Habitat Selection of Three Neotropical Grassland Birds Is Dependent on Vegetation Structure and Resources
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Data Collection
2.2.1. Habitat Mapping
2.2.2. Bird Surveys
2.2.3. Habitat Assessments
2.3. Statistical Analysis
2.3.1. Species Density, Abundance, and Distribution
2.3.2. Associations with Ecological and Disturbance-Mediated Habitat Gradients
2.3.3. Microhabitat Associations
3. Results
3.1. Species Density, Abundance, and Distribution across Grassland Physiognomies
3.2. Broad-Scale Associations with Ecological and Disturbance-Mediated Habitat Gradients
3.3. Finer-Scale Microhabitat Selection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosenberg, K.V.; Kennedy, J.A.; Dettmers, R.; Ford, R.P.; Reynolds, D.; Alexander, J.D.; Beardmore, C.J.; Blancher, J.P.; Bogart, R.E.; Butcher, G.S.; et al. Partners in Flight Landbird Conservation Plan: 2016 Revision for Canada and Continental United States. Partners in Flight Science Committee. 119p. Available online: https://partnersinflight.org/wp-content/uploads/2016/08/pif-continental-plan-final-spread-single.pdf (accessed on 3 April 2024).
- Azpiroz, A.B.; Isacch, J.P.; Dias, R.A.; Di Giacomo, A.S.; Fontana, C.S.; Palarea, C.M. Ecology and Conservation of Grassland Birds in Southeastern South America: A Review. J. Field. Ornithol. 2012, 83, 217–246. [Google Scholar] [CrossRef]
- Reif, J. Long-Term Trends in Bird Populations: A Review of Patterns and Potential Drivers in North America and Europe. Acta Ornithol. 2013, 48, 1–16. [Google Scholar] [CrossRef]
- Sala, O.E.; Chapin, F.S.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global Biodiversity Scenarios for the Year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef]
- Hoekstra, J.M.; Boucher, T.M.; Ricketts, T.H.; Roberts, C. Confronting a Biome Crisis: Global Disparities of Habitat Loss and Protection. Ecol. Lett. 2005, 8, 23–29. [Google Scholar] [CrossRef]
- Klink, C.A.; Machado, R.B. Conservation of the Brazilian Cerrado. Conserv. Biol. 2005, 19, 707–713. [Google Scholar] [CrossRef]
- Marini, M.Â.; Garcia, F.I. Bird Conservation in Brazil. Conserv. Biol. 2005, 19, 665–671. [Google Scholar] [CrossRef]
- Overbeck, G.E.; Vélez-Martin, E.; Scarano, F.R.; Lewinsohn, T.M.; Fonseca, C.R.; Meyer, S.T.; Müller, S.C.; Ceotto, P.; Dadalt, L.; Durigan, G.; et al. Conservation in Brazil Needs to Include Non-forest Ecosystems. Divers. Distrib. 2015, 21, 1455–1460. [Google Scholar] [CrossRef]
- Koper, N.; Nudds, T.D. Progress in Research on Grassland Bird Conservation and Ecology. Avian Conserv. Ecol. 2011, 6. [Google Scholar] [CrossRef]
- Parr, C.L.; Lehmann, C.E.R.; Bond, W.J.; Hoffmann, W.A.; Andersen, A.N. Tropical Grassy Biomes: Misunderstood, Neglected, and under Threat. Trends Ecol. Evol. 2014, 29, 205–213. [Google Scholar] [CrossRef]
- De Carvalho, W.D.; Mustin, K. The Highly Threatened and Little Known Amazonian Savannahs. Nat. Ecol. Evol. 2017, 1, 100. [Google Scholar] [CrossRef]
- Lehmann, C.E.R.; Anderson, T.M.; Sankaran, M.; Higgins, S.I.; Archibald, S.; Hoffmann, W.A.; Hanan, N.P.; Williams, R.J.; Fensham, R.J.; Felfili, J.; et al. Savanna Vegetation-fire-Ccimate Relationships Differ among Continents. Science 2014, 343, 548–552. [Google Scholar] [CrossRef]
- Scholes, R.J.; Archer, S.R. Tree-grass Interactions in Savannas. Annu. Rev. Ecol. Syst. 1997, 28, 517–544. [Google Scholar] [CrossRef]
- Bond, W.J. What Limits Trees in C4 Grasslands and Savannas? Annu. Rev. Ecol. Evol. Syst. 2008, 39, 641–659. [Google Scholar] [CrossRef]
- Brawn, J.D.; Robinson, S.K.; Thompson, F.R., III. The Role of Disturbance in the Ecology and Conservation of Birds. Annu. Rev. Ecol. Syst. 2001, 32, 251–276. [Google Scholar] [CrossRef]
- Tubelis, D.; Cavalcanti, R. Community Similarity and Abundance of Bird Species in Open Habitats of a Central Brazilian Cerrado. Ornitol. Neotrop. 2001, 12, 57–73. [Google Scholar]
- Kutt, A.S.; Woinarski, J.C.Z. The Effects of Grazing and Fire on Vegetation and the Vertebrate Assemblage in a Tropical Savanna Woodland in North-Eastern Australia. J. Trop. Ecol. 2007, 23, 95–106. [Google Scholar] [CrossRef]
- Hovick, T.J.; Mcgranahan, D.A.; Elmore, R.D.; Weir, J.R.; Fuhlendorf, S.D. Pyric-Carnivory: Raptor Use of Prescribed Fires. Ecol. Evol. 2017, 9144–9150. [Google Scholar] [CrossRef]
- Weier, A.; Radford, I.J.; Woolley, L.-A.; Lawes, M.J. Fire Regime Effects on Annual Grass Seeds as Food for Threatened Grass-finch. Fire Ecol. 2018, 14. [Google Scholar] [CrossRef]
- Langstroth, R.P. Forest Islands in an Amazonian Savanna of Northeastern Bolivia. Ph.D. Thesis, University of Wisconsin, Madison, WI, USA, 1996. [Google Scholar]
- Langstroth, R. Biogeography of the Llanos de Moxos: Natural and Anthropogenic Determinants. Geogr. Helv. 2012, 66, 183–192. [Google Scholar] [CrossRef]
- Mayle, F.E.; Langstroth, R.P.; Fisher, R.A.; Meir, P. Long-term Forest-Savannah Dynamics in the Bolivian Amazon: Implications for Conservation. Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 291–307. [Google Scholar] [CrossRef]
- Hanagarth, W. Acerca de La Geoecología de Las Sabanas Del Beni En El Noreste de Bolivia; Instituto de Ecología: La Paz, Bolivia, 1993. [Google Scholar]
- Kingsbury, J.; McKenna, A.; Godsman, K.; McNeil, D. Bolivia Expedition Report 2010; Scotland, Glasgow, 2010, Unpublished Report. Available online: https://armoniabolivia.org/wp-content/uploads/2019/03/Glasgow-2010-Expedition-Report.pdf (accessed on 24 February 2024).
- BirdLife International Alectrurus tricolor. The IUCN Red List of Threatened Species 2017: E.T22700300A110738421. Available online: https://www.iucnredlist.org/species/22700300/110738421 (accessed on 24 February 2024).
- BirdLife International Coryphaspiza melanotis. The IUCN Red List of Threatened Species 2018: E.T22723039A132020897. Available online: https://www.iucnredlist.org/species/22723039/132020897 (accessed on 24 February 2024).
- Denevan, W.M. The Aboriginal Cultural Geography of the Llanos; University of California Press: Berkeley, CA, USA, 1966. [Google Scholar]
- Lombardo, U.; Iriarte, J.; Hilbert, L.; Ruiz-pérez, J.; Capriles, J.M.; Veit, H. Early Holocene Crop Cultivation and Landscape Modification in Amazonia. Nature 2020, 581, 190–195. [Google Scholar] [CrossRef]
- Aguilera, R. La Ganaderia Beniana En Cifras; Federacion de Ganaderos del Beni y Pando: Trinidad, Bolivia, 2004. [Google Scholar]
- Mercado Callau, L.N.; Boorsma, T. Guia Practica Parra Ganaderia de Armonizacion; La Ganaderia Sostenible Para El Beni; Armonia Bolivia: Santa Cruz de la Sierra, Bolivia, 2019. [Google Scholar]
- Parker, T., III; Willis, E. Notes on Three Tiny Grassland Flycatchers, with Comments on the Disappearance of South American Fire-diversified Savannas. Ornithol. Monogr. 1997, 549–555. [Google Scholar] [CrossRef]
- Hesse, A.J. The Blue-throated Macaw in the Wild: A Cause for Concern. Watchbird 1997, 24, 10–15. [Google Scholar]
- Hordijk, I.; Meijer, F.; Nissen, E.; Boorsma, T.; Poorter, L. Cattle Affect Regeneration of the Palm Species Attalea princeps in a Bolivian Forest–Savanna Mosaic. Biotropica 2019, 51, 28–38. [Google Scholar] [CrossRef]
- Peacock, J.; Tonra, C.M.; King, J.; Davies, G.M. Restoration of Gallery Forest Patches Improves Recruitment of Motacu Palms (Attalea princeps) While Diversifying and Increasing Wildlife Populations. PLoS ONE 2021, 16, 1–20. [Google Scholar] [CrossRef]
- Reddy, A.R.; Rasineni, G.K.; Raghavendra, A.S. The Impact of Global Elevated CO₂ Concentration on Photosynthesis and Plant Productivity. Curr. Sci. 2010, 99, 46–57. [Google Scholar]
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; ISBN 9789291691432. [Google Scholar]
- Arieira, J.; Padovani, C.R.; Schuchmann, K.; Landeiro, V.L.; Santos, S.A. Modeling Climatic and Hydrological Suitability for an Encroaching Tree Species in a Neotropical Flooded Savanna. For. Ecol. Manag. 2018, 429, 244–255. [Google Scholar] [CrossRef]
- Levick, S.R.; Richards, A.E.; Cook, G.D.; Schatz, J.; Guderle, M.; Williams, R.J.; Subedi, P.; Trumbore, S.E.; Andersen, A.N. Rapid Response of Habitat Structure and Above-ground Carbon Storage to Altered Fire Regimes in Tropical Savanna. Biogeosciences 2019, 16, 1493–1503. [Google Scholar] [CrossRef]
- BirdLife International. Species Factsheet: Alectrurus tricolor. Available online: http://www.birdlife.org (accessed on 6 June 2022).
- BirdLife International. Emberizoides herbicola. The IUCN Red List of Threatened Species 2018: E.T22723370A132022146. Available online: https://doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22723370A132022146.En (accessed on 3 April 2024).
- Stotz, D.; Fitzpatrick, J.; Parker, T., III; Moskovits, D. Neotropical Birds: Ecology and Conservation; The University of Chicago Press: Chicago, IL, USA, 1996. [Google Scholar]
- Tubelis, D.P.; Cavalcanti, R.B. A Comparison of Bird Communities in Natural and Disturbed Non-wetland Open Habitats in the Cerrado’s Central Region, Brazil. Bird Conserv. Int. 2000, 10, 331–350. [Google Scholar] [CrossRef]
- Borghetti, F.; Barbosa, E.; Ribiero, L.; Ribiero, J.F.; Machado Teles Walter, B. South American savannas. In Savanna Woody Plants and Large Herbivores; Scogings, P.F., Sankaran, M., Eds.; John Wiley and Sons Ltd.: Chichester, West Sussex, UK, 2019; pp. 77–122. [Google Scholar]
- Hamilton, S.K.; Sippel, S.J.; Melack, J.M. Seasonal Inundation Patterns in Two Large Savanna Floodplains of South America: The Llanos de Moxos (Bolivia) and the Llanos Del Orinoco (Venezuela and Colombia). Hydrol. Process. 2004, 18, 2103–2116. [Google Scholar] [CrossRef]
- Haase, R.; Beck, G. Structure and Composition of Savanna Vegetation in Northern Bolivia: A Preliminary Report. Brittonia 1989, 41, 80–100. [Google Scholar] [CrossRef]
- Sarmiento, G. The Ecology of Neotropical Savannas; Harvard University Press: Caimbridge, MA, USA, 1984. [Google Scholar]
- Eiten, G. The Cerrado Vegetation of Brazil. Bot. Rev. 1972, 38, 201–341. [Google Scholar] [CrossRef]
- Ratter, J.A.; Bridgewater, S.; Ribeiro, J.F. Biodiversity patterns of the woody vegetation of the Brazilian cerrado. In Neotropical Savannahs and Seasonally Dry Forests: Plant Diversity Biogeography and Conservation; Pennington, R., Lewis, G., Ratter, J., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 31–66. [Google Scholar]
- Villarroel, D.; Munhoz, C.B.R.; Proenca, C.E.B. Campos y Sabanas Del Cerrado En Bolivia: Delimitación, Síntesis Terminológica y Sus Caracteristicas Fisionómicas. Kempffiana 2016, 12, 47–80. [Google Scholar]
- Buckland, S.; Rexstad, E.; Marques, T.; Oedekoven, C. Distance Sampling: Methods and Applications; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 9783319192185. [Google Scholar]
- Bibby, C.; Burgess, N.; Hill, D.; Mustoe, S. Bird Census Techniques, 2nd ed.; Academic Press Limited: London, UK, 2000. [Google Scholar]
- Askins, R.A.; Chávez-ramírez, F.; Dale, B.C.; Haas, C.A.; Herkert, R.; Knopf, F.L.; Vickery, P.D. Conservation of Grassland Birds in North America: Understanding Ecological Processes in Different Regions Report of the AOU Committee on Conser. Ornithol. Monogr. 2007, 64, 3–46. [Google Scholar] [CrossRef]
- Legge, S.; Garnett, S.; Maute, K.; Heathcote, J.; Murphy, S.; Woinarski, J.C.Z.; Astheimer, L. A Landscape-Scale, Applied Fire Management Experiment Promotes Recovery of a Population of the Threatened Gouldian Finch, Erythrura gouldiae, in Australia’s Tropical Savannas. PLoS ONE 2015, 10, e0137997. [Google Scholar] [CrossRef]
- Cunha, L.; Brown, G.G.; Stanton, D.W.G.; Da Silva, E.; Hansel, F.A.; Jorge, G.; McKey, D.; Vidal-Torrado, P.; Macedo, R.S.; Velasquez, E.; et al. Soil Animals and Pedogenesis: The Role of Earthworms in Anthropogenic Soils. Soil Sci. 2016, 181, 110–125. [Google Scholar] [CrossRef]
- Zangerlé, A.; Renard, D.; Iriarte, J.; Suarez Jimenez, L.E.; Adame Montoya, K.L.; Juilleret, J.; McKey, D. The Surales, Self-Organized Earth-Mound Landscapes Made by Earthworms in a Seasonal Tropical Wetland. PLoS ONE 2016, 11, e0154269. [Google Scholar] [CrossRef] [PubMed]
- Levick, S.R.; Asner, G.P.; Chadwick, O.A.; Khomo, L.M.; Rogers, K.H.; Hartshorn, A.S.; Kennedy-Bowdoin, T.; Knapp, D.E. Regional Insight into Savanna Hydrogeomorphology from Termite Mounds. Nat. Commun. 2010, 1, 65. [Google Scholar] [CrossRef] [PubMed]
- Ocko, S.A.; Heyde, A.; Mahadevan, L. Morphogenesis of Termite Mounds. Proc. Natl. Acad. Sci. USA 2019, 116, 3379–3384. [Google Scholar] [CrossRef]
- Thomas, L.; Buckland, S.T.; Rexstad, E.A.; Laake, J.L.; Strindberg, S.; Hedley, S.L.; Bishop, J.R.B.; Marques, T.A.; Burnham, K.P. Distance Software: Design and Analysis of Distance Sampling Surveys for Estimating Population Size. J. Appl. Ecol. 2010, 47, 5–14. [Google Scholar] [CrossRef]
- Braz, V.S. Ecologia e Conservação Das Aves Campestres Do Bioma Cerrado; Universidade de Brasilia: Brasilia, Brazil, 2008. [Google Scholar]
- Kanegae, M.F. Population Size of Threatened and Endemic Birds of the Cerrado in Estação Ecológica de Itirapina, a Fragmented Area in the State of São Paulo, Brazil. Bird Conserv. Int. 2012, 22, 144–154. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing 3.6.2; R Foundation for Statistical Computing: Vienna, Austria. Available online: https://www.R-Project.Org/2020 (accessed on 3 April 2024).
- McCune, B.; Grace, J.B. Analysis of Ecological Communities; MjM Software Design: Gleneden Beach, OR, USA, 2002. [Google Scholar]
- Croissant, Y. Mlogit: Multinomial Logit Models. R Package Version 1.1-0 2020. Available online: https://cran.r-project.org/web/packages/mlogit/index.html (accessed on 24 February 2024).
- Faraway, J.J. Extending the Linear Model with R—Generalized Linear, Mixed Effects and Nonparametric Regression; Chapman Hall: New York, NY, USA, 2006; ISBN 1584881658. [Google Scholar]
- Barton, K. MuMIn: Multi-model Inference. R Package. 2020. Available online: https://cran.r-project.org/web/packages/MuMIn/index.html (accessed on 24 February 2024).
- Signorell, A. DescTools: Tools for Descriptive Statistics. R Package Version 0.99.36. 2020. Available online: https://cran.r-project.org/web/packages/DescTools/index.html (accessed on 24 February 2024).
- Keating, K.; Cherry, S. Use and Interpretation of Logistic Regression in Habitat-selection Studies. J. Wildl. Manag. 2004, 68, 774–789. [Google Scholar] [CrossRef]
- Silveira, L.F. The Birds of Serra Da Canastra National Park and Adjacent Areas, Minas Gerais, Brazil. Cotinga 1998, 10, 55–63. [Google Scholar]
- Lopes, L.E.; Malacco, G.B.; Alteff, E.F.; De Vasconcelos, M.F.; Hoffmann, D.; Silveira, L.F. Range Extensions and Conservation of Some Threatened or Little Known Brazilian Grassland Birds. Bird Conserv. Int. 2010, 20, 84–94. [Google Scholar] [CrossRef]
- Kanegae, M.F.; Levy, G.; Freitas, S.R. Habitat Use by Sharp-Tailed Tyrant (Culicivora caudacuta), and Cock-Tailed Tyrant (Alectrurus tricolor) in the Cerrado of Southeastern Brazil. Rev. Bras. Ornitol. 2012, 20, 52–58. [Google Scholar]
- Marini, M.Â.; Barbet-Massin, M.; Lopes, L.E.; Jiguet, F. Geographic and Seasonal Distribution of the Cock-Tailed Tyrant (Alectrurus tricolor) Inferred from Niche Modeling. J. Ornithol. 2013, 154, 393–402. [Google Scholar] [CrossRef]
- Padial, J.M.; Heredia, J. Notes on Cock-Tailed Tyrant Alectrurus tricolor in Bolivia. Cotinga 2004, 79–80. [Google Scholar]
- Ferrari, A.; Motta-Junior, J.C.; Siqueira, J.D.O. Seasonal Variation in the Foraging Behavior of Neotropical Tyrant Flycatchers (Tyrannidae) in a Cerrado Fragment, Brazil. Ethol. Ecol. Evol. 2022, 35, 222–239. [Google Scholar] [CrossRef]
- Motta, J.C.; Num, T.; Limpo, C.; Estação, D.A.; Itirapina, E.D.E.; Martins, M.; Filho, D.; Ecologia, D.; Paulo, U.D.S. Taxa de Entrega de Almento Para Ninhego de Alectrurus tricolor Num Campo Limpo de Estacao Ecologica de Itirapina, SP. Poster Apresentado no XVIII Congr. Bras. Ornitol. 2011. Available online: https://www.researchgate.net/publication/283316318_TAXA_DE_ENTREGA_DE_ALIMENTO_PARA_NINHEGO_DE_ALECTRURUS_TRICOLOR_TYRANNIDAE_NUM_CAMPO_LIMPO_DA_ESTACAO_ECOLOGICA_DE_ITIRAPINA_SP (accessed on 24 February 2024).
- Bakker, K.K. The Effect of Woody Vegetation on Grassland Nesting Birds: An Annotated Bibliography. Proc. S. Dak. Acad. Sci. 2003, 82, 119–141. [Google Scholar]
- Da Silva, N.A.P.; Frizzas, M.R.; de Oliveira, C.M. Seasonality in Insect Abundance in the “Cerrado” of Goiás State, Brazil. Rev. Bras. Entomol. 2011, 55, 79–87. [Google Scholar] [CrossRef]
- Wolda, H. Seasonality of Tropical Insects. J. Anim. Ecol. 1980, 49, 277–290. [Google Scholar] [CrossRef]
- Kishimoto-Yamada, K.; Itioka, T. How Much Have We Learned about Seasonality in Tropical Insect Abundance since Wolda (1988)? Entomol. Sci. 2015, 18, 407–419. [Google Scholar] [CrossRef]
- Fujikawa, A.; Tubelis, D.P. Home Ranges and Aspects of the Natural History of the Black-Masked Finch Coryphaspiza melanotis (Gray, 1840) (Aves, Thraupidae) in Central Cerrado, Brazil. J. Nat. Hist. 2019, 53, 2379–2395. [Google Scholar] [CrossRef]
- Billerman, S.; Keeny, B.; Rodewald, P.; Schulenberg, T. (Eds.) Birds of the World; Cornell Laboratory of Ornithology: Ithaca, NY, USA, 2021; Available online: https://birdsoftheworld.org/bow/home (accessed on 3 April 2024).
- Andren, H. Effects of landscape composition on predation rates at habitat edges. In Mosaic Landscapes and Ecological Processes; Springer: Dordrecht, The Netherlands, 1995; pp. 225–255. ISBN 9788490225370. [Google Scholar]
- Siebert, F.; Morris, C.; Chamane, S.; Siebert, S. The Functional Importance of Forbs in Grassland Ecosystems. In Proceedings of the XXIV International Grassland Congress/XI International Rangeland Congress, Virtual, 25–29 October 2021; pp. 1–4. [Google Scholar]
- Brandt, M.J.; Cresswell, W. Breeding Behaviour, Home Range and Habitat Selection in Rock Firefinches Lagonosticta sanguinodorsalis in the Wet and Dry Season in Central Nigeria. IBIS 2008, 150, 495–507. [Google Scholar] [CrossRef]
- Dean, W.R.J. The Distribution and Biology of Nomadic Birds in the Karoo, South Africa. J. Biogeogr. 1997, 24, 769–779. [Google Scholar] [CrossRef]
- Morton, S.R.; Davies, P.H. Food of the Zebra Finch Poephila guttata, and an Examination of Granivory in Birds of the Australian Arid Zone. Aust. J. Ecol. 1983, 8, 235–243. [Google Scholar] [CrossRef]
- Fuhlendorf, S.D.; Harrell, W.C.; Engle, D.M.; Hamilton, R.G.; Davis, C.A.; Leslie, D.M. Should Heterogeneity Be the Basis for Conservation? Grassland Bird Response to Fire and Grazing. Ecol. Appl. 2006, 16, 1706–1716. [Google Scholar] [CrossRef] [PubMed]
- Fuhlendorf, S.D.; Engle, D.M. Restoring Heterogeneity on Rangelands: Ecosystem Management Based on Evolutionary Grazing Patterns. Bioscience 2001, 51, 625. [Google Scholar] [CrossRef]
- Hovick, T.J.; Dwayne Elmore, R.; Fuhlendorf, S.D. Structural Heterogeneity Increases Diversity of Non-Breeding Grassland Birds. Ecosphere 2014, 5, 1–13. [Google Scholar] [CrossRef]
- López-mársico, L.; Lezama, F.; Altesor, A. Heterogeneity Decreases as Time since Fire Increases in a South American Grassland. Appl. Veg. Sci. 2020, 24, e12521. [Google Scholar] [CrossRef]
- Bond, W.J.; Keeley, J.E. Fire as a Global “Herbivore”: The Ecology and Evolution of Flammable Ecosystems. Trends Ecol. Evol. 2005, 20, 387–394. [Google Scholar] [CrossRef] [PubMed]
| Habitat | Area (km2) | Length (km) | Effort (km) | Description † |
|---|---|---|---|---|
| campo cerrado | 0.76 | 2.34 | 4.68 | Lightly wooded grassland with shrubs and trees, ranging 2–5 m in height and not exceeding 15% cover |
| campo sujo | 3.26 | 13.66 | 27.32 | Grassland, with scattered shrubs and occasional small trees not exceeding 2% cover |
| campo limpo | 23.7 | 12.6 | 25.2 | Grassland with occasional shrubs that do not grow taller that the surrounding vegetation and in which tall woody plants are completely absent |
| Density | Population Size | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Habitat | n | D | SE | LCL | UCL | N | SE | LCL | UCL | CV | ER | |||
| Cock-tailed Tyrant | ||||||||||||||
| campo limpo | 23 | 14.9 | 5.9 | 6.9 | 32.0 | 353 | 139 | 164 | 758 | 0.39 | 0.9 | |||
| limpo + sujo | 23 | 7.1 | 3.2 | 3 | 16.9 | 193 | 88 | 81 | 458 | 0.45 | 0.4 | |||
| Black-masked Finch | ||||||||||||||
| campo cerrado | 6 | 21.0 | 9.4 | 5.4 | 81.0 | 16 | 7 | 4 | 62 | 0.45 | 1.1 | |||
| campo sujo | 40 | 25.3 | 4 | 17.8 | 35.8 | 82 | 14 | 58 | 117 | 0.17 | 1.5 | |||
| campo limpo | 28 | 25.2 | 8 | 13.4 | 47.5 | 598 | 190 | 317 | 1127 | 0.32 | 1.1 | |||
| Total | 74 | 25.1 | - | 14.4 | 43.7 | 696 | - | 400 | 1212 | 0.33 | 1.3 | |||
| Wedge-tailed Grass-finch | ||||||||||||||
| campo cerrado | 10 | 34.6 | 14.5 | 11 | 111 | 26 | 11 | 8 | 84 | 0.42 | 2.1 | |||
| campo sujo | 36 | 27.0 | 6.4 | 16.8 | 43.3 | 88 | 21 | 55 | 141 | 0.27 | 1.3 | |||
| campo cimpo | 37 | 27.9 | 5.1 | 19.5 | 39.9 | 661 | 120 | 462 | 946 | 0.18 | 1.5 | |||
| Total | 83 | 27.9 | - | 20.2 | 38.8 | 776 | - | 559 | 1076 | 0.17 | 1.5 | |||
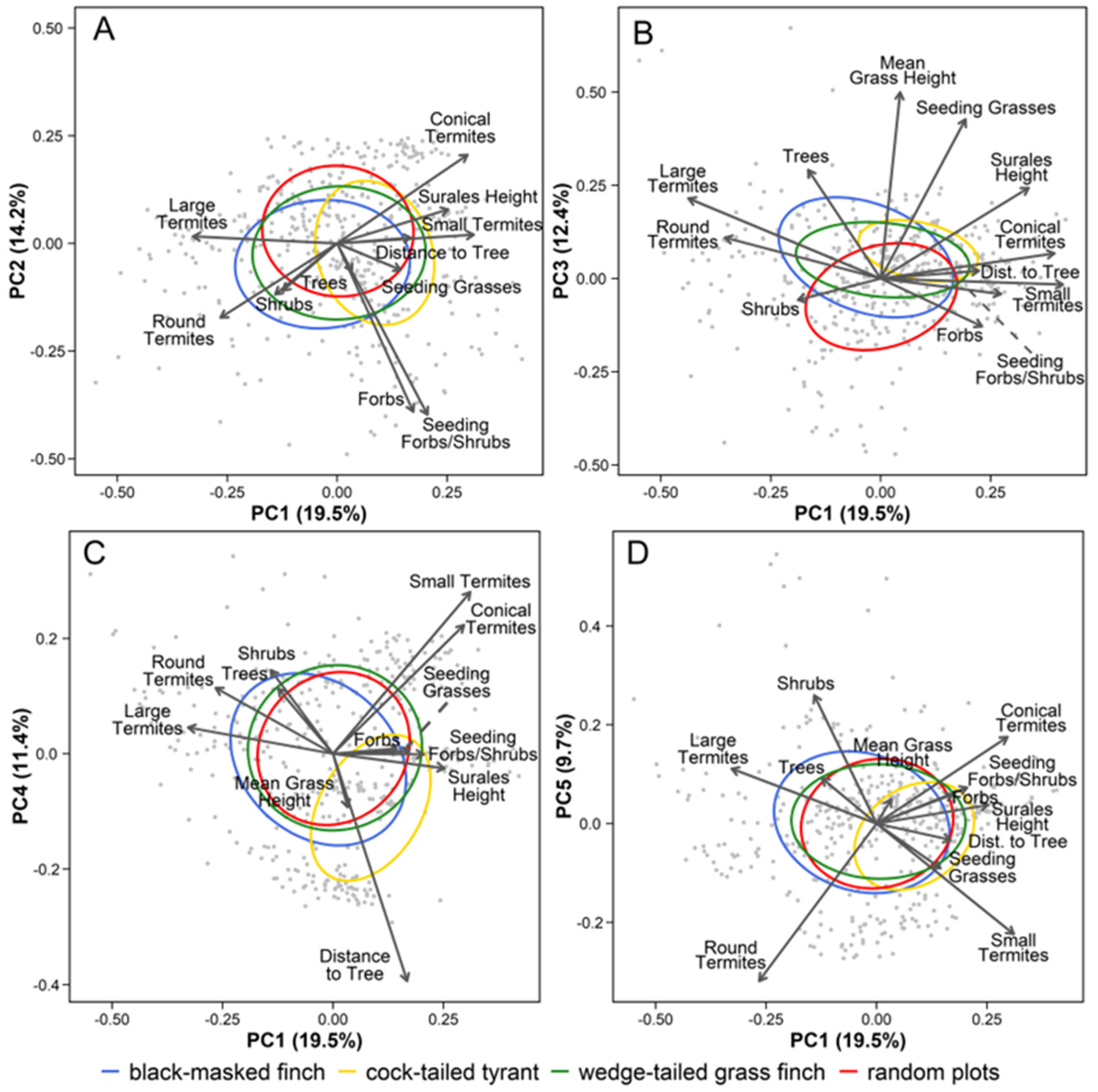
| PCA Axis | Ecological Description | Indicator Variables | Sign | FL | Cor | % Var |
|---|---|---|---|---|---|---|
| PC1 | Flooding gradient | Freq. of large termite mounds * | − | 0.66 | a | 19.5% |
| Freq. of small termite mounds | + | 0.62 | a | |||
| Freq. conical termite mounds * | + | 0.59 | b | |||
| Freq. of round termite mounds | − | 0.53 | b | |||
| Surales height * | + | 0.51 | c | |||
| PC2 | Forb-rich seedy gradient | Freq. of seeding/fruiting forbs/shrubs * | − | 0.80 | d | 14.2% |
| Freq. of forbs | − | 0.78 | d | |||
| PC3 | Tall seedy grassland gradient | Mean grass height * | + | 0.75 | e | 12.4% |
| Freq. of seeding grasses * | + | 0.64 | f | |||
| PC4 | Openness gradient | Distance to nearest tree * | − | 0.79 | g | 11.4% |
| Freq. of small termite mounds | + | 0.56 | a | |||
| PC5 | Shrubby gradient | Freq. of woody shrubs * | + | 0.52 | h | 9.7% |
| Hos–Lem | ||||||||
|---|---|---|---|---|---|---|---|---|
| Model | AIC | ΔAIC | MLik | PR2 | DP | χ2 | df | p |
| Cock-tailed Tyrant | ||||||||
| Occupancy ~ mean grass height + distance to nearest tree | 120.2 | 0.0 | 1.00 | 0.25 | 0.86 | 6.6 | 8 | 0.58 |
| Occupancy ~ surales height + mean grass height + distance to nearest tree | 121.4 | 1.3 | 0.53 | 0.26 | 0.81 | 3.8 | 8 | 0.91 |
| Occupancy ~ surales height + conical termites + mean grass height + distance to nearest tree | 124.3 | 5.1 | 0.08 | - | - | - | - | - |
| Occupancy ~ 1 | 154.2 | 34.1 | 4.4e−8 | - | - | - | - | - |
| Black-masked Finch | ||||||||
| Occupancy ~ surales height + seeding forbs or shrubs + mean grass height + woody shrubs | 291.4 | 0.0 | 1.00 | 0.20 | 0.95 | 4.5 | 8 | 0.81 |
| Occupancy ~ surales height + seeding forbs or shrubs + mean grass height+ distance to nearest tree + woody shrubs | 293.1 | 1.7 | 0.43 | 0.21 | 0.95 | 7.9 | 8 | 0.45 |
| Occupancy ~ surales height + large termites + seeding forbs or shrubs + mean grass height+ distance to nearest tree + woody shrubs | 297.6 | 6.2 | 0.04 | - | - | - | - | - |
| Occupancy ~ 1 | 347.3 | 55.9 | 7.1e−13 | - | - | - | - | - |
| Wedge-tailed Grass-finch | ||||||||
| Occupancy ~ large termites + mean grass height + seeding grass + woody shrubs | 311.3 | 0.0 | 1.00 | 0.22 | 0.98 | 1.4 | 8 | 0.99 |
| Occupancy ~ surales height + large termites + mean grass height + seeding grass + woody shrubs | 312.4 | 1.1 | 0.59 | 0.22 | 0.97 | 2.7 | 8 | 0.95 |
| Occupancy ~ surales height + large termites + mean grass height + seeding grass + distance to nearest tree + woody shrubs | 314.2 | 2.9 | 0.24 | - | - | - | - | - |
| Occupancy ~ 1 | 373.0 | 61.7 | 3.9e−14 | - | - | - | - | - |
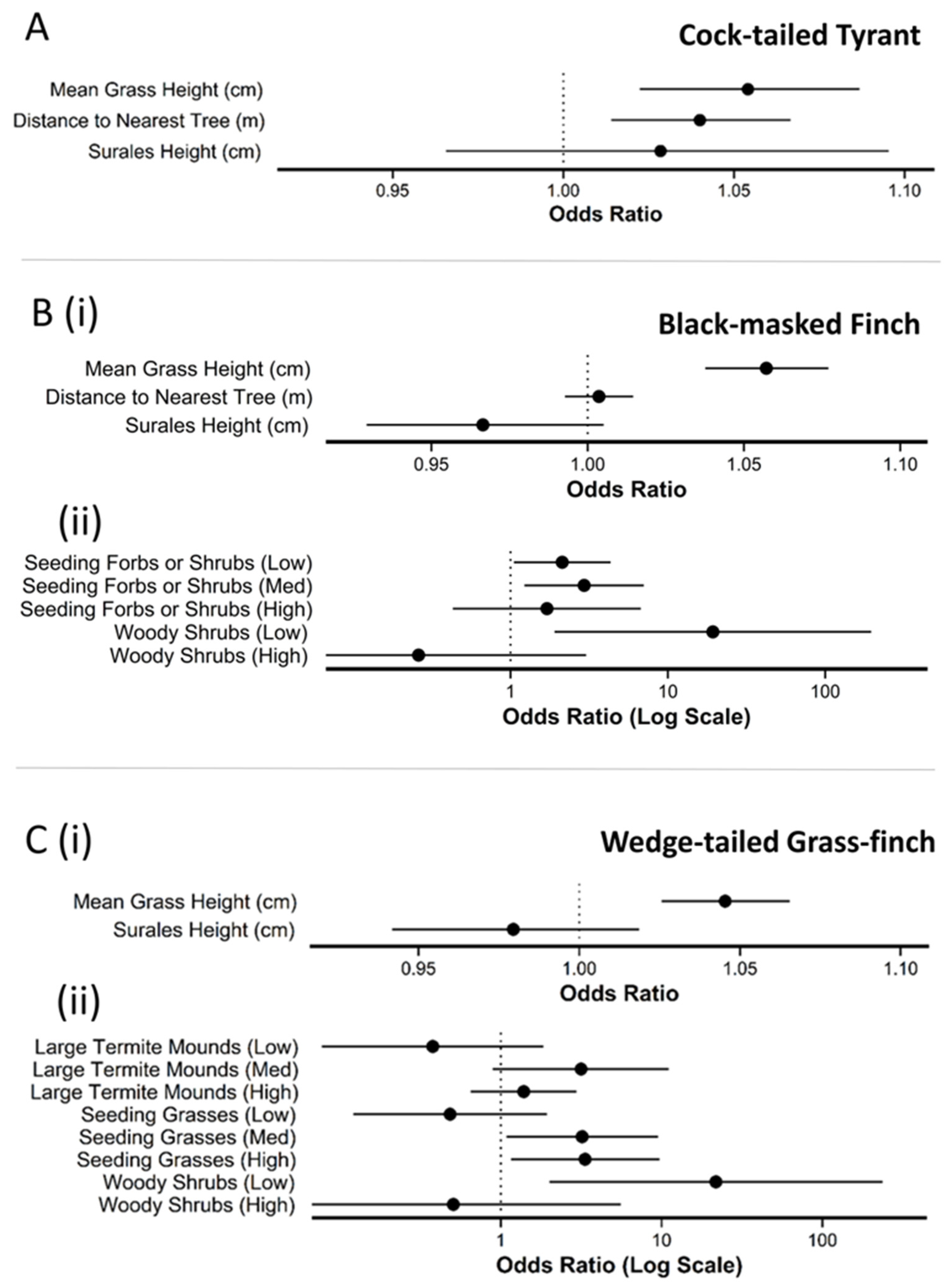
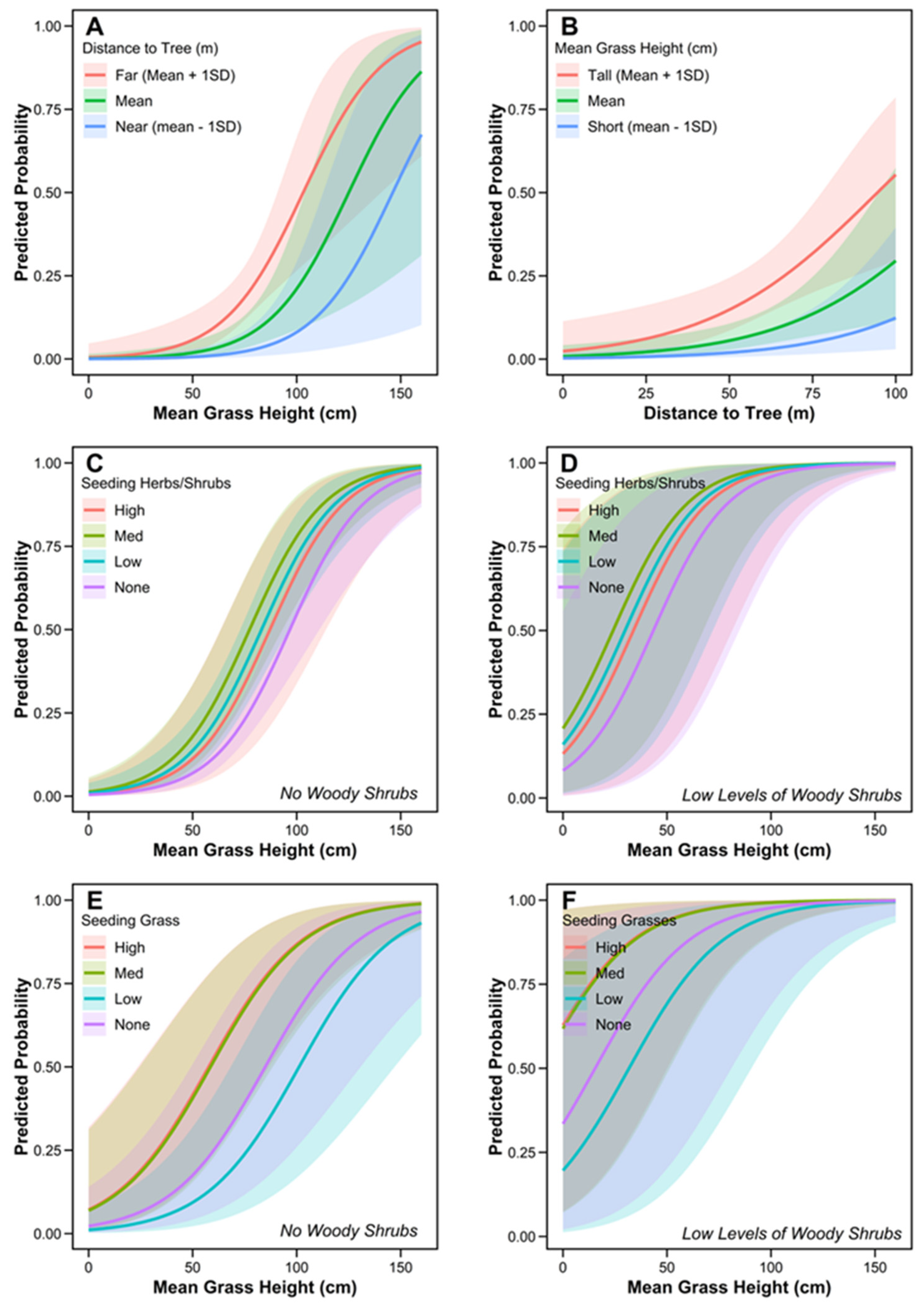
| Variable | β | SE | LCL | UCL | z | p |
|---|---|---|---|---|---|---|
| cock-tailed tyrant | ||||||
| Intercept | −8.3 | 1.5 | −11.2 | −5.4 | 5.6 | *** |
| Mean Grass Height | 5.3e−03 | 1.5e−03 | 2.2e−03 | 8.3e−03 | 3.4 | *** |
| Distance to Nearest Tree | 3.9 e−02 | 1.3e−02 | 1.4e−02 | 6.5e−02 | 3.0 | ** |
| Surales Height | 9.7e−04 | 2.3e−03 | −3.5e−03 | 9.1e−03 | 0.4 | |
| black-masked finch | ||||||
| Intercept | −5.0 | 0.7 | −6.4 | −3.6 | 6.9 | *** |
| Surales Height | −3.4e−03 | 2.0e−03 | −7.3e−03 | 5.1e−04 | 1.7 | . |
| Seeding/Fruiting Forbs/Shrubs (Low) | 0.8 | 3.6e−01 | 4.9e−02 | 1.5 | 2.1 | * |
| Seeding/Fruiting Forbs/Shrubs (Med) | 1.1 | 0.4 | 0.2 | 2.0 | 2.4 | * |
| Seeding/Fruiting Forbs/Shrubs (High) | 0.5 | 0.7 | −0.8 | 2.0 | 0.8 | |
| Mean Grass Height | 5.6e−03 | 9.5e−04 | 3.7e−3 | 7.4e−03 | 5.8 | *** |
| Woody Shrubs (Low) | 3.0 | 1.2 | 0.6 | 5.3 | 2.5 | * |
| Woody Shrubs (High) | −1.3e | 1.2 | −3.8 | 1.1 | 1.1 | |
| Distance to Tree | 3.6e−03 | 5.5e−03 | −7.3e−03 | 1.4e−02 | 0.6 | |
| wedge-tailed grass finch | ||||||
| Intercept | −4.8 | 0.8 | −6.4 | −3.2 | 5.9 | *** |
| Large Termites (Low) | −1.0 | 0.8 | −2.6 | 0.6 | 1.2 | |
| Large Termites (Med) | 1.2 | 0.6 | −0.1 | 2.4 | 1.8 | . |
| Large Termites (High) | 0.3 | 0.4 | −0.4 | 1.1 | 0.9 | |
| Mean Grass Height | 4.4e−03 | 9.6e−03 | 2.5e−03 | 6.3e−03 | 4.6 | *** |
| Seeding Grass (Low) | −0.7 | 0.7 | −2.1 | 0.7 | 1.0 | |
| Seeding Grass (Med) | 1.2 | 0.6 | 0.1 | 2.3 | 2.1 | * |
| Seeding Grass (High) | 1.2 | 0.5 | 0.1 | 2.3 | 2.2 | * |
| Woody Shrubs (Low) | 3.1 | 1.2 | 0.7 | 5.5 | 2.5 | * |
| Woody Shrubs (High) | −0.7 | 1.2 | −3.1 | 1.7 | 0.6 | |
| Surales Height | −2.1e−03 | 1.9e−03 | −6.0e−03 | 1.8e−03 | 1.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peacock, J.; Macleod, R.; Davies, G.M.; Boorsma, T.; Tonra, C.M. Habitat Selection of Three Neotropical Grassland Birds Is Dependent on Vegetation Structure and Resources. Diversity 2024, 16, 229. https://doi.org/10.3390/d16040229
Peacock J, Macleod R, Davies GM, Boorsma T, Tonra CM. Habitat Selection of Three Neotropical Grassland Birds Is Dependent on Vegetation Structure and Resources. Diversity. 2024; 16(4):229. https://doi.org/10.3390/d16040229
Chicago/Turabian StylePeacock, Jo, Ross Macleod, G. Matt Davies, Tjalle Boorsma, and Christopher M. Tonra. 2024. "Habitat Selection of Three Neotropical Grassland Birds Is Dependent on Vegetation Structure and Resources" Diversity 16, no. 4: 229. https://doi.org/10.3390/d16040229
APA StylePeacock, J., Macleod, R., Davies, G. M., Boorsma, T., & Tonra, C. M. (2024). Habitat Selection of Three Neotropical Grassland Birds Is Dependent on Vegetation Structure and Resources. Diversity, 16(4), 229. https://doi.org/10.3390/d16040229






