Abstract
The expansion of human activities across natural environments is now well known. This includes agricultural activities that effectively render many former natural environments sterile habitats for animals. Very often, what remains of the natural habitat are hedgerows that serve as habitat or pathways for movement between habitats for many species, including reptiles. In this study, we describe population changes in the western green lizard, Lacerta bilineata, in a hedgerow system in western France. The results are derived from a univariate diversity analysis of photographic data to identify individual lizards over a 4-year study period. Lizards were sighted from March April to October early November but there was a midsummer gap in sightings during July–August. The annual presence of individual lizards was low, both between and within years, but based on the diversity analysis, the overall stability of the population was high. Female numbers varied and were highest in 2020, but juveniles were highest in 2023; the numbers of males present each year were approximately the same. Individual lizards that were present before the midsummer gap were mostly absent after the midsummer gap and were replaced by new individuals. Incidences of autotomy were low in males and juveniles and were not recorded in females. In general, the results suggest that the lizards move through hedgerow systems but remain in a specific section for reproduction from March to July. Through this study, we also highlight the importance of univariate diversity formulas to obtain robust results in investigations of the demographic aspects of animal populations that are easy to monitor.
1. Introduction
Changes in the population abundance, sex or adult–juvenile ratios and movement patterns are just some of the pressures that animal populations are under due to anthropogenic activities. Since population densities are linked to genetic variability, this suggests that the susceptibility of a population/species to extinction increases as numbers decline in anthropogenically dominated environments [1,2]. Information about species living in such environments is important information for organizations in developing mitigation programs for conservation. One reptile group commonly found in anthropogenically modified landscapes are the lizards, which are usually small species with a rapid population turnover [1,3,4,5]. Hedgerows are key wildlife habitats and the most commonly found in agricultural landscapes [5]. They can be defined as ecotones, as they are often present in a mix of natural and anthropized habitats, mostly farmland [6]. Although widespread in rural habitats in Europe, including France [5], where they are gradually disappearing, there are few detailed ecological studies of reptile populations in hedgerows and how they operate in these systems [7]. A typical example of a hedgerow is the study hedgerow shown in Figure 1. In this study, we ask a central question of whether the hedgerows primarily represent movement pathways to prime habitat or serve as permanent habitat for reptiles. To give insight into this and a series of other questions requires knowledge of the identity of individual reptiles—males, females and juveniles and how long they remain in sections of hedgerows both annually and over the longer term.
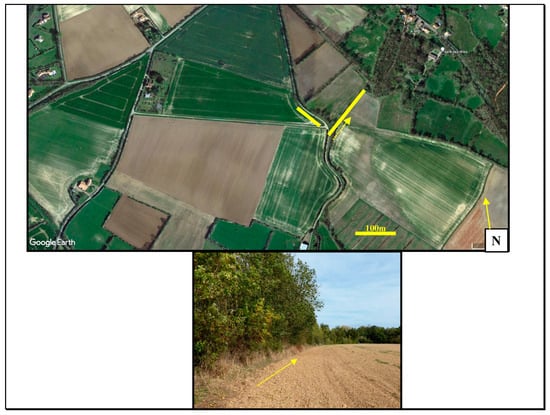
Figure 1.
Google Earth map showing the section of study hedgerow surveyed (colored lines) and the extent of the adjoining hedgerow, woodland and extensive agriculture. A ground view is shown below with arrows indicating the direction of the photograph.
Methods used to estimate lizard numbers include counts of lizard scats [8,9], mark–recapture analyses and distance sampling [10]. A very frequently used method is capture–mark–release–recapture (CMRR), an invasive technique necessitating disturbance to the animal during capture and measurement, which potentially risks distortion of the behavior, population characteristics and even survival rates of individuals [11,12]. Distance sampling is a more recent non-invasive approach [13,14]. However, this technique may fail to capture a segment of the population that is either inactive or concealed within vegetation [15]. The photographic capture–mark–release technique does not require the capture and handling of individuals and hence minimizes the disturbance and stress of handling [12,16]. Moreover, once the focal individuals become adapted to the researchers’ presence, this facilitates data collection [17]. To mitigate the problem of missing individuals in the population, we used a photographic-mark-release technique to study the western green lizard, Lacerta bilineata, using intensive daily sampling during the mornings, when lizards are usually basking in the sun and in full view (Figure 2).
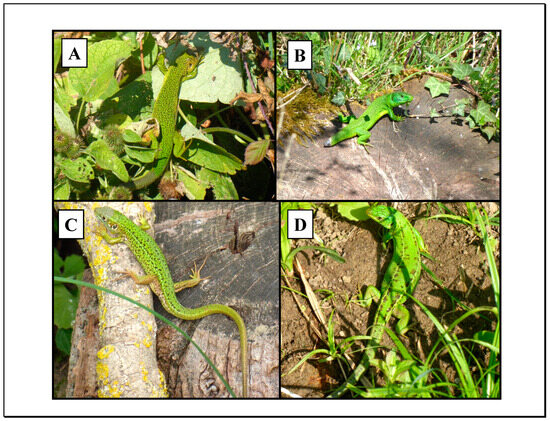
Figure 2.
Examples of the diversity of lizard markings used for identification. Males are in (A,B) with (B) showing a male with recent tail loss. At the bottom are a second year juvenile (C) and a female from 2023 (D).
The western green lizard is a thermophilic species basking in sunlit areas to raise body temperatures to a maximum of around 34 °C [18,19,20], and it is selective of the type of substrate while doing so [18,19]. It is found across Western Europe into northern regions including further north outside its natural range, for instance in southern England and the USA, where populations are able to persist for several years (e.g., [21,22,23]). It is a species frequently found in human-altered landscapes and is able to adapt and even benefit from landscape changes [24]. It occupies a range of habitat types from woodland edges and heathlands [25], with a high presence in hedgerows alongside roads in the wider area around the study area [26]. It is listed in Annex IV of the “Habitat” Directive 92/43/EEC as a species that requires strict protection. For example, it has been proposed that L. bilineata may be less resistant to changes in the environment than lizards of the genus Podarcis, species that are often sympatric [27]. However, as an introduced species in the UK, L. bilineata has spread more widely over an area in the south of England than P. muralis [22]. In the study locality, a long-term research study reported wide annual population fluctuations, but over a longer time period, the populations were rather stable [28].
In this article, we describe population changes in the western green lizard within a hedgerow system in western France. The results were obtained through a univariate diversity analysis of photographic data, identifying individual lizards. More specifically, we asked the following questions.
- (1)
- What was the composition of the lizard population in terms of male/female and juveniles? This is of particular interest, given that the study population is present in an anthropogenically dominated environment.
- (2)
- Do hedgerows represent permanent habitats or movement pathways to prime habitats? This is a frequently asked question in ecological studies concerning hedgerow systems [7] and thus is a question that is important for better understanding the population viability of species inhabiting this type of habitat. We answer this question using data on the annual presence of individuals over the 4-year study period. If the hedgerow is the permanent habitat for most individuals, we should expect to see most individuals in the population present for more than 1 year.
- (3)
- Was the population stable over the 4-year time period in terms of numbers and frequency of presence? This question is of interest, given that a previous long-term study (14 years) over the wider area indicated that the populations fluctuated widely.
- (4)
- What is the annual activity period and is there a midsummer gap, as found in other lacertids? This question originates from a study by [29], where several species of lacertid, including L. bilineata, showed what could be described as a midsummer gap.
In order to explore the above mentioned points, we used a novel approach to population demographics, namely the use of univariate diversity formulas that are frequently used for biodiversity studies and analyses of community ecology. Our study serves to show that through these univariate metrics, it is possible to obtain robust results in investigations of the demographic aspects of animal populations that are easy to monitor and where minimizing their disturbance is a priority.
2. Methods
2.1. Study Area
The study locality, a hedgerow, lies on the edge of the village of Chasnais (46°27 N, 1°53 E) in western France. It was selected because of the number of lizards present that quickly habituated to the observers’ presence, minimizing observer effect [30]. The hedgerow consists of mostly low growing bushes (Rubus fruticosus and Hedera helix), ash trees (Fraxinus excelsior) and oaks (Quercus robur), giving open sunlit patches and shaded areas [19]. Vegetation in the lower segments of the hedgerow where the lizards were found was around 1 to 2 m high and gave around 90% cover. Water was present in a drainage ditch running parallel to the hedge but was devoid of water by June due to desiccation. The total lengths of the surveyed sections were around 260 m. The area is an open system with no barriers to prevent lizards from entering or moving outside the study area (Figure 1), and conversations with elderly hunters indicated that the hedgerows have, for many years (at least since the 1930’s), supported populations of L. bilineata.
2.2. Lizard Sampling
Data were collected between February and November from 2020 to 2023. Allowing for inclement weather, daily sampling was approximately even across seasons and carried out 5–6 days each week for around 45–60 min daily, depending on the weather conditions. Sampling was carried out from around 8:30 a.m. up to around 1:00 p.m. by a single observer. The hedgerow was surveyed six times daily, which means covering a total of 1560 m (=6 × 260 m) each daily session. By surveying the entire study hedgerow an equal number of times daily, and given that lizards were present all along the hedgerow, we assumed this would satisfy the requirement for random encounters. Any lizards detected were photographed several times if possible. All lizards were photographed using a Lumix DMC-TZ70 camera set to Intelligent-Auto mode for rapid use. However, a number of pictures were discarded due to their low quality for identification (e.g., they were out of focus), which was expected with this setting. Identification was by color and markings. When possible, several photographs of each lizard were taken, but only the best quality photographs were used for each daily sighting. We believe that the relatively low numbers of lizards present each year, along with the extensive variation in the shades of green, dorsal spots and lines (Figure 2) minimized identification error. Using one photograph per lizard per daily sampling session was adequate to register that the lizard was present on that day [31]. Examination of the photographs was carried out on a computer, which also made use of the enlargement facility if a closer examination of patterns and markings was needed.
2.3. Statistical Analysis
To calculate individual lizard numbers and the frequency of their sightings, the Shannon–Weiner index was used [32]. The index is formulated to respond to an implicit question of the probability of sighting a single individual lizard two or more times in succession if all lizards in the population are randomly encountered during surveying. The method can be extended for use in combination with the index of equability. In theory, the Shannon–Weiner index has no limit, with high scores indicating a low probability of encountering the same individual lizard twice in a row and hence high diversity (many lizards and a high frequency of their presence). Theoretically, a zero score would indicate that only a single lizard is present. The index is calculated as follows,
where the index score H is derived from pi, which represents the frequency at which each individual lizard was observed. To estimate evenness of individuals’ presence, we calculated E values derived from the H-values from the Shannon–Weiner index using,
where H is the Shannon index value and K the number of individuals in each annual sample. Equability values range from 0 to 1, where 1 indicates that all individuals were sighted an equal number of times. Low E-values indicate the skewed presence of individual lizards. Values of H and E were found for males and females separately and for pooled samples of males and females.
H = −∑[(pi) × loge(pi)]
E = H/loge(K)
To compare differences in the annual frequency of sightings of individual lizards, a Kruskal–Wallis non-parametric test was used. This method combines and ranks the annual sighting frequencies of individuals in each year and then compares their annual rankings using a χ2 test. The null hypothesis was that there were no differences in sighting frequencies among years. Post hoc testing, when appropriate, was carried out using Dunn’s test with Bonferroni correction. A non-parametric Mann–Whitney U-test and z-scores were used to compare lizards’ presence before and after the mid-summer gap.
If lizard sighting frequencies are a measure of the length of time that they were present in the study area, there should be a relationship between the two. This notion was tested by regressing the number of days (Ndays) each lizard was observed between the first and last annual sighting against the numbers of times they were counted (Nc). Days between the first and last sighting were arbitrarily treated as the independent variable (Ndays), and sighting frequencies used as the dependent variable (Nc). The null hypothesis was m = 0, which would indicate no relationship between the variables if the regression coefficient did not differ significantly from 0. To normalize the Nc array and reduce the effect of outliers, a logarithmic (loge) transformation was applied. When a zero count was present, logeNc + 1 was used. This gives an equation of the form
where logeNc or logeNc + 1 is the number of days between sightings and x is the number of corresponding sighting frequencies, with ε being the white noise error [33]. Departures from m = 0 were evaluated using a t-test at n−2 degrees of freedom and, for differences between regression coefficients, a t-test at n − 4 degrees of freedom [34]. In all tests, alpha was set at 5%, with Minitab V17 and various online statistical sites used for data analysis (e.g., [35]).
LogeNc = m ± εxNday + b
3. Results
3.1. General Counts
Full results for individuals’ presence and their sighting frequencies are shown in Table 1 and graphically in Figure 3. Females were more numerous in terms of both individuals and their sighting frequencies only in 2020, with male–female numbers close to equality during 2023 (6 m versus 5 f). Pooled data for all years showed slightly higher numbers of males (25 m versus 21 f). Adult to juvenile ratios varied annually, with juveniles being less frequently present in terms of individuals and their sightings in 2020, although they progressively increased each year to a maximum number in 2023 (Question 1).

Table 1.
Summary statistics for the 4-year period, based on identified lizards.
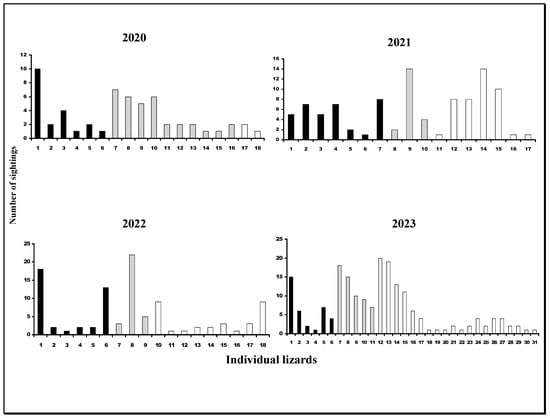
Figure 3.
Annual numbers of lizards identified in each year (x-axis) plotted against the frequency of their sightings. Black bars represent males, grey represents females, and white bars represent juveniles.
3.2. Temporal Presence
Year to year counts indicated that few lizards were present in the study locality for more than 1 year. Individual lizards’ presence for a 2-year period varied: 6% of the population for 2020–2021, 11% for 2022–2023 and 12% for 2021–2022. Most lizards with a 2-year presence were females (three) and juveniles (two), with only one male being sighted during both 2022 and 2023 (Question 2).
The Kruskal–Wallis χ2 Goodness of Fit tests showed that annual differences in the numbers of individual males and their sighting frequencies during the 4-year period were not significant (χ2 (3d.f.) = 1.6, p = 0.66). The mean rank scores of individual lizards’ presence were 9.92 in 2020, 14.57 in 2021, 13 in 2022 and 14 in 2023. Females’ annual presence was lower, with the test indicating that the difference was significant (χ2 (3d.f.) = 8.25, p = 0.036). The post hoc test showed that the critical difference was between 2020 and 2023. The rankings were 2020 = 6.9, 2021 = 10, 2022 = 13.5, 2023 = 16. Thus, male lizards were present in approximately regular numbers during the study period, but females had greater variation in presence.
3.3. Lizard Sightings before and after the Midsummer Gap
The main reproductive period began in late March, lasting until around the end of July (Figure 4). Lizards were generally absent from the hedgerow after this month, appearing again from mid to late September. The number of lizard sightings before the midsummer gap was greater than after the gap during all 4 years: the mean number of sightings before gap was 88.0 ± 43.4; after gap, the mean was 20.0 ± 13.3 (Mann–Whitney U-test: w = 26, p = 0.03). Sightings of individual lizards after the gap were mostly of previously unrecorded individuals. Of these, 73.3% were females, 85% were males and 83.9% were juveniles. Comparisons using z-tests indicated no significant differences between the percentages (z-scores from 0.14 to 1.1; p-values from 0.25–0.88), and hence, the data were pooled and gave an overall percentage of 81.1% of new lizards appearing after the midsummer gap (Question 4).
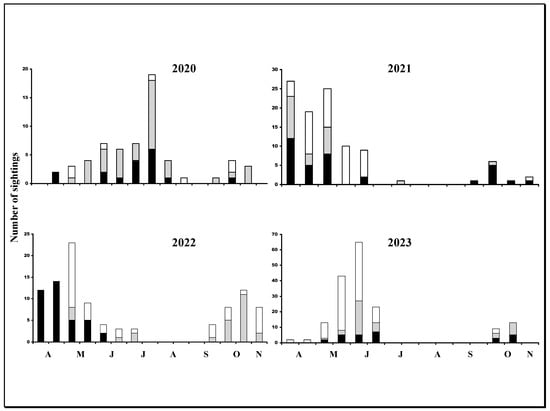
Figure 4.
Annual sighting frequencies of individual lizards by month. Black bars represent males, grey bars represent females, and white bars represent juveniles.
3.4. Annual Sighting Frequencies and Temporal Presence
The results of the analysis of the time between the first and last sightings are shown in Table 2 and graphically in Figure 5. This shows significant positive departures from the hypothetical 0 regression coefficient in males and juveniles, indicating that the time between sightings increased with higher sighting frequencies in males and juveniles. The 0.05 regression coefficient for females indicated non-departure from the theoretical 0 coefficient (no relationship). The comparisons of the regression coefficients for males and juveniles showed good agreement (t = 0.41, p = 0.68), but the high level of scatter in the female data rendered the analysis invalid.

Table 2.
Results from the regression analysis of sighting frequencies as independent variables versus the time between the first and final annual sightings.
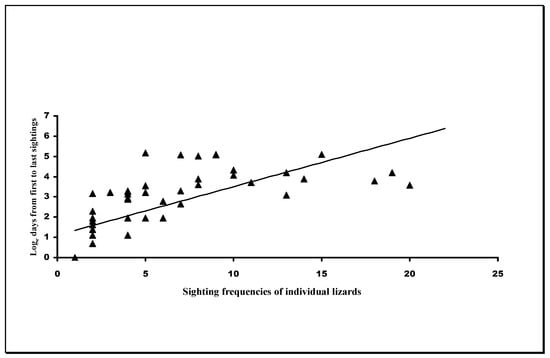
Figure 5.
Graph on semi-logarithmic coordinates, showing the relationship between the number of sighting frequencies of individual lizards and the maximum number of days between their first and final annual sightings within each year. The line running through the data represent the regression equation derived from the pooled male/juvenile data. A number of data points at low numbers of sightings (e.g., below 5 on the x axis) represent more than one data point. See Table 3 and the text for more details.
3.5. Diversity Analysis
Results from the Shannon–Weiner analysis indicated good agreement between years, with H-values from 2.47–2.83, showing that male and female scores were approximately similar (H-values, males = 1.28 ± 0.25 versus females = 1.12 ± 0.65, t = 0.46, p = 0.67). The same was true for the evenness scores (males = 0.78 ± 0.09 versus females = 0.8 ± 0.11, t = 0.30, p = 0.76). Juveniles showed greater annual variation in their H-values, ranging from 2.53 to 0.64, with the annual evenness scores in approximate agreement (0.82–0.92). The full results are shown in Table 3, indicating stable lizard numbers over the 4-year study period (Question 3).

Table 3.
Results from the Shannon–Weiner analysis (H) of numbers of individuals and their relative abundance derived from sighting frequencies. E values indicate the equality of presence calculated from the Shannon–Weiner value H (see text). The evenness score ranges from 0 to 1, with 1 indicating that all individual lizards were sighted an equal number of times. The tests were applied to males and females separately and then to a pooled sample of all groups including juveniles’ sighting frequencies. See the text for further details.
3.6. Autotomy
Tail loss was low in all years. Overall, no females out of a total of 21 individuals identified over the 4-year period showed examples of autotomy, but males (4 of 25; 16%—see the example in Figure 2B) and juveniles (6 of 38; 15.8%) showed similar frequencies of autotomy. This may suggest low predation pressure and/or low intra-specific aggression in the hedgerow, which includes the absence of physical injuries.
4. Discussion
4.1. Univariate Metric Analyses
Our study applied univariate formulas (Shannon–Wiener and evenness formulas), a method normally used to assess and quantify diversity in assemblages of species, to explore the demographic and population characteristics of single lizards throughout the 4 years of the study. In this respect, our approach is original and may be used in future studies, especially when the researchers want to assess the inter-annual stability in population abundance and the structure of single species across a multi-annual time span, and when the need to minimize disturbance (from capturing, marking and recapturing) is high. The method requires individual identification but gives a good insight into the lizard population’s dynamics at a fundamental scale. The results here indicated a general stability in the population structure of males, females and juveniles over the longer 4-year time period, although the sex and juvenile–adult ratios changed annually (Question 1). However, most lizards were seen for only 1 year, which suggests that the study hedgerow was mostly occupied as a temporary habitat for the reproductive period and served as a pathway to other areas, including other sections of the hedgerow (Question 2). The presence of new individuals during autumn (after the midsummer gap) and the absence of many of the adults that were present during the reproductive season is supporting evidence for this notion. This indicates frequent annual movements in and out of the study area, and that L. bilineata individuals move through the hedgerow system and potentially occupy another ecotonal habitat throughout the active year (Question 2). Data collected from across the wider area around the study locality indicated that L. bilineata had a high preference for hedgerows and low-density urban areas, but less for woodland edges [26].
The high scores in the H and E tests in the diversity analysis indicated a range of males and females and, in some years, high numbers of juveniles, especially during 2023. Evenness values gave good insight into individuals’ presence and changes over the 4-year study period, since they are readily converted to percentages. The results ranged from 85% to 90% in the pooled male, female and juvenile samples, although they were lower when the groups were analyzed independently (Table 1). The evenness scores were in good agreement with those found for P. muralis at the study site [36]. Other studies of L. bilineata in less disturbed areas, for instance [37], found a preference for ecotonal zones in L. bilineata that were defined as transition environments separating closed and open habitats, but whether these hedgerows were used temporarily by the various lizard individuals was unknown before the present study. A study of a population in a less disturbed habitat about 40 km south of the present study’s locality indicated that the age span of L. bilineata was potentially around 9 years [25].
4.2. The Midsummer Gap
The midsummer gap found in the recent study of Mediterranean lacertid lizards was confirmed here (Question 4) and was also in agreement with the reproductive period in more southerly areas, such as Greece and Italy, which is from March to July [29]. The midsummer gap that followed the reproductive period was observed annually during the 4-year study and did not exhibit any particular sex bias. The extent of the gap varied, however, being greatest during 2021 and 2023, years that were very hot and dry. Of potential interest was the narrower gap recorded during 2020, when the first spring sightings were later and fewer in number than other years (Figure 4). This was mirrored in a sympatric P. muralis population monitored at the same time that displayed the delayed beginning of activity during 2020 [36]. This was likely due to a colder than normal easterly wind. The individuals recorded after the midsummer gap may represent transients rather than lizards occupying the habitat leading up to hibernation, since most of them were absent during the following year. These changing population dynamics during autumn may involve intra-specific aggression in L. bilineata, with many individuals generally being intolerant of other adults and with juveniles, particularly outside the reproductive season, involving serious injuries and mortalities due to conflicts between adults of both sexes [19,38]. The narrow linear nature of hedgerow systems potentially increases contact between individuals during movement. This result suggests that there were major individual movements within years, including out of the study area, and that at least part of the population was using the hedgerow as a temporary breeding habitat and a movement pathway (Question 2).
A single annual reproductive period in L. bilineata with a later spring emergence and fewer autumn sightings, along with earlier entry into winter dens, must reduce the predation risk in lacertids [39] and is likely to be an adaptive trait. The similar but less definite midsummer gap recorded in the sympatric Podarcis muralis in the same hedgerow [36] also supports a previous study [29], where sightings of small lacertids in comparison with larger species were greater during the midsummer gap. This was attributed to the differences in the capacity of larger lacertids for fasting endurance, which is estimated to be up to 1.65 times greater than that of smaller lacertids [29]. The double reproductive period in P. muralis may explain the shorter gap in this species and hence the need for a longer active season to replenish energy stores previous to the winter dormancy period. However, it is of interest that data from the wider area showed that the numbers of both live and road-killed L. bilineata on roads peaked during August and September [26]. This may reflect greater longer distance movement compared with other months, with hedgerows serving as pathways across the wider area. The present study locality is not close to major road traffic areas, in contrast to the data used in [26]. This is an area of study that could be usefully explored.
4.3. Autotomy
Predation pressure has been cited as a key factor involved in incidences of autotomy, proposing that different levels of pressure may reflect different numbers of incidences [39,40]. The low incidences of autotomy in L. bilineata were, however, unexpected given that, in general, lacertids are amongst the lizard families with higher frequencies of tail autotomy [40]. This might suggest lower predation pressure in hedgerow systems compared with populations in less disturbed habitats, where population densities may be higher. However, the sympatric P. muralis in the study locality showed much higher incidences of autotomy, up to 67% in males and 49% in females, compared with that found in L. bilineata, with 16% in adult males, 15.8% in juveniles and 0 in females. The smaller Podarcis muralis may be subject to a greater predation risk, given a longer annual period of activity, including into the colder months [36]. Both species have similar predators to deal with, including the western whip snake, a saurophagous species frequently seen foraging (adults and juveniles) in the hedgerow [41]. However, juvenile whip snakes are too small to target an adult green lizard, whereas they feed routinely upon Podarcis individuals, and this might also partly explain the differences in predation risks and, therefore, in the frequency of autotomized tails.
4.4. Concluding Remarks
These findings, in conjunction with those from previous studies in the area (e.g., [25]), contribute to our understanding of the role hedgerows play in the life history of L. bilineata. Hedgerows have high multifunctionality for lizards, providing a series of ecological functions to enable a population’s persistence and hence have high conservation value that requires effective conservation approaches to be implemented for biological continuity [42,43,44]. For example, hedgerows’ connectivity potentially enhances gene flow, which would buffer against the risk of inbreeding if the populations are small and at risk of metapopulation extinction. However, gaps in knowledge remain, especially regarding the distance of movements by L. bilineata over longer periods of time. The present study suggests that lizards’ movement may be extensive, given the limited repeat annual sightings, along with adults requiring at least 3 years to reach maturity and potentially reaching an age of 9 years [25]. Moreover, the numbers of L. bilineata living in hedgerows across the wider area was greater than expected compared with other roadside habitats [26], a further indicator of the importance of hedgerows for lizards and other reptile populations. Conservation primarily involves monitoring farming practices that are well known to the local authorities, local farming communities and hunting groups in France. The latter, in particular, are aware of the need for their preservation, since hedgerows are important for their target species. Reptiles can be key indicators of the effects of agricultural intensification, and understanding their population ecology in such areas should be encouraged to understand the impacts of human activities on the environment [25].
Author Contributions
Conceptualization: R.M. and L.L.; methodology, R.M. and L.L.; formal analysis, R.M.; field work/data collection, R.M.; writing original draft preparation, R.M. and L.L.; writing, review and editing, R.M. and L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study did not require ethical approval, since no experimental procedures were carried out.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are shown graphically in the figures and are not publicly available due to an ongoing longitudinal analysis.
Acknowledgments
The authors greatly appreciate the comments by the four anonymous referees that improved on the original submitted draft.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Simberloff, D.; Cox, J. Consequences and costs of conservation corridors. Conserv. Biol. 1987, 1, 63–71. [Google Scholar] [CrossRef]
- Zug, G.R.; Vitt, L.; Caldwell, J.P. Herpetology: An Introductory Biology of Amphibians and Reptiles; Google Books: London, UK, 2001. [Google Scholar]
- Fahrig, L. Rethinking patch size and isolation effects: The habitat amount hypothesis. Glob. Ecol. Biogeorg. 2013, 40, 1649–1663. [Google Scholar] [CrossRef]
- Doherty, T.S.; Balouch, S.; Bell, K.; Burns, T.J.; Feldman, A.; Fist, C.; Garvey, T.F.; Jessop, T.S.; Meiri, S.; Driscoll, D.A. Reptile responses to anthropogenic habitat modification: A global meta-analysis. Glob. Ecol. Biogeorg. 2020, 29, 1265–1279. [Google Scholar] [CrossRef]
- Guiller, G.J.; Legentilhomme, A.; Boissinot, G.; Blouin-Demers, C.; Barbraud, O.; Lourdais, O. Response of farmland reptiles to agricultural intensification: Collapse of the common adder Vipera berus and the western green lizard Lacerta bilineata in a hedgerow landscape. Anim. Conserv. 2022, 25, 849–864. [Google Scholar] [CrossRef]
- Baudry, J.; Jouin, A. De la Haie aux Bocages, Organisation, Dynamique et Gestion; INRA: Paris, France, 2003. [Google Scholar]
- Saint Girons, H. Structure et e’volution d’une petite population de Vipera aspis (L.) dans une re´gion de Bocage de l’ouest de la France. Terre Vie 1996, 51, 223–241. [Google Scholar]
- Jones, L.L.C.; Rorabaugh, J.C.; Winsor, H.; Murphy, J.C.; Rupel, K. Efficacy of low-speed road cruising for lizard detection at two sites in Arizona, USA. Herpetol. Conserv. Biol. 2022, 17, 278–289. [Google Scholar]
- Turner, F.B.; Medica, P.A. The distribution and abundance of the flat-tailed horned lizard (Phrynosoma mcallii). Copeia 1982, 4, 815–823. [Google Scholar] [CrossRef]
- Grant, T.J.; Doherty, P.F., Jr. Monitoring of the flat-tailed horned lizard with methods incorporating detection probability. J. Wild Manag. 2007, 71, 1050–1056. [Google Scholar] [CrossRef]
- Wilson, R.P.; McMahon, C.R. Measuring devices on wild animals: What constitutes acceptable practice? Front. Ecol. Environ. 2006, 4, 147–154. [Google Scholar] [CrossRef]
- Bonnet, X.; Billy, G.; Lakušić, M. Puncture versus capture: Which stresses animals the most? J. Comp. Physiol. B 2020, 190, 341–347. [Google Scholar] [CrossRef]
- Choo, Y.R.; Kudavidanage, E.P.; Amarasinghe, T.R.; Nimalrathna, T.; Chua, M.A.; Webb, E.L. Best practices for reporting individual identification using camera trap photographs. Glob. Ecol. Conserv. 2020, 24, e01294. [Google Scholar] [CrossRef]
- Chan, S.C.Y.; Scott, Y.; Chui, S.; Pretorius, Y.; Karczmarski1, L. Estimating population parameters of African elephants: A photographic mark-recapture application in a South African protected area. Demography and population ecology. Mamm. Biol. 2023, 102, 1231–1247. [Google Scholar] [CrossRef]
- Smolensky, N.L.; Fitzgerald, L.A. Distance sampling underestimates population densities of dune-dwelling lizards. J. Herpetol. 2010, 44, 372–381. [Google Scholar] [CrossRef]
- Perera, A.; Pérez-Mellado, V. Photographic identification as a non-invasive marking technique for lacertid lizards. Herp. Rev. 2004, 35, 349–350. [Google Scholar]
- Welbourne, D.J.; MacGregor, C.; Paull, D.; Lindenmayer, D.B. The effectiveness and cost of camera traps for surveying small reptiles and critical weight range mammals: A comparison with labour-intensive complementary methods. Wildl. Res. 2015, 42, 414–425. [Google Scholar] [CrossRef]
- Meek, R.; Luiselli, L. Living in patchy habitats: Substrate selection by basking sympatric lizards in contrasted anthropogenic habitats in western France. Russ. J. Herp. 2022, 29, 227–236. [Google Scholar] [CrossRef]
- Meek, R.; Luiselli, L. Juveniles are different: Substrate selection in juvenile green lizards Lacerta bilineata. Ethol. Ecol. Evol. 2020, 35, 687–697. [Google Scholar] [CrossRef]
- Rismiller, P.D.; Heldmaier, G. How photoperiod influences body temperature selection in Lacerta viridis. Oecologia 1988, 75, 125–131. [Google Scholar] [CrossRef]
- Deichsel, G.; Gleed-Owen, C.P.; Mayer, W. Lacerta bilineata (western green lizard) and Podarcis muralis (common wall lizard) United Kingdom, Dorset. Herpetol. Rev. 2007, 38, 100–101. [Google Scholar]
- Mole, S.R.C. Changes in relative abundance of the western green lizard Lacerta bilineata and the common wall lizard Podarcis muralis introduced onto Boscombe Cliffs, Dorset, UK. Herpetol. Bull. 2010, 15, 24–29. [Google Scholar]
- Gubanyi, J.A. Breeding colony of western green lacertas (Lacerta bilineata) confirmed in southwestern Topeka (Kansas). Trans. Kansas Acad. Sci. 2000, 103, 191–192. [Google Scholar] [CrossRef]
- Pernat, A.; Sellier, Y.; Preau, C.; Beaune, D. Effet du paturage sur le lizard vert occidental (Lacerta bilineata, Daudin, 1802) (Squamata: Lacertidae) en milieu de landes. Bull. de la Société Herpétologique de Fr. 2017, 161, 57–66. [Google Scholar]
- Saint Girons, H.; Castanet, J.; Bradshaw, S.; Baron, J.P. Demographie comparee de deux populations fran caises de Lacerta viridis (Laurenti, 1768). Rev. Ecol. (Terre Vie) 1989, 44, 361–386. [Google Scholar]
- Meek, R. Temporal distributions, habitat associations and behaviour of the green lizard (Lacerta bilineata) and wall lizard (Podarcis muralis) on roads in a fragmented landscape in Western France. Acta Herpetol. 2014, 9, 179–186. [Google Scholar] [CrossRef]
- Venchi, A. Lacerta bilineata Daudin, 1802. In Anfibi e Rettili del Lazio; Bologna, M.A., Capula, M., Carpaneto, G.M., Eds.; Fratelli Palombi Editori: Rome, Italy, 2000; pp. 82–83. [Google Scholar]
- Meek, R. Temporal trends in Podarcis muralis and Lacerta bilineata populations in a fragmented landscape in western France: Results from a 14 year time series. Herpetol. J. 2020, 30, 20–26. [Google Scholar] [CrossRef]
- Luiselli, L.; Stille, B.; Stille, M.; Buttemer, W.A.; Madsen, T. Mass-related differences in metabolic rate and fasting endurance explain divergence in seasonal activity of Mediterranean lizards. Amphibia-Reptilia 2022, 43, 225–234. [Google Scholar] [CrossRef]
- Diego-Rasilla, F.J. Human influence on the tameness of wall lizard, Podarcis muralis. Ital. J. Zool. 2003, 70, 225–228. [Google Scholar] [CrossRef]
- Welbourne, D.J.; Claridge, A.W.; Paull, D.J.; Ford, F. Camera-traps are a cost-effective method for surveying terrestrial squamates: A comparison with artificial refuges and pitfall traps. PLoS ONE 2020, 15, e0226913. [Google Scholar] [CrossRef]
- Spellerberg, I.F.; Fedor, P.J. A tribute to Claude Shannon (1916–2001) and a plea for more rigorous use of species richness, species diversity and the ‘Shannon–Wiener’ Index. Glob. Ecol. Biogeorg. 2003, 12, 177–179. [Google Scholar] [CrossRef]
- Gotelli, N.J.; Ellison, A.M. A Primer of Ecological Statistics; Sinauer Associates: Sunderland, MA, USA, 2004; p. 510. [Google Scholar]
- Bailey, N.T.J. Statistical Methods in Biology; Cambridge University Press: Cambridge, UK, 1995; p. 255. [Google Scholar]
- Rain, R. Shannon Diversity Index Calculator. Available online: https://www.omnicalculator.com/ecology/shannon-index (accessed on 14 April 2023).
- Meek, R.; Luiselli, L.; Avery, R.A. Aspects of the demography of two Podarcis muralis populations in anthropogenic modified habitats in western France, based on a non-invasive sampling method. Herpetol. J. 2024, in press. [Google Scholar]
- Sacchi, R.; Marchesi, M.; Gentilli, A.; Pellitteri-Rosa, D.; Scali, S.; Borelli, A. Western green lizards (Lacerta bilineata) do not select the composition or structure of the ecotones in Northern Italy. North-West. J. Zool. 2011, 7, 213–221. [Google Scholar]
- Rugiero, L.; Capula, M.; Di Vittorio, M.; Dendi, D.; Meek, R.; Luiselli, L. Ontogenetic habitat use and density of the green lizard (Lacerta bilineata) in contrasted landscapes in France and Italy. Conservation 2021, 1, 1–16. [Google Scholar] [CrossRef]
- Cooper, W.E., Jr.; Perez-Mellado, V.; Vitt, L.J. Ease and effectiveness of costly autotomy vary with predation intensity among lizard populations. J. Zool. 2004, 262, 243–255. [Google Scholar] [CrossRef]
- Bateman, P.W.; Fleming, P.A. To cut a long tail short: A review of lizard caudal autotomy studies carried out over the last 20 years. J. Zool. 2009, 277, 1–14. [Google Scholar] [CrossRef]
- Meek, R.; Luiselli, L. Decision making under risk of predation in the western whip snake, Heirophis viridiflavus. Herpetol. Bull. 2021, 157, 32–34. [Google Scholar] [CrossRef]
- Davies, Z.G.; Pullin, A.S. Are hedgerows effective corridors between fragments of woodland habitat? An evidence-based approach. Landsc. Ecol. 1987, 22, 333–351. [Google Scholar] [CrossRef]
- Lecq, S.; Loisel, A.; Brischoux, F.; Mullin, S.J.; Bonnet, X. Importance of ground refuges for the biodiversity in agricultural hedgerows. Ecol. Ind. 2017, 72, 615–626. [Google Scholar] [CrossRef]
- Lecq, S.; Loisel, A.; Mullin, S.J.; Bonnet, X. Manipulating hedgerow quality: Embankment size influences animal biodiversity in a peri-urban context. Urban For. Urban Green. 2018, 35, 1–7. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).