Low Resource Competition, Availability of Nutrients and Water Level Fluctuations Facilitate Invasions of Australian Swamp Stonecrop (Crassula helmsii)
Abstract
1. Introduction
- Is the cover of C. helmsii limited by nutrient availability? And which nutrients are important?
- Is the cover of C. helmsii limited by interspecific competition? If so, which native species are involved?
- How does the cover of C. helmsii develop over time, and what is its impact on native flora?
2. Materials and Methods
2.1. Selection of Study Sites
2.2. Environmental Data on Water and Soil Chemistry, Precipitation, and Management
2.3. Vegetation Survey and Biomass Measurement
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Conditions for Optimal Growth
4.2. Effect of C. helmsii on Native Flora
4.3. Limitations
4.4. Management of C. helmsii Invasions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dawson, F.; Warman, E. Crassula helmsii (T. Kirk) Cockayne: Is it an aggressive alien aquatic plant in Britain? Biol. Conserv. 1987, 42, 247–272. [Google Scholar] [CrossRef]
- EPPO. Crassula Helmsii (Kirk) Cockayne Report of a Pest Risk Analysis; European and Mediterranean Plant Protection Organization: Paris, France, 2007. [Google Scholar]
- Smith, T. The Environmental Impact of Crassula helmsii; Canterbury Christ Church University: Canterbury, UK, 2015. [Google Scholar]
- Smith, T.; Buckley, P. Biological flora of the British Isles: Crassula helmsii. J. Ecol. 2020, 108, 797–813. [Google Scholar] [CrossRef]
- van der Loop, J.M.M.; Beringen, R.; Leuven, R.S.E.W.; Van Valkenburg, J.L.C.H.; Van Kleef, H.H.; Verhofstad, M.; Odé, B. Risicobeoordeling van Watercrassula (Crassula helmsii) in Europa; FLORON: Nijmegen, The Netherlands, 2020. [Google Scholar]
- Van der Loop, J.M.M.; De Hoop, L.; Van Kleef, H.H.; Leuven, R.S.E.W. Effectiveness of eradication measures ort he invasive Australian swamp stonecrop Crassula helmsii. Manag. Biol. Invasions 2018, 9, 343–355. [Google Scholar] [CrossRef]
- Van der Loop, J.M.; Van de Loo, M.; Vries, W.d.; Van Veenhuisen, L.S.; Van Kleef, H.H.; Leuven, R.S. Lessons learnt from large-scale eradication of Australian swamp stonecrop Crassula helmsii in a protected Natura 2000 site. Manag. Biol. Invasions 2022, 13, 101–117. [Google Scholar] [CrossRef]
- Hussner, A. Growth and photosynthesis of four invasive aquatic plant species in Europe. Weed Res. 2009, 49, 506–515. [Google Scholar] [CrossRef]
- Denys, L.; Packet, J.; Jambon, W.; Scheers, K. Dispersal of the non-native invasive species Crassula helmsii (Crassulaceae) may involve seeds and endozoochorous transport by birds. New J. Bot. 2014, 4, 104–106. [Google Scholar] [CrossRef]
- Byun, C.; de Blois, S.; Brisson, J. Interactions between abiotic constraint, propagule pressure, and biotic resistance regulate plant invasion. Oecologia 2015, 178, 285–296. [Google Scholar] [CrossRef]
- Yannelli, F. Applying competition theory to ensure ecological restoration and prevent plant invasions. Biodiversity 2021, 22, 82–86. [Google Scholar] [CrossRef]
- Dawson, F.H. Natural Habitat and Population Control Mechanism of Crassula helmsii (Australian Swamp Stonecrop) in Australia; Institute of Freshwater Ecology: Dorset, UK, 1989. [Google Scholar]
- Hussner, A. Zur Biologie von Crassula helmsii (Crassulaceae) in Nordrhein-Westfalen. Acta Biol. Benrodis 2007, 14, 77–88. [Google Scholar]
- Brunet, J. Effect of Chemical and Physical Environment on Crassula helmsii Spread; Centre for Ecology and Hydrology, Dorset: Winfrith Newburgh, UK, 2002. [Google Scholar]
- Brouwer, E.; Denys, L.; Lucassen, E.C.H.E.T.; Buiks, M.; Onkelinx, T. Competitive strength of Australian swamp stonecrop (Crassula helmsii) invading moorland pools. Aquat. Invasions 2017, 12, 321–331. [Google Scholar] [CrossRef]
- Van der Loop, J.M.; Tjampens, J.; Vogels, J.J.; Van Kleef, H.H.; Lamers, L.P.; Leuven, R.S. Reducing nutrient availability and enhancing biotic resistance limits settlement and growth of the invasive Australian swamp stonecrop (Crassula helmsii). Biol. Invasions 2020, 22, 3391–3402. [Google Scholar] [CrossRef]
- Van der Loop, J.M.; Van Kleef, H.H.; Van Veenhuisen, L.S.; Lamers, L.L.; Leuven, R.S. The Ecosystem Resilience Approach (ERA) to control the invasive alien species Australian swamp stonecrop (Crassula helmsii). Restor. Ecol. 2023, 31, e13844. [Google Scholar] [CrossRef]
- Stumm, M.; Morgan, J.J. Aquatic Chemistry; John Wiley & Sons: New York, NY, USA, 1996; p. 1022. [Google Scholar]
- KNMI. Cumulative Potential Evaporation Minus Precipitation in the Netherlands. Available online: https://climexp.knmi.nl/data/int_nl.dat (accessed on 20 February 2024).
- Ellenberg, H. Zeigerwerte von Pflanzen in Mitteleuropa. Scr. Geobot 1991, 18, 1–248. [Google Scholar]
- Hill, M.O.; Mountford, J.O.; Roy, D.B.; Bunce, R.G.H. Ellenberg’s Indicator Values for British Plants. ECOFACT Volume 2 Technical Annex; Institute of Terrestrial Ecology: Midlothian, UK, 1999; Volume 2. [Google Scholar]
- Hill, M.O. TWINSPAN, a FORTRAN Program for Arranging Multivariate Data in an Ordered Two-Way Table by Classification of the Individuals and Attributes; Cornell University: Ithaca, NY, USA, 1979; p. 52. [Google Scholar]
- Hillebrand, H.; Blasius, B.; Borer, E.T.; Chase, J.M.; Downing, J.A.; Eriksson, B.K.; Filstrup, C.T.; Harpole, W.S.; Hodapp, D.; Larsen, S. Biodiversity change is uncoupled from species richness trends: Consequences for conservation and monitoring. J. Appl. Ecol. 2018, 55, 169–184. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J. Welcome to the tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Wickham, H. Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009; p. 213. [Google Scholar]
- Ahlmann-Eltze, C.; Patil, I. ggsignif: R Package for Displaying Significance Brackets for ‘ggplot2’. PsyArxiv 2021. [Google Scholar] [CrossRef]
- van Doorn, J.; Lucassen, E.; van Roosmalen, M.; Smolders, A. Carbon limitation and aluminium toxicity prevents dominance of Crassula helmsii on weakly buffered soils. Aquat. Bot. 2024, 191, 103737. [Google Scholar] [CrossRef]
- Brouwer, E.; Van den Broek, T.G.Y. Ganzen brengen de landbouw naar het ven. De Levende Nat. 2010, 111, 60–63. [Google Scholar]
- Mitchell, S.; Diston, D. Managing conflicting water resources requirements in coastal wetlands: Case study Pevensey Levels, UK. Environ. Probl. Coast. Reg. VI Incl. Oil Spill Stud. 2006, 88, 295. [Google Scholar]
- Van Kleef, H.H.; Brouwer, E.; Leuven, R.S.E.W.; Van Dam, H.; De Vries-Brock, A.; Van der Velde, G.; Esselink, H. Effects of reduced nitrogen and sulphur deposition on the water chemistry of moorland pools. Environ. Pollut. 2010, 158, 2679–2685. [Google Scholar] [CrossRef]
- Leuven, R.S.; van der Velde, G.; Kersten, H.L. Interrelations between pH and other physico-chemical factors of Dutch soft waters. Arch. Für Hydrobiol. 1992, 126, 27–51. [Google Scholar] [CrossRef]
- Klavsen, S.K.; Madsen, T.V.; Maberly, S.C. Crassulacean acid metabolism in the context of other carbon-concentrating mechanisms in freshwater plants: A review. Photosynth. Res. 2011, 109, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Spierenburg, P.; Lucassen, E.; Lotter, A.F.; Roelofs, J.G.M. Competition between isoetids and invading elodeids at different concentrations of aquatic carbon dioxide. Freshw. Biol. 2010, 55, 1274–1287. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Lemonnier, P.; Wedow, J. The influence of rising tropospheric carbon dioxide and ozone on plant productivity. Plant Biol. 2020, 22, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Hasler, C.T.; Butman, D.; Jeffrey, J.D.; Suski, C.D. Freshwater biota and rising pCO2? Ecol. Lett. 2016, 19, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Weiss, L.C.; Pötter, L.; Steiger, A.; Kruppert, S.; Frost, U.; Tollrian, R. Rising pCO2 in freshwater ecosystems has the potential to negatively affect predator-induced defenses in Daphnia. Curr. Biol. 2018, 28, 327–332.e3. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Calvin, K.; Dasgupta, D.; Krinner, G.; Mukherji, A.; Thorne, P.; Trisos, C.; Romero, J.; Aldunce, P.; Barret, K. IPCC, 2023: Climate Change 2023: Synthesis Report, Summary for Policymakers. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023. [Google Scholar]
- Levine, J.M.; Adler, P.B.; Yelenik, S.G. A meta-analysis of biotic resistance to exotic plant invasions. Ecol. Lett. 2004, 7, 975–989. [Google Scholar] [CrossRef]
- European and Mediterranean Plant Protection Organization. Data sheets on quarantine pests Crassula helmsii. Bull. OEPP/EPPO 2007, 37, 225–229. [Google Scholar]
- Strong, C. Effective Management of the Invasive Aquatic Plant Australian Swamp Stonecrop (Crassula helmsii) in Wetlands; University of Brighton: Brighton, UK, 2020. [Google Scholar]
- Langdon, S.J.L.; Marrs, R.H.; Hosie, C.A.; McAllister, H.A.; Norris, K.M.; Potter, J.A. Crassula helmsii in UK Ponds: Effects on Plant Biodiversity and Implications for. Weed Technol. 2004, 18, 1349–1352. [Google Scholar] [CrossRef]
- Dean, C. The Ecology, Impacts and Control of Crassula helmsi; Bournemouth University: Fern Barrow, UK, 2015. [Google Scholar]
- Ewald, N.C. Crassula helmsii in the New Forest: Final Report on the Status, Spread and Impact of This Non-Native Invasive Plant, and the Efficacy of Control Techniques following a 3 Year Trial; Prepared on behalf of the New Forest Non-Native Plants Project; Freshwater Habitats Trust: Oxford, UK, 2014. [Google Scholar]
- Winikoff, S.G.; Larkin, D.J.; Meier, S.L.; Finlay, J.C. Vegetation trajectories of restored agricultural wetlands following sediment removal. Restor. Ecol. 2020, 28, 612–622. [Google Scholar] [CrossRef]
- Wang, Y.M.; Wang, Y.X.; Wang, S.Z.; Wang, M.; Chai, W.J. Assessment of topsoil removal as an effective method for vegetation restoration in farmed peatlands. Front. Environ. Sci. 2023, 10, 1110057. [Google Scholar] [CrossRef]
- WallisDeVries, M.F.; Ens, S.H. Effects of Habitat Quality and Isolation on the Colonization of Restored Heathlands by Butterflies. Restor. Ecol. 2010, 18, 390–398. [Google Scholar] [CrossRef]
- Verhagen, R.; Klooker, J.; Bakker, J.P.; Van Diggelen, R. Restoration success of low-production plant communities on former agricultural soils after top-soil removal. Appl. Veg. Sci. 2001, 4, 75–82. [Google Scholar] [CrossRef]
- Torok, P.; Vida, E.; Deak, B.; Lengyel, S.; Tothmeresz, B. Grassland restoration on former croplands in Europe: An assessment of applicability of techniques and costs. Biodivers. Conserv. 2011, 20, 2311–2332. [Google Scholar] [CrossRef]
- Klimkowska, A.; Van der Elst, D.J.D.; Grootjans, A.P. Understanding long-term effects of topsoil removal in peatlands: Overcoming thresholds for fen meadows restoration. Appl. Veg. Sci. 2015, 18, 110–120. [Google Scholar] [CrossRef]
- Hausman, C.E.; Fraser, L.H.; Kershner, M.W.; de Szalay, F.A. Plant community establishment in a restored wetland: Effects of soil removal. Appl. Veg. Sci. 2007, 10, 383–390. [Google Scholar] [CrossRef]
- Van der Loop, J.M.; Van Veenhuisen, L.S.; Van de Loo, M.; Vogels, J.J.; Van Kleef, H.H.; Leuven, R.S. Invasive Australian swamp stonecrop (Crassula helmsii) negatively affects spawning but accelerates larval growth of the endangered natterjack toad (Epidalea calamita). Hydrobiologia 2023, 850, 699–714. [Google Scholar] [CrossRef]
- Brouwer, E.; Bobbink, R.; Roelofs, J.G.M. Restoration of aquatic macrophyte vegetation in acidified and eutrophied softwater lakes: An overview. Aquat. Bot. 2002, 73, 405–431. [Google Scholar] [CrossRef]
- Van Kleef, H.H.; Verberk, W.C.E.P.; Leuven, R.S.E.W.; Esselink, H.; Van der Velde, G.; Van Duinen, G.A. Biological traits successfully predict the effects of restoration management on macroinvertebrates in shallow softwater lakes. Hydrobiologia 2006, 565, 201–216. [Google Scholar] [CrossRef]
- Smolders, A.J.P.; Lucassen, E.C.H.E.T.; Roelofs, J.G.M. The isoetid environment: Biogeochemistry and threats. Aquat. Bot. 2002, 73, 325–350. [Google Scholar] [CrossRef]
- Smolders, A.J.; Lucassen, E.C.; Van der Aalst, M.; Lamers, L.P.; Roelofs, J.G. Decreasing the abundance of Juncus effusus on former agricultural lands with noncalcareous sandy soils: Possible effects of liming and soil removal. Restor. Ecol. 2008, 16, 240–248. [Google Scholar] [CrossRef]
- Funk, J.L.; Cleland, E.E.; Suding, K.N.; Zavaleta, E.S. Restoration through reassembly: Plant traits and invasion resistance. Trends Ecol. Evol. 2008, 23, 695–703. [Google Scholar] [CrossRef]
- Tilman, D. Niche tradeoffs, neutrality, and community structure: A stochastic theory of resource competition, invasion, and community assembly. Proc. Natl. Acad. Sci. USA 2004, 101, 10854–10861. [Google Scholar] [CrossRef]
- Byun, C.; Kettenring, K.M.; Tarsa, E.E.; de Blois, S. Applying ecological principles to maximize resistance to invasion in restored plant communities. Ecol. Eng. 2023, 190, 106926. [Google Scholar] [CrossRef]
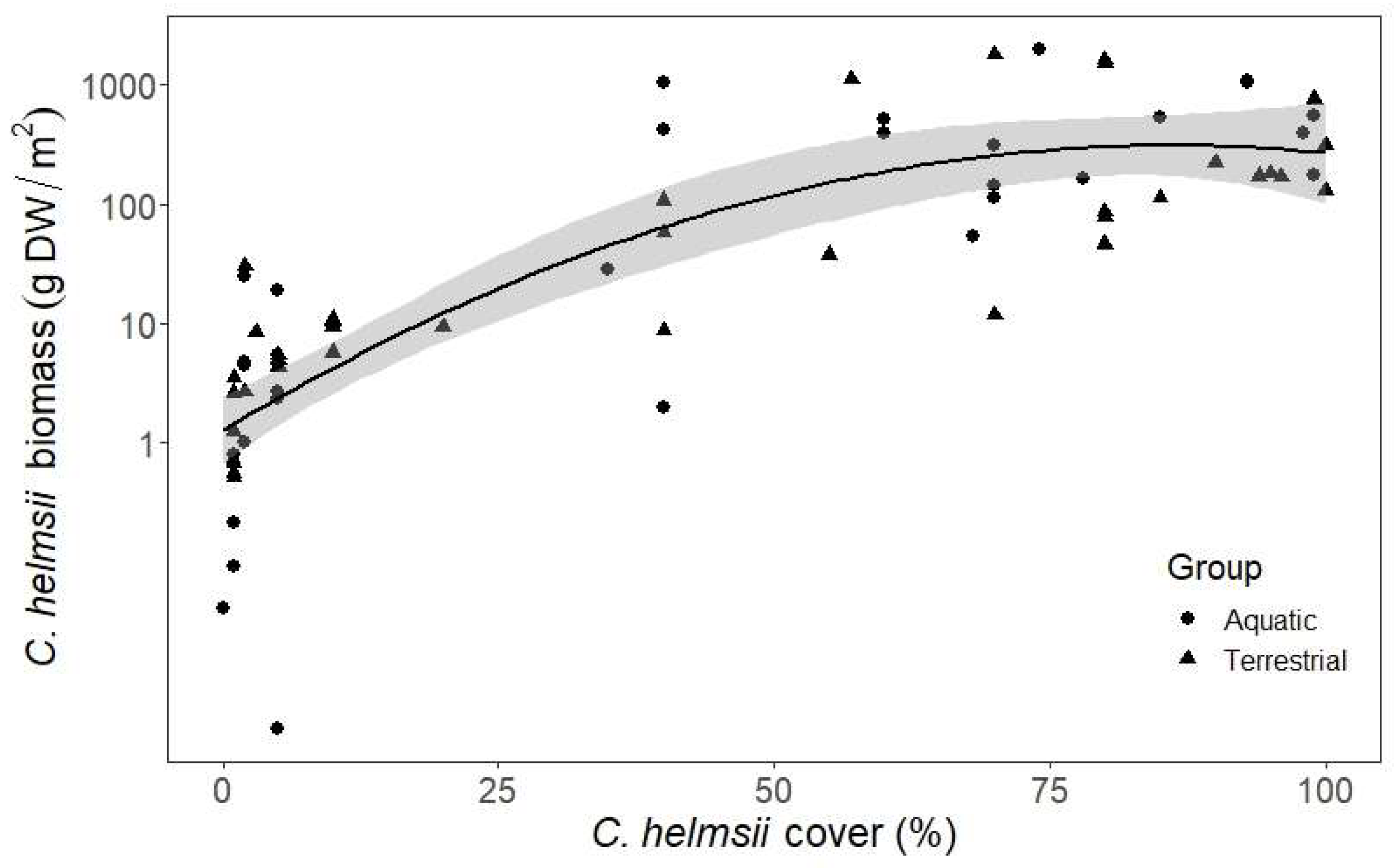
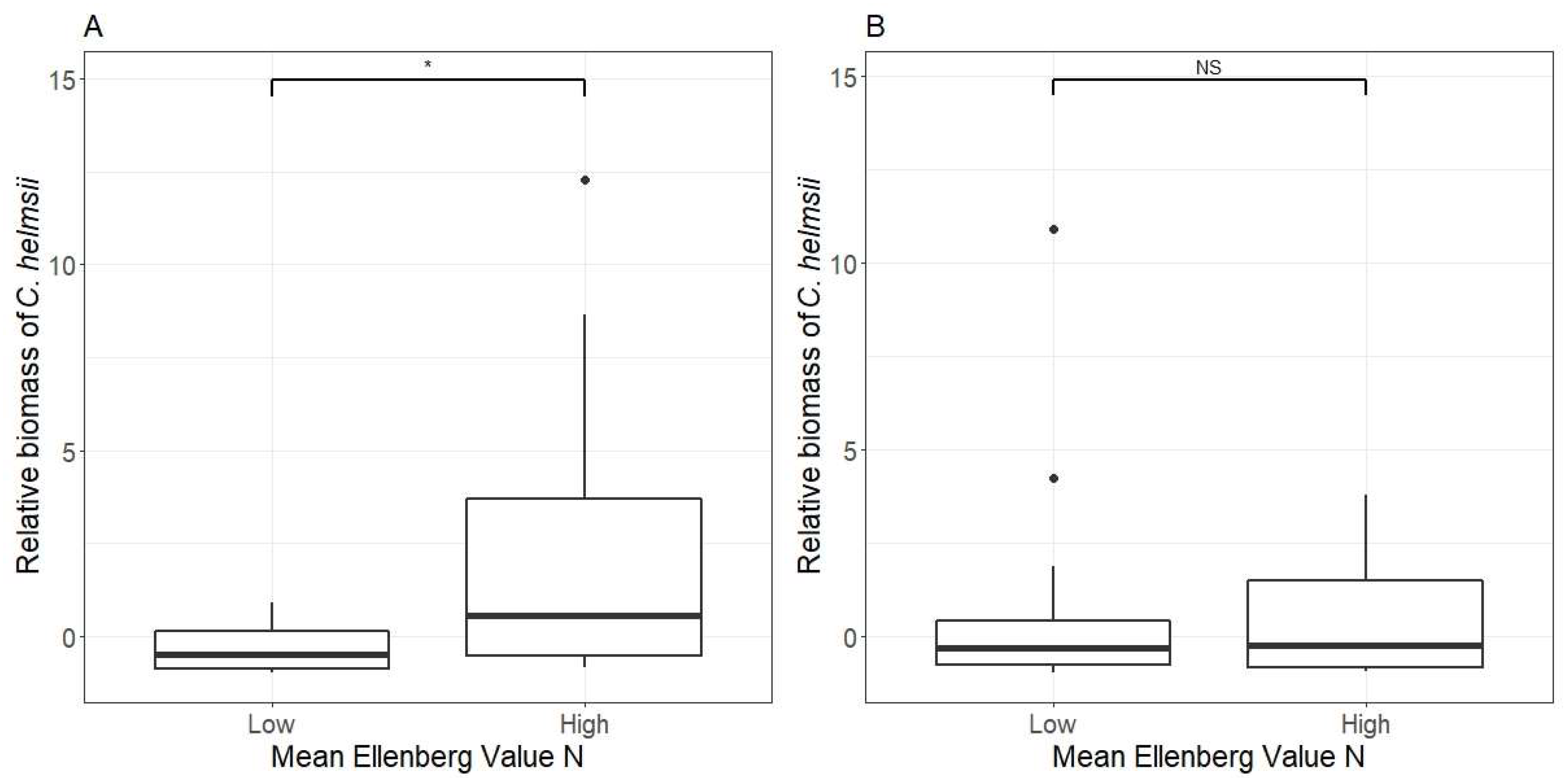

| Subset: | Aquatic Sites | Terrestrial Sites | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C. helmsii Cover: | Low (N = 20) | High (N = 14) | W | p | df | Low (N = 20) | High (N = 17) | W | p | df | ||
| Community | ||||||||||||
| Cover other species | (%) | 79.3 (11.0, 99.0) | 21.3 (1.0, 35.3) | 24 | <0.001 | 32 | 90.0 (48.5, 99.0) | 11.0 (0.0, 24.2) | 3 | <0.001 | 35 | |
| Species richness | 5.0 (2.0, 13.0) | 6.0 (2.3, 12.4) | 147 | 0.819 | 32 | 9.5 (3.9, 16.3) | 4.0 (1.0, 12.4) | 95 | 0.023 | 35 | ||
| Mean Ellenberg value for N | 2.4 (2.0, 7.0) | 4.5 (2.0, 6.2) | 154 | 0.634 | 32 | 2.1 (1.8, 5.7) | 3.82 (1.4, 7.2) | 237 | 0.043 | 35 | ||
| Surface water chemistry | ||||||||||||
| pH | 7.3 (5.6, 8.5) | 6.7 (5.6, 7.3) | 75 | 0.023 | 32 | |||||||
| Alkalinity | (meq/L) | 0.8 (0.1, 3.0) | 0.5 (0.1, 3.1) | 140 | 1.000 | 32 | ||||||
| CO2 | (µmol/L) | 51.6 (1.6, 287) | 225.9 (48.4, 940.5) | 225 | 0.003 | 32 | ||||||
| Total N | (µmol/L) | 13.3 (7.7, 79.3) | 11.1 (2.9, 77.2) | 115 | 0.396 | 32 | ||||||
| Total P | (µmol/L) | 0.7 (0.0, 7.1) | 0.3 (0.0, 7.0) | 135 | 0.872 | 32 | ||||||
| Soil chemistry | ||||||||||||
| Soil organic matter | (%) | 6.5 (0.8, 38.8) | 5.3 (0.9, 37.4) | 106 | 0.950 | 28 | 2.1 (0.8, 41.6) | 10.2 (0.7, 43.4) | 189 | 0.397 | 34 | |
| Total N | (µmol/g DW) | 0.22 (0.04, 0.66) | 0.19 (0.02, 1.70) | 100 | 0.755 | 28 | 0.19 (0.04, 2.81) | 0.21 (0.05, 2.86) | 179 | 0.798 | 35 | |
| Total P | (µmol/g DW) | 2.8 (0.8, 16.0) | 10.9 (1.1, 20.0) | 109 | 0.487 | 28 | 1.8 (0.9, 18.5) | 2.5 (1.0, 28.2) | 197 | 0.153 | 35 | |
| Community | ||||||
|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | |
| Number of relevés: | 7 | 6 | 10 | 28 | 15 | 26 |
| Mean species richness, excluding Crassula helmsii | 3.6 | 6 | 6 | 4.3 | 11.2 | 12.9 |
| Mean C. helmsii cover | 6.1% | 3.5% | 3.8% | 82.7% | 84.4% | 5.7% |
| Eleocharis acicularis | 0.29 | 0.11 | ||||
| Chara sp. | 0.29 | 0.20 | 0.04 | |||
| Pilularia globulifera | 0.29 | 0.83 | 0.18 | 0.13 | 0.08 | |
| Elatine hexandra | 1.00 | 1.00 | 0.20 | 0.18 | 0.04 | |
| Alisma plantago-aquatica | 0.29 | 0.33 | 0.20 | 0.07 | 0.13 | |
| Potamogeton polygonifolius | 0.33 | 0.04 | ||||
| Baldellia ranunculoides subsp. Ranunculoides | 0.83 | 0.07 | ||||
| Lythrum portula | 0.14 | 0.33 | 0.10 | 0.04 | 0.20 | 0.23 |
| Crassula helmsii | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Juncus bulbosus | 0.57 | 0.33 | 0.80 | 0.25 | 0.47 | 0.35 |
| Lysimachia vulgaris | 0.33 | 0.30 | 0.11 | 0.20 | 0.38 | |
| Salix sp. | 0.17 | 0.10 | 0.11 | 0.33 | 0.54 | |
| Eleocharis multicaulis | 0.17 | 0.10 | 0.29 | 0.60 | 0.65 | |
| Hydrocotyle vulgaris | 0.50 | 0.29 | 0.40 | 0.88 | ||
| Hypericum elodes | 0.30 | 0.25 | 0.13 | 0.69 | ||
| Bidens frondosa | 0.10 | 0.21 | 0.73 | 0.27 | ||
| Gnaphalium luteoalbum | 0.47 | 0.08 | ||||
| Mentha aquatica | 0.10 | 0.04 | 0.73 | 0.12 | ||
| Erigeron canadensis | 0.04 | 0.40 | 0.08 | |||
| Digitaria ischaemum | 0.27 | |||||
| Epilobium hirsutum | 0.04 | 0.27 | 0.15 | |||
| Betula pubescens | 0.33 | 0.23 | ||||
| Carex oederi | 0.27 | 0.23 | ||||
| Lotus pedunculatus | 0.27 | 0.42 | ||||
| Agrostis canina | 0.10 | 0.04 | 0.40 | 0.62 | ||
| Leontodon saxatilis | 0.47 | 0.27 | ||||
| Ranunculus flammula | 0.10 | 0.04 | 0.40 | 0.35 | ||
| Lycopus europaeus | 0.17 | 0.18 | 0.80 | 0.81 | ||
| Juncus effusus | 0.18 | 0.13 | 0.42 | |||
| Phragmites australis | 0.20 | 0.18 | 0.07 | 0.31 | ||
| Galium palustre | 0.65 | |||||
| Moss sp. | 0.14 | 0.31 | ||||
| 2020 | |||||||||
| I | II | III | IV | V | VI | N | |||
| 2016 | I | 5 | 1 | 6 | Species-poor aquatic community with Elatine hexandra dominance | ||||
| II | 1 | 1 | 3 | 1 | 6 | Aquatic or recently exposed shores with dominance of Pillularia globulifera | |||
| III | 3 | 2 | 1 | 6 | Aquatic or recently exposed with dominance of Juncus bulbosus | ||||
| IV | 1 | 3 | 6 | 7 | 17 | Mostly species-poor aquatic communities dominated by Crassula helmsii | |||
| V | 1 | 2 | 3 | Moist exposed shores dominated by Crassula helmsii | |||||
| VI | 1 | 7 | 8 | Dry species-rich shores | |||||
| N | 1 | 0 | 4 | 11 | 12 | 18 | 46 | ||
| Subset: | Aquatic Sites | |||||
|---|---|---|---|---|---|---|
| Trend: | Increase | Stable Low | Stable High | Decline | Unknown | |
| Crassula helmsii cover in 2016/2019 period: | Low | Low | High | High | Low | High |
| C. helmsii cover in 2020: | High | Low | High | Low | ||
| N = | 8 | 4 | 6 | 4 | 8 | 4 |
| No dominant species other than C. helmsii | 2/7 | 0/1 | 6/6 | 4/0 | 2 | 4 |
| Elatine hexandra | 4/0 | 0/1 | ||||
| Warnstorfia fluitans | 1/0 | |||||
| Juncus bulbosos | 1/0 | 1/0 | ||||
| Littorella uniflora | 2/2 | 0/1 | ||||
| Pilularia globulifera | 1/0 | |||||
| Algae | 0/1 | 4 | ||||
| Eleocharis palustris | 0/1 | |||||
| Agrostis canina | 0/1 | |||||
| Bidens frondosa | 0/1 | |||||
| Eleocharis multicaulis | 0/1 | |||||
| Hypericum elodes | 0/1 | |||||
| Sphagnum spec. | 0/1 | |||||
| Moss sp. | 0/1 | 0/1 | ||||
| Chara vulgaris | 1 | |||||
| Elodea canadensis | 1 | |||||
| Elodea nuttallii | 2 | |||||
| Hydrocharis morsus-ranae | 1 | |||||
| Hydrocotyle ranunculoides | 1 | |||||
| Subset: | Terrestrial sites | |||||
| Trend: | Increase | Stable low | Stable high | Decline | Unknown | |
| C. helmsii cover in 2016/2019 period: | Low | Low | High | High | Low | High |
| C. helmsii cover in 2020: | High | Low | High | Low | ||
| N = | 4 | 12 | 5 | 5 | 4 | 7 |
| No dominant species other than C. helmsii | 2/4 | 0/3 | 5/5 | 5/2 | 0 | 7 |
| Bidens frondosa | 1/0 | |||||
| Juncus bulbosos | 1/0 | 2/0 | ||||
| Pilularia globulifera | 4/0 | |||||
| Eleocharis multicaulis | 3/0 | |||||
| Juncus effusus | 2/2 | |||||
| Sphagnum sp. | 2/0 | |||||
| Algae | 1/0 | |||||
| Warnstorfia fluitans | 1/0 | 0/1 | ||||
| Agrostis canina | 0/4 | 1 | ||||
| Calamagrostis canescens | 0/1 | |||||
| Hydrocotyle vulgaris | 0/1 | |||||
| Radiola linoides | 0/1 | |||||
| Trifolium repens | 0/1 | |||||
| Moss sp. | 0/2 | 0/1 | ||||
| Salix cinerea | 0/1 | |||||
| Hypericum elodes | 1 | |||||
| Isolepis fluitans | 1 | |||||
| Lycopus europaeus | 1 | |||||
| Potentilla anserina | 1 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Kleef, H.H.; van der Loop, J.M.M.; van Veenhuisen, L.S. Low Resource Competition, Availability of Nutrients and Water Level Fluctuations Facilitate Invasions of Australian Swamp Stonecrop (Crassula helmsii). Diversity 2024, 16, 172. https://doi.org/10.3390/d16030172
van Kleef HH, van der Loop JMM, van Veenhuisen LS. Low Resource Competition, Availability of Nutrients and Water Level Fluctuations Facilitate Invasions of Australian Swamp Stonecrop (Crassula helmsii). Diversity. 2024; 16(3):172. https://doi.org/10.3390/d16030172
Chicago/Turabian Stylevan Kleef, Hein H., Janneke M. M. van der Loop, and Laura S. van Veenhuisen. 2024. "Low Resource Competition, Availability of Nutrients and Water Level Fluctuations Facilitate Invasions of Australian Swamp Stonecrop (Crassula helmsii)" Diversity 16, no. 3: 172. https://doi.org/10.3390/d16030172
APA Stylevan Kleef, H. H., van der Loop, J. M. M., & van Veenhuisen, L. S. (2024). Low Resource Competition, Availability of Nutrients and Water Level Fluctuations Facilitate Invasions of Australian Swamp Stonecrop (Crassula helmsii). Diversity, 16(3), 172. https://doi.org/10.3390/d16030172






