Abstract
The Pallas’s cat (Otocolobus manul) is one of the most understudied taxa in the Felidae family. The species is currently assessed as being of “Least Concern” in the IUCN Red List, but this assessment is based on incomplete data. Additional ecological and genetic information is necessary for the long-term in situ and ex situ conservation of this species. We identified 29 microsatellite loci with sufficient diversity to enable studies into the individual identification, population structure, and phylogeography of Pallas’s cats. These microsatellites were genotyped on six wild Pallas’s cats from the Tibet Autonomous Region and Mongolia and ten cats from a United States zoo-managed population that originated in Russia and Mongolia. Additionally, we examined diversity in a 91 bp segment of the mitochondrial 12S ribosomal RNA (MT-RNR1) locus and a hypoxia-related gene, endothelial PAS domain protein 1 (EPAS1). Based on the microsatellite and MT-RNR1 loci, we established that the Pallas’s cat displays moderate genetic diversity. Intriguingly, we found that the Pallas’s cats had one unique nonsynonymous substitution in EPAS1 not present in snow leopards (Panthera uncia) or domestic cats (Felis catus). The analysis of the zoo-managed population indicated reduced genetic diversity compared to wild individuals. The genetic information from this study is a valuable resource for future research into and the conservation of the Pallas’s cat.
1. Introduction
The Pallas’s cat (Otocolobus manul) is one of the most understudied taxa in the Felidae family [1]. This small felid inhabits the montane grasslands and rocky steppes of central and western Asia, lives at low densities (2–6 individuals/100 km2), and prefers rocky and ravine habitats for use as cover and den sites [2,3]. Large portions of the Pallas’s cat distribution include some of the highest elevational areas in the world [4]. Due to their elusive behavior and the challenges of surveying their remote, rugged habitat, Pallas’s cats are not commonly seen and limited studies have been conducted across their vast range [5]. Most ecological information stems from one in-depth project in central Mongolia conducted between 2005 and 2007 [2,6], as well as anecdotal accounts [7,8] and opportunistic sightings [9,10]. While core populations are thought to occur in Mongolia and China, the species has been reported in Russia, Iran, Afgahanistan, Bhutan, India, Kazakhstan, Kyrgyzstan, Nepal, Pakistan, and Turkmenistan [4].
Pallas’s cats face several threats, including habitat degradation, climate change, wildfires, killings by domestic dogs, and secondary poisoning [5,11]. One of the largest challenges to the conservation of this species is the lack of consistent information across its range [5]. To remedy the lack of information on the Pallas’s status, historical and contemporary records of their occurrence were incorporated into predictive models of the species’ distribution and habitat loss [4,11]. The species’ IUCN Red List (https://www.iucnredlist.org/; accessed 11 November 2022) designation was subsequently changed from “Near Threatened” to “Least Concern” [4]. However, there is concern that the assessment is based on incomplete data, and populations are thought to be declining and increasingly fragmented [4,11]. Further information on the Pallas’s cat will allow for more accurate assessments and the implementation of conservation measures, such as mitigating poaching and creating protected areas [11].
To increase conservation efforts in support of this understudied and vulnerable species, the Association of Zoos and Aquariums (AZA) established a managed population of Pallas’s cats via the import of 20 founders from Russia in the late 1990s and several founders from Mongolia in the 2000s [12,13]. Because the import of additional founders and further expansion of the AZA population size is logistically difficult [12], management recommendations based on genetics are necessary to ensure the long-term sustainability of this zoo-managed population. Typically, animal populations in zoos are small and descended from few founders; thus, these populations often have low genetic diversity [14]. As of 2021, the AZA manages 58 Pallas’s cats [15]. The AZA manages this population through a Species Survival Plan, which utilizes pedigree information recorded in studbooks to produce breeding recommendations that ensure the long-term viability of the population [13,16,17]. Molecularly derived estimates of relatedness should be integrated with this pedigree-related information to provide breeding recommendations that promote genetic diversity [17].
In addition, Pallas’s cats are found on the Tibetan Plateau and in the Himalayas, suggesting an ability to tolerate low oxygen levels [4]. One of the genes potentially underlying this adaptation is endothelial PAS domain protein 1 (EPAS1). EPAS1 is a transcription factor in the hypoxia pathway and serves a vital role in the cellular response to low-oxygen tension [18,19]. Moreover, EPAS1 participates in the molecular adaptation to high altitudes in several species, including humans, Tibetan mastiffs (Canis lupus familaris), Tibetan wolves (Canis lupus chanco), and snow leopards (Panthera unica) [18,19]. Unique variants of this gene in felids were initially determined through a genomic comparative analysis between tigers (Panthera tigris), a low-altitude species, and snow leopards, a high-altitude species [20]. Two non-synonymous SNPs were later found to be polymorphic in two snow leopard populations, and an additional variant was observed in Pallas’s cats in the same region of EPAS1 [19]. The performance of investigations into a sympatric high-altitude specialist, the Pallas’s cat, would enable further exploration of convergent evolution on the Tibetan Plateau.
Though scarce, other recent molecular or morphological studies on the species [4] include the identification of scat samples, the determination of diet [21,22,23], and analyses of individual genomes [24,25]. The current understanding of the species’ genetic diversity is based on pelage coloration, taxonomically suggesting two subspecies: O. m. manul (Pallas, 1776), present in southwest and central Asia, and O. m. nigripectus (Hodgson, 1842), located in the Himalayas [26]. Further investigations into the genetic diversity of the Pallas’s cat are needed to better understand the species’ conservation status and improve in situ and ex situ conservation outcomes. These investigations could lead to the development of species-specific management plans that minimize the loss of fitness and adaptability from a lack of gene flow between populations [27,28], inform breeding recommendations for zoo-managed populations of the species [17], and offer insight into evolutionary processes [29]. The first step in these investigations would be to denote molecular markers of suitable variability for use in individual identification, determination of effective population size and viability, and assessment of population structure and landscape connectivity [30].
In this study, we report the variations of 29 microsatellite loci that have sufficient diversity for use in studies that focus on individual identification, population structure, and phylogeography in Pallas’s cats. These markers and the mitochondrial 12S ribosomal RNA (MT-RNR1) locus were used to provide measures of genetic variation in wild and zoo-managed Pallas’ cats and inform the genetic management of the zoo-managed population. Lastly, we examined the EPAS1 gene to search for putatively adaptive variants in the Pallas’s cat species.
2. Materials and Methods
2.1. Sample Collection
Six scats were collected from Pallas’s cats during noninvasive surveys of snow leopards in Shenza, Tibet Autonomous Region (n = 1) and the Western and Eastern Beauties of Mongolia (n = 2 and n = 3, respectively; Figure 1). These samples were molecularly confirmed to have originated from Pallas’s cat [31,32]. To supplement the small sample size of wild cats (n = 6) in this study, additional samples were obtained from Pallas’s cats housed in AZA-accredited zoos (n = 10). Many of the zoo-managed cats were related through first-order, second-order, third-order, or more distant relationships and were descended from mixed Russian and Mongolian lineages (Figure S1). Whole-blood samples were collected opportunistically from the zoo-managed cats via venipuncture during anesthesia for medical and reproductive procedures (Cincinnati Zoo IACUC protocol # 14-120 and 14-121). DNA was extracted with Qiagen DNeasy Blood and Tissue Kits (Qiagen, Inc., Valencia, CA, USA).
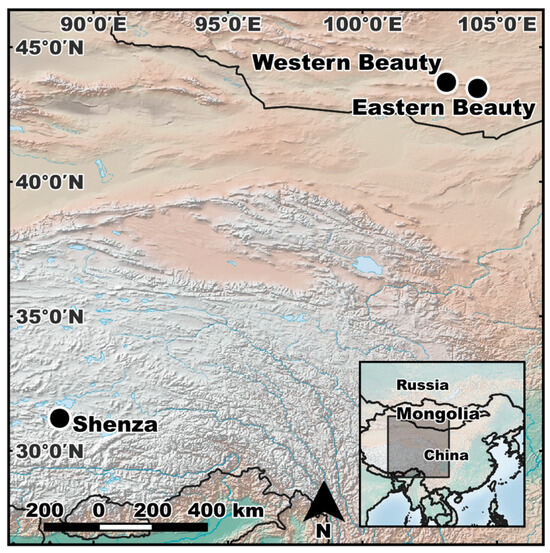
Figure 1.
Sampling sites for the Pallas’s cat (Otocolobus manul) scats included in this study. Scats were collected in Shenza, Tibet Autonomous Region (n = 1) and the Western and Eastern Beauties of Mongolia (n = 2 and n = 3, respectively) during noninvasive genetic surveys.
2.2. Genotyping of Microsatellites
Primers which were designed to amplify 33 dinucleotide microsatellite loci in snow leopards were used to amplify these loci in both wild and zoo-managed Pallas’s cat samples (n = 16). Due to the unknown diversity and utility of these microsatellites in the Pallas’s cat, all 33 microsatellites were amplified and analyzed. As described in [31], the snow leopard-based primers amplified the respective domestic cat (Felis catus) loci from [33,34], but were redesigned to match snow leopard sequences and amplify shorter amplicons to increase genotyping success using scat DNA (i.e., PUN1157 corresponds to FCA1157). Twenty-nine of the snow leopard primer sets successfully amplified loci in the Pallas’s cat samples (Table S1). Multiplex panels of primers (Table S2) were run in 10 μL reactions containing 1 μL DNA template, 5 μL 2× PCR Qiagen Type-It Ready Mix (Qiagen, Inc., Valencia, CA, USA), 0.050 μM of each forward and reverse primer, and PCR-grade water. PCRs were run in a Thermo Scientific Arktik thermal cycler (Thermo Fisher Scientific, Waltham, MA, USA) under the following conditions: denaturation at 95 °C for 5 min, 40 cycles of 95 °C for 30 s, 55 °C for 90 s, and 72 °C for 30 s, and a final extension step of 45 min at 60 °C. Products were confirmed via gel electrophoresis and fractionated on an Applied Biosystems SeqStudio Genetic Analyzer (Thermo Fisher Scientific, Waltham, MA, USA). Microsatellite alleles were scored using SoftGenetics GeneMarker v3.0.0 (SoftGenetics LLC, State College, PA, USA).
In order to detect possible errors, wild individuals (n = 6) were genotyped in triplicate for seven loci (PUN1157, PUN1262, PUN834, PUN100, PUN124, PUN225, and PUN894). These wild individuals showed consistent results with regard to these seven loci, indicating that the error rates for genotyping were low and therefore additional loci were amplified. Zoo-managed individuals (n = 10) were genotyped in duplicate for 12 loci (PUN82, PUN1262, PUN225, PUN894, PUN1270, PUN1138, PUN928, PUN1307, PUN664, PUN1131, PUN924, and PUN44). Individuals were considered to be homozygotes when a single allele peak, excluding stutter peaks, was present in the expected size range with a minimum of 100 relative fluorescence units above the background noise. Individuals were considered heterozygotes when two distinct allele peaks were present, excluding stutter peaks. Samples that failed were reamplified at that locus and reanalyzed up to two times. After considering all replicates, the rate of genotyping success was found to be 90.4% (678 successful genotypes out of 750 total amplifications).
2.3. General Analysis of Microsatellites
Standard measures of microsatellite genetic diversity were performed for zoo-managed and wild individuals (n = 16) in GenAlEx v6.5 [35,36]. These assessments included the probability of identity (PID), the mean number of alleles (AN), the mean effective number of alleles (AE), observed heterozygosity (HO), expected heterozygosity (HE), fixation index (FI, 1—HO/HE), and the number of private alleles (Ap). PID is a measure of individual identity used to determine the probability that two individuals, drawn at random from a population, will share the same genotype at multiple loci [31,37]. An associated measure (PID-sib) describes the probability of the two individuals being related. All loci were evaluated for linkage disequilibrium using GenePop v4.7 [38,39] and deviations from Hardy–Weinberg equilibrium (HWE) using GenAlEx, with significant p-values adjusted for use in multiple comparisons.
2.4. Microsatellite Comparison with Snow Leopards
In order to provide comparable estimates of diversity between the wild Pallas’s cats (n = 6) and snow leopards, standard measures of diversity in the range-wide snow leopard dataset (n = 70) from [31] were reanalyzed with regard to the same microsatellite loci that were successfully amplified in Pallas’s cats. Snow leopard PID and PID-sib were also determined for the top eight most diverse loci in Pallas’s cats.
2.5. Microsatellite Analysis of the Zoo-Managed Population
We estimated pairwise FST values between wild (n = 6) and zoo-managed (n = 10) individuals through an analysis of molecular variance (AMOVA). Principal coordinate analysis (PCoA) was conducted in GenAlEx to visualize clustering. Lastly, using a modified Wang estimator [40] in Coancestry v1.0.1.11 [41], a pairwise relatedness matrix was produced between zoo-managed individuals in order to provide information for breeding recommendations. An estimator value of 1 indicates monozygotic twins; at least 0.5 indicates a parent’s offspring or full sibling; at least 0.25 indicates a second-order relationship; at least 0.125 indicates a third-order relationship; at least 0 indicates a distant degree of relatedness; and equal to or less than 0 indicates no relationship [42]. The modified Wang estimator was chosen to limit the bias caused by small sample sizes and analyses that include both related and unrelated individuals [43].
2.6. Sequencing & Analysis of MT-RNR1
The MT-RNR1 locus was sequenced in all 16 wild and zoo-managed individuals using primers 12SV5F and 12SV5R [44]. The locus was amplified in PCRs containing 1.5 μL of DNA template, 5 μL of KAPA HIFI HotStart Ready Mix (2×) (Kapa Biosystems, Wilmington, MS, USA), 0.16 μL of 20 μM forward primer, 0.16 μL of 20 μM reverse primer, and 5.2 μL of PCR-grade water. The PCRs were run under the following conditions: denaturation at 95 °C for 3 min; 40 cycles of 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s; and a final extension step of 5 min at 72 °C. Successful amplifications were confirmed via gel electrophoresis. The products were then cleaned using Illustra ExProStar PCR and sequence reaction clean-up (MilliporeSigma, Darmstadt, Germany). BigDye reactions contained 1 μL PCR-grade water, 2 μL BigDye v1.1, 2.0 μM forward primer, and 2 μL of cleaned PCR product. Products were then purified using Centri-Spin 20 columns (Thermo Fisher Scientific, Waltham, MA, USA) prior to performing sequencing on an Applied Biosystems SeqStudio Genetic Analyzer.
Sequence files were trimmed and aligned using CLC Genomics Workbench v22 (Qiagen Bioinformatics, Redwood City, CA, USA). In order to supplement the analysis, six additional Pallas’s cat MT-RNR1 sequences were downloaded from the NCBI GenBank database (accession numbers: MH48879.1 (MT-RNR1), KX098454.1 (MT-RNR1), KX098455.1 (MT-RNR1), MH978908.1 (mitochondrion), KR132585.1 (mitochondrion), and NC_028323.1 (mitochondrion)). Sequence MH48879.1 was collected from the Gongga Mountain Nature Reserve in western Sichuan Providence, China [22]. Sequence MH978908.1 was collected from the Inner Mongolia Autonomous region of China [24]. Other sequences were from unknown origins, but KX098454.1 and KX098455.1 were likely collected in India by the Zoological Survey of India.
A preliminary BLAST (Basic Local Alignment Search Tool) analysis was conducted using MT-RNR1 to confirm the identities of all sequenced samples (n = 16). To identify haplotypes and relationships among the haplotypes of the 16 sequenced samples and the 6 additional sequences from GenBank, a minimum spanning haplotype network with a 95% confidence level was constructed using TCS v1.21 [45] and visualized using PopART v1.7 [46].
2.7. Sequencing & Analysis of EPAS1
For EPAS1, the two loci that varied in snow leopards were sequenced in all ten zoo-managed individuals and in the three wild individuals from the Eastern Beauty of Mongolia using primers designed for snow leopards [19]. For the first variant in exon 12, primers EPAS1-133 (5′GATCCGCCATTACATTTTGG) and EPAS1-272 (5′AGGGCCTCTGCCACTTACTT) were used. For the second variant in exon 15, primers EPAS1-156 (5′GGGACAGCCTCTGAGACATC) and EPAS1-321 (5′CTAGCATGGTGGGTCCACTT) were used. The EPAS1 sequences from the three wild individuals from the Eastern Beauty of Mongolia were collected using Illumina (Illumina, Inc., San Diego, CA, USA) ampliconic sequencing with standard barcodes (Illumina forward overhang: 5′TCGTCGGCAGCGTCAGATGTGTATAAGAGACAG; Illumina reverse overhang: 5′GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAG) [19,32]. The EPAS1 loci in the zoo-managed individuals were amplified, sequenced, and aligned using the same methods with MT-RNR1, as previously described. The alignments of the two EPAS1 loci were observed to assess the differences between individuals and in the sequences of the domestic cat, tiger (Panthera tigris), and snow leopard reported by [19].
3. Results
3.1. General Analysis of Microsatellites
When considering both wild (n = 6) and zoo-managed (n = 10) individuals (n = 16), 28 of the 29 microsatellite loci were polymorphic, and 19 loci contained at least five unique alleles (Table S1). No linkage associations were detected via pairwise comparisons of the microsatellite loci within the zoo-managed cats, wild cats, or overall. Significant deviation from HWE (p < 0.001) was only observed in one locus, PUN894, when examining both sets of individuals (zoo-managed and wild), but this issue was not found with individual sets (zoo-managed or wild). The individuals that were sampled were highly unlikely to be the same individual (PID = 2.212 × 10−23) or related (PID-sib = 4.616 × 10−10) across all the loci. Based on PID, the eight most diverse loci were PUN1131, PUN668, PUN1293, PUN1047, PUN100, PUN1307, PUN928, and PUN9013D (Table S2). When these eight microsatellites were examined, the probability of individual (PID = 8.411 × 10−10) and related (PID-sib = 0.0004) identities were sufficient for use in noninvasive genetic surveys as a panel.
The Pallas’s cat had moderate levels of genetic diversity: AN = 5.103, AE = 3.235, HO = 0.571, and HE = 0.623 (Table 1). The measures of genetic diversity for wild (AN = 3.931; AE = 3.057; HO = 0.540; and HE = 0.595) and zoo-managed individuals (AN = 3.552; AE = 2.342; HO = 0.590; and HE = 0.535) were similar, but the wild individuals contained a greater number of total alleles and private alleles, despite having a smaller population size (Table 1). In the zoo-managed population, six loci contained at least five unique alleles. In the wild individuals, the additional locus PUN917 was monomorphic, and nine loci contained at least five unique alleles (Table S1).

Table 1.
Genetic diversity of Pallas’s cats (Otocolobus manul) and snow leopards (Panthera uncia) genotyped at the same 29 microsatellite loci. In the current study, these microsatellites were genotyped in six wild Pallas’s cats from Shenza, Tibet (n = 1) and the Western and Eastern Beauties of Mongolia (n = 2 and n = 3, respectively) and in ten Pallas’s cats from a zoo-managed population with a mixed Russian and Mongolian lineage. Snow leopard genotypes were collected during a previous range-wide study [31]. The data from the study were reanalyzed to compare the same microsatellite loci genotyped in the Pallas’s cat. Means for each number are listed with ± SE. n = sample size, AN = number of alleles, AE = effective number of alleles (1/(∑p2), HO = observed heterozygosity, HE = expected heterozygosity, FI = fixation index (1—HO/HE), AT = total number of alleles, Ap = number of private alleles.
3.2. Microsatellite Comparison with Snow Leopards
With regard to the range-wide snow leopard microsatellite dataset (n = 70), the measures of diversity for the 29 microsatellite loci that were successfully amplified in Pallas’s cats were as follows: AN = 5.724, AE = 2.753, HO = 0.425, and HE = 0.573 (Table 1). When the top eight most diverse microsatellites in Pallas’s cats were examined in the range-wide snow leopard microsatellite dataset, the probabilities of individual (PID = 0.00001) and related (PID-sib = 0.007) identities were also reasonably low, enabling these eight loci to be used for both felids in areas where they coincided. The size ranges of these microsatellites in the two species are similar; thus, these microsatellites should not be used for differentiation (Table S2).
3.3. Microsatellite Analysis of the Zoo-Managed Population
The pairwise FST value between the zoo-managed (n = 10) and wild (n = 6) individuals (FST = 0.143; p = 0.001) and PCoA (Figure S2) indicates divergence between the two sets. The average pairwise relatedness in the zoo-managed population was 0.054 and this value ranged from −0.429 to 0.533, indicating that most of the individuals had some relationship with each other (Table 2). Despite the use of the modified Wang estimator [40,43], the estimates of relatedness appeared to slightly underestimate the relationships documented by the AZA’s North American Regional Pallas’s Cat Studbook (Figure S1) [15].

Table 2.
Pairwise relatedness measured using the modified Wang estimator [40,43] of ten Pallas’s cats (Otocolobus manul) housed in AZA-accredited zoos genotyped at 29 microsatellite loci. These Pallas’s cats are descendants of founders from Russia and Mongolia. Red, orange, yellow, and green highlighted estimator values indicate first-order relationships (rxy ≥ 0.5), second-order relationships (0.5 > rxy ≥ 0.25), third-order relationships (0.25 > rxy ≥ 0.125), and distant relatedness (0.125 > rxy > 0), respectively. The remaining blue estimator values indicate no relationship.
3.4. Analysis of MT-RNR1
In the preliminary sequence alignment analyses of MT-RNR1, all 16 samples sequenced in this study shared >97% identity with Otocolobus manul sequences on GenBank. When the 16 sequences collected from this study were aligned with the 6 available MT-RNR1 sequences from GenBank, two segregating sites were present in the 91 bp sequence (Figure 2 and Figure S3). In the wild individuals sequenced in this study (n = 6), one segregating site was present, and in the zoo-managed individuals sequenced in this study (n = 10), two segregating sites were present. The six wild individuals shared the same haplotype as the three zoo-managed individuals and two individuals from GenBank (MH48879.1, KR132585.1; Figure 2). Two other haplotypes were separated from this primary haplotype by one substitution (Figure 2). The second haplotype included five individuals from the zoo-managed population and the four remaining individuals from GenBank. The third haplotype exclusively comprised two individuals from the zoo-managed population (Figure 2).
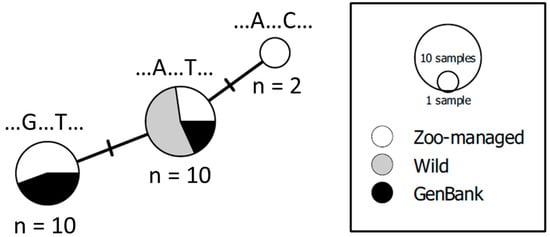
Figure 2.
Minimum spanning network of Pallas’s cats (Otocolobus manul) at the mitochondrial 12S ribosomal RNA (MT-RNR1; 91 bp) locus. Sequences originated from six wild and ten zoo-managed cats sequenced in this study and six additional sequences from NCBI’s GenBank (n = 22). The wild Pallas’s cat scats were collected in Shenza, Tibet Autonomous Region (n = 1) and the Western and Eastern Beauties of Mongolia (n = 2 and n = 3, respectively). The white, gray, and black coloration of the haplotypes correspond to zoo-managed, wild, and GenBank individuals, respectively. Shared haplotypes are split proportionally based on the number of sequences collected from wild specimens, zoo-managed specimens, or sequences available through GenBank. Each solid branch represents one mutational step between haplotypes. For each haplotype, the nucleotide present at each segregating site is listed above.
3.5. Analysis of EPAS1
All ten zoo-managed individuals and the three wild individuals from the Eastern Beauty of Mongolia contained the same variants of EPAS1. While the translated product at exon 12, residue 671 in the snow leopard contained an isoleucine, the translated product in Pallas’s cats had a valine at that position, like the tiger and domestic cat (Figure 3). Similarly, just like the tiger and domestic cat, the Pallas’s cat had a cysteine present in the translated product of exon 15, residue 803 instead of the arginine found in the snow leopard. However, the Pallas’s cat contained a unique substitution at exon 15, residue 806, changing the codon from CCC to TCC. The proline observed in the translated products of the snow leopard, tiger, and domestic cat was substituted for a serine (Figure 3). All three described variants occurred due to nucleotide substitutions in the first codon’s position.

Figure 3.
Haplotype of the translated endothelial PAS domain protein 1 (EPAS1) product identified in ten Pallas’s cats (Otocolobus manul) housed in AZA-accredited zoos and three wild Pallas’s cats from the Eastern Beauty of Mongolia. Both loci were independently sequenced and compared to translated sequences from the domestic cat (Felis catus), tiger (Panthera tigris), and snow leopard (Panthera uncia), which were previously collected by [19]. The outlined box highlights the unique substitution observed in the Pallas’s cat for this gene. Exon and residue numbers are based on human orthologs.
4. Discussion
4.1. Summary of Microsatellite Loci
The 29 sets of microsatellite primers described in this study are a valuable resource for future research into the Pallas’s cat. For instance, the eight most diverse loci have low probabilities of individual (PID = 8.411 × 10−10) and related (PID-sib = 0.0004) identities and can be used for individual genotyping, kinship analyses, and phylogeography studies. Like in Pallas’s cats, the probabilities of individual and related identities were sufficiently low for these eight loci in snow leopards (PID = 0.00001; PID-sib = 0.007), allowing for concurrent studies of both felids. Applications may include studies that estimate abundance, monitor local populations, determine home range sizes, or perform noninvasive genetic sampling [47,48,49]. Once individuals have been identified, the full set of microsatellite primers can be used to describe genetic structure, assess subspecies designations, identify landscape connectivity, and examine populations with possible variation in terms of their adaptation to high altitudes.
4.2. Initial Estimates of Genetic Diversity in the Pallas’s Cat
The Pallas’s cat samples investigated in this study contained moderate genetic diversity when examined at the 29 microsatellite loci. The comparison of these microsatellites between Pallas’s cats (n = 16) and snow leopards across their range (n = 70) indicated greater mean numbers of effective alleles and observed heterozygosity in the Pallas’s cats. The examination of MT-RNR1 sequences (n = 22), which is a standard barcode for species identification that is not expected to be variable, discovered two segregating sites that produced three haplotypes. In comparison, only one MT-RNR1 haplotype was observed in snow leopards from Pakistan, China, Mongolia, and Kyrgyzstan using the same MT-RNR1 primers [32].
4.3. Diversity in the Zoo-Managed Population
The AZA’s managed population contained less genetic diversity in the microsatellites compared to the wild individuals, despite having a higher sample size. The lower genetic diversity in the zoo-managed population was likely due to genetic drift. Since this population was derived from 20–30 founders, a loss of diversity is expected with each generation [17]. Common alleles between the two populations may have been lost over time due to genetic drift in the zoo-managed population, and homozygosity likely increased from inbreeding [50]. The zoo-managed population contained moderate levels of relatedness. In 45 possible pairings of the 10 zoo-managed individuals, 18 (40%) had pairwise relatedness values that indicated third-order relationships at minimum, which slightly underestimated the pedigree information for the analyzed cats (Figure S1). While the estimator values appear to be slightly biased due to a small sample size [43], our genetic data can be incorporated with pedigree information to provide breeding strategies to secure the long-term viability of the zoo-managed population. Further sampling of the population would provide more accurate estimates.
Assisted reproductive technologies for the Pallas’s cat and other felids should continue to be investigated in order to provide a long-term solution if additional living founders cannot be imported into the AZA’s managed population [12,51]. For example, genetic exchange between zoo-managed populations in Europe or Asia, originating from cats in China, may increase overall heterozygosity and introduce new alleles into zoo-managed populations. Alternatively, semen collected from wild (free-ranging) males could be frozen in the field and used to create gene flow between in situ and ex situ populations [52]. Measures of genetic diversity could be performed using the semen prior to artificial insemination. This heterozygosity is valuable in maintaining the fitness of the populations, including disease resistance [53,54]. Pallas’s cats are highly susceptible to feline infections, such as Toxoplasmosa gondii and feline immunodeficiency virus [55,56,57]. Inbreeding depression may serve to increase the severity of these already fatal infections in Pallas’s cats.
4.4. EPAS1 Variant in the Pallas’s Cat
We confirmed a previously unobserved variant of EPAS1 in the Pallas’s cat. A single-nucleotide substitution was present in the ten zoo-managed animals and in three wild cats at exon 15, residue 806. In snow leopards, tigers, and domestic cats, the codon at this residue corresponds to a proline, which contains a nonpolar side chain [58,59], in the translated polypeptide. Meanwhile, serine, which contains a polar side chain [58,59], is present in the translated product in the Pallas’s cats due to the observed substitution. However, this analysis only included individuals from the Eastern Beauty of Mongolia and descendants of founders originating in Russia and Mongolia. The limitations of EPAS1 data were partially due to the difficulty in amplifying the highly degraded DNA found in scat [60]. Specifically, EPAS1 could not be amplified in the scat samples from the Tibet Autonomous Region or Western Beauty of Mongolia.
5. Conclusions
This study describes three sets of molecular markers that are informative for future investigations into the Pallas’s cat and provides initial estimates of genetic diversity in this species overall and in a zoo-managed population. Further information is necessary to confirm the distribution and genetic diversity of this species in order to accurately assess its conservation status. The current analyses were conducted on a small sample of Pallas’s cats taken from limited sampling locations. Thus, the diversity of the species within the core populations of Mongolia and China and across its range remains poorly understood. Future analyses should examine the diversity of the core populations and across the species range by undertaking greater sampling efforts. Additionally, the MT-RNR1 barcode provides a limited estimate of mitochondrial diversity in the species. The full extent of the mitochondrial diversity should be examined through the sequencing of entire mitogenomes. Future investigations of EPAS1 in the Pallas’s cat should be performed between populations of varying elevations to examine potential polymorphisms. While Pallas’s cats are high-altitude specialists, these felids are found across a range of altitudes, such as lower-altitude steppes and higher-altitude mountains [4]. Unique ecotypes across this altitude range would provide insights into the molecular adaptation to hypoxia. The information within this study should encourage further investigations into the Pallas’s cat.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d16040228/s1, Figure S1: Pedigree of the ten zoo-managed Pallas’s cats (Otocolobus manul) sampled in this study and their founding ancestors; Table S1: Description of 29 microsatellites used to genotype six wild and ten zoo-managed Pallas’s cats (Otocolobus manul); Table S2: The nine multiplex panels of the 29 microsatellite primers used to genotype six wild and ten zoo-managed Pallas’s cats (Otocolobus manul); Figure S2: Principal coordinate analysis of six wild and ten zoo-managed Pallas’s cats (Otocolobus manul) genotyped at 29 microsatellite loci; Figure S3: Three observed mitochondrial 12S ribosomal RNA (MT-RNR1) haplotypes in 23 Pallas’s cats (Otocolobus manul).
Author Contributions
Conceptualization, J.E.J.; methodology, J.E.J.; resources, B.M. (Bariushaa Munkhtsog), B.M. (Bayaraa Munkhtsog), Y.Z., W.F.S. and L.A.L.; investigation, J.J.R., A.D.C. and C.E.H.; formal analysis, J.J.R. and J.E.J.; writing—original draft preparation, J.J.R.; writing—review & editing, J.J.R., J.E.J., C.E.H., W.F.S. and L.A.L.; visualization, J.J.R. and C.E.H.; project administration, J.E.J.; supervision, J.E.J.; funding acquisition, J.E.J. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by the Snow Leopard Conservancy, NSF REU Site Award (#1757555), and Duquesne University Department of Biological Sciences.
Institutional Review Board Statement
Blood samples were collected opportunistically from zoo-managed Pallas’s cats anesthetized for annual veterinary health exams conducted concurrently with IACUC-approved reproductive procedures (Cincinnati Zoo IACUC protocol # 14-120 and 14-121).
Data Availability Statement
The original contributions presented in the study are included in the article and Supplementary Material, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank the AZA-accredited zoos (Cincinnati Zoo & Botanical Garden, Columbus Zoo & Aquarium, Dakota Zoo, Great Plains Zoo, Miller Park Zoo, Pueblo Zoo, Red River Zoo) that provided genetic samples from their resident Pallas’s cats. Additionally, we would like to acknowledge Rodney Jackson and Lance Daley’s technical assistance in the field and lab, respectively. All samples were obtained in accordance with the appropriate agencies. The findings and conclusions in this article are those of the author(s) and do not necessarily represent the views of the US Fish and Wildlife Service.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tensen, L. Biases in wildlife and conservation research, using felids and canids as a case study. Glob. Ecol. Conserv. 2018, 15, e00423. [Google Scholar] [CrossRef]
- Ross, S. Providing an Ecological Basis for the Conservation of the Pallas’s Cat (Otocolobus manul). Ph.D. Thesis, University of Bristol, Bristol, UK, 2009. [Google Scholar] [CrossRef]
- Ross, S.; Barashkova, A.; Kirilyuk, V.; Naidenko, S. The behaviour and ecology of the manul. Cat News 2019, 13, 9–13. [Google Scholar]
- Ross, S.; Barashkova, A.; Dhendup, T.; Munkhtsog, B.; Smelansky, I.; Barclay, D.; Moqanaki, E. Otocolobus manul (errata version published in 2020). The IUCN Red List of Threatened Species. 2020. Available online: https://www.researchgate.net/publication/342945167_Otocolobus_manul_errata_version_published_in_2020_The_IUCN_Red_List_of_Threatened_Species (accessed on 11 November 2022).
- Lanz, T.; Breitenmoser-Würsten, C.; Barclay, D.; Nygren, E.; Samelius, G.; Breitenmoser, U. Prologue: Why care about Otocolobus manul? Cat News 2019, 13, 5–8. [Google Scholar]
- Ross, S.; Munkhtsog, B.; Harris, S. Determinants of mesocarnivore range use: Relative effects of prey and habitat properties on Pallas’s cat home-range Size. J. Mammal. 2012, 93, 1292–1300. [Google Scholar] [CrossRef]
- Barashkova, A.N.; Kirilyuk, V.E.; Smelansky, I.E. Significance of protected areas for the Pallas’s cat (Otocolobus manul: Felidae) conservation in Russia. Nat. Conserv. Res. 2017, 2, 113–124. [Google Scholar] [CrossRef][Green Version]
- Greenspan, E.; Giordano, A.J. A rangewide distribution model for the Pallas’s cat (Otocolobus manul): Identifying potential new survey regions for an understudied small cat. Mammalia 2021, 85, 574–587. [Google Scholar] [CrossRef]
- Chalani, M.; Ghoddousi, A.; Ghadirian, T.; Goljani, R. First Pallas’s cat photo-trapped in Khojir National Park, Iran. Cat News 2008, 49, 7. [Google Scholar]
- Pal, R.; Bhattacharya, T.; Sathyakumar, S. First record of Pallas’s cat in Uttarakhand, Nelang Valley, Gangotri National Park, India. Cat News 2019, 69, 24–25. [Google Scholar]
- Barashkova, A.; Smelansky, I.; Kirilyuk, V.; Naidenko, S.; Antonevich, A.; Gritsina, M.; Uulu, K.Z.; Koshkin, M.; Battogtokh, N.; Otgonbayar, B.; et al. Distribution and status of the manul in central Asia and adjacent areas. Cat News 2019, 13, 14–23. [Google Scholar]
- Swanson, W.F. Application of assisted reproduction for population management in felids: The potential and reality for conservation of small cats. Theriogenology 2006, 66, 49–58. [Google Scholar] [CrossRef]
- Kayser, S.; Bladow, R. Population Analysis & Breeding and Transfer Plan—Pallas’ Cat (Otocolobus manul). In AZA Species Survival Plan Red Program; AZA’s Population Management Center, Lincoln Park Zoo: Chicago, IL, USA, 2021; 44p, Available online: https://www.aza.org/population-management-center (accessed on 16 February 2024).
- Leberg, P.L.; Firmin, B.D. Role of inbreeding depression and purging in captive breeding and restoration programmes. Mol. Ecol. 2008, 17, 334–343. [Google Scholar] [CrossRef]
- Kayser, S. AZA Regional Studbook—Pallas’ Cat (Otocolobus manul). 2021; 73p. Available online: http://www.aza.org (accessed on 16 February 2024).
- Hutchins, M.; Wiese, R.J. Beyond genetic and demographic management: The future of the species survival plan and related AAZPA conservation efforts. Zoo Biol. 1991, 10, 285–292. [Google Scholar] [CrossRef]
- Fienieg, E.S.; Galbusera, P. The use and integration of molecular DNA information in conservation breeding programmes: A Review. J. Zoo Aquar. Res. 2013, 1, 44–51. [Google Scholar]
- Tian, H.; Mcknight, S.L.; Russell, D.W. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997, 11, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Janecka, J.E.; Hacker, C.; Broderick, J.; Pulugulla, S.; Auron, P.; Ringling, M.; Nelson, B.; Munkhtsog, B.; Hussain, S.; Davis, B.; et al. Noninvasive Genetics and Genomics Shed Light on the Status, Phylogeography, and Evolution of the Elusive Snow Leopard. In Conservation Genetics in Mammals: Integrative Research Using Novel Approaches; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 83–120. ISBN 9783030333348. [Google Scholar]
- Cho, Y.S.; Hu, L.; Hou, H.; Lee, H.; Xu, J.; Kwon, S.; Oh, S.; Kim, H.M.; Jho, S.; Kim, S.; et al. The tiger genome and comparative analysis with lion and snow leopard genomes. Nat. Commun. 2013, 4, 2433. [Google Scholar] [CrossRef] [PubMed]
- Werhahn, G.; Sherchan, A.M.; Manandhar, P. Eurasian lynx and Pallas’s cat in Dolpa District of Nepal: Genetics, distribution and diet. Cat News 2018, 67, 34–36. [Google Scholar]
- Zhao, D.; Yang, C.; Ma, J.; Zhang, X.; Ran, J. Vertebrate prey composition analysis of the Pallas’s cat (Otocolobus manul) in the Gongga Mountain Nature Reserve, based on fecal DNA. Mammalia 2020, 84, 449–457. [Google Scholar] [CrossRef]
- Hacker, C.E.; Cong, W.; Xue, Y.; Li, J.; Zhang, Y.; Wu, L.; Ji, Y.; Dai, Y.; Li, Y.; Jin, L.; et al. Dietary diversity and niche partitioning of carnivores across the Qinghai–Tibetan Plateau of China using DNA metabarcoding. J. Mammal. 2022, 103, 1005–1018. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, J.; Jiang, E.; Xu, Y.; Ning, F.; Du, Z.; Bai, X. The complete mitochondrial genome of Pallas’s cat (Otocolobus manul). Mitochondrial. DNA B Resour. 2019, 4, 658–659. [Google Scholar] [CrossRef]
- Flack, N.; Drown, M.; Walls, C.; Pratte, J.; Mclain, A.; Faulk, C. Chromosome-level, nanopore-only genome and allele-specific DNA methylation of Pallas’s cat, Otocolobus manul. bioRxiv 2022. [Google Scholar] [CrossRef]
- Kitchener, A.; Breitenmoser-Würsten, C.; Eizirik, E.; Gentry, A.; Werdelin, L.; Wilting, A.; Yamaguchi, N.; Abramov, A.; Christiansen, P.; Driscoll, C.; et al. A revised taxonomy of the felidae. The final report of the cat classification task force of the IUCN/SSC Cat Specialist Group. Cat News 2017, 11, 1–80. [Google Scholar]
- Arif, I.A.; Khan, H.A.; Khan, H.A. Molecular markers for biodiversity analysis of wildlife animals: A brief review. Anim. Biodivers. Conserv. 2009, 32, 9–17. [Google Scholar] [CrossRef]
- Shrestha, B.; Kindlmann, P. Implications of landscape genetics and connectivity of snow leopard in the Nepalese Himalayas for its conservation. Sci. Rep. 2020, 10, 19853. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.G.; Hazzouri, K.M.; Choi, J.Y.; Delaney, P.; Al-Kharafi, M.; Howells, E.J.; Aranda, M.; Burt, J.A. Signatures of selection underpinning rapid coral adaptation to the world’s warmest reefs. Sci. Adv. 2022, 8, 7287. [Google Scholar] [CrossRef] [PubMed]
- DeYoung, R.W.; Honeycutt, R.L. The molecular toolbox: Genetic techniques in wildlife ecology and management. J. Wildl. Manag. 2005, 69, 1362–1384. [Google Scholar] [CrossRef]
- Janecka, J.E.; Zhang, Y.; Li, D.; Munkhtsog, B.; Bayaraa, M.; Galsandorj, N.; Wangchuk, T.R.; Karmacharya, D.; Li, J.; Lu, Z.; et al. Range-wide snow leopard phylogeography supports three subspecies. J. Hered. 2017, 108, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Hacker, C.E.; Jevit, M.; Hussain, S.; Muhammad, G.; Munkhtsog, B.; Munkhtsog, B.; Zhang, Y.; Li, D.; Liu, Y.; Farrington, J.D.; et al. Regional comparison of snow leopard (Panthera uncia) diet using DNA metabarcoding. Biodivers. Conserv. 2021, 30, 797–817. [Google Scholar] [CrossRef]
- Menotti-Raymond, M.; David, V.A.; Lyons, L.A.; Schä, A.A.; Tomlin, J.F.; Hutton, M.K.; O’brien, S.J. A genetic linkage map of microsatellites in the domestic cat (Felis catus). Genomics 1999, 57, 9–23. [Google Scholar] [CrossRef]
- Menotti-Raymond, M.; David, V.A.; Chen, Z.Q.; Menotti, K.A.; Sun, S.; Schaffer, A.A.; Agarwala, R.; Tomlin, J.F.; O’Brien, S.J.; Murphy, W.J. Second-generation integrated genetic linkage/radiation hybrid maps of the domestic cat (Felis catus). J. Hered. 2003, 94, 95–106. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenALEx 6.5: Genetic Analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GENALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Waits, L.P.; Luikart, G.; Taberlet, P. Estimating the probability of identity among genotypes in natural populations: Cautions and guidelines. Mol. Ecol. 2001, 10, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Raymond, M.; Rousset, F. GENEPOP (version 1.2): Population genetics software for exact tests and ecumenicism. J. Hered. 1995, 86, 248–249. [Google Scholar] [CrossRef]
- Rousset, F. GENEPOP’007: A complete re-implementation of the GENEPOP software for Windows and Linux. Mol. Ecol. Resour. 2008, 8, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. An estimator for pairwise relatedness using molecular markers. Genetics 2002, 160, 1203–1215. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Coancestry: A program for simulating, estimating and analysing relatedness and inbreeding coefficients. Mol. Ecol. Resour. 2011, 11, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.; Ritland, K. Estimation of pairwise relatedness with molecular markers. Genetics 1999, 152, 1753–1766. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Estimating pairwise relatedness in a small sample of individuals. Heredity 2017, 119, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Riaz, T.; Shehzad, W.; Viari, A.; Pompanon, F.; Taberlet, P.; Coissac, E. EcoPrimers: Inference of new DNA barcode markers from whole genome sequence analysis. Nucleic Acids Res. 2011, 39, e145. [Google Scholar] [CrossRef]
- Clement, M.; Posada, D.; Crandall, K.A. TCS: A computer program to estimate gene genealogies. Mol. Ecol. 2000, 9, 1657–1659. [Google Scholar] [CrossRef]
- Leigh, J.W.; Bryant, D. POPART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Bischof, R.; Gregersen, E.R.; Brøseth, H.; Ellegren, H.; Flagstad, Ø. Noninvasive genetic sampling reveals intrasex Territoriality in wolverines. Ecol. Evol. 2016, 6, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Treloar, S.; Lohr, C.; Hopkins, A.J.M.; Ottewell, K.; McArthur, S.; Davis, R.A. Scat DNA as a non-invasive method for estimating the abundance of the vulnerable mala (Lagorchestes hirsutus). Wildl. Res. 2023, 51, WR22122. [Google Scholar] [CrossRef]
- Taberlet, P.; Waits, L.P.; Luikart, G. Noninvasive genetic sampling: Look before you leap. Trends Ecol. Evol. 1999, 14, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Huisman, J.; Kruuk, L.E.B.; Ellis, P.A.; Clutton-Brock, T.; Pemberton, J.M. Inbreeding depression across the lifespan in a wild mammal population. Proc. Natl. Acad. Sci. USA 2016, 113, 3585–3590. [Google Scholar] [CrossRef] [PubMed]
- Prochowska, S.; Niżański, W.; Snoeck, F.; Wydooghe, E.; Van Soom, A.; Kochan, J.; Stefanyk, V. How can we introduce ART into wild felid conservation in practice? Joint experience in semen collection from captive wild felids in Europe. Animals 2022, 12, 871. [Google Scholar] [CrossRef] [PubMed]
- Swanson, W.F.; Stoops, M.A.; Magarey, G.M.; Herrick, J.R. Sperm Cryopreservation in Endangered Felids: Developing Linkage of in Situ-Ex Situ Populations. In Spermatology; Roldan, E., Gomenido, M., Eds.; Nottingham University Press: Nottingham, UK, 2007; pp. 417–432. [Google Scholar]
- Boakes, E.H.; Wang, J.; Amos, W. An investigation of inbreeding depression and purging in captive pedigreed populations. Heredity 2007, 98, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Murray, G.G.R.; Woolhouse, M.E.J.; Tapio, M.; Mbole-Kariuki, M.N.; Sonstegard, T.S.; Thumbi, S.M.; Jennings, A.E.; Van Wyk, I.C.; Chase-Topping, M.; Kiara, H.; et al. Genetic susceptibility to infectious disease in East African shorthorn zebu: A genome-wide analysis of the effect of heterozygosity and exotic introgression. BMC Evol. Biol. 2013, 13, 246. [Google Scholar] [CrossRef]
- Pavlova, E.V.; Kirilyuk, V.E.; Naidenko, S.V. Patterns of seroprevalence of feline viruses among domestic cats (Felis catus) and Pallas’ cats (Otocolobus manul) in Daursky Reserve, Russia. Can. J. Zool. 2015, 93, 849–855. [Google Scholar] [CrossRef]
- Lücht, M.; Stagegaard, J.; Conraths, F.J.; Schares, G. Toxoplasma gondii in small exotic felids from zoos in Europe and the Middle East: Serological prevalence and risk factors. Parasit Vectors 2019, 12, 449. [Google Scholar] [CrossRef]
- Naidenko, S.; Demina, T. Pathogens and parasites as potential threats for Pallas’s cats. Cat News 2019, 13, 52–54. [Google Scholar]
- Voet, D.; Voet, J.G.; Pratt, C.W. Fundamentals of Biochemistry; John Wiley & Sons, Inc.: New York, NY, USA, 1999. [Google Scholar]
- Morris, J.; Hartl, D.; Knoll, A.; Lue, R.; Michael, M.; Berry, A.; Biewener, A.; Farrell, R.; Holbrook, N.M.; Heitz, J.; et al. Biology How Life Works, 3rd ed.; W. H. Freeman and Company: New York, NY, USA, 2013. [Google Scholar]
- Deagle, B.E.; Eveson, J.P.; Jarman, S.N. Quantification of damage in DNA recovered from highly degraded samples—A case study on DNA in faeces. Front. Zool. 2006, 3, 11. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).