The Potential of Foraging Chacma Baboons (Papio ursinus) to Disperse Seeds of Alien and Invasive Plant Species in the Amathole Forest in Hogsback in the Eastern Cape Province, South Africa
Abstract
1. Introduction
2. Material and Methods
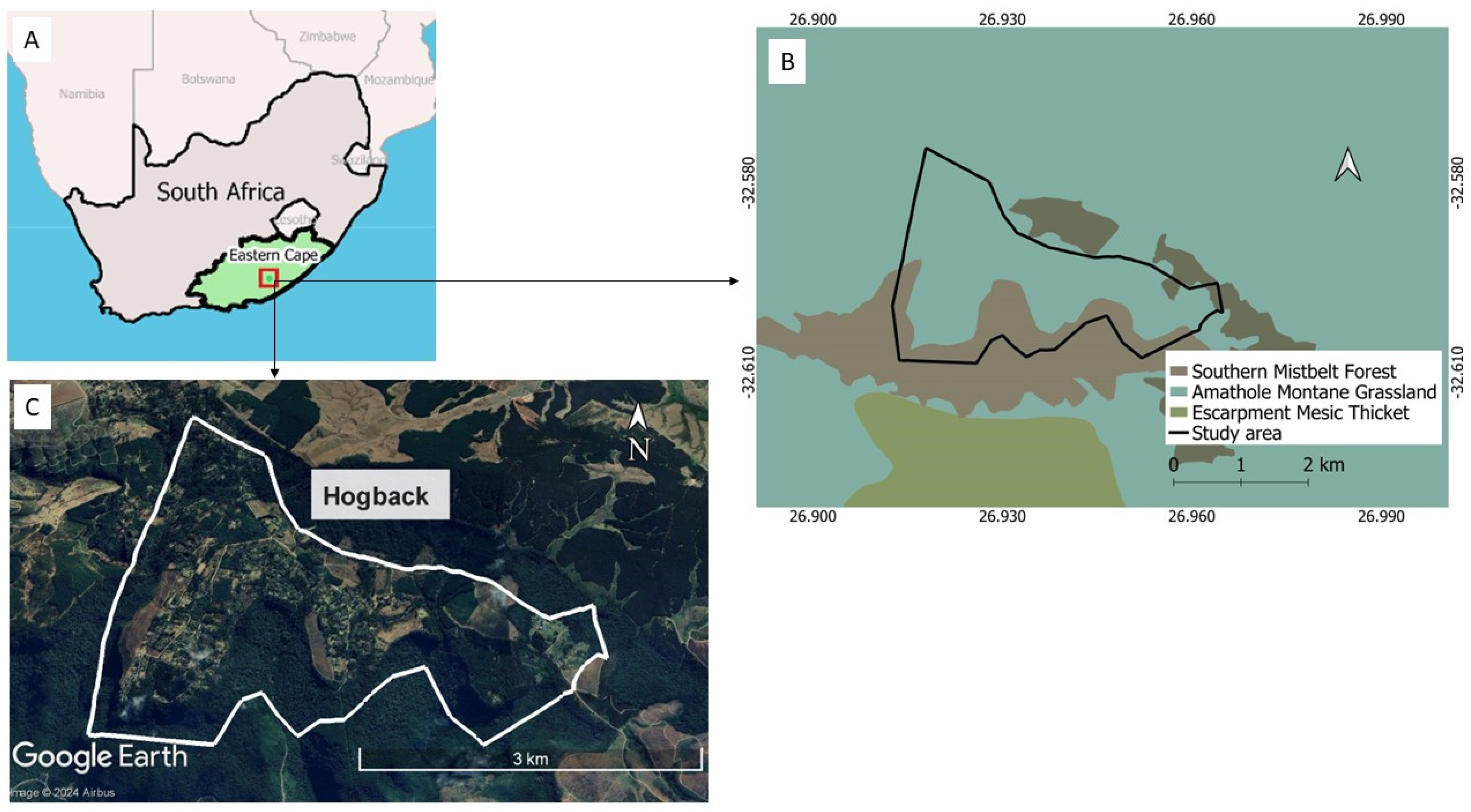
2.1. Study Site
2.2. Study Species
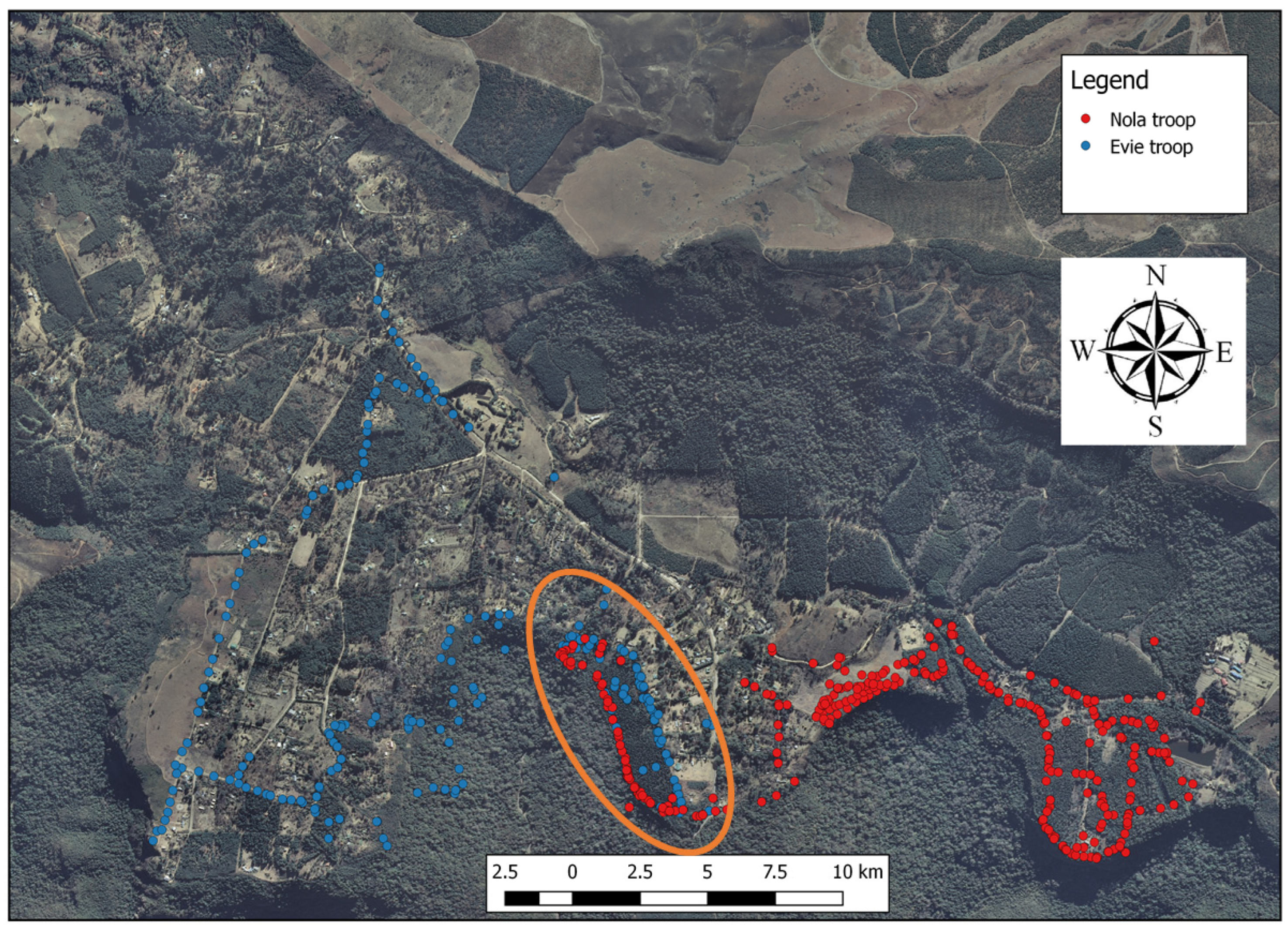
2.3. Data Collection: Foraging Visits, Occurrence of Alien Plant Species, and Faecal Sampling
3. Statistical Analysis
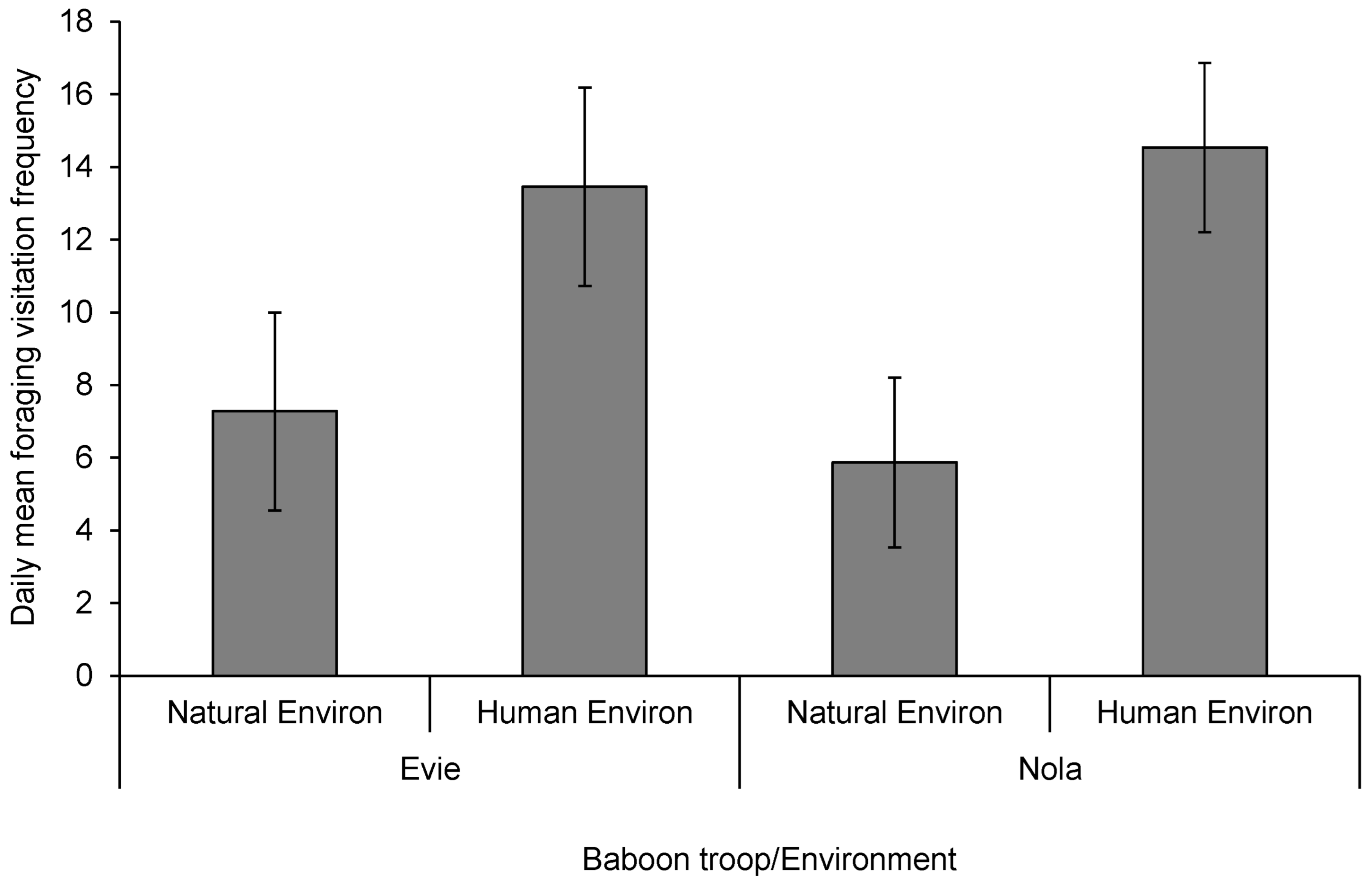
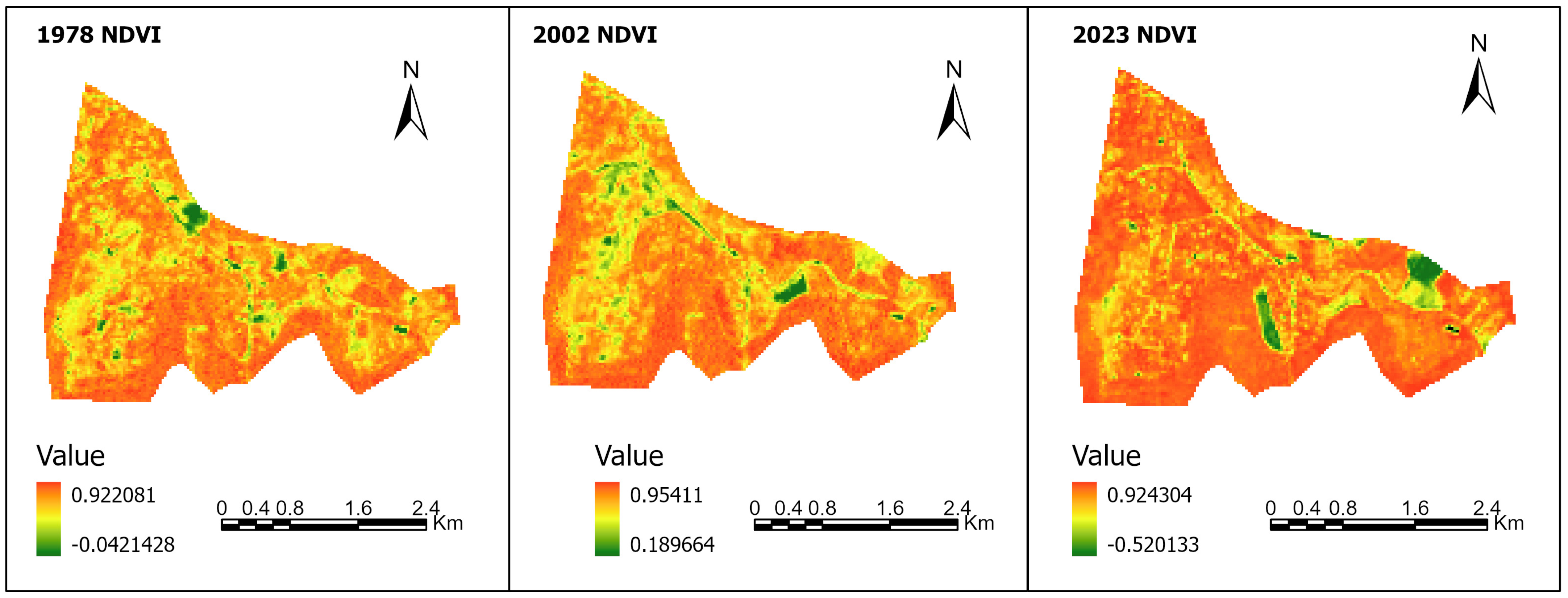
4. Results
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Terborgh, J.; Pitman, N.; Silman, M.; Schichter, H.; Nunez, P.V. Maintenance of tree diversity in tropical forests. In Seed Dispersal and Frugivory: Ecology, Evolution and Conservation; Levey, D.J., Silva, W.R., Galetti, M., Eds.; CABI Publishing: New York, NY, USA, 2002; pp. 351–364. [Google Scholar]
- Howe, H.F.; Smallwood, J. Ecology of Seed Dispersal. Annu. Rev. Ecol. Syst. 1982, 13, 201–228. [Google Scholar] [CrossRef]
- Gómez, J.M.; Verdú, M. Mutualism with plants drives primate diversification. Syst. Biol. 2012, 61, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Herrera, C.M. Seed dispersal by vertebrates. In Plant-Animal Interactions: An Evolutionary Approach; Herrera, C.M., Pellmyr, O., Eds.; Blackwell Publishing: Oxford, UK, 2003; pp. 185–208. [Google Scholar]
- Jordano, P. Fruits and frugivory. In Seeds: The Ecology of Regeneration in Natural Plant Communities; Fenner, M., Ed.; CABI Publishers: Wallingford, UK, 2000; pp. 125–166. [Google Scholar]
- Bufalo, F.S.; Galleti, M.; Culot, L. Seed Dispersal by Primates and Implications for the Conservation of a Biodiversity Hotspot, the Atlantic Forest of South America. Int. J. Primatol. 2016, 37, 333–349. [Google Scholar] [CrossRef]
- Chapman, C.A.; Russo, S.E. Primate seed dispersal. In Primates in Perspective; Oxford University Press: New York, NY, USA, 2007; pp. 510–525. [Google Scholar]
- Sengupta, A.; McConkey, K.R.; Radhakrishna, S. Primates, provisioning and plants: Impacts of human cultural behaviours on primate ecological functions. PLoS ONE 2015, 10, e0140961. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, A.; Radhakrishna, S. Fruit trait preference in rhesus macaques (Macaca mulatta) and its implications for seed dispersal. Int. J. Primatol. 2015, 36, 999–1013. [Google Scholar] [CrossRef]
- Chapman, C.A. Primate seed dispersal: Coevolution and conservation implications. Evol. Anthropol. Issues News Rev. 1995, 4, 74–82. [Google Scholar] [CrossRef]
- Chaves, O.M.; Bicca-Marques, J.C.; Chapman, C.A. Quantity and quality of seed dispersal by a large arboreal frugivore in small and large Atlantic forest fragments. PLoS ONE 2018, 3, e0193660. [Google Scholar] [CrossRef]
- Johnson, C.E.; Tafoya, K.A.; Beck, P.; Concilio, A.; White, K.E.; Quirós, R. Primate richness and abundance is driven by both forest structure and conservation scenario in Costa Rica. PLoS ONE 2023, 18, e0290742. [Google Scholar] [CrossRef]
- Reed, K.E.; Bidner, L.R. Primate communities: Past, present, and possible future. Am. J. Phys. Anthropol. 2004, 125, 2–39. [Google Scholar] [CrossRef]
- Swedell, L. Baboon Ecology. Available online: http://www.imfene.org/baboon-biology/baboon-ecology (accessed on 7 July 2017).
- Tew, E.; Landman, M.; Kerley, G.I.H. The Contribution of the Chacma Baboon to Seed Dispersal in the Eastern Karoo, South Africa. Afr. J. Wildl. Res. 2018, 48, 1–8. [Google Scholar] [CrossRef]
- Bueno, R.S.; Guevara, R.; Ribeiro, M.C.; Culot, L.; Bufalo, F.S.; Galetti, M. Functional redundancy and complementarities of seed dispersal by the last neotropical megafrugivores. PLoS ONE 2013, 8, e56252. [Google Scholar] [CrossRef] [PubMed]
- Julliot, C. Impact of seed dispersal by red howler monkeys Alouatta seniculus on the seedling population in the understory of tropical rain forest. J. Ecol. 1997, 85, 431–440. [Google Scholar] [CrossRef]
- Lambert, J.E. Seed handling in chimpanzees (Pan troglodytes) and redtail monkeys (Cercopithecus ascanius): Implications for understanding hominoid and cercopithecine fruit-processing strategies and seed dispersal. Am. J. Phys. Anthropol. 1999, 109, 365–386. [Google Scholar] [CrossRef]
- Stevenson, P.R. Estimates of the number of seeds dispersed by a population of primates in a lowland forest in western Amazonia. In Seed Dispersal: Theory and Its Application in a Changing World; Dennis, A.J., Schupp, E.W., Green, R.J., Westcott, D.A., Eds.; Biddles Ltd.: King’s Lynn, UK, 2007; pp. 340–362. [Google Scholar]
- Wrangham, R.W.; Chapman, C.A.; Chapman, L.J. Seed dispersal by forest chimpanzees. J. Trop. Ecol. 1994, 10, 355–368. [Google Scholar] [CrossRef]
- Kunz, B.K.; Linsenmair, K.E. Fruit Traits in Baboon Diet: A comparison with plant species characteristics in west Africa. Biotropica 2010, 42, 363–371. [Google Scholar] [CrossRef]
- Schurr, F.M.; Spiegel, O.; Steinitz, O.; Trakhtenbrot, A.; Tsoar, A.; Nathan, R. Long-distance seed dispersal. Annu. Plant Rev. 2009, 38, 204–237. [Google Scholar] [CrossRef]
- Segal, C. Foraging Behaviour and Diet in Chacma Baboons in Suikerbosrand Nature Reserve. Master’s Thesis, University of the Witwatersrand, Johannesburg, South Africa, 2008. [Google Scholar]
- Slater, K.; du Toit, J.T. Seed dispersal by chacma baboons and synoptic ungulates in southern African savannas. South Afr. J. Wildl. Res. 2002, 32, 75–79. [Google Scholar]
- Albert, A.; Savini, T.; Huynen, M.C. The role of Macaca spp (primates Cercopithecidae) in seed dispersal networks. Raffles Bull. Zool. 2013, 61, 423–434. [Google Scholar]
- Gautier-Hion, A. La dissemination des graines par les cercopithecides forestiers Africains. Terre Vie 1984, 39, 159–165. [Google Scholar] [CrossRef]
- Gautier-Hion, A.; Duplantier, J.M.; Quris, R.; Feer, F.; Sourd, C.; Decous, J.P.; Doubost, G.; Emmons, L.; Erard, C.; Hecketsweiler, P.; et al. Fruit characters as a basis of fruit choice and seed dispersal in a tropical forest vertebrate community. Oecologia 1985, 65, 324–337. [Google Scholar] [CrossRef]
- Kunz, B.K. Frugivory and Seed Dispersal: Ecological Interactions between Baboons, Plants, and Dung Beetles in the Savanna-Forest Mosaic of West Africa. Ph.D. Thesis, University of Würzburg, Würzburg, Germany, 2008. [Google Scholar]
- Barnes, M.E. Seed predation, germination and seedling establishment of Acacia erioloba in northern Botswana. J. Arid. Environ. 2001, 49, 541–554. [Google Scholar] [CrossRef]
- Linden, B.; Linden, J.; Fischer, F.; Linsenmair, K.E. Seed dispersal by South Africa’s only forest-dwelling guenon, the samango monkey (Cercopithecus mitis). Afr. J. Wildl. Res. 2015, 45, 88–99. [Google Scholar] [CrossRef]
- Spennemann, D.H.R. Frugivory and seed dispersal revisited: Codifying the plant-centred net benefit of animal-mediated interactions. Flora 2020, 263, 151534. [Google Scholar] [CrossRef]
- Chave, J.; Muller-Landau, H.C.; Levin, S.A. Comparing classical community models: Theoretical consequences for patterns of diversity. Am. Nat. 2002, 159, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Bitani, N.; Shivambu, T.C.; Shivambu, N.; Downs, C.T. An impact assessment of alien invasive plants in South Africa generally dispersed by native avian species. NeoBiota 2022, 74, 189–207. [Google Scholar] [CrossRef]
- Mokotjomela, T.M.; Musil, C.F.; Esler, K.J. Do frugivorous birds concentrate their foraging activities on those alien plants with the most abundant and nutritious fruits in the South African Mediterranean-climate region? Plant Ecol. 2013, 214, 49–59. [Google Scholar] [CrossRef]
- Trakhtenbrot, A.; Nathan, R.; Perry, G.; Richardson, D.M. The importance of long distance dispersal in biodiversity conservation. Divers. Distrib. 2005, 11, 173–181. [Google Scholar] [CrossRef]
- Traveset, A.; Richardson, D.M. Biological invasions as disruptors of plant reproductive mutualisms. Trends Ecol. Evol. 2006, 21, 208–216. [Google Scholar] [CrossRef]
- Richardson, D.M.; Pyšek, P.; Rejma’nek, M.; Barbour, M.G.; Panetta, F.D.; West, C.J. Naturalization and invasion of alien plants—Concepts and definitions. Divers. Distrib. 2000, 6, 93–107. [Google Scholar] [CrossRef]
- Rejmanek, M. Species richness and resistance to invasions. In Biodiversity and Ecosystem Processes in Tropical Forests; Orians, G.H., Dirzo, R., Cushman, J.H., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 153–172. [Google Scholar]
- Rejmanek, M.; Richardson, D.M.; Pysek, P. Plant invasions and invasibility of plant communities. In Vegetation Ecology; van der Maarel, E., Ed.; Oxford: Blackwell, UK, 2005; pp. 332–355. [Google Scholar]
- Debussche, M.; Isenmann, P. Fleshy fruit characters and the choices of bird and mammal seed dispersers in a Mediterranean region. Oikos 1989, 56, 327–338. [Google Scholar] [CrossRef]
- Richardson, D.M.; Rejmánek, M. Trees and shrubs as invasive alien species—A global review: Global review of invasive trees & shrubs. Divers. Distrib. 2011, 17, 788–809. [Google Scholar] [CrossRef]
- Pebsworth, P. Feeding ecology of chacma baboons (Papio ursinus) living in a human-modified environment. Afr. J. Ecol. 2020, 58, 319–326. [Google Scholar] [CrossRef]
- Muller-Landau, H.C.; Hardesty, B.D. Seed dispersal of woody plants in tropical forests: Concepts, Examples, and Future Directions. In Biotic Interactions in the Tropics: Their Role in the Maintenance of Species Diversity; Burslem, D., Pinard, M., Hartley, S., Eds.; Cambridge University Press: Cambridge, UK, 2005; pp. 267–309. [Google Scholar]
- Fleming, T.H.; Kress, W.J. The Ornaments of Life: Coevolution and Conservation in the Tropics; The University of Chicago Press: Chicago, IL, USA, 2013; p. 588. [Google Scholar]
- Mucina, L.; Rutherford, M.C. (Eds.) The Vegetation of South Africa, Lesotho and Swaziland; Strelitzia 19; South African National Biodiversity Institute: Pretoria, South Africa, 2006. [Google Scholar]
- Leaver, J.; Cherry, M.I. Forest product harvesting in the Eastern Cape, South Africa: Impacts on habitat structure. S. Afr. J. Sci. 2020, 116, 1–9. [Google Scholar] [CrossRef]
- Chapman, C.A.; Lawes, M.J.; Eeley, H.A.C. What hope for African primate diversity? Afr. J. Ecol. 2006, 44, 116–133. [Google Scholar] [CrossRef]
- Eeley, H.A.C.; Lawes, M.J.; Piper, S.E. The influence of climate change on the distribution of indigenous forest in KwaZulu-Natal, South Africa. J. Biogeogr. 1999, 26, 595–617. [Google Scholar] [CrossRef]
- Lawes, M.J.; Mealin, P.E.; Piper, S.E. Patch occupancy and potential metapopulation dynamics of three forest mammals in fragmented Afromontane forest in South Africa. Conserv. Biol. 2000, 14, 1088–1098. [Google Scholar] [CrossRef]
- Mokotjomela, T.M.; Vukeya, L.R.; Pamla, L.; Scott, Z. The critical role of coastal protected areas in buffering impacts of extreme climatic conditions on bird diversity and their ecosystem services’ provisioning in the Eastern Cape Province, South Africa. Ecol. Evol. 2023, 13, e10452. [Google Scholar] [CrossRef] [PubMed]
- von Maltitz, G.; Mucina, L.; Geldenhuys, C.J.; Lawes, M.; Eeley, H.; Aidie, H.; Vink, D.; Fleming, G.; Bailey, C. Classification System for South African Indigenous Forests. An Objective Classification for the Department of Water Affairs and Forestry; Report ENV-P-C 2003-017; Environmentek, CSIR: Pretoria, South Africa, 2003. [Google Scholar]
- Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (IPBES). Summary for Policymakers of the Thematic Assessment Report on Invasive Alien Species and Their Control of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. IPBES Secretariat. 2023. Available online: https://zenodo.org/records/10521002 (accessed on 22 December 2023).
- Van Wilgen, B.W.; Zengeya, T.A.; Richardson, D.M. A review of the impacts of biological invasions in South Africa. Biol. Invasions 2022, 24, 27–50. [Google Scholar] [CrossRef]
- Mucina, L.; Geldenhuys, C.J. (Eds.) Afrotemperate, subtropical and azonal forests. In The Vegetation of South Africa, Lesotho and Swaziland; Strelitzia 19; South African National Biodiversity Institute: Pretoria, South Africa, 2006; pp. 585–614. [Google Scholar]
- Pamla, L. The Importance of Habitat Use in the Foraging Behaviour of Village Chacma Baboons (Papio Ursinus, Cercopithecidae: Primates) in Hogsback, Eastern Cape. Master’s Thesis, University of Fort Hare, Alice, South Africa, 2016. [Google Scholar]
- Richardson, D.M. Forestry trees as invasive aliens. Conserv. Biol. 1998, 12, 18–26. [Google Scholar] [CrossRef]
- Quix, J.C. The role of alien plants in the composition of fruit-eating bird assemblages in Brazilian urban ecosystems. Orsis 2007, 22, 87–104. [Google Scholar]
- Mokotjomela, T.M. A Comparison of Bird Foraging Preferences for Fruits of Indigenous and Alien Shrubs and Seed Dispersal Potentials in the Cape Floristic Region. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2012. [Google Scholar]
- Driver, A.; Sink, K.J.; Nel, J.N.; Holness, S.; Van Niekerk, L.; Daniels, F.; Jonas, Z.; Majiedt, P.A.; Harris, L.; Maze, K. National Biodiversity Assessment 2011: An assessment of South Africa’s Biodiversity and Ecosystems. Synthesis Report; South African National Biodiversity Institute and Department of Environmental Affairs: Pretoria, South Africa, 2011. [Google Scholar]
- Webster, J.P.; Gower, C.M.; Knowles, S.C.; Molyneux, D.H.; Fenton, A. One health–an ecological and evolutionary framework for tackling Neglected Zoonotic Diseases. Evol. Appl. 2016, 9, 313–333. [Google Scholar] [CrossRef]
- Xulu, S.; Peerbhay, K.; Gebreslasie, M. Remote sensing of forest health and vitality: A South African perspective. South. For. A J. For. Sci. 2018, 81, 91–102. [Google Scholar] [CrossRef]
- Dye, P.; Versfeld, D. Managing the hydrological impacts of South African plantation forests: An overview. For. Ecol. Manag. 2007, 251, 121–128. [Google Scholar] [CrossRef]
- Scott, D.F.; Le Maitre, D.C.; Fairbanks, D.H.K. Forestry and streamflow reductions in South Africa: A reference system for assessing extent and distribution. Water SA 1998, 24, 187–199. [Google Scholar]
- Yeiser, J.L.; Ezell, A.W. Competition control in slash pine (Pinus ellliottii Engelm) plantations. In Slash Pine: Still Growing and Growing! Proceedings of the Slash Pine Symposium. United States, Department of Agriculture, Forest Service; Dickens, E.D., Barnett, J.P., Hubbard, W.G., Jokela, E.J., Eds.; Southern Research Station: Asheville, NC, USA, 2004; pp. 23–25. [Google Scholar]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Rad, S.M.; Ray, A.K.; Barghi, S. Water pollution and agriculture pesticide. Clean Technol. 2022, 4, 1088–1102. [Google Scholar] [CrossRef]
- Owen-Smith, N. Foraging responses of kudus to seasonal changes in food resources: Elasticity in constraints. Ecology 1994, 75, 1050–1062. [Google Scholar] [CrossRef]
- Kerr, R. The Animal Kingdom or Zoological System of the Celebrated Sir Charles Linnaeus; John Murry: London, UK, 1792. [Google Scholar]
- Chowdhury, S.; Brown, J.; Swedell, L. Anthropogenic effects on the physiology and behaviour of chacma baboons in the Cape Peninsula of South Africa. Conserv. Physiol. 2020, 8, coaa066. [Google Scholar] [CrossRef]
- Slater, K. Home range utilization by chacma baboon (Papio ursinus) troops on Suikerbosrand Nature Reserve, South Africa. PLoS ONE 2018, 13, e0194717. [Google Scholar] [CrossRef]
- Hill, C.M. People, crops, and primates: A conflict of interests. In Commensalism and Conflict: The Human–Primate Interface; Paterson, J.D., Wallis, J., Eds.; The American Society of Primatologists: Norman, OK, USA, 2005; pp. 40–59. [Google Scholar]
- Stone, O.M.; Laffan, S.W.; Curnoe, D.; Herries, A.I. The spatial distribution of chacma baboon (Papio ursinus) habitat based on an environmental envelope model. Int. J. Primatol. 2013, 34, 407–422. [Google Scholar] [CrossRef]
- Pebsworth, P.A.; MacIntosh, A.J.; Morgan, H.R.; Huffman, M.A. Factors influencing the ranging behavior of chacma baboons (Papio hamadryas ursinus) living in a human-modified habitat. Int. J. Primatol. 2012, 33, 872–887. [Google Scholar] [CrossRef]
- McCann, R.; Bracken, A.M.; Christensen, C.; Fuertbauer, I.; King, A.J. The relationship between GPS sampling interval and estimated daily travel distances in chacma baboons (Papio ursinus). Int. J. Primatol. 2021, 42, 589–599. [Google Scholar] [CrossRef]
- Altmann, J. Observational study of behaviour: Sampling methods. Behaviour 1974, 49, 227–265. [Google Scholar] [CrossRef] [PubMed]
- Mokotjomela, T.M.; Musil, C.F.; Esler, K.J. Frugivorous birds visit fruits of emerging alien shrub species more frequently than those of native shrub species in the South African Mediterranean climate region. S. Afr. J. Bot. 2013, 86, 73–78. [Google Scholar] [CrossRef]
- Vazquez, D.P.; Morris, W.F.; Jordano, P. Interaction frequency as a surrogate for the total effect of animal mutualists on plants. Ecol. Lett. 2005, 8, 1088–1094. [Google Scholar] [CrossRef]
- Vukeya, L.R.; Mokotjomela, T.M.; Powrie, L.W.; Nenungwi, L. Determining the critical recruitment needs for the declining population of Olea europaea subsp. africana (Mill.) PS Green in Free State, South Africa. Ecol. Evol. 2023, 13, e10177. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.; Tucker, G.; Fasham, M.; Shrewry, M.; Shaw, P. Handbook of Biodiversity Methods: Survey, Evaluation and Monitoring; Cambridge University Press: New York, NY, USA, 2005. [Google Scholar]
- Hamilton, W.J. Baboon Sleeping Site Preferences and Relationships to Primate Grouping Patterns. Am. J. Primatol. 1982, 3, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Howe, H.F. Scatter-and clump-dispersal and seedling demography: Hypothesis and implications. Oecologia 1989, 79, 417–426. [Google Scholar] [CrossRef]
- Steuer, P.; Südekum, K.; Müller DW, H.; Franz, R.; Kaandorp, J.; Clauss, M.; Hummel, J. Is there an influence of body mass on digesta mean retention time in herbivores? A comparative study on ungulates. Comp. Biochem. Physiol. A 2011, 160, 355–364. [Google Scholar] [CrossRef]
- McQualter, K.; Chase, M.; Fennessy, J.; McLeod, S.; Leggett, K. Home ranges, season alranges and daily movements of giraffe (Giraffa camelopardalis giraffa) in Northern Botswana. Afr. J. Ecol. 2015, 54, 99–102. [Google Scholar] [CrossRef]
- Msweli, L. Role and Effects of Wild Southern African Ungulates on Seed Dispersal of Selected Alien Invasive Plants. Ph.D. Thesis, University of KwaZulu-Natal-Afrique du Sud, Durban, South Africa, 2021. [Google Scholar]
- Saïd, S.; Servanty, S. The influence of landscape structure on female roe deer home-range size. Landsc. Ecol. 2005, 20, 1003–1012. [Google Scholar] [CrossRef]
- du Toit, J. Homerange-body mass relations: A field study on African browsing ruminants. Oecologia 1990, 85, 301–303. [Google Scholar] [CrossRef]
- Urban, M.; Berger, C.; Mudau, T.E.; Heckel, K.; Truckenbrodt, J.; Onyango Odipo, V.; Smit, I.P.; Schmullius, C. Surface moisture and vegetation cover analysis for drought monitoring in the southern Kruger National Park using Sentinel-1, Sentinel-2, and Landsat-8. Remote Sens. 2018, 10, 1482. [Google Scholar] [CrossRef]
- Vukeya, L.R.; Mokotjomela, T.M.; Malebo, N.J.; Smith, D.A.E.; Oke, S. The vegetation cover dynamics and potential drivers of habitat change over 30 years in the Free State National Botanical Garden, South Africa. Reg. Environ. Chang. 2023, 23, 24. [Google Scholar] [CrossRef]
- Takahashi, M.Q.; Rothman, J.M.; Cords, M. The role of non-natural foods in the nutritional strategies of monkeys in a human-modified mosaic landscape. Biotropica 2023, 55, 106–118. [Google Scholar] [CrossRef]
- Mokotjomela, T.M.; Nemurangoni, T.; Mundalamo, T.; Jaca, T.P.; Kuhudzai, A.G. The value of dump sites for monitoring biological invasions in South Africa. Biol. Invasions 2022, 24, 971–986. [Google Scholar] [CrossRef]
- Martínez, I.; García, D.; Obeso, J.R. Differential seed dispersal patterns generated by a common assemblage of vertebrate frugivores in three fleshy-fruited trees. Ecoscience 2008, 15, 189–199. [Google Scholar] [CrossRef]
- Sobral, M.; Larrinaga, A.R.; Guitian, J. Fruit-size preferences in wild and naive Eurasian blackbirds (Turdus merula) feeding on one seed hawthorn (Crataegus Monogyna). Auk 2010, 127, 532–539. [Google Scholar] [CrossRef]
- Sebastián-González, E.; Pires, M.M.; Donatti, C.I.; Guimarães, P.R., Jr.; Dirzo, R. Species traits and interaction rules shape a species-rich seed-dispersal interaction network. Ecol. Evol. 2017, 7, 4496–4506. [Google Scholar] [CrossRef]
- Hill, R.A. Ecological and Demographic Determinants of Time Budgets in Baboons: Implications for Cross-Populational Models of Baboon Socioecology. Ph.D. Thesis, University of Liverpool, Liverpool, UK, 1999. [Google Scholar]
- Kunz, B.K.; Linsenmair, K.E. The role of the olive baboon (Papio anubis, Cercopithecidae) as seed disperser in a savannah-forest mosaic of West Africa. J. Trop. Ecol. 2008, 24, 235–246. [Google Scholar] [CrossRef]
- Lambert, J.E. Exploring the link between animal frugivory and plant strategies: The case of primate fruit processing and post-dispersal fate. In Seed Dispersal and Frugivory: Ecology, Evolution and Conservation; Levey, D.J., Silva, W.R., Galetti, M., Eds.; CAB International: Wallingford, UK, 2002; pp. 365–379. [Google Scholar]
- Lambert, J.E.; Garber, P.A. Evolutionary and Ecological Implications of Primate Seed Dispersal. Am. J. Primatol. 1998, 45, 9–28. [Google Scholar] [CrossRef]
- Altmann, S.A. Foraging for Survival; University of Chicago Press: Chicago, IL, USA, 1998. [Google Scholar]
- Lotter, W.D.; Thatcher, L.; Rossouw, L.; Reinhardt, C.F. The influence of baboon predation and time in water on germination and early establishment of Opuntia stricta (Australian pest pear) in Kruger National Park. Koedoe 1999, 42, 43–50. [Google Scholar] [CrossRef]
- Venter, S.M.; Witkowski, E.T.F. Baobab (Adansonia digitata L.) fruit production in communal and conservation land-use types in Southern Africa. For. Ecol. Manag. 2011, 261, 630–639. [Google Scholar] [CrossRef]
- Kleyheeg, E.; van Dijk, J.G.; Nolet, B.A.; Tsopoglou-Gkina, D.; Woud, T.; Boonstra, D.; Soons, M.B. Daily movement distances and home range sizes of mallards (Anas platyrhynchos) are strongly affected by landscape configuration. Seed Dispersal A Gen. Duck 2017, 101. [Google Scholar]
- Cowie, B.W. Bugweed Biocontrol: New Insights into the Biological Control Agents of Solanum Mauritianum, Gargaphia Decoris and Anthonomus Santacruzi. Master’s Thesis, University of Witwatersrand, Johannesburg, South Africa, 2016. [Google Scholar]
- Cipollini, M.L.; Levey, D.J. Secondary metabolites of fleshy vertebrate-dispersed fruits: Adaptive hypotheses and implications for seed dispersal. Am. Nat. 1997, 150, 346–372. [Google Scholar] [CrossRef]
- Mokotjomela, T.M.; Downs, C.T.; Esler, K.; Knight, J. Seed dispersal effectiveness: A comparison of four bird species feeding on seeds of invasive Acacia cyclops in South Africa. S. Afr. J. Bot. 2016, 105, 259–263. [Google Scholar] [CrossRef]
- Vukeya, L.R.; Mokotjomela, T.M.; Malebo, N.J.; Saheed, O. Seed dispersal phenology of encroaching woody species in the Free State National Botanical Garden, South Africa. Afr. J. Ecol. 2022, 60, 723–735. [Google Scholar] [CrossRef]
- Le Roux, J.J.; Clusella-Trullas, S.; Mokotjomela, T.M.; Mairal, M.; Richardson, D.M.; Skein, L.; Wilson, J.R.; Weyl, O.L.; Geerts, S. Biotic interactions as mediators of biological invasions: Insights from South Africa. Biol. Invasions S. Afr. 2020, 35, 387–427. [Google Scholar]
- Tolley, K.A.; Da Silva, J.M.; Jansen van Vuuren, B.; Bishop, J.; Dalton, D.; Du Plessis, M.; Labuschagne, K.; Kotze, A.; Masehela, T.; Suleman, E. South African National Biodiversity Assessment 2019: Technical Report Volume 7: Genetic Diversity. Available online: http://researchspace.csir.co.za/dspace/handle/10204/11471 (accessed on 22 December 2023).
- Perry, G.L.; Enright, N.J.; Miller, B.P.; Lamont, B.B. Do plant functional traits determine spatial pattern? A test on species-rich shrublands, Western Australia. J. Veg. Sci. 2013, 24, 441–452. [Google Scholar] [CrossRef]
- Bunney, K. Seed dispersal in South African Trees: With a Focus on the Megafaunal Fruit and Their Dispersal Agents. Master’s Thesis, University of Cape Town, Cape Town, South Africa, 2014. [Google Scholar]
- Corlett, R.T. Frugivory and seed dispersal by vertebrates in tropical and subtropical Asia: An update. Glob. Ecol. Conserv. 2017, 11, 1–22. [Google Scholar] [CrossRef]
- Webster, J. Ethical and animal welfare considerations in relation to species selection for animal experimentation. Animals 2014, 4, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, E.T.F.; Garner, R.D. Seed production, seed bank dynamics, resprouting and long-term response to clearing of the alien invasive Solanum mauritianum in a temperate to subtropical riparian ecosystem. S. Afr. J. Bot. 2008, 74, 476–484. [Google Scholar] [CrossRef]
- Lesica, P.; Shelly, J.S. Competitive effects of Centaurea maculosa on the population dynamics of Arabis fecunda. Bull. Torrey Bot. Club 1996, 123, 111–121. [Google Scholar] [CrossRef]


| Plant Species | Hypothesis Test | ||||
|---|---|---|---|---|---|
| χ2 | Df | p-Value | |||
| Overall test model | 217.328 | 5 | 0.000 | ||
| B | Std. Error | ||||
| Rubus cuneifolius | −0.920 | 0.1089 | 71.358 | 1 | 0.000 |
| Cotoneaster pannosus | −1.008 | 0.1124 | 80.436 | 1 | 0.000 |
| Sersia trilobata *** | −1.224 | 0.1220 | 100.808 | 1 | 0.000 |
| Pine patula | −0.041 | 0.0831 | 0.248 | 1 | 0.618 |
| Solanum mauratianum | −0.248 | 0.0878 | 7.976 | 1 | 0.005 |
| Acacia mearnsii | 0 a | - | - | - | - |
| Troop | Predicted Distance in km [78] | Actual Distance (km) | Average (km) |
|---|---|---|---|
| Evie | 4.4 | 7.14 | 5.8 |
| Nola | 4.4 | 6.33 | 5.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pamla, L.; Vukeya, L.R.; Mokotjomela, T.M. The Potential of Foraging Chacma Baboons (Papio ursinus) to Disperse Seeds of Alien and Invasive Plant Species in the Amathole Forest in Hogsback in the Eastern Cape Province, South Africa. Diversity 2024, 16, 168. https://doi.org/10.3390/d16030168
Pamla L, Vukeya LR, Mokotjomela TM. The Potential of Foraging Chacma Baboons (Papio ursinus) to Disperse Seeds of Alien and Invasive Plant Species in the Amathole Forest in Hogsback in the Eastern Cape Province, South Africa. Diversity. 2024; 16(3):168. https://doi.org/10.3390/d16030168
Chicago/Turabian StylePamla, Lwandiso, Loyd R. Vukeya, and Thabiso M. Mokotjomela. 2024. "The Potential of Foraging Chacma Baboons (Papio ursinus) to Disperse Seeds of Alien and Invasive Plant Species in the Amathole Forest in Hogsback in the Eastern Cape Province, South Africa" Diversity 16, no. 3: 168. https://doi.org/10.3390/d16030168
APA StylePamla, L., Vukeya, L. R., & Mokotjomela, T. M. (2024). The Potential of Foraging Chacma Baboons (Papio ursinus) to Disperse Seeds of Alien and Invasive Plant Species in the Amathole Forest in Hogsback in the Eastern Cape Province, South Africa. Diversity, 16(3), 168. https://doi.org/10.3390/d16030168






