska Chełmskie (PLH060023) designated under the EU Habitats Directive, A. vorticulus should be officially included in the lists of species protected by European law. Our survey showed that the fen pools are valuable secondary habitats preserving mollusc communities, which are also characteristic of the least transformed fens.
There are no man-made boundaries for aquatic organisms including molluscs and wildfowls that participate in their distribution, especially among wetlands isolated by land. One of the main threats to freshwater molluscs is the fragmentation of their habitats. Therefore the boundaries of the protected site PLH60023 as the Special Area of Conservation (SAC), which includes three separate, fragmented enclaves, should be extended and cover an area such as a special protection area for birds, i.e., PLB060002.
Calcareous fens are listed in Annex I of the European Union (EU) Habitats Directive (Council Directive 92/43/EEC, habitat code 7230, 7210 and 7140) as habitats requiring, special conservation measures. Therefore, some steps should be taken to reduce water pollution, fragmentation of aquatic environments, draining and other anthropogenic impacts that threaten calcareous fen habitats and aquatic organisms including molluscs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d16060350/s1, Table S1: Number of sampling sites, location of sampling sites, geographic coordinates and the type of calcareous fen habitats; Table S2: Date of sampling, number of sampling sites and mollusc specimens in calcareous fen habitats.
Author Contributions
Conceptualisation, P.B., A.T., I.L. and E.B.; methodology, P.B., A.T., W.P., P.S., I.L. and E.B.; software, I.L.; validation, I.L. and A.T.; formal analysis, P.B., A.T. and I.L.; investigation, A.T., P.B., W.P., P.S. and E.B.; resources, A.T. and P.B.; data curation, P.B., A.T. and I.L.; writing—original draft preparation, I.L.; writing—review and editing, I.L. and P.B.; visualisation, I.L.; supervision, P.B.; project administration, A.T. and P.B.; funding acquisition, A.T. All authors have read and agreed to the published version of the manuscript.
Funding
The material was collected during odonatological studies financed by the Doctoral Study of the Faculty of Biology and Biotechnology of Maria Curie-Skłodowska University and the individual grant for young employees and PhD students of this faculty entitled “Dragonflies (Odonata) as indicators of the condition of calcareous fens” (no. BS-M-11-010-17-1-18).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank the Regional Directorate for Environmental Protection in Lublin for permission to conduct research in nature reserves (permit numbers WPN.6205.1.52.2015.MO and WPN.6205.1.3.2018.MO). The authors are grateful to the anonymous Reviewers and Section “Freshwater Biodiversity” Editor-in-Chief for their valuable suggestions and comments, which significantly improved the quality of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Living Planet Report 2020. WWF (2020) Living Planet Report 2020—Bending the Curve of Biodiversity Loss; Almond, R.E.A., Grooten, M., Petersen, T., Eds.; WWF: Gland, Switzerland, 2020. [Google Scholar]
- Living Planet Report 2022. CWWF (2022) Living Planet Report 2022—Building a Naturepositive Society; Almond, R.E.A., Grooten, M., Juffe Bignoli, D., Petersen, T., Eds.; WWF: Gland, Switzerland, 2022. [Google Scholar]
- Bijkerk, E.; Regan, S.; Paul, M.; Johnston, P.M.; Coxon, C.; Gill, L.W. The challenge of developing ecohydrological metrics for vegetation communities in calcareous fen wetland systems. Front. Earth Sci. 2022, 10, 917233. [Google Scholar] [CrossRef]
- Singh, P.; Jiroušek, M.; Hájková, P.; Horsák, M.; Hájek, M. The future of carbon storage in calcareous fens depends on the balance between groundwater discharge and air temperature. Catena 2023, 231, 107350. [Google Scholar] [CrossRef]
- Štokmane, M.; Spuņģis, V. The influence of vegetation structure on spider species richness, diversity and community organization in the Apšuciems calcareous fen, Latvia. Anim. Biodivers. Conserv. 2016, 39, 221–236. [Google Scholar] [CrossRef]
- Štokmane, M.; Cera, I. Revision of the calcareous fen arachnofauna: Habitat affinities of the fen-inhabiting spiders. ZooKeys 2018, 802, 67–108. [Google Scholar] [CrossRef] [PubMed]
- Buczyńska, E.; Tarkowski, A.; Sugier, P.; Płaska, W.; Zawal, A.; Janicka, A.; Buczyński, P. Caddisflies (Trichoptera) of Protected Calcareous Fen Habitats: Assemblages, Environmental Drivers, Indicator Species, and Conservation Issues. Insects 2023, 14, 850. [Google Scholar] [CrossRef]
- Council Directive 92/43/EEC; Council Directive 92/43/EEC of 21 May 1992 on the Conservation of Natural Habitats and Wild Fauna and Flora. Council of the European Communities: Brussels, Belgium, 1992.
- Horsák, M.; Rádkowá, V.; Syrovátka, V.; Bojková, J.; Křoupalová, V.; Schenková, J.; Zajacová, J. Drivers of aquatic macroinvertebrate richness in spring fens in relation to habitat specialization and dispersal mode. J. Biogeogr. 2015, 42, 2112–2121. [Google Scholar] [CrossRef]
- Böhm, M.; Dewhurst-Richman, N.I.; Seddon, M.; Ledge, S.E.H.; Albrecht, C.; Allen, D.; Bogan, A.E.; Cordeiri, J.; Cummings, K.S.; Cuttelod, A.; et al. The conservation status of the world’s freshwater molluscs. Hydrobiologia 2021, 848, 3231–3254. [Google Scholar] [CrossRef]
- Ormerod, S.J.; Durance, I.; Terrier, A.; Swanson, A.M. Priority Wetland Invertebrates as Conservation Surrogates. Conserv. Biol. 2010, 24, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Directive 2009/147/EC; Directive 2009/147/EC of the European Parliament and of the Council of 30 November 2009 on the Conservation of Wild Birds (Codified Version); Official Journal of the European Union (L20/7). Publications Office of the European Union: Luxembourg, 2009; pp. 1–19.
- Piechocki, A.; Wawrzyniak-Wydrowska, B. Guide to Freshwater and Marine Mollusca of Poland; Bogucki Wydawnictwo Naukowe: Poznań, Poland, 2016. [Google Scholar]
- Górny, M.; Grüm, L. Metody Stosowane w Zoologii Gleby; Państwowe Wydawnictwo Naukowe: Warszawa, Poland, 1981. [Google Scholar]
- McCune, B.; Grace, J.B. Analysis of Ecological Communities; MjM Software Design: Gleneden Beach, OR, USA, 2002. [Google Scholar]
- Ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5), 2nd ed.; Microcomputer Power: Ithaca, NY, USA, 2002. [Google Scholar]
- Painter, D. Macroinvertebrate distributions and the conservation value of aquatic Coleoptera\Mollusca and Odonata in the ditches of traditionally managed and grazing fen at Wicken Fen\UK. J. Appl. Ecol. 1999, 36, 33–48. [Google Scholar] [CrossRef]
- Horsák, M.; Cernohorsky, N. Mollusc diversity patterns in Central European fens: Hotspots and conservation priorities. J. Biogeogr. 2008, 35, 1215–1225. [Google Scholar] [CrossRef]
- Schenková, V.; Horsák, M.; Hájek, M.; Hájková, P.; Díté, D. Mollusc assemblages of Scandinavian fens: Species composition in relation to environmental gradients and vegetation. Ann. Zool. Fenn. 2015, 52, 1–16. [Google Scholar] [CrossRef]
- Lewin, I. Mollusc communities of lowland rivers and oxbow lakes in agricultural areas with anthropogenically elevated nutrient concentration. Folia Malacol. 2014, 22, 87–159. [Google Scholar] [CrossRef]
- Jurkiewicz-Karnkowska, E. Diversity of aquatic malacofauna of temporary water bodies within the lower Bug river floodplain. Folia Malacol. 2011, 19, 9–18. [Google Scholar] [CrossRef][Green Version]
- Watson, A.M.; Ormerod, S.J. The distribution of three uncommon freshwater gastropods in the drainage ditches of British grazing marshes. Biol. Conserv. 2004, 118, 455–466. [Google Scholar] [CrossRef]
- Buczyński, P. Dragonflies (Odonata) of Anthropogenic Waters in Middle-Eastern Poland; Wydawnictwo Mantis: Olsztyn, Poland, 2015. [Google Scholar]
- Hájek, M.; Horsáková, V.; Hájková, P.; Coufal, R.; Díte, D.; Němec, T.; Horsák, M. Habitat extremity and conservation management stabilise endangered calcareous fens in a changing world. Sci. Total Environ. 2020, 719, 134693. [Google Scholar] [CrossRef]
- Økland, J. Factors regulating the distribution of fresh-water snails (Gastropoda) in Norway. Malacologia 1983, 24, 277–288. [Google Scholar]
- Hunter, R.D. Effects of low pH and low calcium concentration on the pulmonate snail Planorbella trivolvis: A laboratory study. Can. J. Zool. 1990, 68, 1382–1389. [Google Scholar] [CrossRef]
- Horsák, M.; Hájek, M. Composition and species richness of molluscan communities in relation to vegetation and water chemistry in the Western Carpathian spring fens: The poor–rich gradient. J. Moll. Stud. 2003, 69, 349–357. [Google Scholar] [CrossRef]
- Horsák, M.; Hájek, M.; Hájková, P.; Cameron, R.; Cernohorsky, N.; Apostolova, I. Mollusc communities in Bulgarian fens: Predictive power of the environment, vegetation, and spatial structure in an isolated habitat. Naturwissenschaften 2011, 98, 671–681. [Google Scholar] [CrossRef]
- Dodds, W.K.; Whiles, M.R. Freshwater Ecology: Concepts and Environmental Applications of Limnology, 3rd ed.; Elsevier: London, UK; Academic Press: Cambridge, MA, USA, 2020; ISBN 978-0-12-813255-5. [Google Scholar]
- Lodge, D.M. Macrophyte-gastropod associations: Observations and experiments on macrophyte choice by gastropods. Freshw. Biol. 1985, 15, 695–708. [Google Scholar] [CrossRef]
- Thomas, J.D.; Kowalczyk, C. Utilization of dissolved organic matter (DOM), from living macrophytes, by pulmonate snails: Implications to the “food web” and “module” concepts. Comp. Biochem. Phys. A 1997, 117, 105–119. [Google Scholar] [CrossRef]
- Lajtner, J.; Kozak, A.; Špoljar, M.; Kuczyńska-Kippen, N.; Dražina, T.; Perić, M.S.; Tkalčec, I.; Gottstein, S.; Zrinščak, I. Gastropod Assemblages Associated with Habitat Heterogeneity and Hydrological Shifts in Two Shallow Waterbodies. Water 2022, 14, 2290. [Google Scholar] [CrossRef]
- Cheng, H.; Feng, M.; Zhang, P.; Zhang, H.; Wang, H.; Xu, J.; Zhang, M. Effects of Warming on Aquatic Snails and Periphyton in Freshwater Ecosystems with and without Predation by Common Carp. Water 2023, 15, 153. [Google Scholar] [CrossRef]
- Cuttelod, A.; Seddon, M.; Neubert, E. European Red List of Non-Marine Molluscs; Publications Office of the European Union: Luxembourg, 2011. [Google Scholar]
- Piechocki, A. Gastropoda aquatica. Ślimaki wodne. In Czerwona Lista Zwierząt Ginących i Zagrożonych w Polsce. Red List of Threatened Animals in Poland; Głowaciński, Z., Ed.; Polish Academy of Sciences, Institute of Nature Conservation: Cracow, Poland, 2002; pp. 34–37. [Google Scholar]
- Terrier, A.; Castella, E.; Falkner, G.; Killeen, I. Species account for Anisus vorticulus (Troschel,1834) (Gastropoda: Planorbidae), a species listed in annexes II and IIV of the Habitats Directive. J. Conchol. 2006, 39, 193–205. [Google Scholar]
- Zettler, M.L. Some ecological peculiarities of Anisus vorticulus (Troschel 1834) (Gastropoda: Planorbidae) in northeast Germany. J. Conchol. 2013, 41, 389–398. [Google Scholar]
- Van Damme, D. Anisus Vorticulus. The IUCN Red List of Threatened Species 2012: e.T155966A738056; International Union for Conservation of Nature and Natural Resources: Gland, Switzerland, 2012. [Google Scholar] [CrossRef]
- Dz, U. 2021. Journal of Laws of the Republic of Poland, item 1752. Regulation of the Minister of Climate and Environment of 2 September 2021 on the special habitat protection area Torfowisko Chełmskie (PLH060023).
- Dyduch-Falniowska, A.; Zając, K. Bivalvia Małże. In Czerwona Lista Zwierząt Ginących i Zagrożonych w Polsce. Red List of Threatened Animals in Poland; Głowaciński, Z., Ed.; Polish Academy of Sciences: Warsaw, Poland; Institute of Nature Conservation Cracow: Kraków, Poland, 2002; pp. 23–26. [Google Scholar]
- Beran, L.; Horsák, M. Distribution of Bithynia leachii (Sheppard, 1823) and Bithynia troschelii (Paasch, 1842) (Gastropoda: Bithyniidae) in the Czech Republic. Malacol. Bohemoslov. 2009, 8, 19–23. [Google Scholar] [CrossRef]
- Gojšina, V.; Marković, V.; Karan-Žnidaršič, T. New Insight on the Presence of Several Freshwater Gastropod Species Considered Rare in Serbia. Acta Zool. Bul. 2024, 76, 43–48. [Google Scholar]
- Killeen, I.; Aldridge, D.; Oliver, G. Freshwater Bivalves of Britain and Ireland; FSC, AIDGSP Occasional Publication 82; Field Studies Council: Bonn, Germany, 2004. [Google Scholar]
- van Leeuwen, C.H.A.; der Velde, G. Prerequisites for Flying Snails: External Transport Potential of Aquatic Snails by Waterbirds. Freshw. Sci. 2012, 31, 963–972. [Google Scholar] [CrossRef]
- Gittenberger, E. Long-distance dispersal of molluscs: ‘Their distribution at first perplexed me much’. J. Biogeogr. 2012, 39, 10–11. [Google Scholar] [CrossRef]
- Lewin, I.; Stępień, E.; Szlauer-Łukaszewska, A.; Pakulnicka, J.; Stryjecki, R.; Pešić, V.; Bańkowska, A.; Szućko-Kociuba, I.; Michoński, G.; Krzynówek, Z.; et al. Drivers of the Structure of Mollusc Communities in the Natural Aquatic Habitats along the Valley of a Lowland River: Implications for Their Conservation through the Buffer Zones. Water 2023, 15, 2059. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).