Diorygma tiantaiense sp. nov. and a Checklist and Key to Diorygma Species from China
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimens and Morphology
2.2. Chemistry
2.3. DNA Extraction and PCR Sequencing
2.4. Phylogenetic Analysis
3. Results and Discussion
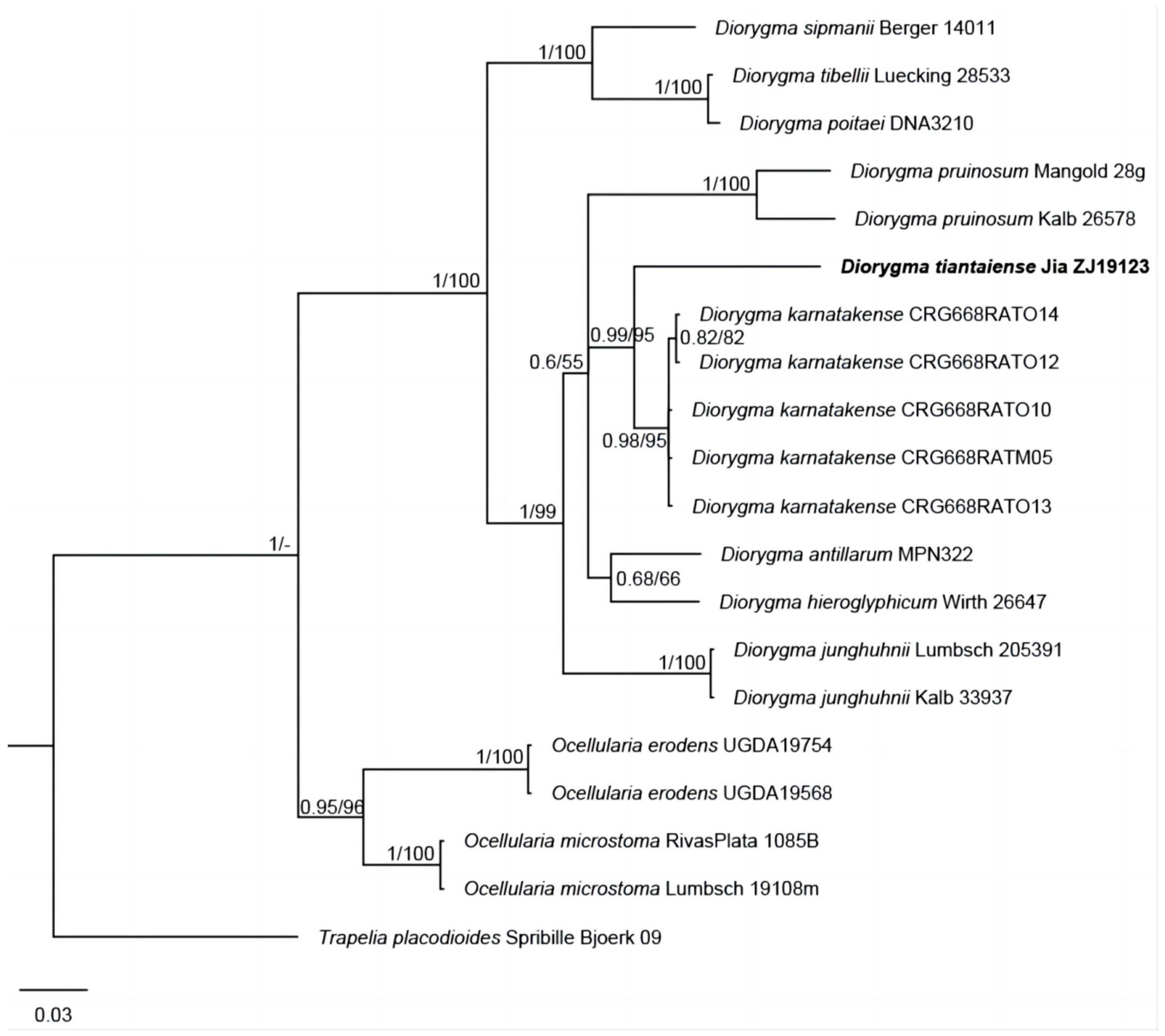
3.1. Phylogenetic Results
3.2. Taxonomy
- Key to the species of Diorygma known from China

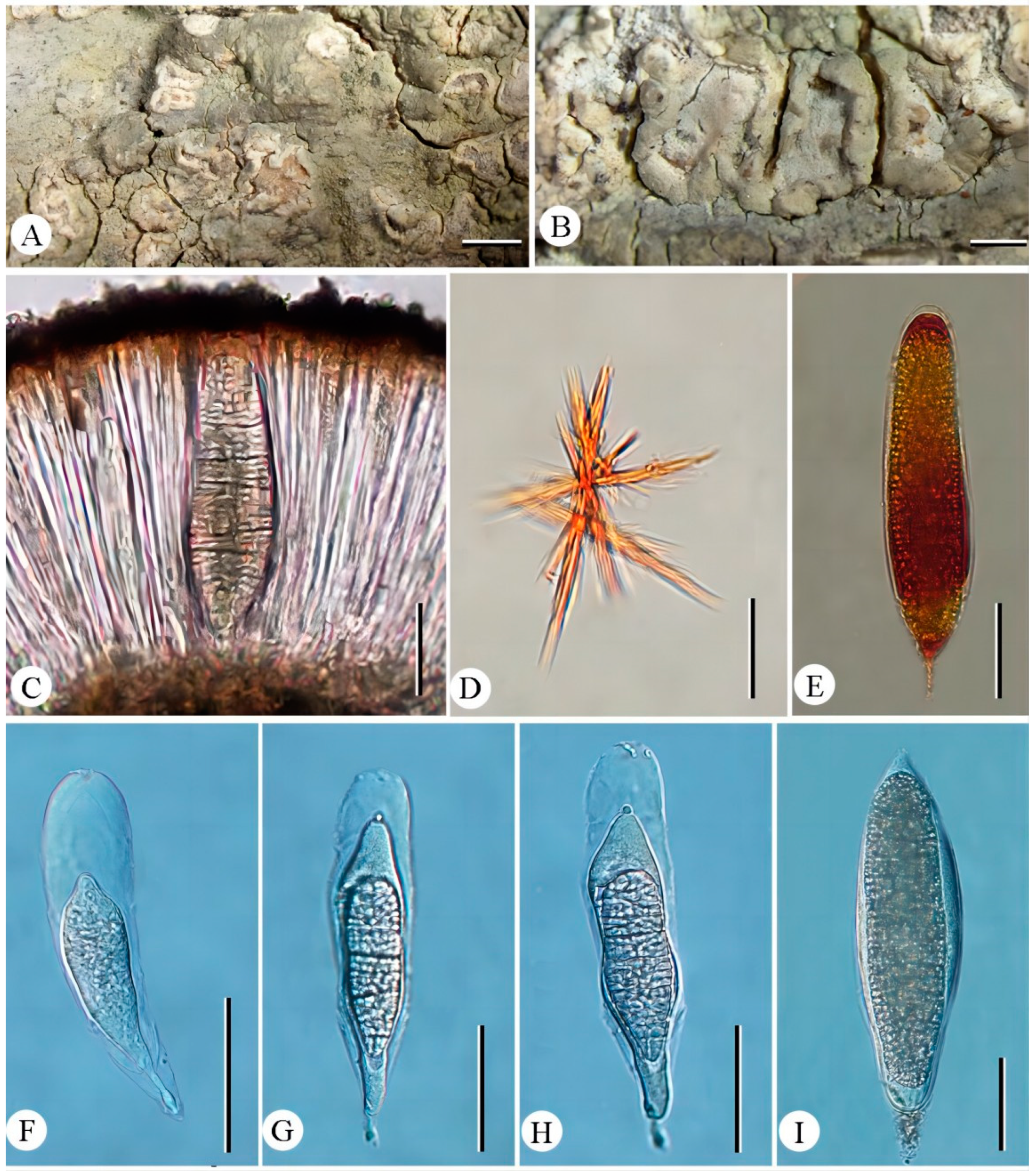
- Diorygma tiantaiense Z.F. Jia, sp. nov. (Figure 2).MycoBank: MB 852800Diagnosis: Differs from Diorygma karnatakense by its ascocarps ± raised when mature, whitish to greyish disc, divergent exciple, ascospores 1/ascus, I–, and the absence of salazinic acid.Type: China, Zhejiang Province, Tiantai County, Huading National Forest Park, on bark of Rhododendron simsii Planch., 29°15′16″ N, 121°05′30″ E, alt. 950 m, 27/IV/2019, Z.F. Jia ZJ19123 (Holotype, LCUF; GenBank MW750692 for LSU).Etymology: The species epithet refers to the locality of holotype.Description: Thallus corticolous, crustose, pale grey to greenishgrey, 100–130 µm thick, surface uneven to slightly rugose, without soralia or isidia; algal layer 40–60 µm thick; medulla poorly developed. Ascocarps numerous, oval or oblong, flexuous and branched, immersed in the thallus when young, becoming open and ± raised when mature, whitish to greyish, rounded at the ends, 0.5–3 × 0.4–2 mm; disc surrounded by entire raised thalline margins, open, rarely convex, with thick and white pruina, sometimes sparse; exciple divergent, laterally uncarbonized, basally and laterally brownish, consisting of a weakly and irregularly or brownish hyphal tissue intermingled with parts of the substrate; hymenium 150–220 µm high, not inspersed, I–; epithecium usually distinctly developed, consisting of intermingled anastomosing, hyaline or brownish paraphysis tips with short ± globular cells, hyaline granules, and dead hyphae; paraphyses 1–2 µm diam., with a gelatinous wall, often anastomosing, especially in the upper part of the hymenium and near the asci, sometimes branched at the tip. Ascospores 1/ascus, hyaline, muriform, dense spore locules of equal size, 120–210 × 35–60 µm, I–, with thin halo.Chemistry: K+ red, P+ yellow to red; norstictic acid.Ecology and distribution: This species is distributed in a subtropical forest in the southeast of China, growing on bark. The type location is in China.Additional specimen examined: China. Zhejiang Province, Tiantai County, Huading National Forest Park, on bark of Rhododendron simsii, 29°15′16″ N, 121°05′30″ E, alt. 950 m, 27/IV/2019, Z.F. Jia ZJ19124, ZJ19125 (LCUF).Discussions: Diorygma tiantaiense is characterized by the oval or oblong apothecia, the large muriform ascospores (20–210 × 35–60 µm) and the presence of norstictic acid only. The species is shown as sister to the clade consisting of D. karnatakense, but different in the latter having concealed and brownish black disc, convergent exciple, I+ in hymenium, ascospores 1–8/ascus, longer ascospores (75–220 × 18.5–51.5 µm), I+ blue violet, and the presence of salazinic acids [36]. Morphologically, it resembles D. africanum Kalb, Staiger & Elix, D. reniforme (Fée) Kalb, Staiger & Elix, D. salvadoriense Kalb, Staiger & Elix, and D. soozanum (Zahlbr.) M. Nakan. & Kashiw. in ascocarps, but is distinguished from those species by D. africanum having protocetraric acid and the absence of norstictic acid, D. reniforme having larger ascospores (110–230 × 35–80 µm) and the presence of norstictic, protocetraric, and salazinic acids, D. salvadoriense having wider ascospores (150–200 × 50–75µm) and the presence of norstictic and salazinic acids, D. soozanum having thick white pruinate discs and the presence of norstictic and connorstictic acids [3].
- Diorygma erythrellum (Mont. & Bosch) Kalb, Staiger & Elix, Symb. Bot. Upsal. 34(1): 150, 2004.≡ Ustalia erythrella Mont. & Bosch, in Junghuhn, Pl. Jungh. 4: 478, 1856.≡ Graphina erythrella (Mont. & Bosch) Zahlbr. Cat. Lich. Univers. 2: 405 1923.Thallus crustose, greenish grey; surface smooth; pseudocortex well developed. Ascocarps lirellae, 0.5–5 × 0.3–0.5 mm, ± raised, sometimes branched, isabelline. Disc narrow to open, with a thin white pruina; Exciple divergent, uncarbonized, brown. Hymenium clear, 120–150 µm high. Ascospores 8/ascus, hyaline, muriform, spore locules of equal size, 10–12/2–4 locular, 40–53 × 12–15 µm.Chemistry: Norstictic, connorstictic and stictic acids.Ecology and distribution: This species is a pantropical species, which is distributed in tropical to subtropical forests, growing on bark. Known from China, Java, the Philippines, Australia, New Caledonia, Thailand, and Sumatra [3].Specimens: China. Fujian Province: Wuyishan City, Mt. Wuyishan, alt. 540 m, 27/V/2007, Z.F. Jia FJ457 (LCUF). Hainan Province: Qiongzhong County, Mt. Wuzhishan, alt. 700 m, 21/VII/2009, J. Li HN09077 (LCUF).Discussions: This species is similar to Diorygma fuscum Jian Li bis & Z.F. Jia, but differs in the latter having opened discs with a thick and white pruina, and stictic acid as major chemistry, with norstictic acid absent [6]. The size of ascospores in specimens from China is smaller than the previously reported of Kalb et al. in 2004 [3]. Position in key of Feuerstein et al. (2014): couplet 22(21) Ascospores 30–65 × 12–20 µm; thallus with smooth cortex [4].
- Diorygma fuscum Jian Li bis & Z.F. Jia, Mycotaxon 131(3): 718, 2016.Thallus crustose, pale grey to olivegrey, surface uneven to slightly rugose or warty; pseudocortex indistinctly developed, partly lacking. Ascocarps lirellate, 1–4 × 0.3–2 mm, sometimes branched, immersed to ± raised, whitish. Disc with thick and white pruina. Exciple divergent, laterally uncarbonized, carbonization sometimes at the basal position. Hymenium clear, 100–180 µm high, I+ weakly bluish violet. Ascospores 8/ascus, hyaline to brownish, muriform, spore locules of equal size, 10–14/3–4-locular, 40–60 × 12–18 µm, I+ violet, with thin halo.Chemistry: Sticti, constictic, hypostictic and hypoconstictic acids.Ecology and distribution: This species is distributed in subtropical forests, growing on bark. Known from China [6].Specimens examined: Type: China. Fujian Province, Jianou City, Fangdao Town, Wanmulin, alt. 310 m, on bark, 3/VI/2007, Q.F. Meng FJ1280 (HMAS-L 137193); alt. 540 m, 2/VI/2007, J. Li FJ1066 (HMAS-L 137199).Discussions: This species is similar to D. pruinosum (Eschw.) Kalb et al., which differs in the latter having 1-spored asci, larger ascospores, and the presence of protocetraric acids [3]. This species is similar to D. poitaei, but differs in having opened discs, a slightly carbonized proper exciple at the base, and the presence of stictic acid (major), while D. poitaei contains hypostictic and hypoconstictic acids (major), α-acetylhypoconstictic, constictic, and stictic acids (minor, trace or absent) [3].
- Diorygma hieroglyphicum (Pers.) Staiger & Kalb, in Kalb et al., Symb. Bot. Upsal. 34(1): 151, 2004.≡ Opegrapha hieroglyphica Pers., Ann. Wetter. Gesellsch. Ges. Naturk. 2(1): 16, 1810.Thallus crustose, whitish grey or greenish, surface rough and matt, sometimes verrucose; pseudocortex indistinctly developed, with crystallization. Ascocarps lirellate, 0.5–3 × 0.4–0.8 mm, oblong, numerous or branched, immersed, whitish. Discs narrow to open and covered with whitish or yellowish pruina. Exciple divergent, uncarbonized, and indistinctly developed. Hymenium clear, 100–150 μm high, I+ weakly blue to violet blue. Ascospores 1/ascus, hyaline, muriform, oblong, with thick wall, spore locules of equal size, 20–30/6–10-locular, 80–150 × 20–40 µm, I+ violet blue.Chemistry: K+ red, P+ yellow to red; stictic, norstictic, constictic and cryptostictic acids.Ecology and distribution: This species is a pantropical species, which is distributed in tropical to subtropical coastal rainforests, growing on bark. Known from China, Africa, Singapore, Papua New Guinea, Philippines, Australia, and Southwest Pacific Island Countries [3,37].Specimens examined: China. Fujian Province: Wuyishan City, Mt. Wuyishan, alt. 500 m, 27/V/2007, Z.F. Jia FJ450 (LCUF). Hainan Province: Mangrove near the Qiongshan city, alt. 0m, 24/V/2007, Q.F. Meng M402 (HMAS-L 128726), M405 (HMAS-L 128727); Baoting contry, Mt. Qixianling, alt. 110 m, 25/VII/2009, M. Liu HN09386 (HMAS-L 115519); Qiongzhong County, Limushan National Forest Park, alt. 630 m, 24/IX/2008, Z.F. Jia HN003 (LCUF). Yunnan Province: Mengla County, Rainforest Valley Xishuangbanna National Park of Tropical Rainforests, alt. 570 m, 21/VIII/2011, Q. Ren YN-R-08 (LCUF); Pingbian County, Dawei Mountain Sand Pearl Bottom, alt. 900 m, 23/VIII/2011, Z.F. Jia 11-419, 11-421, 11-452 (LCUF).Discussions: This species is similar to Diorygma pruinosum, but differs in the latter having obviously opened discs, and stictic acid absent [3]. It is morphologically similar to D. megasporum Kalb, Staiger & Elix, but differs in the latter having ascospores 2–6/ascus [3]. Position in key of Feuerstein et al. (2014): couplet 36 (34) Stictic acid present in addition to norstictic acid; ascospores 95–150(–170) × 30–45 µm [4].
- Diorygma hololeucum (Mont. & Bosch) Kalb, Staiger & Elix, Symb. Bot. Upsal. 34(1): 155, 2004.≡ Graphis hololeuca Mont. & Bosch, Pl. Jungh. 4: 473, 1856.Thallus crustose, white, creamy white to grey, surface rough and matt; pseudocortex indistinctly developed or thin, with crystallization. Ascocarps lirellate, 1–7 × 0.5–1.5 mm, scattered, elongated to oblong, numerous or branched, raised or adnate, whitish. Discs open, covered with a thick and white pruina. Exciple divergent, uncarbonized, poorly developed. Hymenium clear, 180–250 μm high, I+ weakly violet blue. Ascospores 2–4(–6 or –8)/ascus, muriform, hyaline, oblong, with thin gelatinous wall at ends, spore locules of equal size, 25–40/5–8-locular, 120–200 × 30–40 µm, I+ violet blue.Chemistry: K–, P–; protocetraric acid.Ecology and distribution: This species is a pantropical species, which is distributed in tropical to subtropical rainforests, growing on bark. Known from China, Philippines, Papua New Guinea, Malaysia, Indonesia [3]; and Australia [38].Specimens examined: China. Hainan Province: Ledong County, Mt. Jianfengling Core Area, alt. 950 m, 2/X/2008, J. Li HN081449 (HMAS-L 117001).Discussions: This species is similar to Diorygma megasporum, but differs in the latter having immersed ascocarps, with narrow to slightly opened discs, and containing stictic acid, α-acetylconstictic acid, and constictic acid [3]. Position in key of Feuerstein et al. (2014): couplet 12(11) Ascospores 125(–250) × 30–40(–50) µm [4].
- Diorygma isabellinum (Zahlbr.) Z.F. Jia & Lücking, MycoKeys 25: 24, 2017.≡ Graphina isabellina Zahlbr., in Handel-Mazzetti, Symb. Sinic. 3: 58, 1930.Thallus crustose, creamy white, somewhat yellowish, surface rough and warty. Ascocarps lirellate, 2–4.5 × 0.2–0.35 mm, elongate, single and rarely branched. Labia obvious. Discs closed to slightly open and proper margin conspicuous; Exciple uncarbonized. Hymenium clear, 160–180 µm high, I–. Ascospores 1/ascus, muriform, hyaline, ellipsoid, 110–120 × 35–48 µm, I+ violet.Chemistry: Norstictic and connorstictic acids.Ecology and distribution: This species is a subtropical species, growing on bark. Known from China [7,39].Specimens examined: China. Hunan Province: Changsha City, Mt. Yuelushan, alt. 250 m, 27/I/1918, Handel-Mazzetti 11437 (W).Discussions: Diorygma isabellinum was reported in China as Graphina isabellina Zahlbr. [39], and then was recombined as D. isabellinum [7]. This species is similar to D. junghuhnii (Mont. & Bosch) Kalb, Staiger & Elix, but the latter differs in having I+ blue-violet hymenium and smaller ascospores sized (60–)80–125 × 21–42 µm [3].
- Diorygma junghuhnii (Mont. & Bosch) Kalb, Staiger & Elix, Sym. Bot. Upsal. 34(1): 157, 2004.≡ Graphis junghuhnii Mont. & Bosch, Pl. Jungh. 4: 471, 1856.= Graphis mendax Nyl., Annls Sci. Nat., Bot., sér. 4 11: 244 1859.Thallus crustose, whitish, greyish or greenish, surface rough and matt, verrucose; pseudocortex absent or thin, with crystallization. Ascocarps lirellate, 1–6 × 0.5 mm, scattered, elongated to nearly round, single or irregular branched, immersed, whitish. Discs open, covered with greyish to brownish pruina. Exciple slightly divergent, indistinctly developed, uncarbonized. Hymenium clear, 100–130 μm high, I+ blue. Ascospores 1/ascus, muriform, hyaline, oblong, with thin gelatinous wall at ends, spore locules of equal size, 25–30/6–10-locular, 70–125 × 20–40 µm, I+ violet blue.Chemistry: K+ red, P+ yellow to red; norstictic and connorstictic acids.Ecology and distribution: This species is a pantropical species, which is distributed in tropical to subtropical rainforests, growing on bark. Known from China, Togo (Central and Southern West Africa), Tanzania (East Africa), Philippines, Australia, Fiji, Costa Rica, Guatemala, Guyana, Brazil, etc. [3]; Thailand, Australia, and South Pacific island countries [38].Specimens examined: China. Fujian Province: Wuyishan City, Mt. Wuyishan, alt. 500 m, 27/V/2007, Z.F. Jia FJ445, FJ446 (LCUF). Guangdong Province: Xinyi City, Mt. Datianding, alt. 1700 m, 5/XI/2010, H.Y. Wang 20107250, 20107750, 20107958 (SDNU). Guangxi Province: Longsheng County, Huaping, alt. 900 m, 6/VI/2001, J.B. Chen 20032-1-3 (HMAS-L 030808-10). Hainan Province: Ledong Country, The tropical arboretum in the Mt. Jianfengling, alt. 650 m, 30/IX/2008, Z.F. Jia HN080692 (HMAS-L 127471); Wuzhishan City, Mt. Wuzhishan, alt. 680 m–880 m, 28/IX/2008, J. Li, HN081254 (HMAS-L 127474), HN081259 (HMAS-L 117008).Discussion: Diorygma junghuhnii is similar to D. soozanum, but differs in the latter having narrow to open discs covered with white pruina, and I+ weakly blue in hymenium [3]. It is similar to D. macgregorii (Vain.) Kalb, Staiger & Elix in appearance and the chemical substance, but differs in the latter having larger spores (135–185 × 40–63 μm) [3]. Position in key of Feuerstein et al. (2014): couplet 41(40) Hymenium completely I+ blue-violet; ascospores (60–)80–125 × 21–42 µm [4].
- Diorygma macgregorii (Vain.) Kalb, Staiger & Elix, Sym. Bot. Upsal. 34(1): 159, 2004.≡ Helminthocarpon pervarians var. macgregorii Vain., Ann. Acad. Sci. Fenn., Ser. A 15(6): 266, 1921.Thallus crustose, creamy white, grey or greenish grey, surface rough and matt with small granula; pseudocortex absent or thin, with crystallization. Ascocarps lirellate, 1–5 × 0.5–2 mm, scattered, oval to elongated, branched, distinctly raised. Discs distinctly open, covered with white or greyish pruina. Exciple divergent, indistinctly developed, uncarbonized. Hymenium clear, 140–200 μm high, I+ weakly violet blue near exciple. Ascospores 1/ascus, muriform, hyaline, oblong, with the central cells larger than the peripheral ones, 135–180 × 35–50 µm, I+ violet blue.Chemistry: K+ red, P+ yellow to red; norstictic, connorstictic, cryptostictic and stictic acids.Ecology and distribution: This species is a pantropical species, which is distributed in tropical to subtropical rainforests, growing on bark. Known from China, Southeast Asia, and Papua New Guinea [3].Specimens examined: China. Hunan Province: Sangzhi County, Mt. Badagongshan, alt. 1400 m, 19/VIII/1997, J.B. Chen, D.P. Wang, S.L. Wang 9633 (HMAS-L 121023). Hainan Province: Changjiang County, Mt. Bawangling, alt. 980 m, 18/V/2007, Q.F. Meng M488 (HMAS-L 128742); Ledong Coutry, Mt. Jianfengling, alt. 180 m, 1/X/2008, J. Li, HN081360 (HMAS-L 117074). Guizhou province: Tongren City, Mt. Fanjingshan, alt. 1570 m, 1/IX/1963, J.C. Wei 621 (HMAS-L 047731).Discussion: This species is similar to Diorygma hieroglyphicum, but differs in the latter having immersed lirellae with narrow to open discs and smaller spores sized 95–150(–170) × 30–45 μm [3]. It is similar to D. junghuhnii, but differs in the latter having smaller spores sized (60–)80–125 × 21–42 µm [3].
- Diorygma megasporum Kalb, Staiger & Elix, Symb. Bot. Upsal. 34(1): 160, 2004.Thallus crustose, whitish, creamy white, yellowish, or weakly grayish green, surface rough and matt, with small granula; pseudocortex indistinctly developed or thin, with crystallization. Ascocarps lirellate, 0.5–5 × 0.1–0.7 mm, scattered, sub round to elongated, branched, immersed. Discs narrow to slightly open, and covered with a thick greyish pruina. Exciple divergent, uncarbonized, poorly developed. Hymenium clear, 170–200 μm high, I+ violet blue on upper parts and exciple. Ascospores 2–6/ascus, muriform, hyaline, irregular round, with thick wall, spore locules of equal size, 30–60/7–12-locular, 80–200 × 20–50 µm, I– or I+ violet blue.Chemistry: K+ yellow, P+ yellow to orange; stictic, α-acetylconstictic and constictic acids.Ecology and distribution: This species is a pantropical species, growing on bark. Known from China and India [3].Specimens examined: China. Guizhou Province: Tongren City, Mt. Fanjing Mountain, Scissor Gorge, alt. 1570 m, 6/IX/1963, J.C. Wei 0675 (HMAS-L 047752).Discussions: This species is similar to Diorygma hololeucum, but differs in the latter having protocetraric acid, and adnate ascocarps with open discs [3]. This species is recorded containing chemical components such as norstictic and hypostictic acids by Kalb et al. (2004); however, we did not detect these components in Chinese specimens. Position in key of Feuerstein et al. (2014): couplet 49(48) Ascospores 231–244 × 59–76 µm [4].
- Diorygma pruinosum (Eschw.) Kalb, Staiger & Elix, Sym. Bot. Upsal. 34(1): 166, 2004.≡ Leiogramma pruinosum Eschw., in von Martius, Icon. Plant. Cryptog. 2: 12, 1828.≡ Cyclographina pruinosa (Eschw.) D. D. Awasthi, Norw. J. Bot. 26: 175, 1979.Thallus crustose, creamy white, greenish or pale grey, surface rough and matt, partially cracked; pseudocortex distinctly developed and partially indistinctly developed, with crystallization. Ascocarps lirellate, 1–3 × 0.2–1 mm, scattered, elongated to round, single or branched, immersed to ± raised. Discs wide open and covered with a thick and white pruina. Exciple slightly divergent, indistinctly developed, uncarbonized to slightly carbonized. Hymenium clear, 110–190 μm high, I+ weakly violet blue. Ascospores 1/ascus, muriform, hyaline, oblong, spore locules of equal size, 25–40/6–10-locular, 90–170 × 20–50 µm, I+ violet blue.Chemistry: K–, P–; protocetraric acid.Ecology and distribution: This species is a pantropical species, which distributed in tropical to subtropical forests, growing on bark. Known from China, Cameroon, Nigeria (West Coast African country), Kenya (Equatorial East African country), and Tanzania Nigeria (East African countries), Indonesia, Singapore, Papua New Guinea (Western Pacific countries), Australia, Scotland, Brazil, etc. [3]; Australia, Philippines, and Solomon Islands [37].Specimens examined: China. Fujian Province: Jian’ou City, Fangdao Town, Wanmulin, alt. 600 m, 2/VI/2007, Q.F. Meng FJ645 (LCUF). Hainan Province: Ledong Country, Mt. Jianfengling, Rainforest Valley, alt. 670 m, 16/V/2007, Q.F. Meng M231 (HMAS-LHMAS-L128760), M333 (128737), M362 (HMAS-L128761).Discussion: This species is similar to Diorygma hololeucum, but differs in the latter having 2 or more spores with larger spores sized 125–230(–250) × 30–45(–50) µm [3]. It is similar to D. macgregorii in disc and the size of spores, but differs in the latter having norstictic acid [3]. Position in key of Feuerstein et al. (2014): couplet 15(13) Ascospores up to 150 µm long (or very rarely to 170 µm long), 95–150(–170) × 19–50 µm; peripheral and central ascospore locules of more or less equal size [4].
- Diorygma soozanum (Zahlbr.) M. Nakan. & Kashiw. [as ‘soozana’], in Nakanishi, Kashiwadani & Moon, Bull. Natn. Sci. Mus., Tokyo, B 29(2): 86, 2003.≡ Graphina soozana Zahlbr., Feddes Repert. Spec. Nov. Regni veg. 31: 215, 1933.Thallus crustose, creamy white to pale grey, surface slightly rough and matt, cracked partialy; pseudocortex distinctly developed, with small crystallization. Ascocarps lirellate, 1–5 × 0.4–0.6 mm, scattered, oblong to enlarged, single or branched, prominently raised. Discs narrow to open, covered with white pruina. Exciple slightly divergent, indistinctly developed, uncarbonized. Hymenium clear, 130–160 μm high, I+ weakly violet blue. Ascospores 1/ascus, muriform, hyaline, oblong, spore locules of equal size, 20–30/7–8-locular, 110–140 × 35–45 µm, I+ violet blue.Chemistry: K+ red, P+ yellow to red; norstictic and connorstictic acids.Ecology and distribution: This species is a pantropical species, which is distributed in tropical to subtropical rainforests, growing on bark. Known from China and Japan [3,20].Specimens examined: China. Fujian Province: Wuyishan City, Mt. Wuyishan, alt. 500 m, 27/V/2007, Z.F. Jia FJ470, FJ453, FJ454 (LCUF); Jian’ou City, Fangdao Town, Wanmulin, alt. 320 m, 3/VI/2007, Q.F. Meng FJ942, FJ943 (LCUF). Sichuan Province: Dujiangyan City, Xiangshui Cave, alt. 1750 m, 13/VIII/1997, J.C. Wei 97141 (HMAS-L 055260). Yunnan Province: Luxi City, Mt. Santai, alt. 1340 m, 28/XI/1980, Y.M. Jiang 552-2 (HMAS-L 047681).Discussion: This species is similar to Diorygma tuberculosum (Stirt.) Kalb, Staiger & Elix, but differs in the latter having small and unequal spores, I– [3]. It is similar to D. junghuhnii, but differs in the latter having immersed ascocarps, with thickly pruinose discs, and I+ distinctly bluish violet in hymenium [3]. Position in key of Feuerstein et al. (2014): couplet 41(40) Hymenium weakly I+ blue-violet (mostly laterally); ascospores 110–145 × 36–45 μm [4].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eschweiler, F.G. Systema lichenum, genera exhibens rite distincta, pluribus novis adaucta. Norimbergae 1824, 25, 1–26. [Google Scholar]
- Staiger, B. Die Flechtenfamilie Graphidaceae. Bibl. Lichenol. 2002, 85, 1–526. [Google Scholar]
- Kalb, K.; Staiger, B.; Elix, J.A. A monograph of the lichen genus Diorygma—A first attempt. Symb. Bot. Ups. 2004, 34, 133–181. [Google Scholar]
- Feuerstein, S.C.; Cunha-Dias, I.P.R.; Aptroot, A.; Eliasaro, S.; CÁCeres, M.E.d.S. Three new Diorygma (Graphidaceae) species from Brazil, with a revised world key. Lichenol. 2014, 46, 753–761. [Google Scholar] [CrossRef]
- Sutjaritturakan, J.; Saipunkaew, W.; Boonpragob, K.; Kalb, K. New species of Graphidaceae (Ostropales, Lecanoromycetes) from southern Thailand. Phytotaxa 2014, 189, 312–324. [Google Scholar] [CrossRef]
- Li, J.; Jia, Z.F. Diorygma fuscum sp. nov. from China. Mycotaxon 2016, 131, 717–721. [Google Scholar] [CrossRef]
- Jia, Z.F.; Lücking, R. Resolving the species of the lichen genus Graphina Müll. Arg. in China, with some new combinations. MycoKeys 2017, 25, 13–29. [Google Scholar] [CrossRef][Green Version]
- Sipman, H.J.M. Diorygma upretii spec. nov., a poleotolerant lichen in the tropics. Cryptogam Biodivers. Assess. 2018, 1–5. [Google Scholar] [CrossRef]
- Aptroot, A.; Feuerstein, S. New Graphidaceae from South and Central Brazil. Arch. Lichenol. 2020, 16, 1–11. [Google Scholar]
- Wijayawardene, N.N.; Hyde, K.D.; Dai, D.Q.; Sánchez-García, M.; Goto, B.T.; Saxena, R.K.; Erdoğdu, M.; Selçuk, F.; Rajeshkumar, K.C.; Aptroot, A.; et al. Outline of Fungi and fungus-like taxa—2021. Mycosphere 2022, 13, 53–453. [Google Scholar] [CrossRef]
- Aptroot, A.; Lücking, R.; Cáceres, M.E.S. New species and records of Graphidaceae and Gomphillaceae (lichenized fungi) from Brazil. Plant Fungal Syst. 2023, 68, 249–261. [Google Scholar] [CrossRef]
- Schumm, F.; Aptroot, A. Rondônia. Braz. Lichens 2024, 7, 1–668. [Google Scholar]
- Aptroot, A.; Lücking, R.; Cáceres, M.E.S. New Species, Records and Combinations of Graphidaceae (Lichenized Fungi) from Brazil. Bryologist 2024, 127, 22–55. [Google Scholar] [CrossRef]
- Kraichak, E.; Huang, J.-P.; Nelsen, M.; Leavitt, S.D.; Lumbsch, H.T. A revised classification of orders and families in the two major subclasses of Lecanoromycetes (Ascomycota) based on a temporal approach. Bot. J. Linn. Soc. 2018, 188, 233–249. [Google Scholar] [CrossRef]
- Lücking, R. Stop the Abuse of Time! Strict Temporal Banding is not the Future of Rank-Based Classifications in Fungi (Including Lichens) and Other Organisms. Crit. Rev. Plant Sci. 2019, 38, 199–253. [Google Scholar] [CrossRef]
- Zahlbruckner, A. Flechten der Insel Formosa. Repert. Nov. Specierum Regni Veg. 1933, 31, 194–224. [Google Scholar] [CrossRef]
- Lamb, I.M. Index Nominum Lichenum inter Annos 1932 et 1960 Divulgatorum; The Ronald Press Company: New York, NY, USA, 1963; p. 809. [Google Scholar]
- Wang, Z.; Lai, M. A Checklist of the Lichens of Taiwan. Taiwania 1973, 18, 83–104. [Google Scholar]
- Wu, J.N.; Qian, Z.G. Lichens. In Cryptogamic Flora of the Yangtze Delta and Adjacent Regions; Xu, B.S., Ed.; Shanghai Scientific & Technical Publisher: Shanghai, China, 1989; pp. 158–226. [Google Scholar]
- Nakanishi, M.; Kashiwadani, H.; Moon, K.H. Taxonomical notes on Japanese Graphidaceae (Ascomycotina), including some new combinations. Bull. Natl. Sci. Mus. Ser. B 2003, 29, 83–90. [Google Scholar]
- Meng, Q.F.; Wei, J.C. A lichen genus Diorygma (Graphidaceae, Ascomycota) in China. Mycosystema 2008, 27, 525–531. [Google Scholar]
- Wei, J.C.; Jia, Z.F.; Wu, X.L. An investigation of lichen diversity from Hainan Island of China and prospect of the R & D of their resources. J. Fungal Res. 2013, 11, 224–238. [Google Scholar]
- Jia, Z.F.; Wei, J.C. Flora Lichenum Sinicorum Vol. 13 Ostropales(I) Graphidaceae (1); Science Press: Beijing, China, 2016; pp. 1–210. [Google Scholar]
- Orange, A.; James, P.; White, F. Microchemical Methods for the Identification of Lichens; British Lichen Society: London, UK, 2001; p. 101. [Google Scholar]
- Culberson, C.F. Improved conditions and new data for identification of lichen products by standardized thin-layer chromatographic method. J. Chromatogr. A 1972, 72, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Culberson, C.F.; Kristinsson, H.-D. A standardized method for the identification of lichen products. J. Chromatogr. A 1970, 46, 85–93. [Google Scholar] [CrossRef]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Hillis, D.M.; Bull, J.J. An Empirical Test of Bootstrapping as a Method for Assessing Confidence in Phylogenetic Analysis. Syst. Biol. 1993, 42, 182–192. [Google Scholar] [CrossRef]
- Rikkinen, J.; Tuovila, H.; Beimforde, C.; Seyfullah, L.; Perrichot, V.; Schmidt, A.R. Chaenothecopsis neocaledonica sp. nov.: The first resinicolous mycocalicioid fungus from an araucarian conifer. Phytotaxa 2014, 173, 49–60. [Google Scholar] [CrossRef][Green Version]
- Beimforde, C.; Tuovila, H.; Schmidt, A.R.; Lee, W.G.; Gube, M.; Rikkinen, J. Chaenothecopsis schefflerae (Ascomycota: Mycocaliciales): A widespread fungus on semi-hardened exudates of endemic New Zealand Araliaceae. New Zealand J. Bot. 2017, 55, 387–406. [Google Scholar] [CrossRef]
- Thrower, S.L. Hong Kong Lichens; An Urban Council Publication: Hong Kong, China, 1988; pp. 1–193. [Google Scholar]
- Ansil, P.A.; Rajeshkumar, K.C.; Sharma, B.; Lücking, R.; Hawksworth, D.L. Phylogenetic placement and reappraisal of Diorygma karnatakense including the new synonym, Diorygma dandeliense, from Maharashtra, India. Lichenol. 2023, 55, 59–67. [Google Scholar] [CrossRef]
- Archer, A.W. The lichen family Graphidaceae in Australia. Bibl. Lichenol. 2006, 94, 1–191. [Google Scholar]
- Archer, A.W.; Elix, J.A. New species and new reports in the Australian Graphidaceae. Telopea 2007, 11, 451–462. [Google Scholar] [CrossRef]
- Zahlbruckner, A. Lichenes (Übersicht über sämtliche aus China bekannten Flechten). In Symbolae Sinicae Botanisch Ergebinsse der Expedition der Akademie der Wissenschaften in Wien Nach Südwest-China 1914–1918, Teil 3; Handdel-Mazzetti, H., Ed.; Julius Apringer Verlag: Wien, Austria, 1930; pp. 1–254. [Google Scholar]
| Species | Specimen | nuLSU |
|---|---|---|
| Diorygma antillarum | MPN322 | JX046465 |
| Diorygma hieroglyphicum | Wirth 26647 | AY640015 |
| Diorygma junghuhnii | Lumbsch 20539l | JX421474 |
| Diorygma junghuhnii | Kalb 33937 | AY640018 |
| Diorygma karnatakense | CRG668RATO14 | OP235520 |
| Diorygma karnatakense | CRG668RATO12 | OP235518 |
| Diorygma karnatakense | CRG668RATO10 | OP235517 |
| Diorygma karnatakense | CRG668RATM05 | OP235516 |
| Diorygma karnatakense | CRG668RATO13 | OP235519 |
| Diorygma poitaei | DNA3210 | HQ639627 |
| Diorygma pruinosum | Kalb 26578 | AY640014 |
| Diorygma pruinosum | Mangold 28g | JX421476 |
| Diorygma sipmanii | Berger 14011 | AY640020 |
| Diorygma tiantaiense | Jia ZJ19123 | MW750692 |
| Diorygma tibellii | Luecking 28533 | JX421475 |
| Ocellularia erodens | UGDA19568 | MK542902 |
| Ocellularia erodens | UGDA19754 | MK542900 |
| Ocellularia microstoma | Lumbsch 19108m | JX421575 |
| Ocellularia microstoma | RivasPlata 1085B | JX421577 |
| Trapelia placodioides | Spribille Bjoerk 09 | MH627046 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, C.; Li, Y.; Xu, J.; Zhao, X.; Jia, Z. Diorygma tiantaiense sp. nov. and a Checklist and Key to Diorygma Species from China. Diversity 2024, 16, 213. https://doi.org/10.3390/d16040213
Cui C, Li Y, Xu J, Zhao X, Jia Z. Diorygma tiantaiense sp. nov. and a Checklist and Key to Diorygma Species from China. Diversity. 2024; 16(4):213. https://doi.org/10.3390/d16040213
Chicago/Turabian StyleCui, Can, Yujie Li, Jiahui Xu, Xin Zhao, and Zefeng Jia. 2024. "Diorygma tiantaiense sp. nov. and a Checklist and Key to Diorygma Species from China" Diversity 16, no. 4: 213. https://doi.org/10.3390/d16040213
APA StyleCui, C., Li, Y., Xu, J., Zhao, X., & Jia, Z. (2024). Diorygma tiantaiense sp. nov. and a Checklist and Key to Diorygma Species from China. Diversity, 16(4), 213. https://doi.org/10.3390/d16040213






