Soil Moisture and Litter Coverage Drive the Altitude Gradient Pattern of Soil Arthropods in a Low-Elevation Mountain
Abstract
1. Introduction
2. Study Area and Research Methods
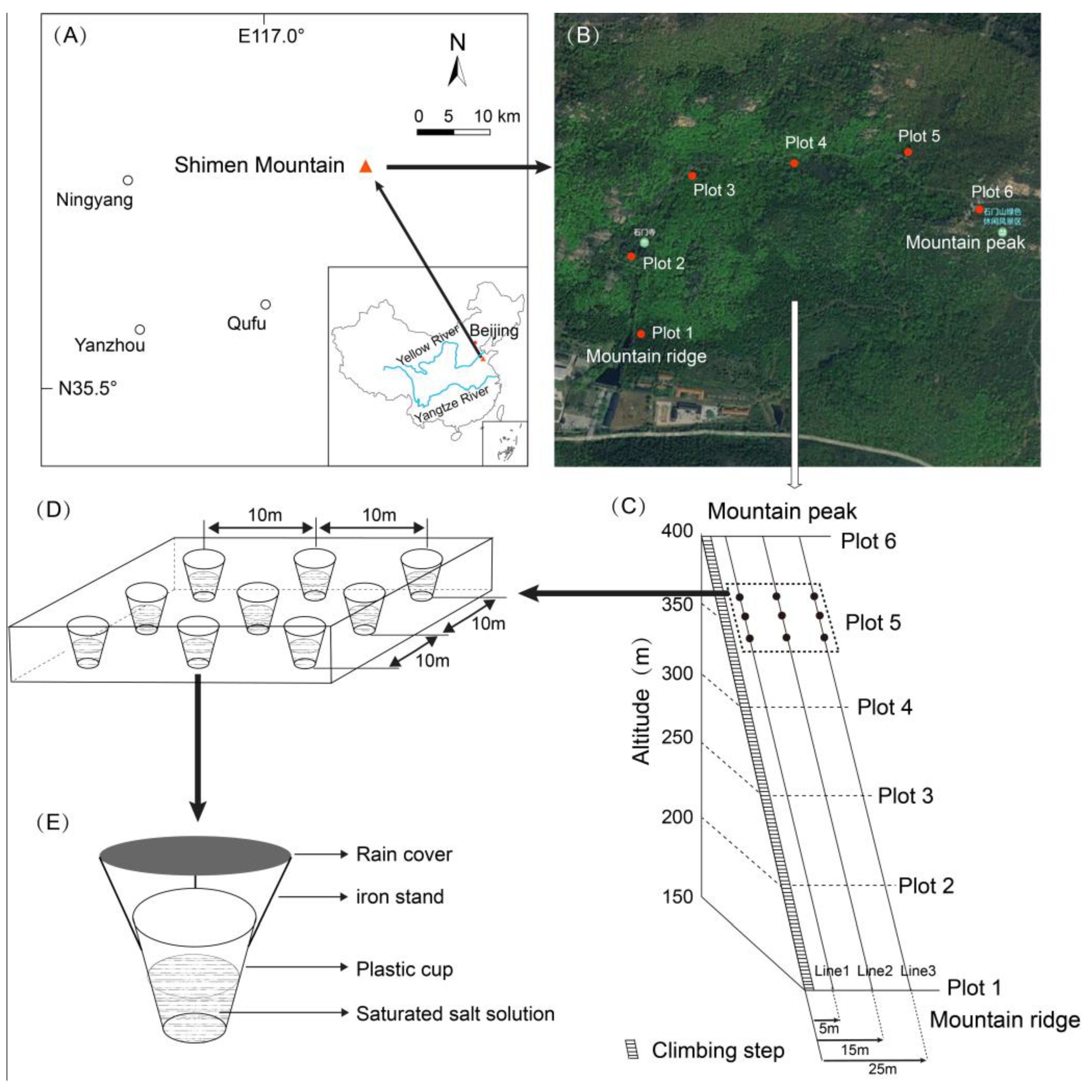
2.1. Plot Description
2.2. Research Methods
2.2.1. Sampling Plot Setting, Soil Arthropod Collection and Treatment
2.2.2. Environmental Factor Determination
2.2.3. Data Analysis
3. Results
3.1. Environmental Factors
3.2. Species Composition of Soil Arthropods
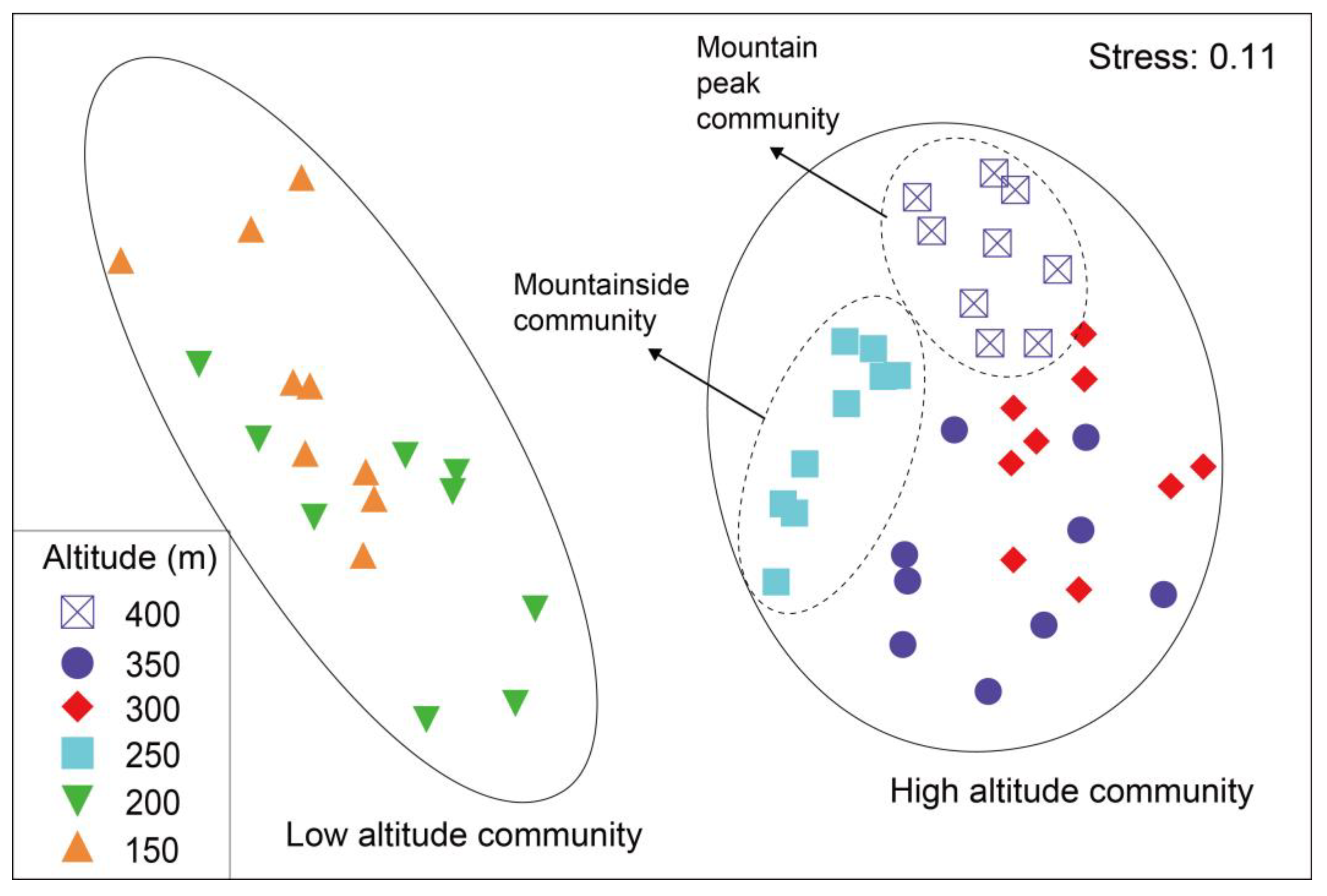
3.3. Soil Arthropod Community Structure
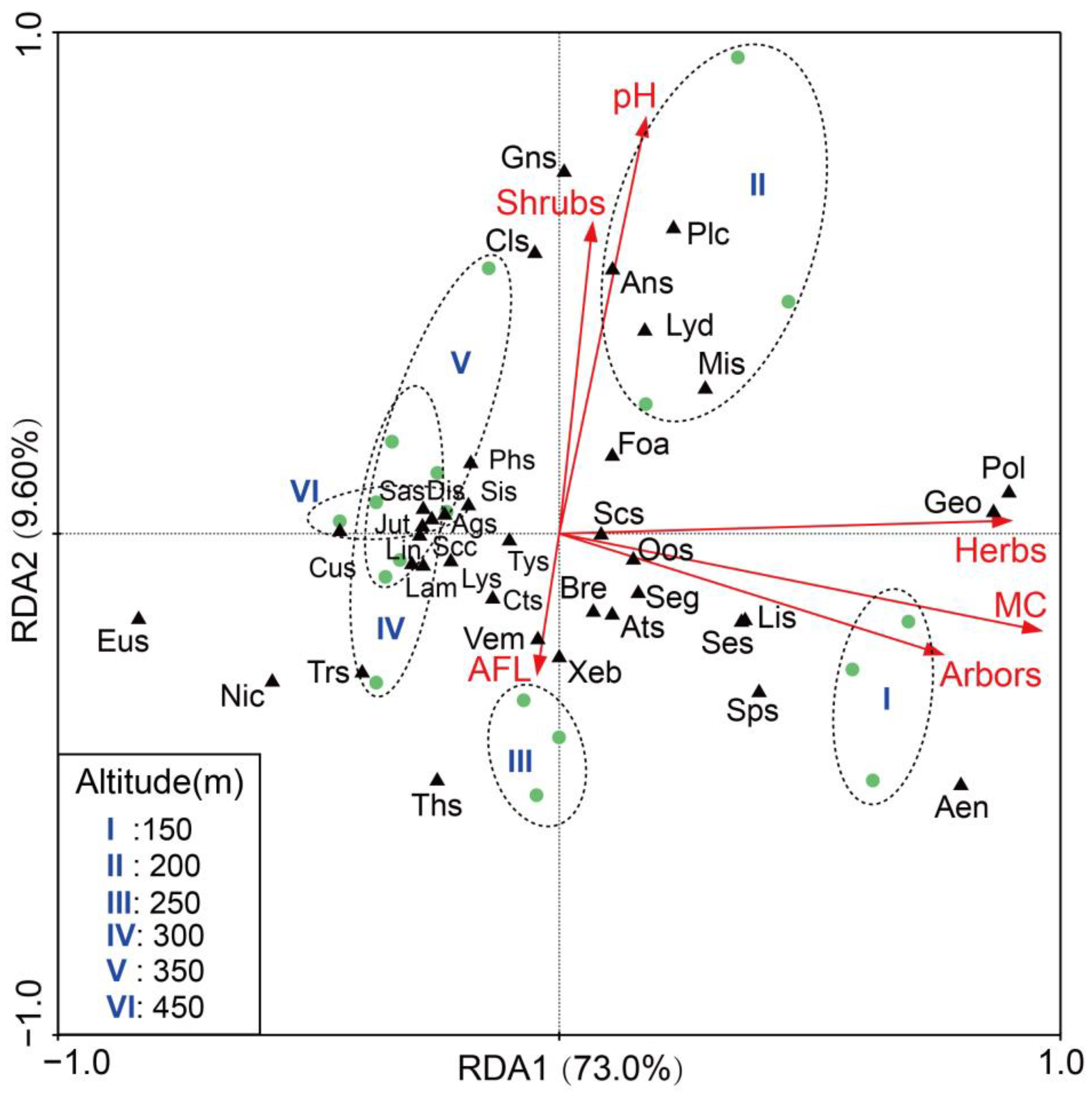
3.4. Impact of Environmental Factors on Soil Arthropods
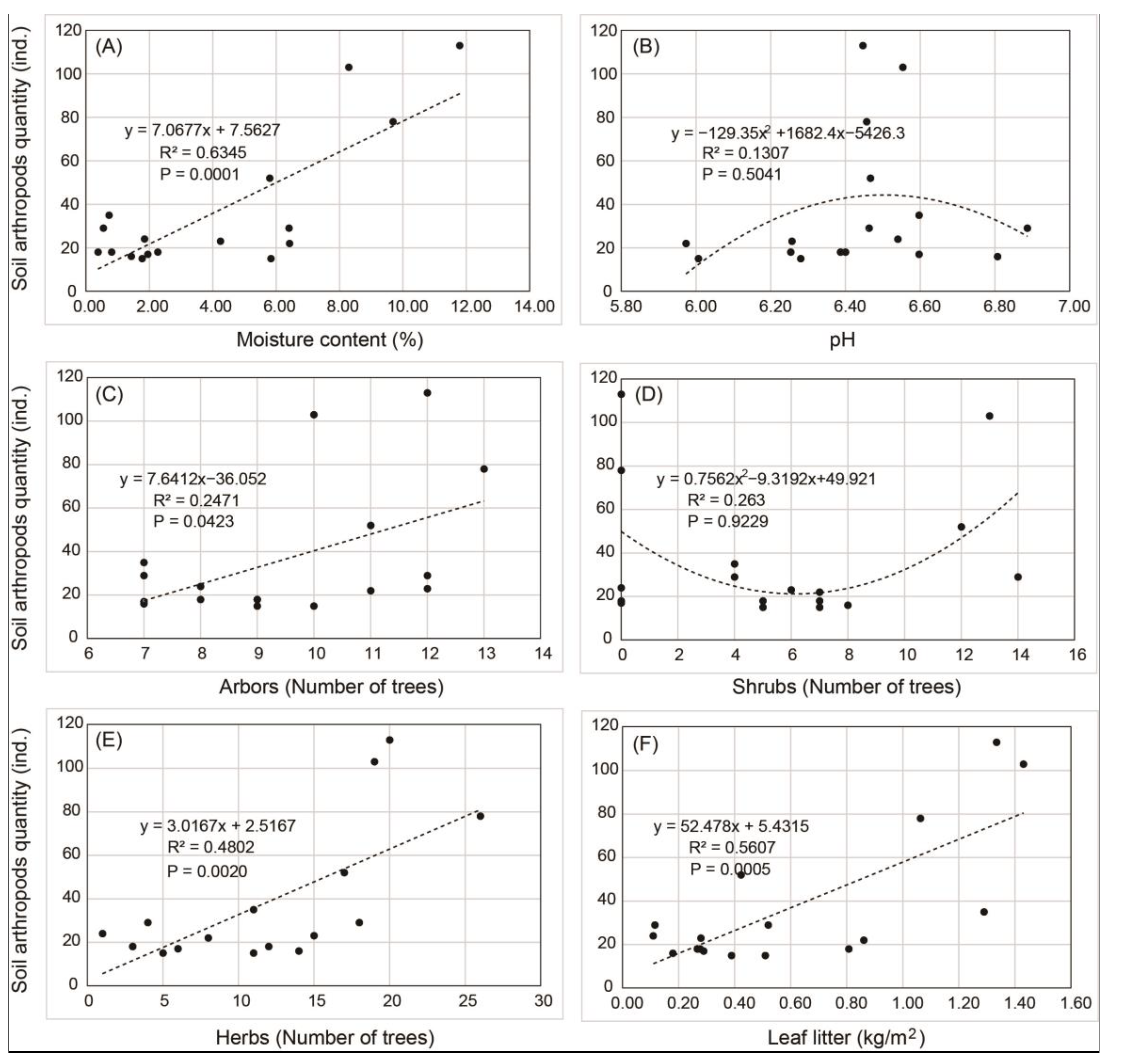
3.5. Fitting Analysis and Pearson Correlation Analysis of Soil Arthropod Quantity and Environmental Factors
3.6. Effects of Human Tourism and Mountaineering Activities on Soil Arthropods
4. Discussion
4.1. Analysis of Soil Arthropod Community Characteristics and Influencing Factors at Different Altitudes
4.2. Effects of Human Interference on Soil Fauna
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orgiazzi, A.; Bardgett, R.D.; Barrios, E.; Behan-Pelletier, V.; Briones, M.J.I.; Chotte, J.L.; De Deyn, G.B.; Eggleton, P.; Fierer, N.; Fraser, T.; et al. Global Soil Biodiversity Atlas; European Commission, Publications Office of the European Union: Luxembourg, 2016. [Google Scholar]
- Yin, W.Y. Review and Prospect of Soil Zoology. Biol. Bull. 2001, 8, 1–3. [Google Scholar]
- Sun, X.; Xie, Z.J.; Qiao, Z.H.; Gao, M.X.; Yin, R.; Chang, L.; Wu, D.H.; Liu, M.Q.; Zhu, Y.G. Research advances in trait-based approaches in soil fauna community ecology. Chin. J. Appl. Ecol. 2024. accepted. Available online: https://link.cnki.net/urlid/21.1253.Q.20240206.1058.002 (accessed on 25 March 2024).
- Yan, J.; Wu, J.H. Study Advances in Plant Diversity Effects on Soil Fauna. Soils 2018, 50, 231–238. [Google Scholar] [CrossRef]
- Liu, X.J.; Wu, Q.Q.; Li, Y.Y.; Ruan, H.H.; Ding, X.N.; Cao, G.H.; Shen, C.Q. Interaction effects of stand development and seasonality on soil arthropods community in poplar plantations. J. Nanjing For. Univ. 2023, 47, 224–230. [Google Scholar]
- Xu, H.J.; Yu, L.Z.; Huang, X.R.; Zhu, J.J.; Yang, J.Y.; Gao, S.L.; Wang, Y.J. Biodiversity of macro ground-dwelling arthropods in secondary forests and plantation forests of montane region of eastern Liaoning Province. Chin. J. Ecol. 2015, 34, 727–735. [Google Scholar] [CrossRef]
- Menta, C.; Conti, F.D.; Fondón, C.L.; Staffilani, F.; Remelli, S. Soil arthropods responses in agroecosystem: Implications of different management and cropping systems. Agronomy 2020, 10, 982. [Google Scholar] [CrossRef]
- Liu, S.Q. The Characteristics of Soil Fauna Communities and the Division of Functional Groups in Different Altitudinal Gradients of Picea Schrenkiana in Western Tianshan Mountains. Master Thesis, Yili Normal University, Yili, China, 2024. [Google Scholar]
- Wang, S.J.; Ruan, H.H.; Wang, J.S.; Xu, Z.K.; Wu, Y.Y. Composition structure of soil fauna community under the typical vegetations in the Wuyi Mountains. Acta Ecol. Sin. 2010, 30, 5174–5184. [Google Scholar]
- Ding, Z.Q.; Xu, G.R.; Zhang, S.; Zhang, Y.X.; Ma, K.M. Altitudinal pattern of soil fauna plant interaction in Dongling Mountain, Beijing. Acta Ecol. Sin. 2022, 42, 2741–2750. [Google Scholar] [CrossRef]
- Sanders, N.J.; Rahbek, C. The patterns and causes of elevational diversity gradients. Ecography 2012, 35, 1–3. [Google Scholar] [CrossRef]
- Jiang, Y.F.; Yin, X.Q.; Wang, F.B. Composition and spatial distribution of soil mesofauna along an elevation gradient on the north slope of the Changbai Mountains, China. Pedosphere 2015, 25, 811–824. [Google Scholar] [CrossRef]
- Wang, R.H.; Liu, Q.Y.; Wang, X.Y.; Zhao, Z.Y.; Dou, Y.J. Responses of soil mite community diversity to altitude gradients in Luya Mountain, China. J. Shanxi Univ. 2022, 45, 1138–1150. [Google Scholar] [CrossRef]
- Liu, D.D.; Wu, H.T.; Yu, H.X.; Sun, X.; Liu, D.; Cheng, P.; Bai, X.Y.; Dai, G.H.; Zhang, Z.S.; Wang, W.F. Distribution pattern of soil Oribatida and Collembola diversity along altitudinal gradient in the Changbai Mountains. Sci. Geog. Sin. 2023, 43, 1299–1309. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, H.T. Research progresses in effects of climate warming on soil fauna community structure. Chin. J. Ecol. 2020, 39, 655–664. [Google Scholar] [CrossRef]
- Yang, C.C.; Tao, Y.; Jia, D.X. Climate Profile of Qufu City in 2020 and Its Impact Assessment. Mod. Agric. Sci. Technol. 2021, 17, 185–187. [Google Scholar] [CrossRef]
- Duan, M.C.; Qin, R.X.; Zhang, H.B.; Chen, B.X.; Jin, B.; Zhang, S.B.; Ren, S.P.; Jin, S.Q.; Zhu, S.H.; Hua, J.N.; et al. Comprehensive comparison of different sampling methods for arthropod diversity in farmland. Biodivers. Sci. 2021, 29, 477–487. [Google Scholar] [CrossRef]
- Yin, W.Y. Atlas of Soil Fauna in China; Science Press: Beijing, China, 1998. [Google Scholar]
- Li, H.X.; Sui, J.Z.; Zhou, S.X. Insect Classification and Retrieval; Agriculture Press: Beijing, China, 1987. [Google Scholar]
- Bao, S. Agro-Chemical Analyses of Soils; China Agriculture Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Scheunemann, N.; Russell, D.J. Hydrological regime and forest development have indirect effects on soil fauna feeding activity in Central European hardwood floodplain forests. Nat. Conserv. 2023, 53, 257–278. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analyses and Interpretation, 2nd ed.; PRIMER-E: Plymouth, UK, 2021; 172p. [Google Scholar]
- Zhang, J.; Yuan, Z.; Wang, H.Y.Y.; Cao, J.; Zheng, Z.X.; Ye, B.B. Study on the relationship between plankton community structure change and environmental factors in Dafangying Reservoir. J. Anhui Agric. Univ. 2024. [Google Scholar] [CrossRef]
- Liu, D.D.; Liu, D.; Yu, H.X.; Wu, H.T. Strong variations and shifting mechanisms of altitudal diversity and abundance patterns in soil oribatid mites (Acari: Oribatida) on the Changbai Mountain, China. Appl. Soil Ecol. 2023, 186, 104808. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Cao, M.; Xu, G.R. Diversity distribution patterns of Collembola in litter layers along three typical climate zones in Yunnan Province. Acta Ecol. Sin. 2020, 40, 5008–5017. [Google Scholar]
- Wang, H.Y.; Zhu, Y.H. The study of the characteristics of soil fauna community and soil humus characteristics at different elevations in Lushan. Hubei Agric. Sci. 2022, 7, 25–30. [Google Scholar] [CrossRef]
- Zang, J.C.; Huang, W.J.; Zang, Y.J.; Bin, L.; Zhang, Y.L.; Song, M.C. Community structure and diversity of soil fauna at different altitudes in Alpine grassland in northern Tibet. J. Northwest Sci.-Tech. Univ. Agric. For. 2023, 51, 72–81. [Google Scholar] [CrossRef]
- Chen, H.; Luo, S.W.; Li, G.X.; Jiang, W.Y.; Qi, W.; Hu, J.; Ma, M.J.; Du, G.Z. Large-scale patterns of soil nematodes across grasslands on the Tibetan Plateau: Relationships with Climate, Soil and Plants. Diversity 2021, 13, 369. [Google Scholar] [CrossRef]
- Liu, S. Distribution Pattern and Influencing Factors of Soil Oribatid Mites Diversity along Altitudinal Gradient in Evergreen Broad-leaved Forest of Tianmu Mountain. Ph.D. Thesis, Harbin Normal University, Harbin, China, 2022. [Google Scholar] [CrossRef]
- Luo, M.J.; Li, S.S.; Qiang, D.H.; Liu, C.H. Relationship between soil fauna community composition and soil physical and chemical properties in Nanniwan wetland. Ecol. Environ. Sci. 2018, 27, 1432–1439. [Google Scholar] [CrossRef]
- Sun, L.N. The Characteristics of Soil Faunal in Longwan Nature Reserve Forest and the Division of Functional Groups. Ph.D. Thesis, Harbin Normal University, Harbin, China, 2013. [Google Scholar]
- Feng, J.; Qiao, Z.H.; Yan, Q.B.; Yao, H.F.; Wang, B.; Sun, X. Effects of urbanization and greenspace types on community structure and functional traits of soil Collembola. Acta Ecol. Sin. 2024, 6, 1–15. [Google Scholar] [CrossRef]
- Cao, L.L.; Ruan, H.H.; Li, Y.Y.; Ni, J.P.; Wang, G.B.; Cao, G.H.; Shen, C.Q.; Xu, Y.M. Variations of surface soil macrofauna in different aged Metasequoia glyptostroboides plantations. J. Nanjing For. Univ. 2024. accepted. Available online: http://kns.cnki.net/kcms/detail/32.1161.S.20231115.1415.002.html (accessed on 24 March 2024).
- Wenninger, E.J.; Inouye, R.S. Insect community response to plant diversity and productivity in a sagebrush-steppe ecosystem. J. Arid Environ. 2008, 72, 24–33. [Google Scholar] [CrossRef]
- Agnieszka, J.; Bartłomiej, W.; Edyta, S.; Agnieszka, K.B.; Wojciech, B.; Anna, K.I.; Marcin, C.; Marcin, P. How applied reclamation treatments and vegetation type affect on soil fauna in a novel ecosystem developed on a spoil heap of carboniferous rocks. Eur. J. Soil Biol. 2023, 119, 103571. [Google Scholar] [CrossRef]
- Yan, J.C.; Cui, D.; Lv, L.Q.; Jiang, Z.C.; Zhang, M.R.; Liu, J.H.; Cao, J.; Wang, Q.L. Distribution characteristics of soil fauna communities in the forest-grassland ecotone of the West Tianshan Nature Reserve. Chin. J. Ecol. 2024. accepted. Available online: https://link.cnki.net/urlid/21.1148.Q.20240124.1721.008 (accessed on 24 March 2024).
- Cao, Y.; Gao, M.X.; Zhang, X.P.; Dong, C.X. Distribution characteristics of soil macro-faunal communities along a latitudinal gradient in farmland of Heilongjiang Province. Acta Ecol. Sin. 2017, 37, 1677–1687. [Google Scholar] [CrossRef][Green Version]
- Wu, Q.; Wu, H.T.; Sun, X.; Lin, Y.L.; Liu, D.D.; Kang, Y.J.; Liu, J.P. Community composition and surface aggregation characteristics of soil collembola in Changbai Mountain Tundra. Environ. Ecol. 2023, 5, 39–46. [Google Scholar]
- Huang, M. Research on Taxonomy and Diversity of Soil Oribatid Mites in Agricultural Region of Heilongjiang Province. Master Thesis, Chinese Academic Science (Northeast Institute of Geography and Agroecology, Chinese Academic Science), Changchun, China, 2023. [Google Scholar]
- Wang, L.L.; Lu, L. Effects of tourism disturbances on activities of soil enzymes and soil macrofauna in Taiping Lake national wetland park. Wetl. Sci. 2013, 11, 212–218. [Google Scholar] [CrossRef]
- Tang, J.R. Relationships between Soil Fauna Community Characteristics and Environmental Factors in Natural Forests of Mountain Parks in the Main Urban Area of Chongqing. Master Thesis, Southwest University, Chongqing, China, 2022. [Google Scholar] [CrossRef]
- Van-Klink, R.; Schrama, M.; Nolte, S.; Bakker, J.P.; WallisDeVries, M.F.; Berg, M.P. Defoliation and soil compaction jointly drive large-herbivore grazing effects on plants and soil arthropods on clay soil. Ecosystems 2015, 18, 671–685. [Google Scholar] [CrossRef]
- Ye, Y.; Jiang, Y.X. Changes of Community Structure and Functional Groups of Soil fauna under Tourism Disturbance—Taking Heishiding, Jiulong Lake and Northridge Mountain as an Example. J. Zhaoqing Univ. 2017, 38, 52–57. [Google Scholar]
- Dong, C.X.; Wang, B.J.; Xue, Z.; Tang, N.; Miao, Z.P. Response of soil macro-fauna communities to tourism disturbance-Taking Sanqing Mountain as an example. J. Shangrao Norm. Univ. 2022, 3, 71–76. [Google Scholar] [CrossRef]
- Wang, Z.M.; Zhao, Y.M.; Zhong, S.G.; Chen, G. Indicative effect of soil fauna on heavy metal pollution. J. Environ. Hyg. 2022, 6, 463–472. [Google Scholar] [CrossRef]
- Xing, S.W.; Xu, J.M.; Huang, B.; Gao, J.T.; Han, L. Effect of heavy metal pollution on the community structure and diversity of soil fauna in tea garden located in a tungsten mining area. Ecol. Environ. Sci. 2021, 30, 1903–1915. [Google Scholar] [CrossRef]


| Moisture Content (%) | Soil pH | Arbors (Number of Trees) | Shrubs (Number of Trees) | Herbs (Number of Trees) | Leaf Litter (kg/m2) | |
|---|---|---|---|---|---|---|
| Insecta | 0.324 | −0.021 | 0.285 | −0.566 * | 0.304 | 0.403 |
| Arachnida | −0.495 * | 0.302 | −0.175 | 0.184 | −0.117 | −0.424 |
| Myriapoda | −0.250 | 0.030 | −0.295 | 0.068 | −0.085 | −0.290 |
| Chilopoda | −0.247 | −0.166 | −0.219 | 0.228 | −0.128 | −0.180 |
| Crustacea | 0.755 ** | 0.163 | 0.437 | 0.191 | 0.622 ** | −0.675 ** |
| Total quantity | 0.796 ** | 0.173 | 0.497 * | 0.025 | 0.693 ** | 0.749 ** |
| Sample Line | Species Richness | Abundance |
|---|---|---|
| Line 1 | 3.44 ± 0.26 a | 14.67 ± 6.25 |
| Line 2 | 3.94 ± 0.32 a | 19.11 ± 7.78 |
| Line 3 | 5.11 ± 0.46 b | 30.78 ± 10.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, H.; Shang, J.; Qi, Q.; Cao, B.; Kong, Y.; Li, Y.; Chen, J.; Yi, X. Soil Moisture and Litter Coverage Drive the Altitude Gradient Pattern of Soil Arthropods in a Low-Elevation Mountain. Diversity 2024, 16, 263. https://doi.org/10.3390/d16050263
Qin H, Shang J, Qi Q, Cao B, Kong Y, Li Y, Chen J, Yi X. Soil Moisture and Litter Coverage Drive the Altitude Gradient Pattern of Soil Arthropods in a Low-Elevation Mountain. Diversity. 2024; 16(5):263. https://doi.org/10.3390/d16050263
Chicago/Turabian StyleQin, Haiming, Jingwen Shang, Qin Qi, Bo Cao, Yong Kong, Yujian Li, Junfeng Chen, and Xianfeng Yi. 2024. "Soil Moisture and Litter Coverage Drive the Altitude Gradient Pattern of Soil Arthropods in a Low-Elevation Mountain" Diversity 16, no. 5: 263. https://doi.org/10.3390/d16050263
APA StyleQin, H., Shang, J., Qi, Q., Cao, B., Kong, Y., Li, Y., Chen, J., & Yi, X. (2024). Soil Moisture and Litter Coverage Drive the Altitude Gradient Pattern of Soil Arthropods in a Low-Elevation Mountain. Diversity, 16(5), 263. https://doi.org/10.3390/d16050263







