Exceptional In Situ Preservation of Chondrocranial Elements in a Coniacian Mosasaurid from Colombia
Abstract
1. Introduction
2. Materials and Methods
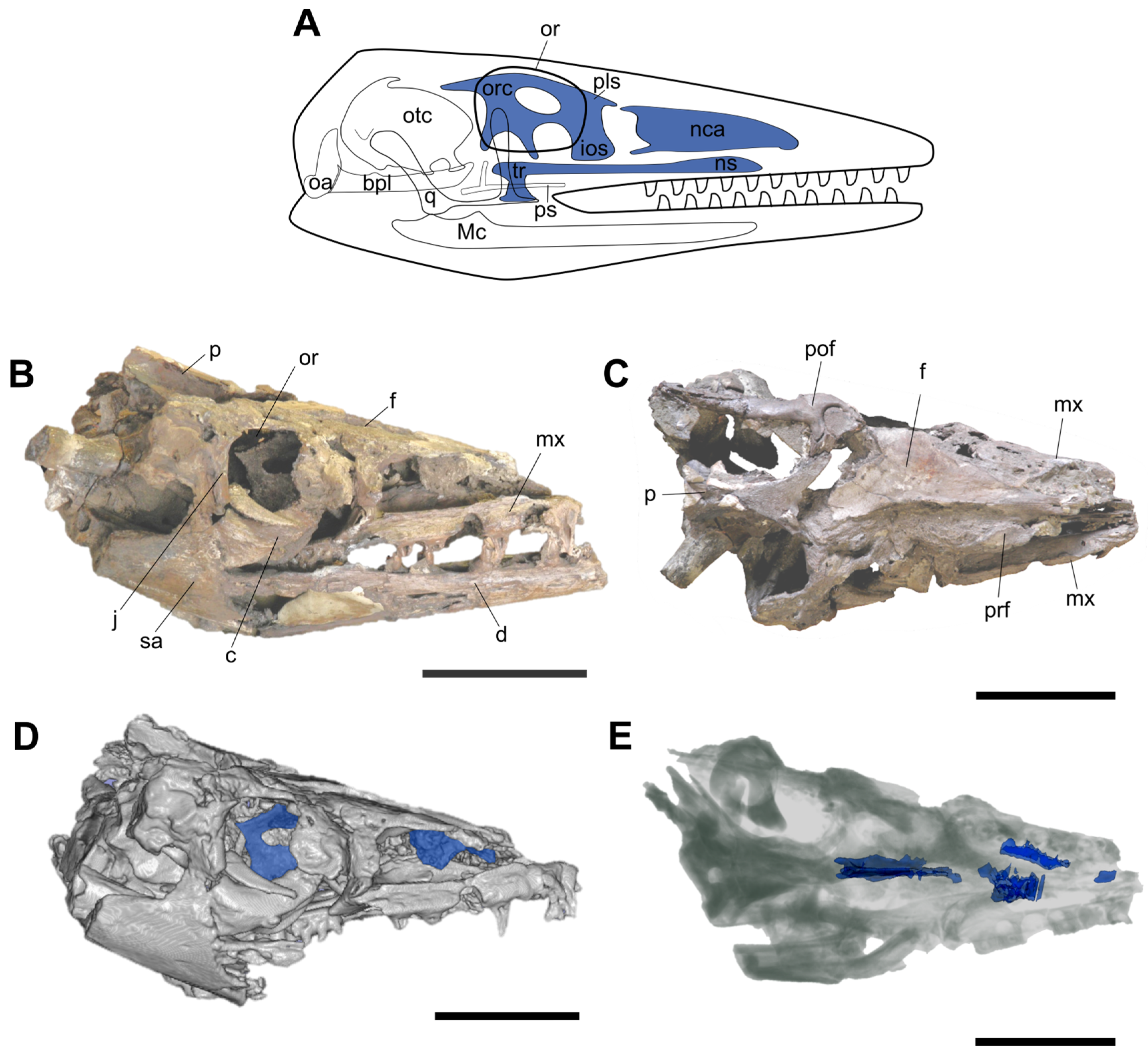
3. Anatomical Description
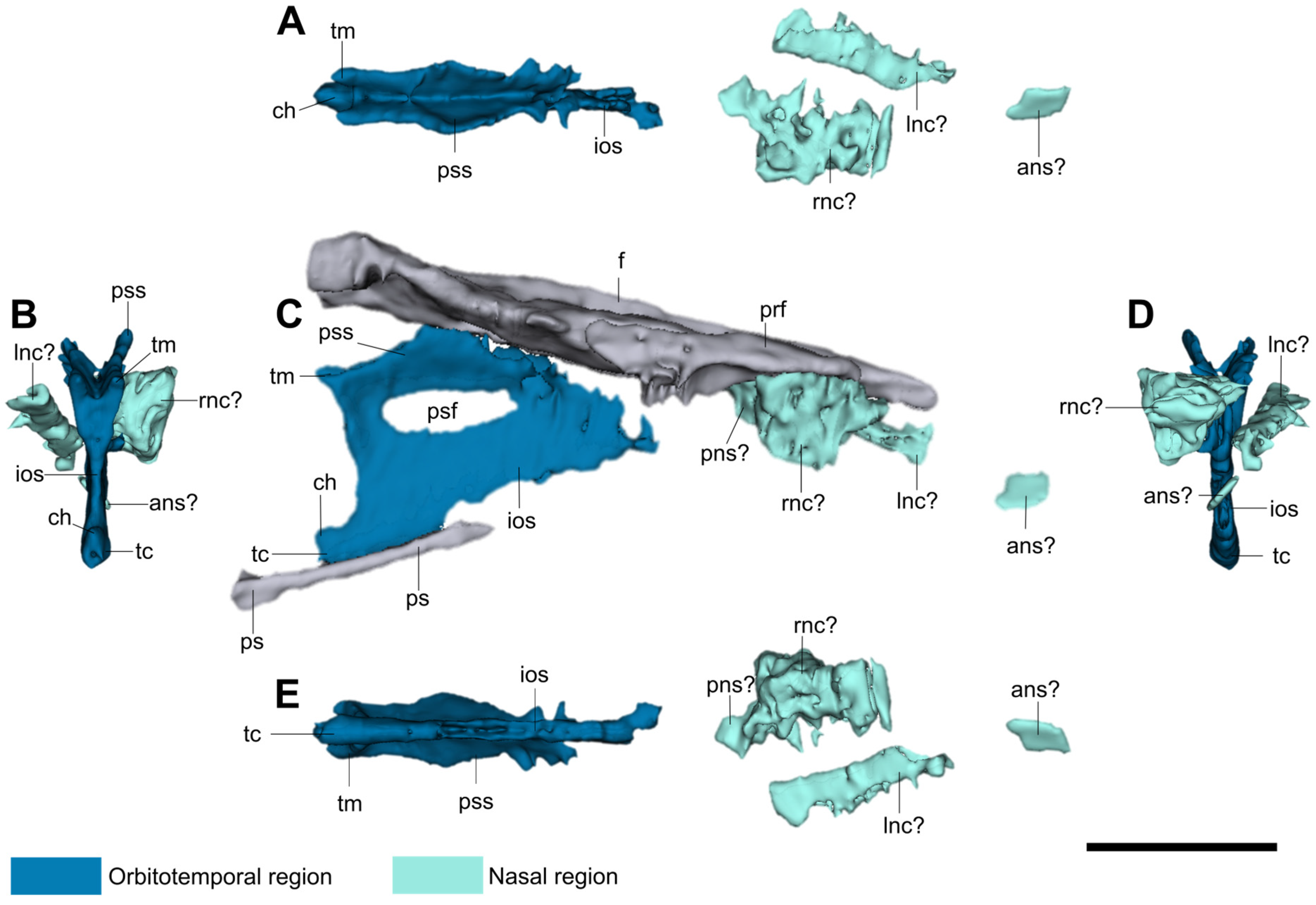
3.1. Nasal Region
3.2. Orbitotemporal Region
4. Functional Implications
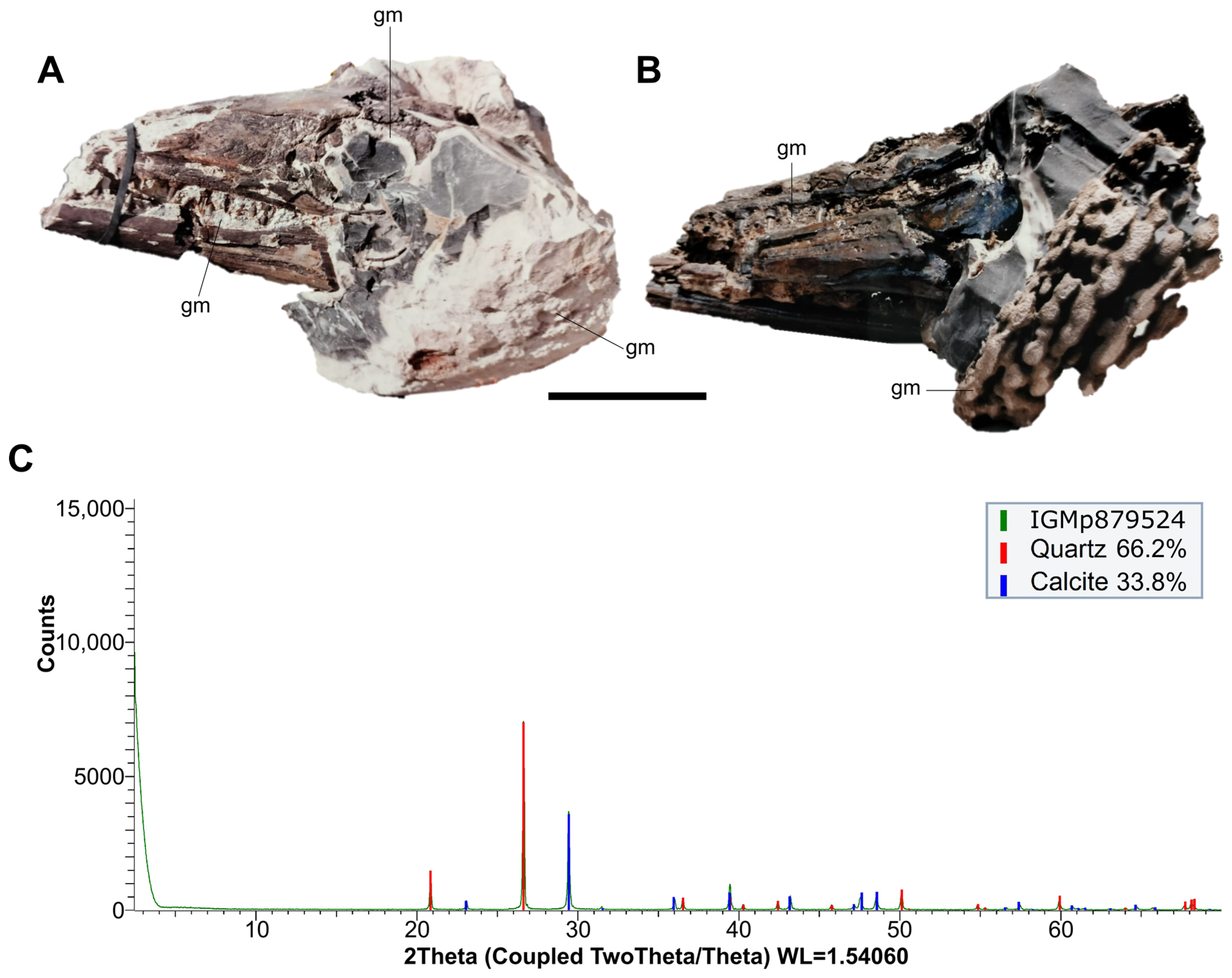
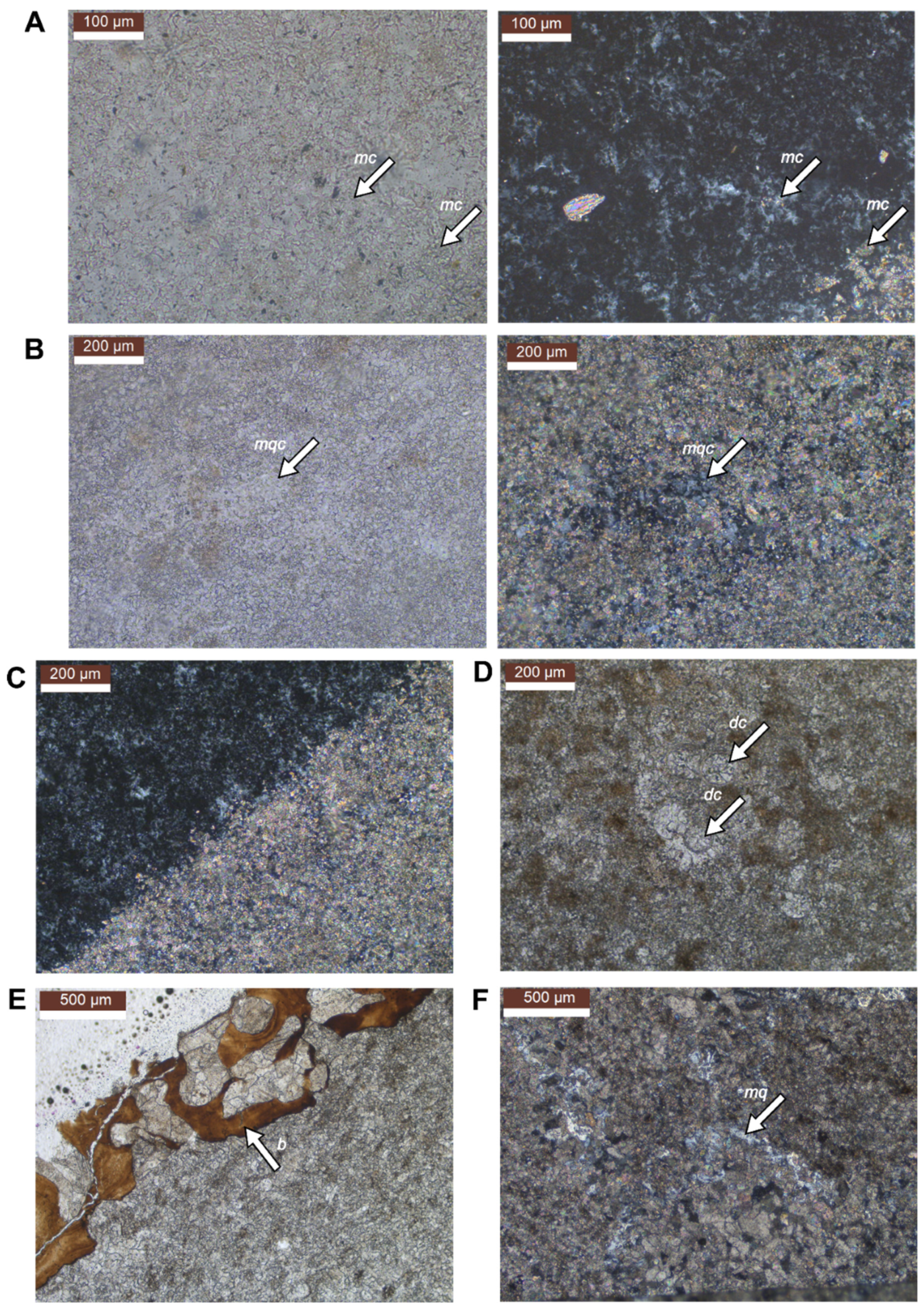
5. Preservation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Beer, G.R. The early development of the chondrocranium of the lizard. Q. J. Microsc. Sci. 1930, 73, 707–739. [Google Scholar]
- Bellairs, A.D.; Kamal, A.M. The chondrocranium and the development of the skull in recent reptiles. In Biology of the Reptilia, Volume 11, Morphology F; Gans, C., Parsons, T.S., Eds.; Academic Press: New York, NY, USA, 1981; Volume 11, pp. 1–283. [Google Scholar]
- Jones, M.E.; Gröning, F.; Aspden, R.M.; Dutel, H.; Sharp, A.; Moazen, M.; Fagan, M.J.; Evans, S.E. The biomechanical role of the chondrocranium and the material properties of cartilage. Vertebr. Zool. 2020, 70, 699–715. [Google Scholar] [CrossRef]
- Kardong, K. Comparative Anatomy, Function, Evolution, 8th ed.; Mc Graw Hill: New York, NY, USA, 2019; pp. 1–814. [Google Scholar]
- Bellairs, A.D. The anterior brain-case and interorbital septum of Sauropsida, with a consideration of the origin of snakes. Zool. J. Linn. Soc. 1949, 41, 485–512. [Google Scholar] [CrossRef]
- Shrivastava, R.K. The structure and development of the chondrocranium of Varanus. Part I. The development of the ethmoidal region. Okajima Folia Anat. Jpn. 1963, 39, 55–83. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, R.K. The structure and development of the chondrocranium of Varanus. Part II. The development of the orbito-temporal region. J. Morphol. 1964, 115, 97–108. [Google Scholar] [CrossRef]
- Shrivastava, R.K. The structure and development of the chondrocranium of Varanus. Part III. The otic and occipital regions, basal plate, viscerocranium, and certain features of the osteocranium. Morphol. Jahrb. 1964, 106, 147–187. [Google Scholar]
- Yaryhin, O.; Werneburg, I. The origin of orbitotemporal diversity in lepidosaurs: Insights from tuatara chondrocranial anatomy. Vertebr. Zool. 2020, 69, 169–181. [Google Scholar] [CrossRef]
- Camp, C.L. California Mosasaurs; Memoirs of the University of California; University of California Press: Berkeley, CA, USA, 1942; Volume 13, pp. 1–68. [Google Scholar]
- Russel, D. Systematics and morphology of American mosasaurs (Reptilia, Sauria). Bull. Peabody Mus. Nat. Hist. 1967, 23, 1–241. [Google Scholar]
- Páramo-Fonseca, M.E. Mosasauroids from Colombia. Bull. Soc. Geol. Fr. 2012, 183, 103–109. [Google Scholar] [CrossRef]
- Terraza-Melo, R. “Formación La Luna”: Expresión espuria en la geología colombiana. In Estudios Geológicos y Paleontológicos Sobre el Cretácico en la Región del Embalse del río Sogamoso, Valle Medio del Magdalena, 1st ed.; Etayo-Serna, F., Ed.; Servicio Geológico Colombiano: Bogotá, Colombia, 2019; Compilación de los Estudios Geológicos Oficiales en Colombia Volume XXIII; pp. 303–362. [Google Scholar]
- Morales, L.G.; Podesta, D.J.; Hatfield, W.C.; Tanner, H.; Jones, S.H.; Barker, M.H.S.; O’Donoghue, D.J.; Mohler, C.E.; Dubois, E.P.; Jacobs, C.; et al. General Geology and oil occurrence of Middle Magdalena Valley, Colombia. In Habitat of Oil; American Association of Petroleum Geologist: Tulsa, OK, USA, 1958. [Google Scholar]
- Thorez, J. Practical Identification of Clay Minerals; Institute of Mineralogy—Liege State University: Liege, Belgium, 1976; pp. 1–90. [Google Scholar]
- Bonilla, G.; Sarmiento, G.A.; Gaviria, S. Proveniencia y transformación diagenética de minerales arcillosos del Maastrichtiano—Paleoceno al norte de Bogotá, Cordillera Oriental de Colombia. Geol. Column 2011, 36, 179–195. [Google Scholar]
- Bellairs, A.D. Observations on the snout of Varanus, and a comparison with that of other lizards and snakes. J. Anat. 1949, 83, 116–146. [Google Scholar] [PubMed]
- Konishi, T. A mosasaur (Squamata: Mosasauridae) sneeze: A hypothesis concerning salt excretion in the top predators of the Cretaceous Seas. In Proceedings of the 75th Annual Meeting of the Society of Vertebrate Paloentology, Dallas, TX, USA, 14–17 October 2015; MacKenzie, A., Maxwell, E., Miller-Camp, J., Eds.; 2015. [Google Scholar]
- Yaryhin, O.; Werneburg, I. Tracing the developmental origin of a lizard skull: Chondrocranial architecture, heterochrony, and variation in lacertids. J. Morphol. 2018, 279, 1058–1087. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.E.; Gröning, F.; Dutel, H.; Sharp, A.; Fagan, M.J.; Evans, S.E. The biomechanical role of the chondrocranium and sutures in a lizard cranium. J. R. Soc. Interface 2017, 14, 20170637. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yaryhin, O.; Koyabu, D.; Werneburg, I. Morphological associtaion betwwen muscle attachments and ossification sites in the late cartilaginous skull of tuatara embryos. J. Morphol. 2022, 283, 908–931. [Google Scholar] [CrossRef] [PubMed]
- Yaryhin, O.; Klembara, J.; Pichugin, Y.; Kaucka, M.; Werneburg, I. Limb reduction insquamate reptiles correlates with the reduction of the chondrocranium: A case study on serpentiform anguids. Dev. Dyn. 2021, 250, 1300–1317. [Google Scholar] [CrossRef] [PubMed]
- Montoya Arenas, D.M. Formación La Paja: Descripción de la sección tipo. Influencia de los tapices microbiales en su génesis. In Estudios Geológicos y Paleontológicos Sobre el Cretácico en la Región del Embalse del Río Sogamoso, Valle Medio del Magdalena, 1st ed.; Etayo-Serna, F., Ed.; Servicio Geológico Colombiano: Bogotá, Colombia, 2019; Compilación de los Estudios Geológicos Oficiales en Colombia Volume XXIII; pp. 55–156. [Google Scholar]
- Trewin, N.H.; Fayers, S.R. Sedimentary Rocks|Chert. In Encyclopedia of Geology; Richard, C., Selley, L., Cocks, M., Ian, R., Palmer, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 3, pp. 154–196. [Google Scholar] [CrossRef]
- Knauth, L.P. Chapter 7. Petrogenesis of Chert. In Silica: Physical Behavior, Geochemistry, and Materials Applications, 1st ed.; Heaney, P., Prewit, C., Gibbs, G., Eds.; De Gruyter: Berlin, Germany, 1994; pp. 233–258. [Google Scholar] [CrossRef]
- McNamara, M.E.; Orr, P.J.; Kearns, S.L.; Alcala, L.; Anadon, P.; Penalver Molla, E. Soft tissue preservation in Miocene frogs from Libros, Spain: Insights into the genesis of decay microenvironments. PALAIOS 2009, 24, 107–117. [Google Scholar] [CrossRef]
- Muscente, A.D.; Schiffbauer, J.D.; Broce, J.; Laflamme, M.; O’Donnell, K.; Boag, T.H.; Meyer, M.; Hawkins, A.D.; Huntley, J.W.; McNamara, M.; et al. Exceptionally preserved fossil assemblages through geologic time and space. Gondwana Res. 2017, 48, 164–188. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Páramo-Fonseca, M.E.; Narváez-Rincón, J.A.; Benavides-Cabra, C.D.; Yanez-Leaño, C.F. Exceptional In Situ Preservation of Chondrocranial Elements in a Coniacian Mosasaurid from Colombia. Diversity 2024, 16, 285. https://doi.org/10.3390/d16050285
Páramo-Fonseca ME, Narváez-Rincón JA, Benavides-Cabra CD, Yanez-Leaño CF. Exceptional In Situ Preservation of Chondrocranial Elements in a Coniacian Mosasaurid from Colombia. Diversity. 2024; 16(5):285. https://doi.org/10.3390/d16050285
Chicago/Turabian StylePáramo-Fonseca, María Eurídice, José Alejandro Narváez-Rincón, Cristian David Benavides-Cabra, and Christian Felipe Yanez-Leaño. 2024. "Exceptional In Situ Preservation of Chondrocranial Elements in a Coniacian Mosasaurid from Colombia" Diversity 16, no. 5: 285. https://doi.org/10.3390/d16050285
APA StylePáramo-Fonseca, M. E., Narváez-Rincón, J. A., Benavides-Cabra, C. D., & Yanez-Leaño, C. F. (2024). Exceptional In Situ Preservation of Chondrocranial Elements in a Coniacian Mosasaurid from Colombia. Diversity, 16(5), 285. https://doi.org/10.3390/d16050285





