Ascaridoid Nematodes Infection in Anadromous Fish Coilia nasus from Yangtze River
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
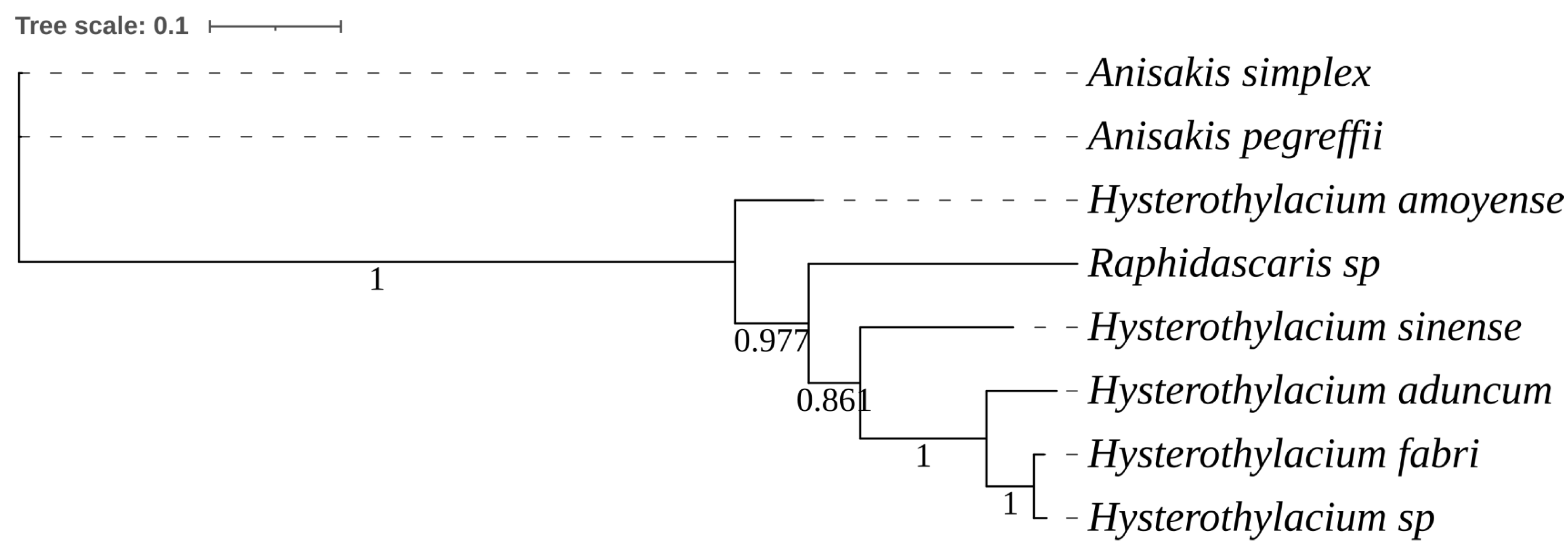
2.2. Molecular Identification
2.3. Data Processing
2.3.1. Terms and Indicators
2.3.2. Statistical Analysis
3. Results
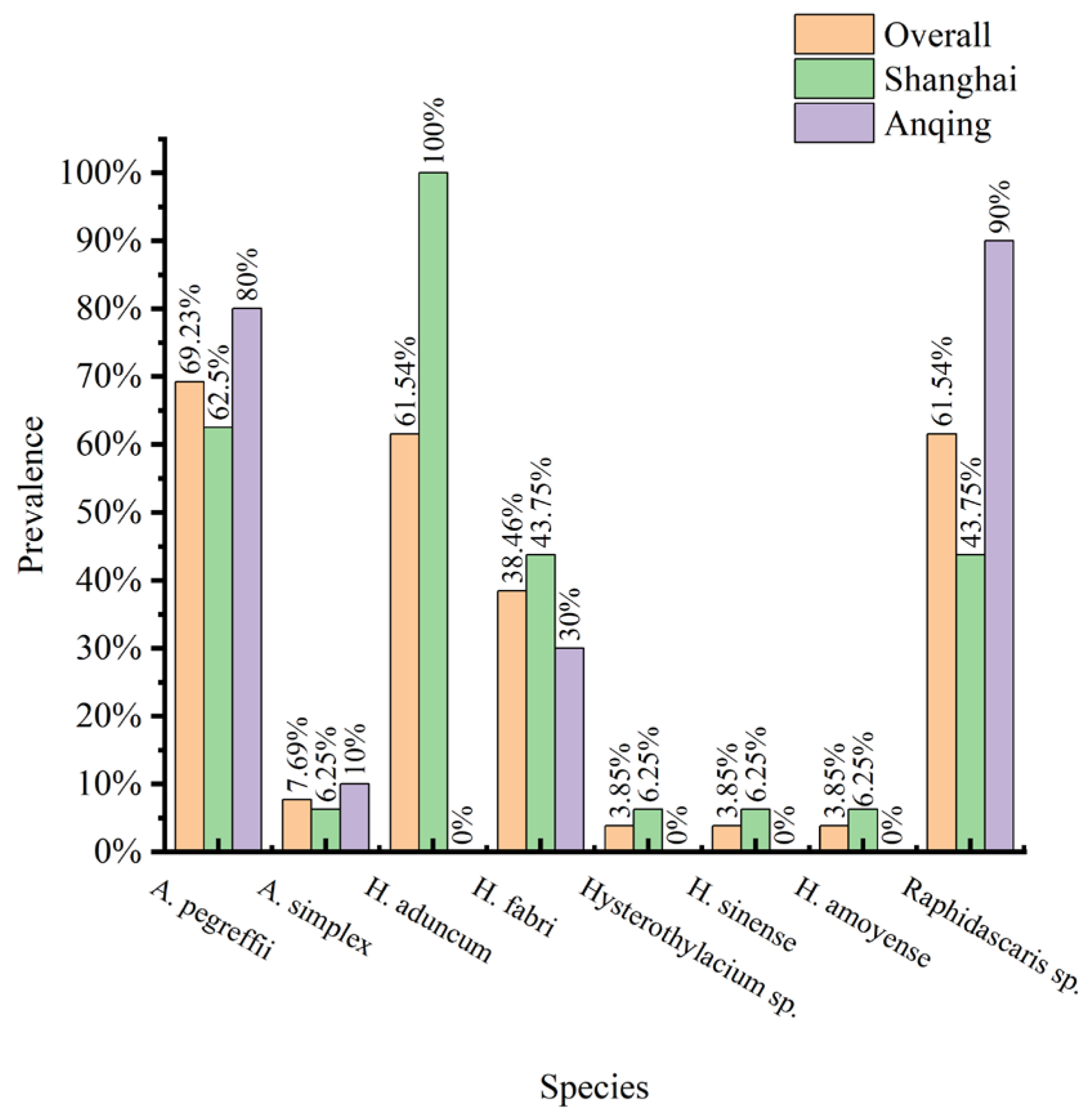
3.1. Nematode Infection in C. nasus
3.2. Infection Difference among Organs
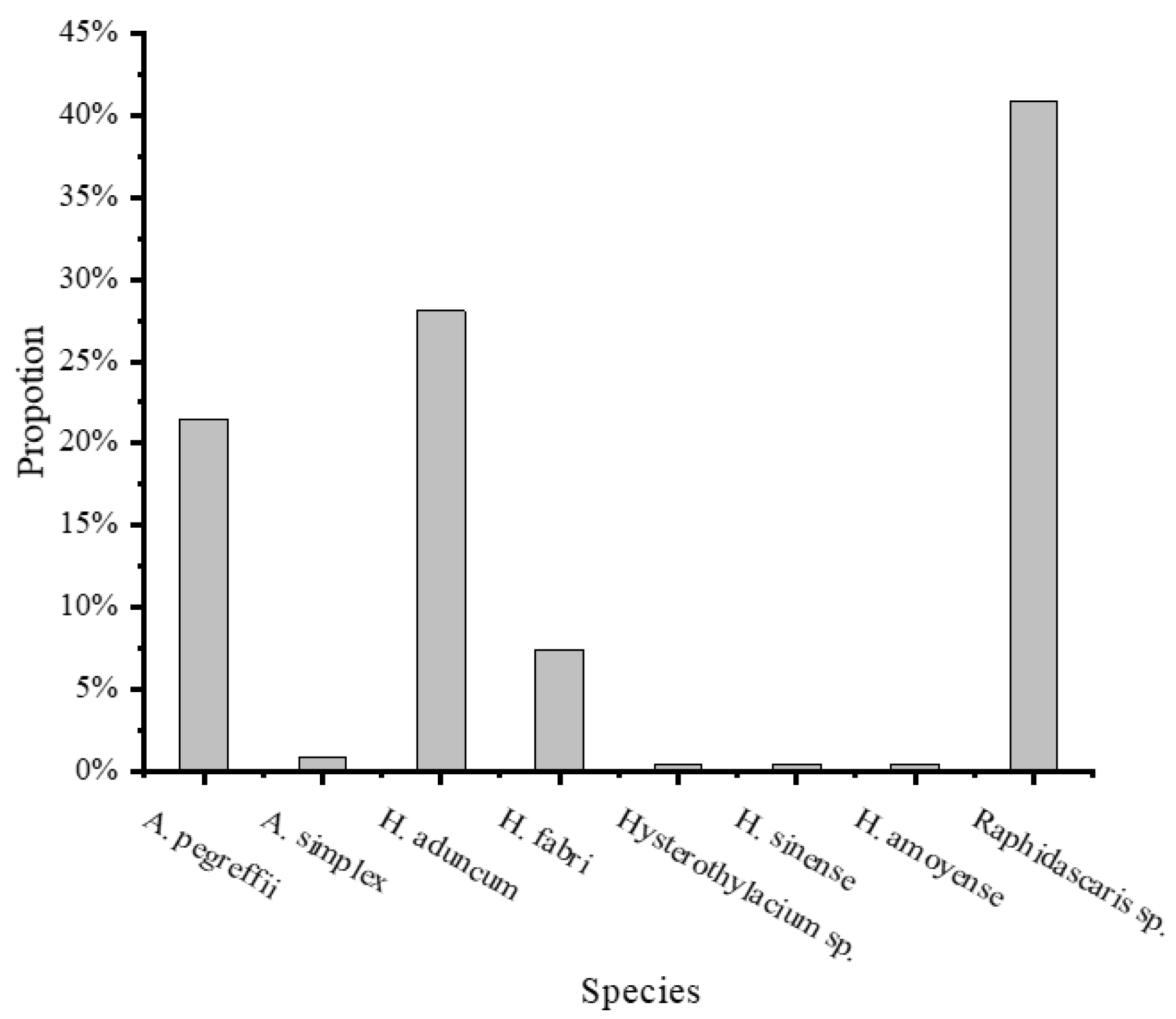
3.3. Composition of Nematode Species
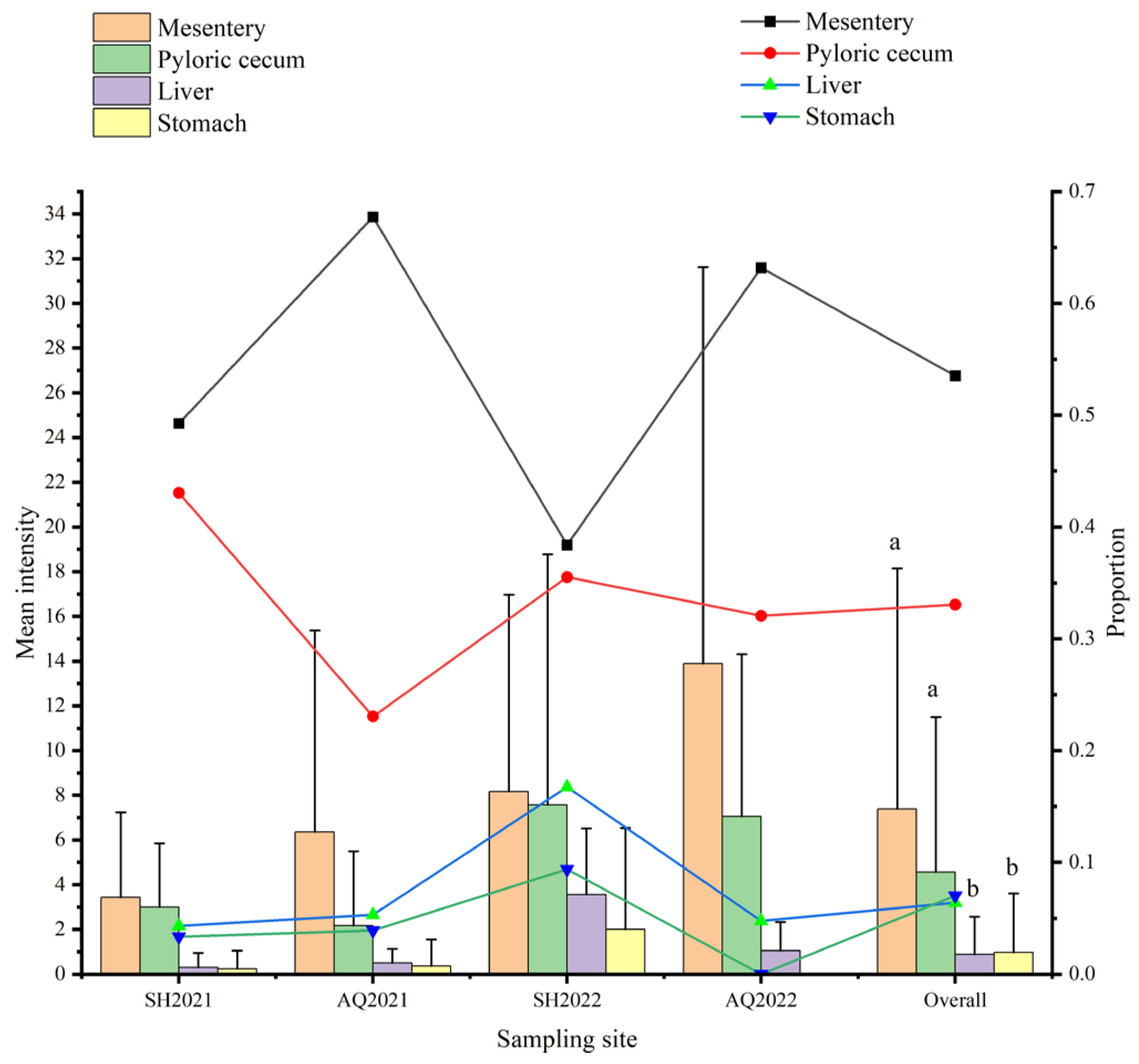
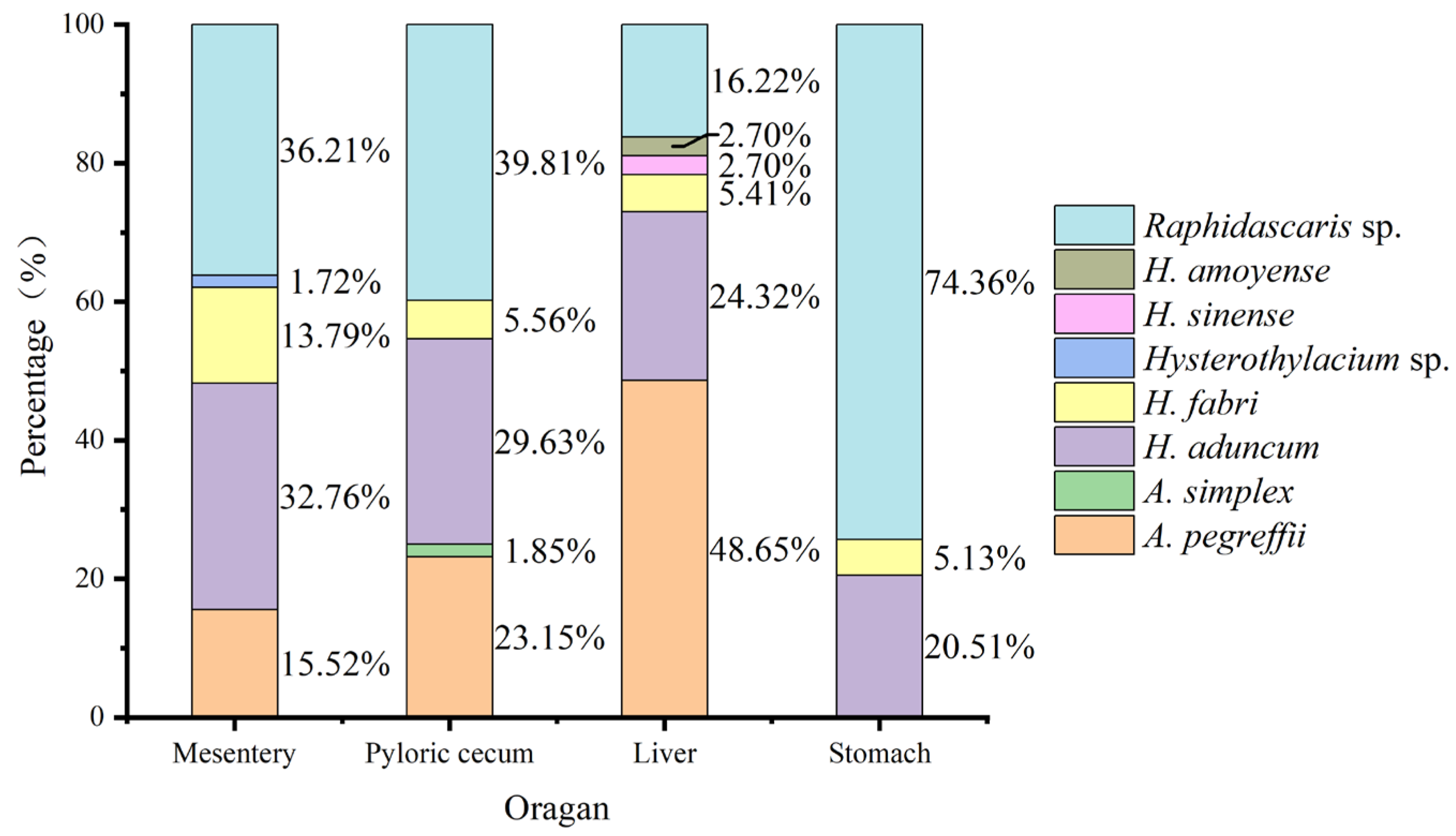
3.4. Organ Tropism
3.5. Community Structure of Nematodes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hochberg, N.S.; Hamer, D.H.; Hughes, J.M.; Wilson, M.E. Anisakidosis: Perils of the Deep. Clin. Infect. Dis. 2010, 51, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Xu, Z.; Chen, H.-X.; Guo, N.; Li, L. Anisakid and Raphidascaridid nematodes (Ascaridoidea) infection in the important marine food-fish Lophius litulon (Jordan) (Lophiiformes: Lophiidae). Int. J. Food Microbiol. 2018, 284, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Cavallero, S.; Magnabosco, C.; Civettini, M.; Boffo, L.; Mingarelli, G.; Buratti, P.; Giovanardi, O.; Fortuna, C.M.; Arcangeli, G. Survey of Anisakis Sp. and Hysterothylacium Sp. in sardines and anchovies from the North Adriatic Sea. Int. J. Food Microbiol. 2015, 200, 18–21. [Google Scholar] [CrossRef]
- Li, L.; Zhao, J.-Y.; Chen, H.-X.; Ju, H.-D.; An, M.; Xu, Z.; Zhang, L.-P. Survey for the presence of ascaridoid larvae in the cinnamon flounder Pseudorhombus Cinnamoneus (Temminck & Schlegel) (Pleuronectiformes: Paralichthyidae). Int. J. Food Microbiol. 2017, 241, 108–116. [Google Scholar] [CrossRef]
- Klimpel, S.; Palm, H.W.; Rückert, S.; Piatkowski, U. The life cycle of Anisakis simplex in the Norwegian Deep (Northern North Sea). Parasitol. Res. 2004, 94, 1–9. [Google Scholar] [CrossRef]
- Murphy, T.M.; Berzano, M.; O’Keeffe, S.M.; Cotter, D.M.; McEvoy, S.E.; Thomas, K.A.; Maoiléidigh, N.P.; Whelan, K.F. Anisakid larvae in Atlantic Salmon (Salmo salar L.) grilse and post-smolts: Molecular identification and histopathology. J. Parasitol. 2010, 96, 77–82. [Google Scholar] [CrossRef]
- Shamsi, S.; Barton, D.P.; Zhu, X. Description and characterisation of terranova Pectinolabiatan. Sp. (Nematoda: Anisakidae) in great hammerhead shark, Sphyrna mokarran (Rüppell, 1837), in Australia. Parasitol. Res. 2019, 118, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, S.; Briand, M.J.; Justine, J.-L. Occurrence of Anisakis (Nematoda: Anisakidae) larvae in unusual hosts in Southern Hemisphere. Parasitol. Int. 2017, 66, 837–840. [Google Scholar] [CrossRef]
- Dezfuli, B.S.; Bosi, G.; DePasquale, J.A.; Manera, M.; Giari, L. Fish innate immunity against intestinal helminths. Fish Shellfish Immunol. 2016, 50, 274–287. [Google Scholar] [CrossRef]
- Liu, K.; Yin, D.; Shu, Y.; Dai, P.; Yang, Y.; Wu, H. Transcriptome and metabolome analyses of Coilia nasus in response to Anisakidae parasite infection. Fish Shellfish Immunol. 2019, 87, 235–242. [Google Scholar] [CrossRef]
- Mattiucci, S.; Nascetti, G. Chapter 2 Advances and trends in the molecular systematics of Anisakid nematodes, with implications for their evolutionary ecology and host—Parasite co-evolutionary processes. In Advances in Parasitology; Academic Press: Cambridge, MA, USA, 2008; Volume 66, pp. 47–148. [Google Scholar]
- Levsen, A.; Berland, B. Anisakis Species. In Fish Parasites: Pathobiology and Protection; CABI: Wallingford, UK, 2012; pp. 298–309. [Google Scholar] [CrossRef]
- Shamsi, S.; Barton, D.P. A critical review of Anisakidosis cases occurring globally. Parasitol. Res. 2023, 122, 1733–1745. [Google Scholar] [CrossRef]
- Jiang, T.; Yang, J.; Liu, H.; Shen, X. Life History of Coilia nasus from the Yellow Sea inferred from Otolith Sr:Ca Ratios. Environ. Biol. Fishes 2012, 95, 503–508. [Google Scholar] [CrossRef]
- Feng, X.; Yang, X.; Ruan, J.; Wang, Y.; Zhou, Y.; Xu, D.; Fang, D. Molecular cloning and characteristics of DnaJa1and DnaJb1 in Coilia nasus: Possible function involved in oogenesis during spawning migration. BMC Dev. Biol. 2019, 19, 9. [Google Scholar] [CrossRef] [PubMed]
- Dou, S.Z.; Yamada, Y.; Okamura, A.; Tanaka, S.; Shinoda, A.; Tsukamoto, K. Observations on the spawning behavior of artificially matured Japanese eels Anguilla japonica in captivity. Aquaculture 2007, 266, 117–129. [Google Scholar] [CrossRef]
- Yuan, C. Spawning migration of Coilia nasus. Bull. Biol. 1987, 12, 1–3. (In Chinese) [Google Scholar]
- Jiang, T.; Yang, J.; Lu, M.J.; Liu, H.B.; Chen, T.T.; Gao, Y.W. Discovery of a spawning area for anadromous Coilia nasus Temminck et Schlegel, 1846 in Poyang Lake, China. J. Appl. Ichthyol. 2017, 33, 189–192. [Google Scholar] [CrossRef]
- Xuan, Z.; Jiang, T.; Liu, H.; Chen, X.; Yang, J. Mitochondrial DNA and microsatellite analyses reveal strong genetic differentiation between two types of estuarine tapertail anchovies (Coilia) in Yangtze River Basin, China. Hydrobiologia 2021, 848, 1409–1431. [Google Scholar] [CrossRef]
- Liang, C.; Pauly, D. Growth and mortality of exploited fishes in China’s coastal seas and their uses for yield-per-recruit analyses. J. Appl. Ichthyol. 2017, 33, 746–756. [Google Scholar] [CrossRef]
- Ma, J.; Li, B.; Zhao, J.; Wang, X.; Hodgdon, C.T.; Tian, S. Environmental influences on the spatio-temporal distribution of Coilia nasus in the Yangtze River Estuary. J. Appl. Ichthyol. 2020, 36, 315–325. [Google Scholar] [CrossRef]
- Xu, H.-N.; Sun, C.-B.; Tong, Y.-R.; Tao, J.-Y. The biological indicator of the spawning migration of anchovy (Coilia ectenes Jordan et Seale) in Yangtze River. J. Nanjing Univ. (Nat. Sci.) 1978, 3, 85–91. (In Chinese) [Google Scholar]
- Li, W.X.; Song, R.; Wu, S.G.; Zou, H.; Nie, P.; Wang, G.T. Seasonal occurrence of helminths in the anadromous fish Coilia nasus (Engraulidae): Parasite indicators of fish migratory movements. J. Parasitol. 2011, 97, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Li, W.X.; Zou, H.; Wu, S.G.; Song, R.; Wang, G.T. Richness and diversity of helminth communities in the Japanese grenadier anchovy, Coilia nasus, during its anadromous migration in the Yangtze River, China. J. Parasitol. 2012, 98, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Dai, P.; Guo, Q.; Tian, J.; Ma, F.; Wang, Y.; Liu, K. Nematode infection status and temporal characteristic of estuarine tapertail anchovy Coilia nasus in Anqing section of the Yangtze River. J. Dalian Ocean Univ. 2022, 37, 464–470. (In Chinese) [Google Scholar] [CrossRef]
- Cheng, X.; Yang, Y.; Ying, C.; Ma, F.; Deng, Y.; Liu, K. Investigation on Anisakidae nematode parasitic status of Coilia nasus at the early stages of banning fishing in Yangtze River. Trans. Oceanol. Limnol. 2023, 45, 142–149. (In Chinese) [Google Scholar] [CrossRef]
- Williams, J.D.; Boyko, C.B. The global diversity of parasitic isopods associated with crustacean hosts (Isopoda: Bopyroidea and Cryptoniscoidea). PLoS ONE 2012, 7, e35350. [Google Scholar] [CrossRef] [PubMed]
- Liu, K. Research on Immune Adaptive Response of Coilia nasus Infected with Nematode in Yangtze River Based on Multi-Omics Techniques. Ph.D. Thesis, Anhui Normal University, Wuhu, China, 2019. [Google Scholar]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology Meets Ecology on Its Own Terms: Margolis et al. Revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Lü, J.-Y.; Zeng, H.; Yang, D.-W.; Chen, Z.-S. Studies on community ecology of helminthes parasitic in Mugil cephalus in Guangdong Province, China. Acta Ecol. Sin. 2001, 21, 1003–1008. (In Chinese) [Google Scholar]
- Dai, P.; Yan, Y.; Zhu, X.; Tian, J.; Ma, F.; Liu, K. Status of Coilia nasus resources in the National Aquatic Germplasm Resources Conservation Area in the Anqing Section of the Yangtze River. J. Fish. Sci. China 2020, 27, 1267–1276. (In Chinese) [Google Scholar]
- Dai, P.; Ma, F.-J.; Tian, J.-L.; Wang, Y.-P.; Yang, Y.-P.; Liu, K. Community structure and infection characteristics of nematodes in the Coilia nasus in Anqing section of the Yangtze River. Acta Hydrobiol. Sin. 2023, 47, 917–923. (In Chinese) [Google Scholar]
- Shamsi, S.; Turner, A.; Wassens, S. Description and genetic characterization of a new Contracaecum Larval Type (Nematoda: Anisakidae) from Australia. J. Helminthol. 2018, 92, 216–222. [Google Scholar] [CrossRef]
- Shamsi, S.; Pearce, L.; Zhu, X. Characterisation of nematode larvae found in a vulnerable native Australian fish, the Southern Pygmy Perch, Nannoperca Australis Günther. Mar. Freshw. Res. 2023, 74, 1095–1101. [Google Scholar] [CrossRef]
- Song, R. The Ecological Investigation of the Helminths in Coilia nasus. Master’s Thesis, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan, China, 2011. (In Chinese). [Google Scholar]
- Xi, B.-W.; Wang, G.-T.; Wu, S.-G.; Gao, D.; Zou, H.; Yao, W.-J.; Nie, P. Community structure of the intestinal helminths of the Chinese hooksnout carp, Opsariichthys Bidens (g(u)nther), from the Danjangkou reservio. Acta Hydrobiol. Sin. 2009, 33, 177–182. (In Chinese) [Google Scholar] [CrossRef]
- Balbuena, J.A.; Karlsbakk, E.; Kvenseth, A.M.; Saksvik, M.; Nylund, A. Growth and emigration of third-stage larvae of Hysterothylacium aduncum (Nematoda: Anisakidae) in larval herring Clupea harengus. J. Parasitol. 2000, 86, 1271–1275. [Google Scholar] [CrossRef] [PubMed]
- Larsen, A.H.; Bresciani, J.; Buchmann, K. Interactions between Ecto- and Endoparasites in Trout Salmo Trutta. Vet. Parasitol. 2002, 103, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-L. Anisakidae Nematodes on Digestive Tract Tissue Damage and the Effect of Immune Response of Coilia nasus. Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2022. (In Chinese). [Google Scholar]
- Ying, C.; Fang, X.; Wang, H.; Yang, Y.; Xu, P.; Liu, K.; Yin, G. Anisakidae parasitism activated immune response and induced liver fibrosis in wild anadromous Coilia nasus. J. Fish Biol. 2022, 100, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-X.; Wang, G.-T. Helminth Communities in Coilia nasus from Anadromous, Freshwater and Landlocked Stock. Chin. J. Zool. 2014, 49, 233–243. (In Chinese) [Google Scholar] [CrossRef]
- Li, M.; Jiang, T.; Chen, T.; Liu, H.; Yang, J. Otolith microchemistry of the estuarine tapertail anchovy Coilia nasus from the Anqing section of the Yangtze River and its significance for migration ecology. Acta Ecol. Sin. 2017, 37, 2788–2795. (In Chinese) [Google Scholar]
- Cao, Z.; Liu, J.; He, F.; Lin, R.; Zhu, X. Overview of Anisakis disease. J. Trop. Med. 2004, 4, 494–497. (In Chinese) [Google Scholar]
- Shamsi, S.; Francis, N.; Masiga, J.; Barton, D.P.; Zhu, X.; Pearce, L.; McLellan, M. Occurrence and characterisation of Eustrongylides species in Australian Native birds and fish. Food Waterborne Parasitol. 2023, 30, e00189. [Google Scholar] [CrossRef]
- Li, L. Systematics of the Genus Hysterothylacium from Marine Fishes from Yellow Seea, China (Ascaridoidea: Raphidascaridae). Master’s Thesis, Hebei Normal University, Shijiazhuang, China, 2007. (In Chinese). [Google Scholar]
- Li, X.; Cao, T.; Du, A.; Cao, W. Preliminary investigation on infection of Anisakis larvae in Marine fish in the East China Sea. Chin. J. Vet. Med. 2009, 45, 76–77. (In Chinese) [Google Scholar]
- Xu, Z. Preliminary Studies on Ecology of Parasitic Nematodes from Several Marine Fish from Yellow Sea, China. Master’s Thesis, Hebei Normal University, Shijiazhuang, China, 2007. (In Chinese). [Google Scholar]
- Bouree, P.; Paugam, A.; Petithory, J.-C. Anisakidosis: Report of 25 Cases and Review of the Literature. Comp. Immunol. Microbiol. Infect. Dis. 1995, 18, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Takano, T.; Iwaki, T.; Waki, T.; Murata, R.; Suzuki, J.; Kodo, Y.; Kobayashi, K.; Ogawa, K. Species composition and infection levels of Anisakis (Nematoda: Anisakidae) in the Skipjack Tuna Katsuwonus Pelamis (Linnaeus) in the Northwest Pacific. Parasitol. Res. 2021, 120, 1605–1615. [Google Scholar] [CrossRef] [PubMed]
- Hossen, M.S.; Shamsi, S. Zoonotic nematode parasites infecting selected edible fish in New South Wales, Australia. Int. J. Food Microbiol. 2019, 308, 108306. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, S. The occurrence of Anisakis spp. in Australian Waters: Past, present, and future trends. Parasitol. Res. 2021, 120, 3007–3033. [Google Scholar] [CrossRef] [PubMed]
- Palm, H.W.; Reimann, N.; Spindler, M.; Plötz, J. The role of the rock cod Notothenia coriiceps Richardson, 1844 in the life-cycle of Antarctic parasites. Polar Biol. 1998, 19, 399–406. [Google Scholar] [CrossRef]
- Klöser, H.; Plötz, J.; Palm, H.; Bartsch, A.; Hubold, G. Adjustment of anisakid nematode life cycles to the high antarctic food web as shown by Contracaecum radiatum and C. osculatum in the Weddell Sea. Antarct. Sci. 1992, 4, 171–178. [Google Scholar] [CrossRef][Green Version]
- Ning, J. Host-Parasitic Relationships Between Antarctic Icefish (Channichthyidae) And its Parasites, with Focusing on Nematode and Copepod. Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2021. (In Chinese). [Google Scholar]
- Zuo, S.; Kania, P.W.; Mehrdana, F.; Marana, M.H.; Buchmann, K. Contracaecum osculatum and other anisakid nematodes in Grey Seals and Cod in the Baltic Sea: Molecular and ecological links. J. Helminthol. 2018, 92, 81–89. [Google Scholar] [CrossRef]
- Palomba, M.; Marchiori, E.; Tedesco, P.; Fioravanti, M.; Marcer, F.; Gustinelli, A.; Aco-Alburqueque, R.; Belli, B.; Canestrelli, D.; Santoro, M.; et al. An update and ecological perspective on certain sentinel helminth endoparasites within the Mediterranean Sea. Parasitology 2023, 150, 1139–1157. [Google Scholar] [CrossRef]

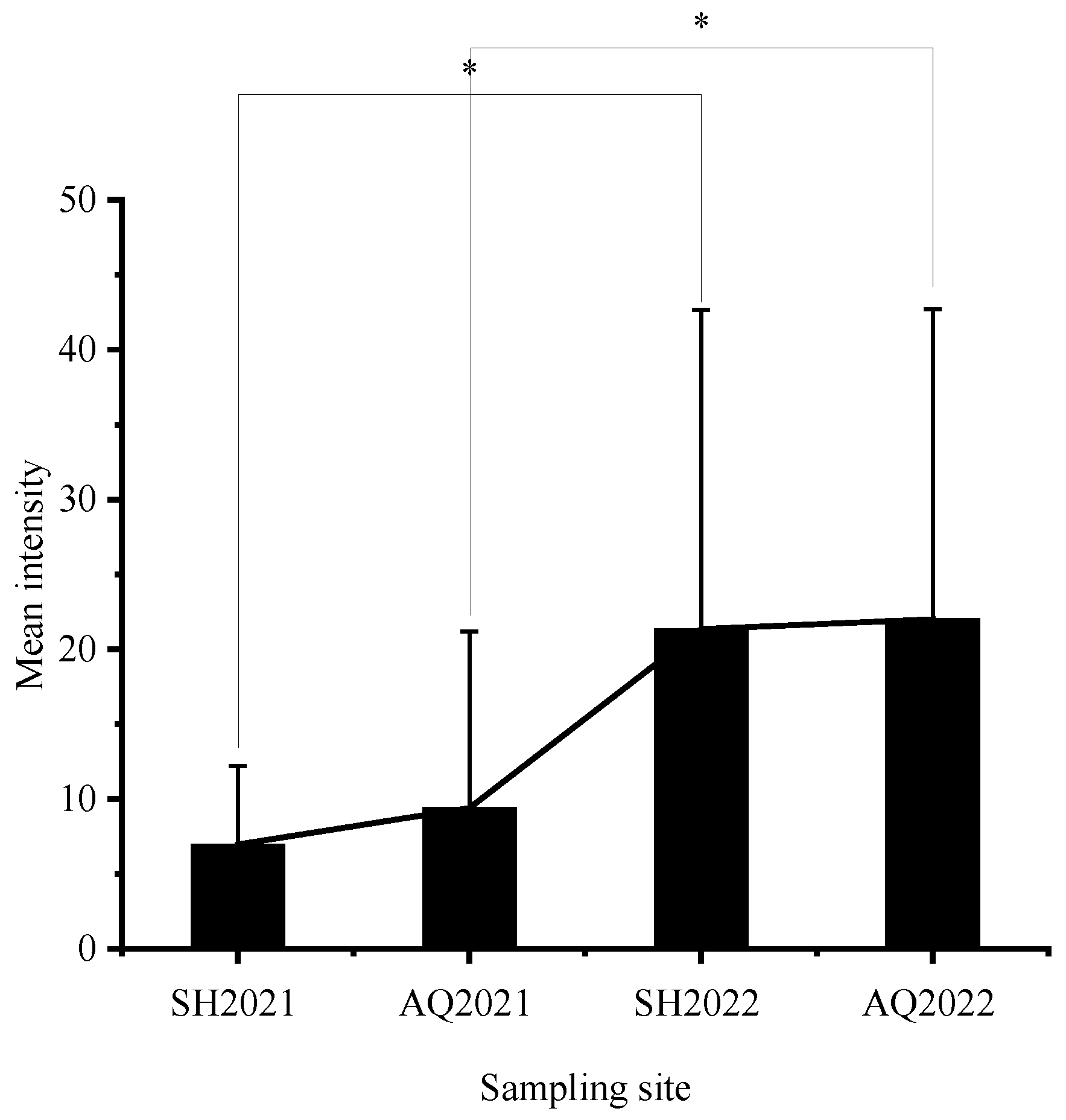
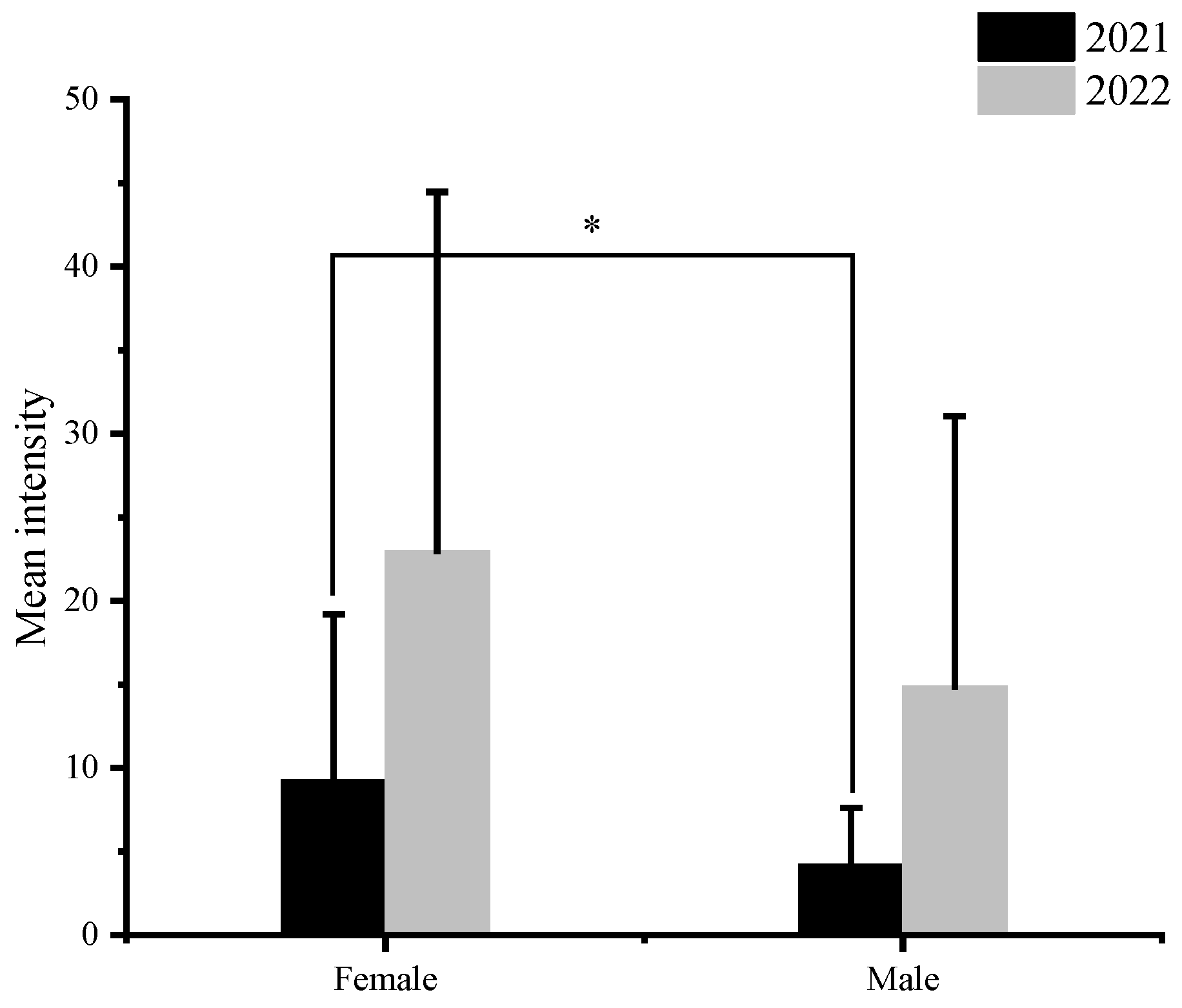
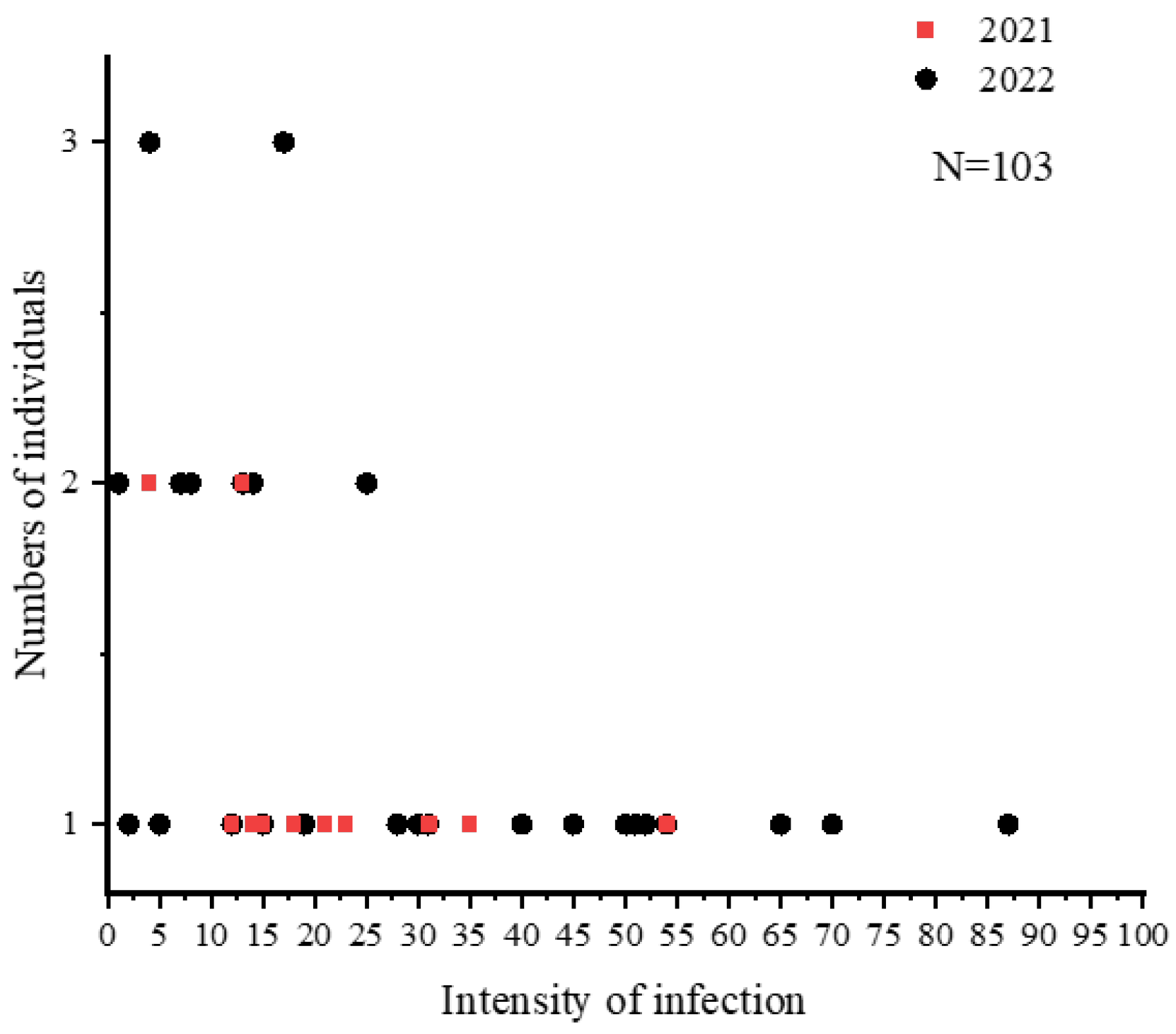
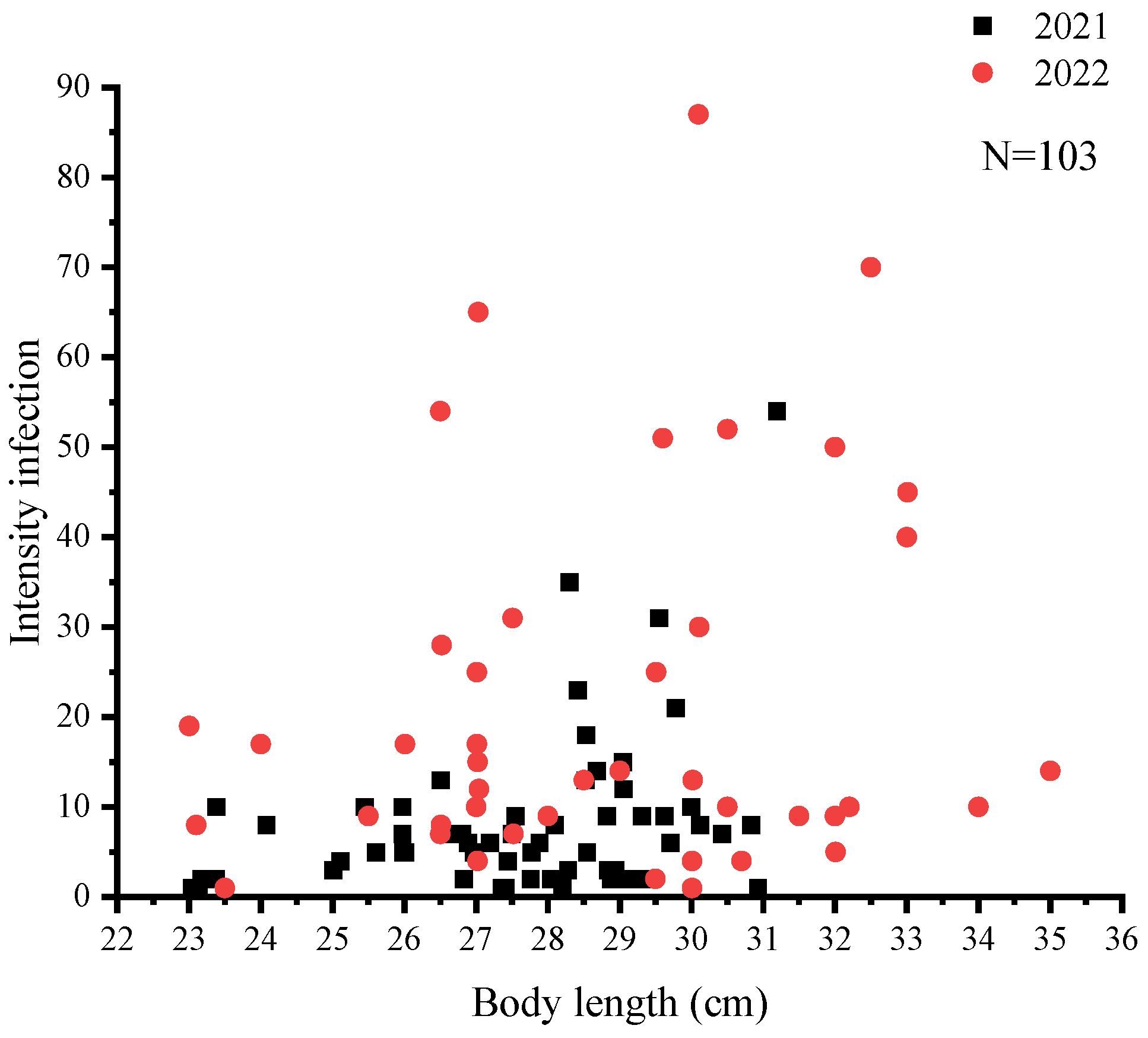
| Site | Year | N | Body Length (cm) | Female to Male Ratio | Prevalence (%) | Mean Abundance |
|---|---|---|---|---|---|---|
| Shanghai section | 2021 | 30 | 26.93 ± 2.26 | 4.00:1 | 100 | 6.97 ± 5.26 |
| Anqing section | 2021 | 30 | 27.40 ± 2.28 | 3.29:1 | 100 | 9.40 ± 11.78 |
| Shanghai section | 2022 | 23 | 28.16 ± 3.12 | 4.75:1 | 100 | 21.35 ± 21.29 |
| Anqing section | 2022 | 20 | 29.50 ± 2.64 | 5.67:1 | 100 | 22.00 ± 20.72 |
| Nematode Species | No. of Individuals Sequenced | Sequence Identity (%) | GenBank Acc. No. | Closely Related Nematode in GenBank | |
|---|---|---|---|---|---|
| Species (Accession) | Sequence Similarity (%) | ||||
| Hysterothylacium aduncum | 68 | 99.4–100 | PP029287 | Hysterothylacium aduncum (MH211517) | 98.3–100 |
| Hysterothylacium fabri | 18 | 99.4–100 | PP029274 | Hysterothylacium fabri (MH211492) | 99.6–100 |
| Hysterothylacium amoyense | 1 | - | PP029291 | Hysterothylacium amoyense (MT269312) | 99.89 |
| Hysterothylacium sinense | 1 | - | PP029292 | Hysterothylacium sinense (MH211574) | 100 |
| Hysterothylacium sp. | 1 | - | PP034301 | Hysterothylacium sp. (MF061683) | 100 |
| Anisakis simplex | 2 | 100 | PP029277 | Anisakis simplex (MT355320) | 100 |
| Anisakis pegreffii | 52 | 99.9–100 | PP029289 | Anisakis pegreffii (MH211473) | 99.6–100 |
| Raphidascaris sp. | 99 | 99.0–100 | PP034302 | Raphidascaris sp. (MW370774) | 99.5–100 |
| Nematode Species | Mean Value | Variance | Distribution Index | d | Aggregate Index | Infection Index |
|---|---|---|---|---|---|---|
| Anisakis pegreffii | 2.000 | 4.560 | 2.280 | 3.677 | 0.025 | 1.385 |
| Anisakis simplex | 0.077 | 0.074 | 0.960 | −0.072 | −0.040 | 0.006 |
| Hysterothylacium aduncum | 2.615 | 7.206 | 2.755 | 4.737 | 0.026 | 1.609 |
| Hysterothylacium fabri | 0.692 | 1.022 | 1.476 | 1.589 | 0.028 | 0.266 |
| Hysterothylacium sp. | 0.038 | 0.038 | 1.000 | 0.071 | - | 0.001 |
| Hysterothylacium sinense | 0.038 | 0.038 | 1.000 | 0.071 | - | 0.001 |
| Hysterothylacium amoyense | 0.038 | 0.038 | 1.000 | 0.071 | - | 0.001 |
| Raphidascaris sp. | 3.808 | 32.882 | 8.636 | 13.779 | 0.078 | 2.343 |
| Nematode Species | Mean Value | Variance | Distribution Index | d | Aggregate Index | Infection Index |
|---|---|---|---|---|---|---|
| Anisakis pegreffii | 1.625 | 4.783 | 2.944 | 4.012 | 0.078 | 1.016 |
| Anisakis simplex | 0.063 | 0.063 | 1.000 | 0.092 | - | 0.004 |
| Hysterothylacium aduncum | 4.250 | 4.600 | 1.082 | 0.313 | 0.001 | 4.250 |
| Hysterothylacium fabri | 0.750 | 1.000 | 1.333 | 0.939 | 0.030 | 0.328 |
| Hysterothylacium sp. | 0.063 | 0.063 | 1.000 | 0.092 | - | 0.004 |
| Hysterothylacium sinense | 0.063 | 0.063 | 1.000 | 0.092 | - | 0.004 |
| Hysterothylacium amoyense | 0.063 | 0.063 | 1.000 | 0.092 | - | 0.004 |
| Raphidascaris sp. | 3.938 | 44.863 | 11.394 | 13.103 | 0.168 | 1.723 |
| Nematode Species | Mean Value | Variance | Distribution Index | d | Aggregate Index | Infection Index |
|---|---|---|---|---|---|---|
| Anisakis pegreffii | 2.600 | 4.044 | 1.556 | 1.168 | 0.022 | 2.080 |
| Anisakis simplex | 0.100 | 0.100 | 1.000 | 0.120 | - | 0.010 |
| Hysterothylacium fabri | 0.600 | 1.156 | 1.926 | 1.765 | 0.185 | 0.180 |
| Raphidascaris sp. | 3.600 | 16.489 | 4.580 | 4.957 | 0.102 | 3.240 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.; Wang, L.; Xi, B.; Ying, C.; Liu, K. Ascaridoid Nematodes Infection in Anadromous Fish Coilia nasus from Yangtze River. Diversity 2024, 16, 167. https://doi.org/10.3390/d16030167
Zhou Q, Wang L, Xi B, Ying C, Liu K. Ascaridoid Nematodes Infection in Anadromous Fish Coilia nasus from Yangtze River. Diversity. 2024; 16(3):167. https://doi.org/10.3390/d16030167
Chicago/Turabian StyleZhou, Qingjie, Lijun Wang, Bingwen Xi, Congping Ying, and Kai Liu. 2024. "Ascaridoid Nematodes Infection in Anadromous Fish Coilia nasus from Yangtze River" Diversity 16, no. 3: 167. https://doi.org/10.3390/d16030167
APA StyleZhou, Q., Wang, L., Xi, B., Ying, C., & Liu, K. (2024). Ascaridoid Nematodes Infection in Anadromous Fish Coilia nasus from Yangtze River. Diversity, 16(3), 167. https://doi.org/10.3390/d16030167






