The Lichen Genus Letrouitia (Brigantiaeaceae, Ascomycota) in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimens and Morphology
2.2. Chemistry
2.3. DNA Extraction and PCR Sequencing
2.4. Phylogenetic Analysis
3. Results
3.1. Phylogenetic Results
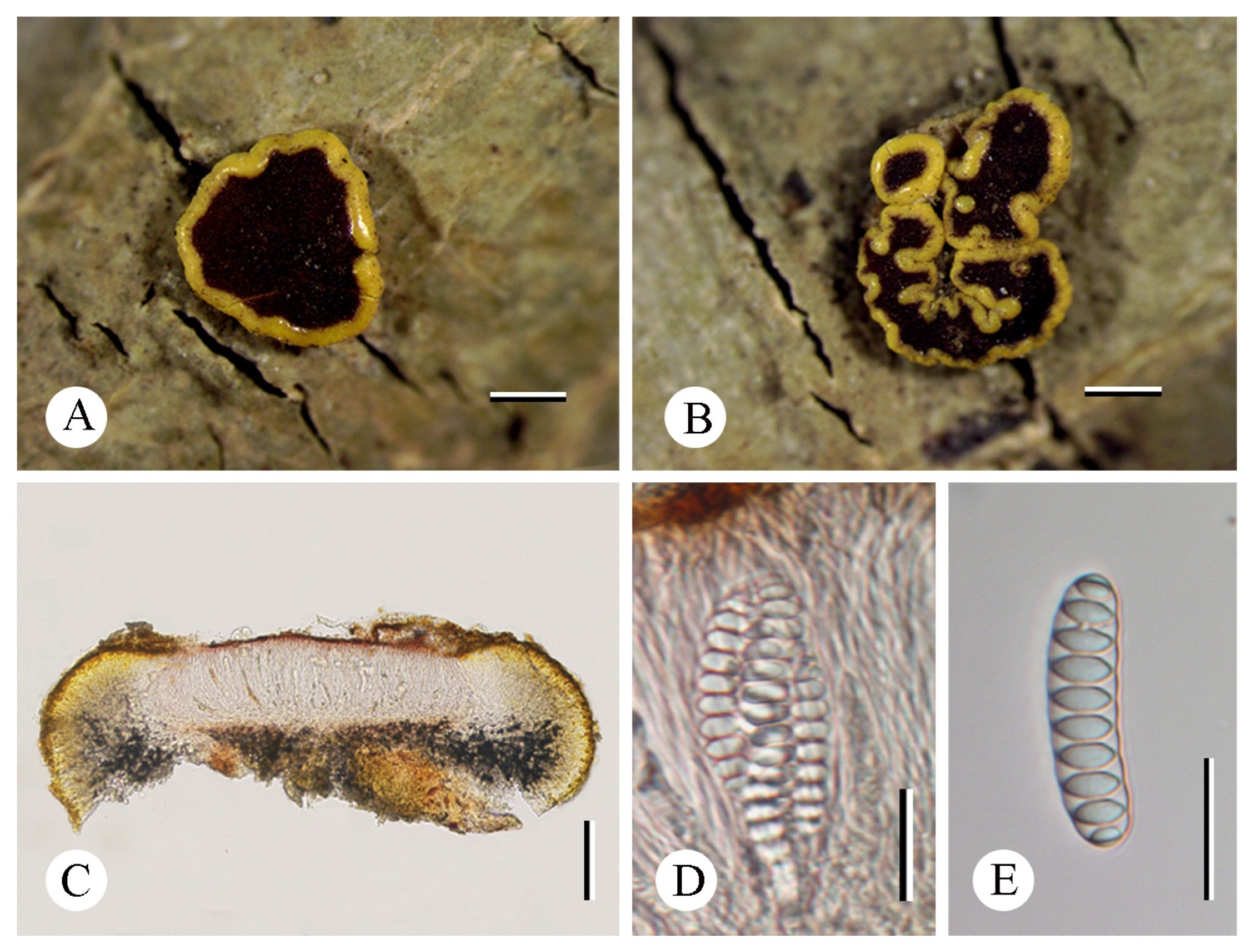
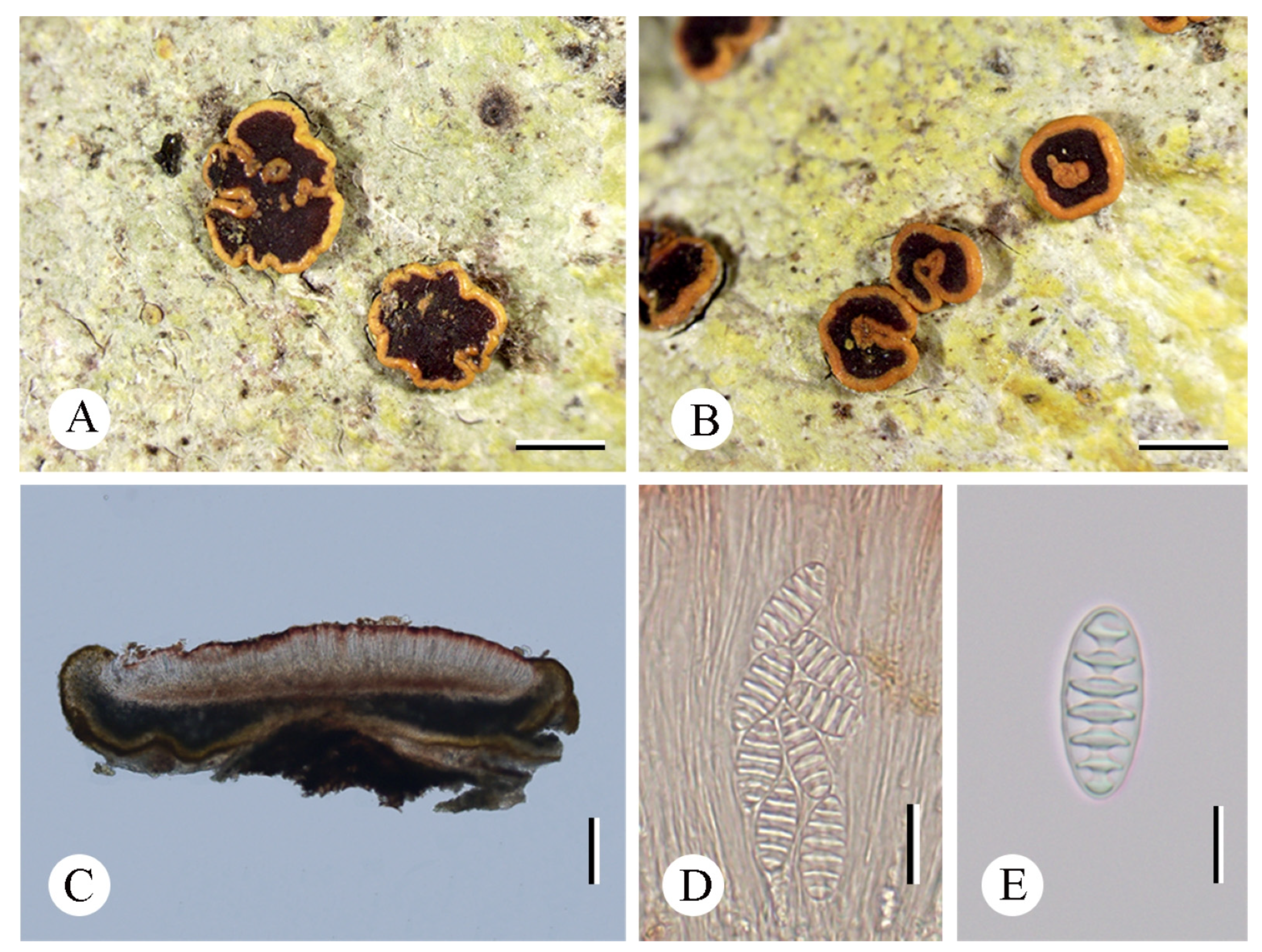
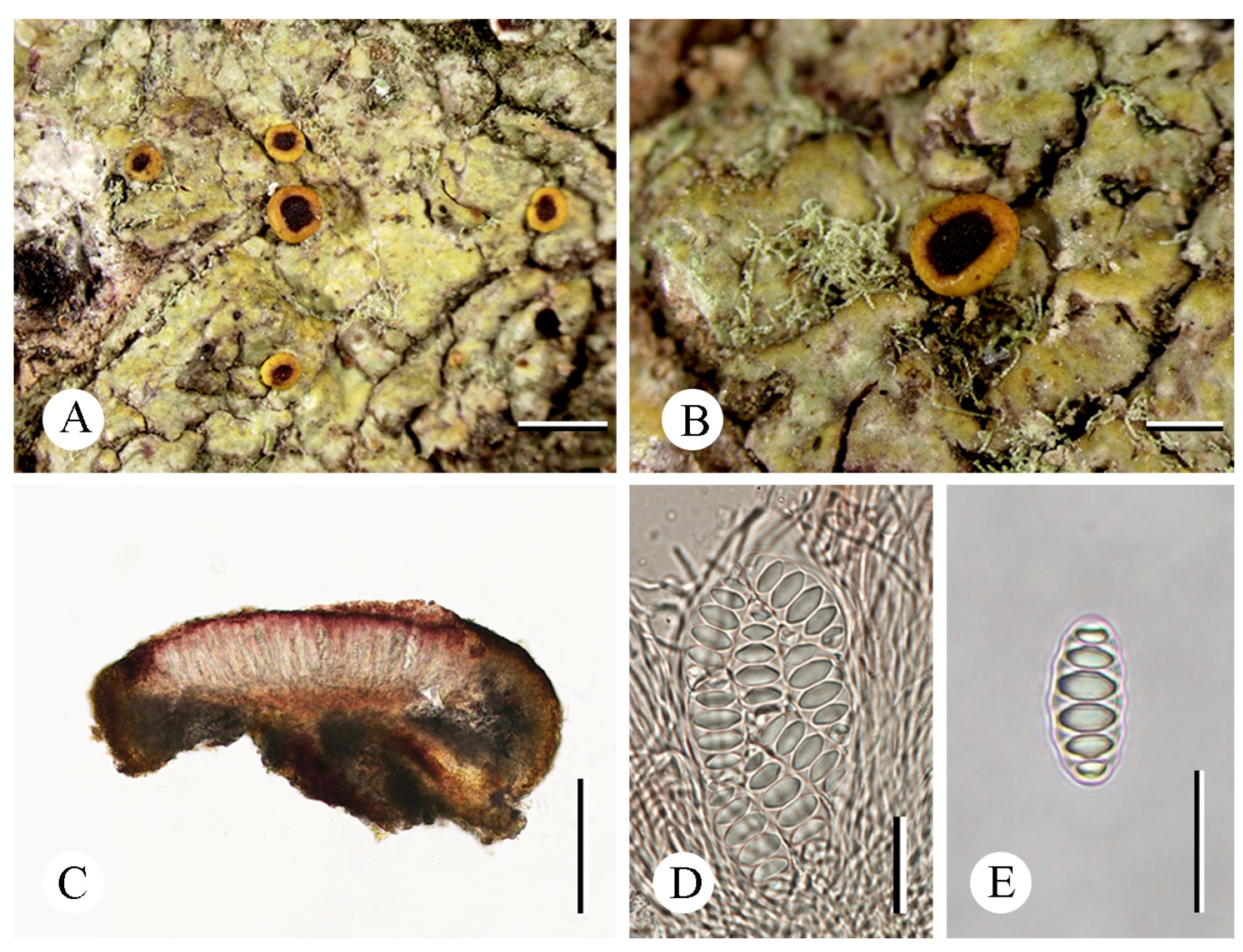
3.2. Taxonomy
3.3. Previously Reported Species of China
| Key to the species of Letrouitia known in China |
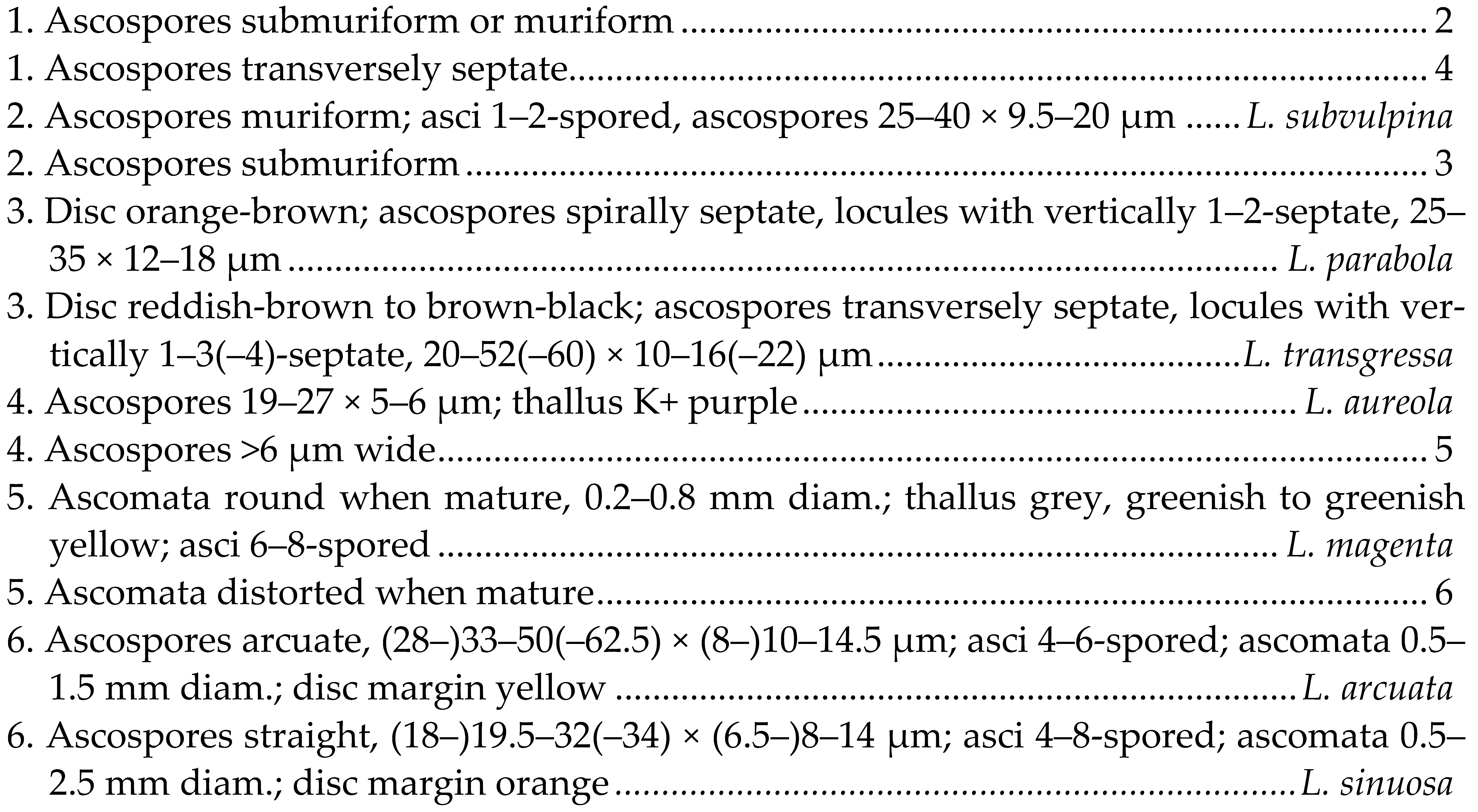 |
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santesson, J. Neuere Probleme der Flechtenchemie. Dtsch. Bot. Ges. Neue Folge 1970, 4, 5–21. [Google Scholar]
- Hafellner, J.; Bellemere, A. Elektronenoptische Untersuchungen an Arten der Flechtengattung Letrouitia gen. nov. Nova Hedwig. 1981, 35, 263–312. [Google Scholar]
- Eriksson, O.; Baral, H.-O.; Currah, R.S.; Hansen, K.; Kurtzman, C.P.; Rambold, G.; Læssøe, T. Outline of Ascomycota—2001. Myconet 2001, 7, 1–88. [Google Scholar]
- Wijayawardene, N.N.; Hyde, K.D.; Dai, D.Q.; Sánchez-García, M.; Goto, B.T.; Saxena, R.K.; Erdoğdu, M.; Selçuk, F.; Rajeshkumar, K.C.; Aptroot, A.; et al. Outline of Fungi and fungus-like taxa—2021. Mycosphere 2022, 13, 53–453. [Google Scholar] [CrossRef]
- Hafellner, J. Monographie der Flechtengattung Letrouitia (Lecanorales, Teloschistineae). Nova Hedwig. 1981, 35, 645–729. [Google Scholar]
- Awasthi, D.D.; Srivastava, P. Lichen genera Brigantiaea and Letrouitia from India. Proc. Indian Acad. Sci. (Plant Sci.) 1989, 99, 165–177. [Google Scholar] [CrossRef]
- Shi, H.X.; Qian, Z.G.; Wang, X.Y.; Liu, D.; Zhang, Y.Y.; Ye, X.; Harada, H.; Wang, L.S. The genus Letrouitia (Letrouitiaceae: Lichenized Ascomycota) new to Cambodia. Mycobiology 2015, 43, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Ekanayaka, A.H. New and known discolichens from Asia and eastern Europe. Asian J. Mycol. 2019, 2, 48–86. [Google Scholar] [CrossRef]
- Gogoi, R.; Joseph, S.; Nayaka, S.; Yasmin, F. Additions to the lichen biota of Assam State, India. J. Threat. Taxa 2019, 11, 13765–13781. [Google Scholar] [CrossRef]
- Lücking, R.; Hodkinson, B.P.; Leavitt, S.D. The 2016 classification of lichenized fungi in the Ascomycota and Basidiomycota—Approaching one thousand genera. Bryologist 2017, 119, 361–416. [Google Scholar] [CrossRef]
- Johansson, S.; Schting, U.; Elix, J.A.; Wardlaw, J.H. Chemical variation in the lichen genus Letrouitia (Ascomycota, Letrouitiaceae). Mycol. Prog. 2005, 4, 139–148. [Google Scholar] [CrossRef]
- Gaya, E.; Navarro-Rosinés, P.; Llimona, X.; Hladun, N.; Lutzoni, F. Phylogenetic reassessment of the Teloschistaceae (lichen-forming Ascomycota, Lecanoromycetes). Mycol. Res. 2008, 112, 528–546. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Nguyen, T.T.; Dzung, N.A.; Jayalal, U.; Oh, S.-O.; Hur, J.-S. New records of corticolous lichens from Vietnam. Mycotaxon 2013, 123, 479–489. [Google Scholar] [CrossRef]
- Aptroot, A.; Sparrius, L.B. New microlichens from Taiwan. Fungal Divers. 2003, 14, 1–50. [Google Scholar]
- Kondratyuk, S.; Lőkös, L.; Tschabanenko, S.; Haji Moniri, M.; Farkas, E.; Wang, X.; Oh, S.O.; Hur, J.S. New and noteworthy lichen-forming and lichenicolous fungi. Acta Bot. Hung. 2013, 55, 275–349. [Google Scholar] [CrossRef]
- Culberson, C.F.; Kristinsson, H. A standardized method for the identification of lichen products. J. Chromatogr. A 1970, 46, 85–93. [Google Scholar] [CrossRef]
- Culberson, C.F. Improved conditions and new data for identification of lichen products by standardized thin-layer chromatographic method. J. Chromatogr. A 1972, 72, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Orange, A.; James, P.; White, F. Microchemical Methods for the Identification of Lichens; British Lichen Society: London, UK, 2010; p. 101. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, S.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. Guide Methods Appl. 1990, 1, 315–322. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes-application to identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- Scheidegger, C.; Sperisen, C.; Zoller, S. PCR primers for the amplification of mitochondrial small subunit ribosomal DNA of lichen-forming Ascomycetes. Lichenologist 1999, 31, 511–516. [Google Scholar] [CrossRef]
- Kondratyuk, S.Y.; Lőkös, L.; Jang, S.H.; Hur, J.S.; Farkas, E. Phylogeny and taxonomy of Polyozosia, Sedelnikovaea and Verseghya of the Lecanoraceae (Lecanorales, lichen-forming Ascomycota). Acta Bot. Hung. 2019, 61, 137–184. [Google Scholar] [CrossRef]
- Miadlikowska, J.; Kauff, F.; Hofstetter, V.; Fraker, E.; Grube, M.; Hafellner, J.; Reeb, V.; Hodkinson, B.P.; Kukwa, M.; Lücking, R.; et al. New insights into classification and evolution of the Lecanoromycetes (Pezizomycotina, Ascomycota) from phylogenetic analyses of three ribosomal RNA- and two protein-coding genes. Mycologia 2006, 98, 1088–1103. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nature Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Hillis, D.M.; Bull, J.J. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst. Biol. 1993, 42, 182–192. [Google Scholar] [CrossRef]
- Kondratyuk, S.Y.; Upreti, D.K.; Mishra, G.K.; Nayaka, S.; Hur, J.S. New and noteworthy lichen-forming and lichenicolous fungi 10. Acta Bot. Hung. 2020, 62, 69–108. [Google Scholar] [CrossRef]
- Elix, J.A.; Kondratyuk, S.Y. Two new species of Letrouitia (Letrouitiaceae: Ascomycota) from Australia. Australas. Lichenol. 2008, 62, 16–19. Available online: https://www.anbg.gov.au/abrs/lichenlist/AL_62.pdf (accessed on 5 January 2023).
- Asahina, Y. Lichenologische notizen (V). J. Jpn. Bot. 1934, 10, 352–357. [Google Scholar] [CrossRef]
- Asahina, Y. Lichenologische notizen (XXV). J. Jpn. Bot. 1944, 20, 129–134. [Google Scholar] [CrossRef]
- Wang Yang, J.R.; Lai, M.J. A checklist of the lichens of Taiwan. Taiwania 1973, 18, 83–104. [Google Scholar]
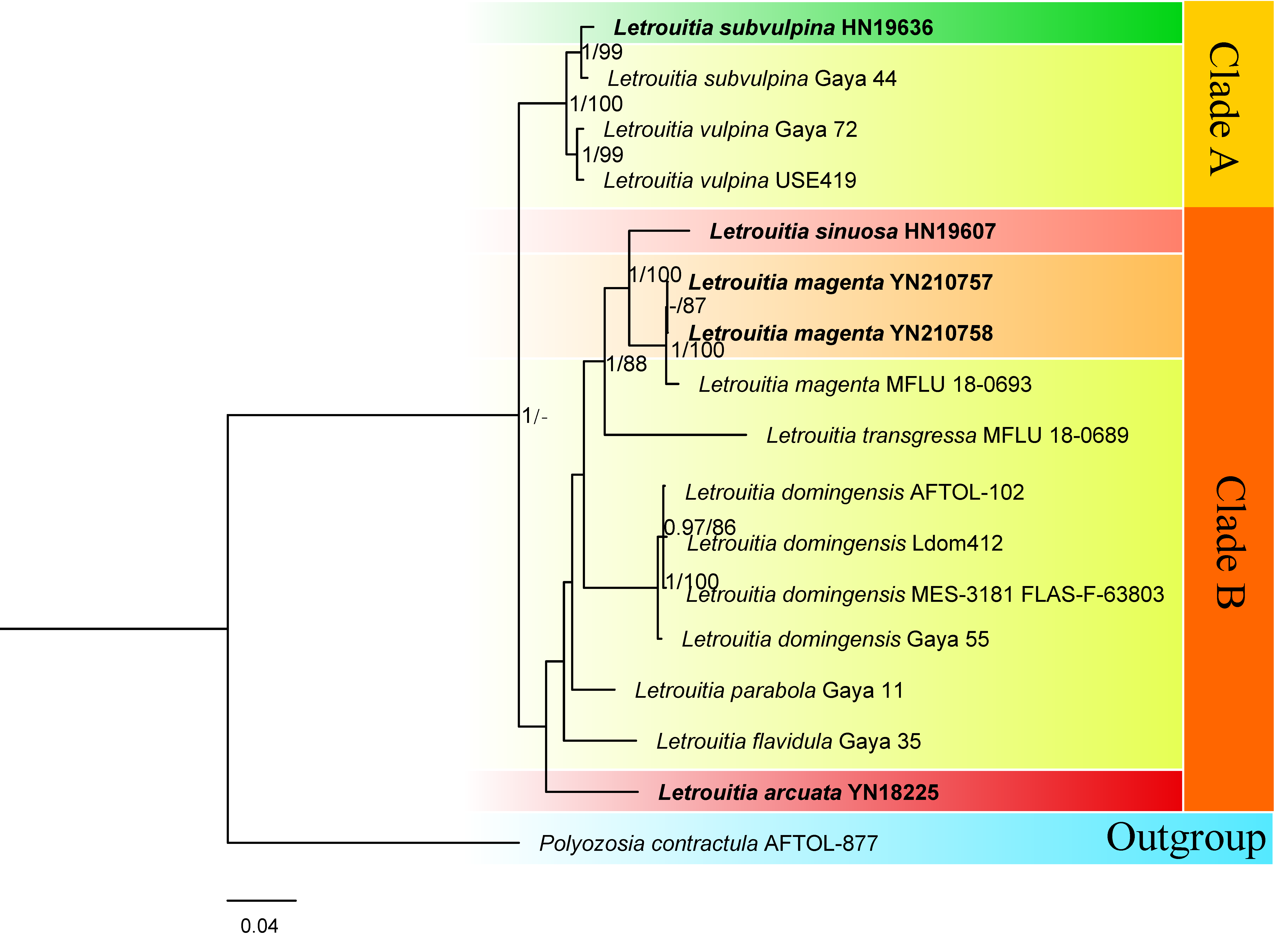
| Species | Specimen | Locality | ITS | nuLSU | mtSSU |
|---|---|---|---|---|---|
| Polyozosia contractula | AFTOL-877 | – | HQ650604 | DQ986746 | DQ986898 |
| Letrouitia arcuata | YN18225 | China Yunnan | OR395215 | OR395220 | – |
| Letrouitia domingensis | AFTOL-102 | – | HQ650700 | AY584648 | AY584619 |
| Letrouitia domingensis | Gaya 55 | Dominican Republic | JQ301673 | JQ301569 | JQ301505 |
| Letrouitia domingensis | MES-3181 FLAS-F-63803 | Belize | ON383441 | – | – |
| Letrouitia flavidula | Gaya 35 | Costa Rica | JQ301674 | – | JQ301506 |
| Letrouitia magenta | MFLU 18-0693 | Thailand | MK499353 | MK499365 | – |
| Letrouitia magenta | YN210757 | China Yunnan | OR395216 | OR395221 | OR395225 |
| Letrouitia magenta | YN210758 | China Yunnan | OR395217 | OR395222 | OR395226 |
| Letrouitia parabola | Gaya 11 | USA | JQ301675 | JQ301570 | JQ301507 |
| Letrouitia sinuosa | HN19607 | China Hainan | OR395219 | OR395224 | OR395227 |
| Letrouitia subvulpina | Gaya 44 | Costa Rica | JQ301676 | – | – |
| Letrouitia subvulpina | HN19636 | China Hainan | OR395218 | OR395223 | – |
| Letrouitia transgressa | MFLU 18-0689 | Thailand | MK499352 | MK499364 | – |
| Letrouitia vulpina | Gaya 72 | Reunion | JQ301677 | JQ301571 | JQ301509 |
| Letrouitia vulpina | USE419 | France | KC179452 | KC179209 | KC179543 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, C.; Dou, M.; Jiang, S.; Jia, Z. The Lichen Genus Letrouitia (Brigantiaeaceae, Ascomycota) in China. Diversity 2024, 16, 254. https://doi.org/10.3390/d16050254
Cui C, Dou M, Jiang S, Jia Z. The Lichen Genus Letrouitia (Brigantiaeaceae, Ascomycota) in China. Diversity. 2024; 16(5):254. https://doi.org/10.3390/d16050254
Chicago/Turabian StyleCui, Can, Mingzhu Dou, Shuhao Jiang, and Zefeng Jia. 2024. "The Lichen Genus Letrouitia (Brigantiaeaceae, Ascomycota) in China" Diversity 16, no. 5: 254. https://doi.org/10.3390/d16050254
APA StyleCui, C., Dou, M., Jiang, S., & Jia, Z. (2024). The Lichen Genus Letrouitia (Brigantiaeaceae, Ascomycota) in China. Diversity, 16(5), 254. https://doi.org/10.3390/d16050254






