The Changing Biogeography of the Ligurian Sea: Seawater Warming and Further Records of Southern Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Ligurian Sea Temperature
2.2. Fish Species Identification
2.3. DNA Extraction, Amplification, and Sequencing for Kyphosus vaigiensis
2.4. Fish Species Ranges
3. Results
3.1. Temperature
3.2. Warm-Water Fishes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lenoir, J.; Svenning, J.C. Climate-related range shifts—A global multidimensional synthesis and new research directions. Ecography 2015, 38, 15–28. [Google Scholar] [CrossRef]
- Blowes, S.A.; Supp, S.R.; Antão, L.H.; Bates, A.; Bruelheide, H.; Chase, J.M.; Moyes, F.; Magurran, A.; McGill, B.; Myers-Smith, I.H.; et al. The geography of biodiversity change in marine and terrestrial assemblages. Science 2019, 366, 339–345. [Google Scholar] [CrossRef]
- Antão, L.H.; Bates, A.E.; Blowes, S.A.; Waldock, C.; Supp, S.R.; Magurran, A.E.; Dornelas, M.; Schipper, A.M. Temperature-related biodiversity change across temperate marine and terrestrial systems. Nat. Ecol. Evol. 2020, 4, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, J.; Bertrand, R.; Comte, L.; Bourgeaud, L.; Hattab, T.; Murienne, J.; Grenouillet, G. Species better track climate warming in the oceans than on land. Nat. Ecol. Evol. 2020, 4, 1044–1059. [Google Scholar] [CrossRef] [PubMed]
- Hodapp, D.; Roca, I.T.; Fiorentino, D.; Garilao, C.; Kaschner, K.; Kesner-Reyes, K.; Schneider, B.; Segschneider, J.; Kocsis, Á.T.; Kiessling, W.; et al. Climate change disrupts core habitats of marine species. Glob. Chang. Biol. 2023, 29, 3304–3317. [Google Scholar] [CrossRef] [PubMed]
- Poloczanska, E.S.; Burrows, M.T.; Brown, C.J.; García Molinos, J.; Halpern, B.S.; Hoegh-Guldberg, O.; Kappel, C.V.; Moore, P.J.; Richardson, A.J.; Schoeman, D.S.; et al. Responses of marine organisms to climate change across oceans. Front. Mar. Sci. 2016, 3, 62. [Google Scholar] [CrossRef]
- Schuster, J.M.; Stuart-Smith, R.D.; Edgar, G.J.; Bates, A.E. Tropicalization of temperate reef fish communities facilitated by urchin grazing and diversity of thermal affinities. Glob. Ecol. Biogeogr. 2022, 31, 995–1005. [Google Scholar] [CrossRef]
- Vergés, A.; McCosker, E.; Mayer-Pinto, M.; Coleman, M.A.; Wernberg, T.; Ainsworth, T.; Steinberg, P.D. Tropicalisation of temperate reefs: Implications for ecosystem functions and management actions. Funct. Ecol. 2019, 33, 1000–1013. [Google Scholar] [CrossRef]
- Zarzyczny, K.M.; Rius, M.; Williams, S.T.; Fenberg, P.B. The ecological and evolutionary consequences of tropicalisation. Trends Ecol. Evol. 2024, in press. [CrossRef]
- Osland, M.J.; Stevens, P.W.; Lamont, M.M.; Brusca, R.C.; Hart, K.M.; Waddle, J.H.; Langtimm, C.A.; Williams, C.M.; Keim, B.D.; Terando, A.J.; et al. Tropicalization of temperate ecosystems in North America: The northward range expansion of tropical organisms in response to warming winter temperatures. Glob. Chang. Biol. 2021, 27, 3009–3034. [Google Scholar] [CrossRef]
- Pessarrodona, A.; Vergés, A.; Bosch, N.E.; Bell, S.; Smith, S.; Sgarlatta, M.P.; Wernberg, T. Tropicalization unlocks novel trophic pathways and enhances secondary productivity in temperate reefs. Funct. Ecol. 2022, 36, 659–673. [Google Scholar] [CrossRef]
- Montero-Serra, I.; Edwards, M.; Genner, M.J. Warming shelf seas drive the subtropicalization of European pelagic fish communities. Glob. Chang. Biol. 2015, 21, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Kaimuddin, A.H.; Laë, R.; Tito De Morais, L. Fish species in a changing world: The route and timing of species migration between tropical and temperate ecosystems in Eastern Atlantic. Front. Mar. Sci. 2016, 3, 162. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Morri, C.; Sartoni, G.F.; Wirtz, P. Sublittoral epibenthic communities around Funchal (Ilha da Madeira, NE Atlantic). Bol. Mus. Munic. Funchal 1998, 5, 59–80. [Google Scholar]
- Bianchi, C.N.; Morri, C. Global sea warming and “tropicalization” of the Mediterranean Sea: Biogeographic and ecological aspects. Biogeographia 2003, 24, 319–327. [Google Scholar] [CrossRef]
- Schroeder, K.; Chiggiato, J.; Josey, S.A.; Borghini, M.; Aracri, S.; Sparnocchia, S. Rapid response to climate change in a marginal sea. Sci. Rep. 2017, 7, 4065. [Google Scholar] [CrossRef]
- Diffenbaugh, N.S.; Pal, J.S.; Giorgi, F.; Gao, X. Heat stress intensification in the Mediterranean climate change hotspot. Geophys. Res. Lett. 2007, 34, L11706. [Google Scholar] [CrossRef]
- Cos, J.; Doblas-Reyes, F.; Jury, M.; Marcos, R.; Bretonnière, P.A.; Samsó, M. The Mediterranean climate change hotspot in the CMIP5 and CMIP6 projections. Earth Syst. Dyn. 2022, 13, 321–340. [Google Scholar] [CrossRef]
- Kubin, E.; Menna, M.; Mauri, E.; Notarstefano, G.; Mieruch, S.; Poulain, P.M. Heat content and temperature trends in the Mediterranean Sea as derived from Argo float data. Front. Mar. Sci. 2023, 10, 1271638. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Morri, C. Marine biodiversity of the Mediterranean Sea: Situation, problems and prospects for future research. Mar. Pollut. Bull. 2000, 40, 367–376. [Google Scholar] [CrossRef]
- Bianchi, C.N. Biodiversity issues for the forthcoming tropical Mediterranean Sea. Hydrobiologia 2007, 580, 7–21. [Google Scholar] [CrossRef]
- Zenetos, A.; Gofas, S.; Verlaque, M.; Çinar, M.E.; García Raso, J.E.; Bianchi, C.N.; Morri, C.; Azzurro, E.; Bilecenoglu, M.; Froglia, C.; et al. Alien species in the Mediterranean Sea by 2010. A contribution to the application of European Union’s Marine Strategy Framework Directive (MSFD). Part 1. Spatial distribution. Mediterr. Mar. Sci. 2010, 11, 381–493. [Google Scholar] [CrossRef]
- Zenetos, A.; Gofas, S.; Morri, C.; Rosso, A.; Violanti, D.; García Raso, J.E.; Çinar, M.E.; Almogi-Labin, A.; Ates, A.S.; Azzurro, E.; et al. Alien species in the Mediterranean Sea by 2010. A contribution to the application of European Union’s Marine Strategy Framework Directive (MSFD). Part 2. Introduction trends and pathways. Mediterr. Mar. Sci. 2012, 13, 328–352. [Google Scholar] [CrossRef]
- Schultz, L.; Wessely, J.; Dullinger, S.; Albano, P.G. The climate crisis affects Mediterranean marine molluscs of conservation concern. Divers. Distrib. 2024, 30, e13805. [Google Scholar] [CrossRef]
- Frölicher, T.L.; Fischer, E.M.; Gruber, N. Marine heatwaves under global warming. Nature 2018, 560, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Garrabou, J.; Gómez-Gras, D.; Medrano, A.; Cerrano, C.; Ponti, M.; Schlegel, R.; Bensoussan, N.; Turicchia, E.; Sini, M.; Gerovasileiou, V.; et al. Marine heatwaves drive recurrent mass mortalities in the Mediterranean Sea. Glob. Chang. Biol. 2022, 28, 5708–5725. [Google Scholar] [CrossRef] [PubMed]
- Boudouresque, C.F.; Astruch, P.; André, S.; Belloni, B.; Blanfuné, A.; Charbonnel, É.; Cheminée, A.; Cottalorda, J.M.; Dupuy de la Grandrive, R.; Marengo, M.; et al. The heatwave of summer 2022 in the North-Western Mediterranean Sea: Some species were winners. Water 2024, 16, 219. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Azzola, A.; Bertolino, M.; Betti, F.; Bo, M.; Cattaneo-Vietti, R.; Cocito, S.; Montefalcone, M.; Morri, C.; Oprandi, A.; et al. Consequences of the marine climate and ecosystem shift of the 1980–90s on the Ligurian Sea biodiversity (NW Mediterranean). Eur. Zool. J. 2019, 86, 458–487. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Morri, C.; Chiantore, M.; Montefalcone, M.; Parravicini, V.; Rovere, A. Mediterranean Sea biodiversity between the legacy from the past and a future of change. In Life in the Mediterranean Sea: A Look at Habitat Changes; Stambler, N., Ed.; Nova Science: New York, NY, USA, 2012; pp. 1–55. [Google Scholar]
- Rossi, L. Considerazioni zoogeografiche sul bacino N.W. del Mediterraneo, con particolare riguardo al Mar Ligure. In Archivio Botanico e Biogeografico Italiano; XLV-4a Serie; Tipo-Lito Valbonesi: Forli, Italy, 1969; Volume 14, pp. 139–152. [Google Scholar]
- Bianchi, C.N.; Morri, C. Range extensions of warm-water species in the northern Mediterranean: Evidence for climatic fluctuations? Porcup. Newsl. 1993, 5, 156–159. [Google Scholar]
- Bianchi, C.N.; Morri, C. Southern species in the Ligurian Sea (northern Mediterranean): New records and a review. Boll. Ist. Mus. Biol. Univ. Genova 1994, 58–59, 181–197. [Google Scholar]
- Astraldi, M.; Bianchi, C.N.; Gasparini, G.P.; Morri, C. Climatic fluctuations, current variability and marine species distribution: A case study in the Ligurian Sea (north-west Mediterranean). Oceanol. Acta 1995, 18, 139–149. [Google Scholar]
- Morri, C.; Bianchi, C.N. Recent changes in biodiversity in the Ligurian Sea (NW Mediterranean): Is there a climatic forcing? In Structure and Processes in the Mediterranean Ecosystems; Faranda, F.M., Guglielmo, L., Spezie, G., Eds.; Springer: Milano, Italy, 2001; pp. 375–384. [Google Scholar]
- Vacchi, M.; Morri, C.; Modena, M.; La Mesa, G.; Bianchi, C.N. Temperature changes and warm-water species in the Ligurian Sea: The case of the ornate wrasse Thalassoma pavo (Linnaeus, 1758). Arch. Oceanogr. Limnol. 2001, 22, 149–154. [Google Scholar]
- Bianchi, C.N.; Caroli, F.; Guidetti, P.; Morri, C. Seawater warming at the northern reach for southern species: Gulf of Genoa, NW Mediterranean. J. Mar. Biol. Assoc. UK 2018, 98, 1–12. [Google Scholar] [CrossRef]
- Parravicini, V.; Azzurro, E.; Kulbicki, M.; Belmaker, J. Niche shift can impair the ability to predict invasion risk in the marine realm: An illustration using Mediterranean fish invaders. Ecol. Lett. 2015, 18, 246–253. [Google Scholar] [CrossRef]
- D’Amen, M.; Smeraldo, S.; Azzurro, E. Salinity, not only temperature, drives tropical fish invasions in the Mediterranean Sea, and surface-only variables explain it better. Coral Reefs 2023, 42, 467–472. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Boudouresque, C.F.; Francour, P.; Morri, C.; Parravicini, V.; Templado, J.; Zenetos, A. The changing biogeography of the Mediterranean Sea: From the old frontiers to the new gradients. Boll. Mus. Ist. Biol. Univ. Genova 2013, 75, 81–84. [Google Scholar]
- NOAA Physical Sciences Laboratory. Available online: www.esrl.noaa.gov/psd/cgi-bin/data/timeseries/timeseries1.pl (accessed on 5 January 2024).
- Bianchi, C.N.; Morri, C. Uomo, clima e biodiversità marina: Esempi dal Mar Ligure. Uomo Nat. 2004, 9, 15–23. [Google Scholar]
- Bruschi, A.; Sgorbini, S. Banche dati ambientali: Idrologia del mar Mediterraneo. Acqua Aria 1986, 6, 565–578. [Google Scholar]
- Astraldi, M.; Gasparini, G.P.; Sparnocchia, S. The seasonal and interannual variability in the Ligurian-Provençal Basin. In Seasonal and Interannual Variability of the Western Mediterranean Sea; Coastal and Estuarine Studies 46; La Violette, P.E., Ed.; American Geophysical Union: Washington DC, USA, 1994; pp. 93–113. [Google Scholar]
- Gatti, G.; Bianchi, C.N.; Montefalcone, M.; Venturini, S.; Diviacco, G.; Morri, C. Observational information on a temperate reef community helps understanding the marine climate and ecosystem shift of the 1980–90s. Mar. Pollut. Bull. 2017, 114, 528–538. [Google Scholar] [CrossRef]
- Tortonese, E. Fauna d’Italia XI: Osteichthyes (Pesci Ossei), Parte Seconda; Calderini: Bologna, Italy, 1975; pp. 1–636. [Google Scholar]
- Bañón, R.; de Carlos, A. Preliminary evidence about the colonisation process of Kyphosus species (Perciformes: Kyphosidae) in the subtropical–temperate Northeast Atlantic Ocean and Mediterranean Sea. J. Mar. Sci. Eng. 2022, 10, 1237. [Google Scholar] [CrossRef]
- Al Mabruk, S.A.; Abdulghani, A.; Nour, O.M.; Adel, M.; Crocetta, F.; Doumpas, N.; Kleitou, P.; Tiralongo, F. The role of social media in compensating for the lack of field studies: Five new fish species for Mediterranean Egypt. J. Fish Biol. 2021, 99, 673–678. [Google Scholar] [CrossRef]
- Knudsen, S.W.; Clements, K.D. Revision of the fish family Kyphosidae (Teleostei: Perciformes). Zootaxa 2013, 3751, 1–101. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, K.E. Kyphosidae: Sea chubs. In The Living Marine Resources of the Western Central Atlantic; FAO Species Identification Guide for Fishery Purposes and American Society of Ichthyologists and Herpetologists Special Publication No. 5; Carpenter, K.E., Ed.; FAO: Rome, Italy, 2002; Volume 3, pp. 1684–1687. [Google Scholar]
- Mannino, A.M.; Balistreri, P.; Iaciofano, D.; Galil, B.S.; Lo Brutto, S. An additional record of Kyphosus vaigiensis (Quoy & Gaimard, 1825) (Osteichthyes, Kyphosidae) from Sicily clarifies the confused situation of the Mediterranean kyphosids. Zootaxa 2015, 3963, 45–54. [Google Scholar] [PubMed]
- Orsi-Relini, L. Note on recent revisions of the taxonomy of Kyphosidae. Biol. Mar. Medit. 2017, 24, 206–208. [Google Scholar]
- Knudsen, S.W.; Clements, K.D. World-wide species distributions in the family Kyphosidae (Teleostei: Perciformes). Mol. Phylogenet. Evol. 2016, 101, 252–266. [Google Scholar] [CrossRef]
- Bañón, R.; Barros-García, D.; de Carlos, A. Integrative taxonomy supports the presence of two species of Kyphosus (Perciformes: Kyphosidae) in Atlantic European waters. Sci. Mar. 2017, 81, 467–475. [Google Scholar] [CrossRef]
- Tiralongo, F.; Lillo, A.O.; Tibullo, D.; Tondo, E.; Lo Martire, C.; D’Agnese, R.; Macali, A.; Mancini, E.; Giovos, I.; Coco, S.; et al. Monitoring uncommon and non-indigenous fishes in Italian waters: One year of results for the AlienFish project. Reg. Stud. Mar. Sci. 2019, 28, 100606. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Astrin, J.J.; Stüben, P.E. Phylogeny in cryptic weevils: Molecules, morphology and new genera of western Palaearctic Cryptorhynchinae (Coleoptera: Curculionidae). Invertebr. Syst. 2008, 22, 503–522. [Google Scholar] [CrossRef]
- Fischer, W.; Bauchot, M.L.; Schneider, M. (Eds.) Fiches FAO D’identification des Espèces pour les Besoins de la Pêche. (Révision 1). Méditerranée et Mer Noire. Zone de Pêche 37; FAO: Rome, Italy, 1987; Volume 2, pp. 761–1530. [Google Scholar]
- Whitehead, P.J.P.; Bauchot, M.L.; Hureau, J.C.; Nielsen, J.; Tortonese, E. (Eds.) Fishes of the North-Eastern Atlantic and the Mediterranean; UNESCO: Paris, France, 1984; Volume 2, pp. 511–1002. [Google Scholar]
- Golani, D.; Azzurro, E.; Dulčić, J.; Massuti, E.; Orsi-Relini, L. Atlas of Exotic Fishes in the Mediterranean Sea, 2nd ed.; Commission Internationale pour l’Exploration Scientifique de la Mer Méditerranée: Monte Carlo, Monaco, 2021; pp. 1–365. [Google Scholar]
- Kiyağa, V.B.; Mavruk, S.; Özyurt, C.E.; Akamca, E.; Coşkun, Ç. Range extension of Kyphosus vaigiensis (Quoy & Gaimard, 1825) in the northeastern Mediterranean, İskenderun Bay, Turkey. Turk. J. Zool. 2019, 43, 644–649. [Google Scholar]
- Goren, M.; Galil, B.S.; Gevili, R.; Stern, N. First record of the brassy chub Kyphosus vaigiensis (Quoy & Gaimard, 1825) in the Eastern Mediterranean (Osteichthyes: Perciformes: Kyphosidae). Zool. Middle East 2016, 62, 319–322. [Google Scholar]
- Vella, N.; Vella, A.; Agius Darmanin, S. The first record of the lowfin chub Kyphosus vaigiensis (Quoy & Gaimard, 1825) from Malta. J. Black Sea/Medit. Environ. 2016, 22, 175–181. [Google Scholar]
- Glamuzina, B.; Tutman, P.; Kozul, V.; Glavic, N.; Skaramuca, B. The first recorded occurrence of the mottled grouper, Mycteroperca rubra (Serranidae), in the southeastern Adriatic Sea. Cybium 2002, 26, 156–158. [Google Scholar]
- Relini Orsi, L.; Costa, M.R.; Relini, M. First record of the yellow sea chub Kyphosus incisor in the Mediterranean. Mar. Biodivers. Rec. 2010, 3, e4. [Google Scholar] [CrossRef]
- Cottalorda, J.M.; Dominici, J.M.; Harmelin, J.G.; Harmelin Vivien, M.; Louisy, P.; Francour, P. Étude et Synthèse des Principales Données Disponibles sur les Espèces de «Mérous» de la Réserve Naturelle de Scandola et de Ses Environs Immédiats; Ecomers: Nice, France, 2012; pp. 1–48. [Google Scholar]
- Psomadakis, P.N.; Giustino, S.; Vacchi, M. Mediterranean fish biodiversity: An updated inventory with focus on the Ligurian and Tyrrhenian seas. Zootaxa 2012, 3263, 1–46. [Google Scholar] [CrossRef]
- Azzurro, E.; Peña-Rivas, L.; Lloris, D.; Bariche, M. First documented occurrence of Kyphosus incisor in the Mediterranean Sea. Mar. Biodivers. Rec. 2013, 6, e98. [Google Scholar] [CrossRef]
- Peña-Rivas, L.; Azzurro, E. A new record of Kyphosus incisor for the Mediterranean Sea. Mediterr. Mar. Sci. 2013, 14, 475. [Google Scholar]
- Michailidis, N.; Rousou, M. First record of the brassy chub Kyphosus vaigiensis (Quoy & Gaimard, 1825) from Cyprus. Mediterr. Mar. Sci. 2017, 18, 355–384. [Google Scholar]
- Macali, A.; Tiralongo, F. New record of the skipjack tuna, Katsuwonus pelamis (Linnaeus, 1758) in the Mediterranean Sea. Mediterr. Mar. Sci. 2017, 18, 534–556. [Google Scholar]
- Gwilliam, M.P.; Winkler, A.C.; Potts, W.M.; Santos, C.V.; Sauer, W.H.H.; Shaw, P.W.; McKeown, N.J. Integrated genetic and morphological data support eco-evolutionary divergence of Angolan and South African populations of Diplodus hottentotus. J. Fish Biol. 2018, 92, 1163–1176. [Google Scholar] [CrossRef]
- Pollard, D.A.; Francour, P. Mycteroperca rubra, mottled grouper. In The IUCN Red List of Threatened Species; e.T14054A42691814; International Union for the Conservation of Nature: London, UK, 2018. [Google Scholar]
- Tiralongo, F.; Crocetta, F.; Riginella, E.; Lillo, A.O.; Tondo, E.; Macali, A.; Mancini, E.; Russo, F.; Coco, S.; Paolillo, G.; et al. Snapshot of rare, exotic and overlooked fish species in the Italian seas: A citizen science survey. J. Sea Res. 2020, 164, 101930. [Google Scholar] [CrossRef]
- Groud, L.L.; Chaoui, L.; Kara, M.H. A new record of the brassy chub, Kyphosus vaigiensis (Actinopterygii: Perciformes: Kyphosidae), from the Mediterranean Sea. Acta Ichthyol. Piscat. 2021, 51, 219–223. [Google Scholar] [CrossRef]
- Desiderà, E.; Mazzoldi, C.; Navone, A.; Panzalis, P.; Gervaise, C.; Guidetti, P.; Di Iorio, L. Reproductive behaviours and potentially associated sounds of the mottled grouper Mycteroperca rubra: Implications for conservation. Diversity 2022, 14, 318. [Google Scholar] [CrossRef]
- Fitori, A.; El-Fituri, A.; Golani, D. First record of the brassy chub Kyphosus vaigiensis (Pisces: Kyphosidae) from the Mediterranean coast of Libya. Acta Adriat. 2022, 63, 123–126. [Google Scholar] [CrossRef]
- Puerto, M.A.; Saber, S.; de Urbina, J.M.O.; Gómez-Vives, M.J.; García-Barcelona, S.; Macías, D. Spawning area of the tropical skipjack tuna, Katsuwonus pelamis (Scombridae), in the western Mediterranean Sea. Sci. Mar. 2022, 86, e051. [Google Scholar] [CrossRef]
- Evans, J.; Arndt, E.; Schembri, P.J. Atlantic fishes in the Mediterranean: Using biological traits to assess the origin of newcomer fishes. Mar. Ecol. Prog. Ser. 2020, 643, 133–143. [Google Scholar] [CrossRef]
- Ligas, A.; Sartor, P.; Sbrana, M.; de Ranieri, S. A new record of Kyphosus saltatrix (Pisces: Kyphosidae) along the Italian coasts (north-western Mediterranean). Mar. Biodivers. Rec. 2011, 4, e6. [Google Scholar] [CrossRef]
- Elbaraasi, H.; Bograra, O.; Elsilini, O.; Bojwari, J. First record of the Bermuda sea chub, Kyphosus saltatrix (Actinopterygii: Perciformes: Kyphosidae), in the coastal waters of Libya. Acta Ichthyol. Piscat. 2013, 43, 251–253. [Google Scholar] [CrossRef][Green Version]
- Parravicini, V.; Mangialajo, L.; Mousseau, L.; Peirano, A.; Morri, C.; Montefalcone, M.; Francour, P.; Kulbicki, M.; Bianchi, C.N. Climate change and warm-water species at the northwestern boundary of the Mediterranean Sea. Mar. Ecol. 2015, 36, 897–909. [Google Scholar] [CrossRef]
- Mercalli, L. Il Clima Che Cambia; BUR Rizzoli: Milan, Italy, 2019; pp. 1–355. [Google Scholar]
- Dittberner, G.J. Climatic change: Volcanoes, man-made pollution, and carbon dioxide. IEEE T. Geosci. Elect. 1978, 16, 50–61. [Google Scholar] [CrossRef]
- Fatima, F.; Fatima, N.; Amjad, T.; Anjum, A.; Afzal, T.; Riaz, J.; Razzaq, H. A review on acid rain: An environmental threat. Pure Appl. Biol. 2021, 10, 301–310. [Google Scholar] [CrossRef]
- Grennfelt, P.; Engleryd, A.; Forsius, M.; Hov, Ø.; Rodhe, H.; Cowling, E. Acid rain and air pollution: 50 years of progress in environmental science and policy. Ambio 2020, 49, 849–864. [Google Scholar] [CrossRef]
- Simmons, A.J.; Berrisford, P.; Dee, D.P.; Hersbach, H.; Hirahara, S.; Thépaut, J.N. A reassessment of temperature variations and trends from global reanalyses and monthly surface climatological datasets. Q. J. R. Meteorol. Soc. 2017, 143, 101–119. [Google Scholar] [CrossRef]
- Akasofu, S.I. On the present halting of global warming. Climate 2013, 1, 4–11. [Google Scholar] [CrossRef]
- Sippel, S.; Meinshausen, N.; Fischer, E.M.; Székely, E.; Knutti, R. Climate change now detectable from any single day of weather at global scale. Nat. Clim. Chang. 2020, 10, 35–41. [Google Scholar] [CrossRef]
- McHenry, J.; Welch, H.; Lester, S.E.; Saba, V. Projecting marine species range shifts from only temperature can mask climate vulnerability. Glob. Chang. Biol. 2019, 25, 4208–4221. [Google Scholar] [CrossRef] [PubMed]
- Gaylord, B.; Gaines, S.D. Temperature or transport? Range limits in marine species mediated solely by flow. Am. Nat. 2000, 155, 769–789. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.J.; Fulton, C.J.; McC Hogg, A.; Joyce, K.E.; Radford, B.T.M.; Fraser, C.I. Climate-driven changes to ocean circulation and their inferred impacts on marine dispersal patterns. Glob. Ecol. Biogeogr. 2016, 25, 923–939. [Google Scholar] [CrossRef]
- Raitsos, D.E.; Beaugrand, G.; Georgopoulos, D.; Zenetos, A.; Pancucci-Papadopoulou, A.M.; Theocharis, A.; Papathanassiou, E. Global climate change amplifies the entry of tropical species into the Eastern Mediterranean Sea. Limnol. Oceanogr. 2010, 55, 1478–1484. [Google Scholar] [CrossRef]
- Azzola, A.; Furfaro, G.; Trainito, E.; Doneddu, M.; Montefalcone, M. Seawater warming favours the northward range expansion of Lessepsian species in the Mediterranean Sea: The cephalaspidean Lamprohaminoea ovalis. J. Mar. Biol. Assoc. UK 2022, 102, 167–173. [Google Scholar] [CrossRef]
- Bennett, S.; Santana-Garcon, J.; Marbà, N.; Jorda, G.; Anton, A.; Apostolaki, E.T.; Cebrian, J.; Geraldi, N.R.; Krause-Jensen, D.; Lovelock, C.E.; et al. Climate-driven impacts of exotic species on marine ecosystems. Glob. Ecol. Biogeogr. 2021, 30, 1043–1055. [Google Scholar] [CrossRef]
- Guidetti, P.; Bianchi, C.N.; La Mesa, G.; Modena, M.; Morri, C.; Sara, G.; Vacchi, M. Abundance and size structure of Thalassoma pavo (Pisces: Labridae) in the western Mediterranean Sea: Variability at different spatial scales. J. Mar. Biol. Assoc. UK 2002, 82, 495–500. [Google Scholar] [CrossRef]
- Vacchi, M.; Sara, G.; Morri, C.; Modena, M.; La Mesa, G.; Guidetti, P.; Bianchi, C.N. Dynamics of marine populations and climate change: Lessons from a Mediterranean fish. Porcup. Mar. Nat. Hist. Soc. Newsl. 1999, 3, 13–17. [Google Scholar]
- Sara, G.; Bianchi, C.N.; Morri, C. Mating behaviour of the newly-established ornate wrasse Thalassoma pavo (Osteichthyes: Labridae) in the Ligurian Sea (north-western Mediterranean). J. Mar. Biol. Assoc. UK 2005, 85, 191–196. [Google Scholar] [CrossRef]
- Merotto, L.; Pesaro, S. Pesci Foresti: Nuovi Inquilini di un Mare Sempre Più Caldo; Tuss: Genoa, Italy, 2022; pp. 1–208. [Google Scholar]
- Tortonese, E. Il «Sarago faraone» del Mediterraneo: Diplodus cervinus (Lowe) (Pisces, Sparidae). Doriana 1965, 4, 155. [Google Scholar]
- Tortonese, E. I Pesci e i Cetacei del Mare Ligure; Mario Bozzi: Genoa, Italy, 1965; pp. 1–216. [Google Scholar]
- Sbragaglia, V.; Espasandín, L.; Jarić, I.; Vardi, R.; Ramírez, F.; Coll, M. Tracking ongoing transboundary marine distributional range shifts in the digital era. Mar. Ecol. Progr. Ser. 2024, 78, 103–114. [Google Scholar] [CrossRef]
- Tassara, P. (Lega Navale di Quinto, Genoa, Italy). Personal communication, 2023.
- Wesselmann, M.; Anton, A.; Duarte, C.M.; Hendriks, I.E.; Agusti, S.; Savva, I.; Apostolaki, E.T.; Marbà, N. Tropical seagrass Halophila stipulacea shifts thermal tolerance during Mediterranean invasion. Proc. R. Soc. B Biol. Sci. 2020, 287, 20193001. [Google Scholar] [CrossRef]
- Wesselmann, M.; Chefaoui, R.M.; Marbà, N.; Serrao, E.A.; Duarte, C.M. Warming threatens to propel the expansion of the exotic seagrass Halophila stipulacea. Front. Mar. Sci. 2021, 8, 759676. [Google Scholar] [CrossRef]
- García-Escudero, C.A.; Tsigenopoulos, C.S.; Manousaki, T.; Tsakogiannis, A.; Marbà, N.; Vizzini, S.; Duarte, C.M.; Apostolaki, E.T. Population genomics unveils the century-old invasion of the seagrass Halophila stipulacea in the Mediterranean Sea. Mar. Biol. 2024, 171, 40. [Google Scholar] [CrossRef]
- Forti, A. La propagazione dell’Halophila stipulacea (Forsk) Asch. anche nel Mediterraneo. Nuovo G. Bot. Ital. 1927, 34, 714–716. [Google Scholar]
- Gambi, M.C.; Barbieri, F.; Bianchi, C.N. New record of the alien seagrass Halophila stipulacea (Hydrocharitaceae) in the western Mediterranean: A further clue to changing Mediterranean Sea biogeography. Biodivers. Rec. 2008, 2, e84. [Google Scholar] [CrossRef]
- Pica, D.; Galanti, L.; Pola, L. First records of the seagrass Halophila stipulacea in Sardinia (Tyrrhenian Sea, Italy). Mediterr. Mar. Sci. 2021, 22, 183–184. [Google Scholar]
- Cnudde, S.; Boudouresque, C.F.; Marengo, M.; Pergent, G.; Thibaut, T. First record of the Red Sea seagrass Halophila stipulacea in Corsica. Sci. Rep. Port-Cros Nat. Park 2023, 37, 503–507. [Google Scholar]
- Thibaut, T.; Blanfuné, A.; Boudouresque, C.F.; Holon, F.; Agel, N.; Descamps, P.; Deter, J.; Pavy, T.; Delaruelle, G.; Verlaque, M. Distribution of the seagrass Halophila stipulacea: A big jump to the northwestern Mediterranean Sea. Aquat. Bot. 2022, 176, 103465. [Google Scholar] [CrossRef]
- Guerra-García, J.M.; Revanales, T.; Saenz-Arias, P.; Navarro-Barranco, C.; Ruiz-Velasco, S.; Pastor-Montero, M.; Sempere-Valverde, J.; Chebaane, S.; Vélez-Ruiz, A.; Martínez-Laiz, G.; et al. Quick spreading of the exotic amphipod Laticorophium baconi (Shoemaker, 1934): Another small stowaway overlooked? Mediterr. Mar. Sci. 2023, 24, 644–665. [Google Scholar] [CrossRef]
- Tortonese, E. I pesci a distribuzione circumtropicale presenti nel Mediterraneo. Mem. Biol. Mar. Oceanogr. 1982, 12, 191–203. [Google Scholar]
- Champion, C.; Brodie, S.; Coleman, M.A. Climate-driven range shifts are rapid yet variable among recreationally important coastal-pelagic fishes. Front. Mar. Sci. 2021, 8, 622299. [Google Scholar] [CrossRef]
- Matsumoto, W.M.; Skillman, R.A.; Dizon, A.E. Synopsis of Biological Data on Skipjack Tuna, Katsuwonus pelamis; FAO Fisheries Synopsis; FAO: Rome, Italy, 1984; Volume 136, pp. 1–92. [Google Scholar]
- Nota, A.; Ignoto, S.; Bertolino, S.; Tiralongo, F. First record of Caranx crysos (Mitchill, 1815) in the Ligurian Sea (northwestern Mediterranean Sea) suggests northward expansion of the species. Ann. Ser. Hist. Nat. 2023, 33, 55–60. [Google Scholar]
- Di Blasi, D.; ·Bava, S.; Desiderà, E.; Merotto, L.; Poli, F.; Guidetti, P. The northernmost records of Caranx crysos (Osteichthyes: Carangidae) in the NW Mediterranean Sea. Thalassas 2024, 40, in press. [Google Scholar] [CrossRef]
- Pavičić, M.; Šiljić, J.; Duganđžić, P.; Skaramuca, B. New record of blue runner, Caranx crysos (Mitchill, 1815), in the Adriatic sea. Ribar. Croat. J. Fish. 2014, 72, 125–127. [Google Scholar]
- Lo Brutto, S. The case of a rudderfish highlights the role of natural history museums as sentinels of bio-invasions. Zootaxa 2017, 4254, 382–386. [Google Scholar] [CrossRef]
- Orsi Relini, L. Non native marine fish in Italian waters. In Fish Invasion of the Mediterranean Sea: Changes and Renewal; Golani, D., Golani-Appelbaum, B., Eds.; Pensoft: Sofia, Bulgaria, 2010; pp. 265–290. [Google Scholar]
- Cattaneo Vietti, R.; Albertelli, G.; Aliani, S.; Bava, S.; Bavestrello, G.; Benedetti Cecchi, L.; Bianchi, C.N.; Bozzo, E.; Capello, M.; Castellano, M.; et al. The Ligurian Sea: Present status, problems and perspectives. Chem. Ecol. 2010, 26 (Suppl. S1), 319–340. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Verlaque, M. An overview of species introduction and invasion processes in marine and coastal lagoon habitats. Cah. Biol. Mar. 2012, 53, 309–317. [Google Scholar]
- Mancini, I.; Bianchi, C.N.; Morri, C.; Azzola, A.; Oprandi, A.; Robello, C.; Montefalcone, M. A marine invasion story: Caulerpa cylindracea (Chlorophyta, Ulvophyceae) in the marine protected area of Portofino (Ligurian Sea). Biol. Mar. Medit. 2024, in press.
- Vergés, A.; Steinberg, P.D.; Hay, M.E.; Poore, A.G.B.; Campbell, A.H.; Ballesteros, E.; Heck, K.L., Jr.; Booth, D.J.; Coleman, M.A.; Feary, D.A.; et al. The tropicalization of temperate marine ecosystems: Climate-mediated changes in herbivory and community phase shifts. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140846. [Google Scholar] [CrossRef] [PubMed]
- Santana-Garcon, J.; Bennett, S.; Marbà, N.; Vergés, A.; Arthur, R.; Alcoverro, T. Tropicalization shifts herbivore pressure from seagrass to rocky reef communities. Proc. R. Soc. B Biol. Sci. 2023, 290, 20221744. [Google Scholar] [CrossRef]
- Dimitriadis, C.; Marampouti, C.; Calò, A.; Di Franco, A.; Giakoumi, S.; Di Franco, E.; Di Lorenzo, M.; Gerovasileiou, V.; Guidetti, P.; Pey, A.; et al. Evaluating the long term effectiveness of a Mediterranean marine protected area to tackle the effects of invasive and range expanding herbivorous fish on algal resources of rocky reefs through a multifaceted approach. Mar. Environ. Res. 2024, 193, 106293. [Google Scholar] [CrossRef] [PubMed]
- Pajuelo, J.G.; Lorenzo, J.M.; Domínguez, R.; Ramos, A.; Gregoire, M. On the population ecology of the zebra seabream Diplodus cervinus cervinus (Lowe 1838) from the coasts of the Canarian archipelago, North West Africa. Environ. Biol. Fishes 2003, 67, 407–416. [Google Scholar] [CrossRef]
- Heemstra, P.C.; Randall, J.E. FAO Species Catalogue, Vol. 16. Groupers of the World (Family Serranidae, Subfamily Epinephelinae). An Annotated and Illustrated Catalogue of the Grouper, Rockcod, Hind, Coral Grouper and Lyretail Species Known to Date; FAO Fisheries Synopsis; FAO: Rome, Italy, 1993; Volume 125, pp. 1–382. [Google Scholar]
- Barrett, S.; Stavins, R. Increasing participation and compliance in international climate change agreements. Int. Environ. Agreem. Politics Law Econ. 2003, 3, 349–376. [Google Scholar] [CrossRef]
- Nordhaus, W. Dynamic climate clubs: On the effectiveness of incentives in global climate agreements. Proc. Natl. Acad. Sci. USA 2021, 118, e2109988118. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, R.; Hovi, J.; Sprinz, D.F.; Sælen, H.; Underdal, A. Institutional and environmental effectiveness: Will the Paris Agreement work? Wiley Interdiscip. Rev. Clim. 2019, 10, e583. [Google Scholar] [CrossRef]
- Burke, A.; Fishel, S. A coal elimination treaty 2030: Fast tracking climate change mitigation, global health and security. Earth Syst. Gov. 2020, 3, 100046. [Google Scholar] [CrossRef]
- Dechezleprêtre, A.; Fabre, A.; Kruse, T.; Planterose, B.; Chico, A.S.; Stantcheva, S. Fighting Climate Change: International Attitudes toward Climate Policies; Working Paper No. 30265; National Bureau of Economic Research: Cambridge, MA, USA, 2023; pp. 1–50. [Google Scholar]
- Franta, B. Weaponizing economics: Big Oil, economic consultants, and climate policy delay. Environ. Politics 2022, 31, 555–575. [Google Scholar] [CrossRef]
- Wei, Y.M.; Han, R.; Wang, C.; Yu, B.; Liang, Q.M.; Yuan, X.C.; Chang, J.; Zhao, Q.; Liao, H.; Tang, B.; et al. Self-preservation strategy for approaching global warming targets in the post-Paris Agreement era. Nat. Commun. 2020, 11, 1624. [Google Scholar] [CrossRef] [PubMed]
- Fekete, H.; Kuramochi, T.; Roelfsema, M.; den Elzen, M.; Forsell, N.; Höhne, N.; Luna, L.; Hans, F.; Sterl, S.; Olivier, J.; et al. A review of successful climate change mitigation policies in major emitting economies and the potential of global replication. Renew. Sustain. Energy Rev. 2021, 137, 110602. [Google Scholar] [CrossRef]
- Al Khourdajie, A.; Finus, M. Measures to enhance the effectiveness of international climate agreements: The case of border carbon adjustments. Eur. Econ. Rev. 2020, 124, 103405. [Google Scholar] [CrossRef]
- Nunes, L.J. The rising threat of atmospheric CO2: A review on the causes, impacts, and mitigation strategies. Environments 2023, 10, 66. [Google Scholar] [CrossRef]
- Core Writing Team; Lee, H.; Romero, J. (Eds.) Summary for Policymakers. In Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2023; pp. 1–34. [Google Scholar]
- Pettorelli, N.; Graham, N.A.; Seddon, N.; da Cunha Bustamante, M.M.; Lowton, M.J.; Sutherland, W.J.; Koldewey, H.J.; Prentice, H.C.; Barlow, J. Time to integrate global climate change and biodiversity science-policy agendas. J. Appl. Ecol. 2021, 58, 2384–2393. [Google Scholar] [CrossRef]
- Valente, S.; Moro, S.; Di Lorenzo, M.; Milisenda, G.; Maiorano, L.; Colloca, F. Mediterranean fish communities are struggling to adapt to global warming. Evidence from the western coast of Italy. Mar. Environ. Res. 2023, 191, 106176. [Google Scholar] [CrossRef]
- Bay, R.A.; Rose, N.H.; Logan, C.A.; Palumbi, S.R. Genomic models predict successful coral adaptation if future ocean warming rates are reduced. Sci. Adv. 2017, 3, e1701413. [Google Scholar] [CrossRef]
- Ross, P.M.; Scanes, E.; Byrne, M.; Ainsworth, T.D.; Donelson, J.M.; Fool, S.A.; Hutchings, P.; Thiyagarajan, V.; Parker, L.M. Surviving the Anthropocene: The resilience of marine animals to climate change. Oceanogr. Mar. Biol. Annu. Rev. 2023, 61, 35–80. [Google Scholar]
- Hall-Spencer, J.M. Changing seas: Adaptation of the fisheries in the Mediterranean basin. Focus 2024, 20, 1–3. [Google Scholar]
- Brown, C.J.; Sauders, M.I.; Possingham, H.P.; Richardson, A.J. Managing for interactions between local and global stressors of ecosystems. PLoS ONE 2013, 8, e65765. [Google Scholar] [CrossRef] [PubMed]
- Geraldi, N.R.; Anton, A.; Santana-Garcon, J.; Bennett, S.; Marbà, N.; Lovelock, C.E.; Apostolaki, E.T.; Cebrian, J.; Krause-Jensen, D.; Martinetto, P.; et al. Ecological effects of non-native species in marine ecosystems relate to co-occurring anthropogenic pressures. Glob. Chang. Biol. 2020, 26, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Fox, H.E.; Mascia, M.B.; Basurto, X.; Costa, A.; Glew, L.; Heinemann, D.; Karrer, L.B.; Lester, S.E.; Lombana, A.V.; Pomeroy, R.S.; et al. Reexamining the science of marine protected areas: Linking knowledge to action. Conserv. Lett. 2012, 5, 1–10. [Google Scholar] [CrossRef]
- Agardy, T. Justified ambivalence about MPA effectiveness. ICES J. Mar. Sci. 2018, 75, 1183–1185. [Google Scholar] [CrossRef]
- Guidetti, P.; Baiata, P.; Ballesteros, E.; Di Franco, A.; Hereu, B.; Macpherson, E.; Micheli, F.; Pais, A.; Panzalis, P.; Rosenberg, A.A.; et al. Large-scale assessment of Mediterranean Marine Protected Areas effects on fish assemblages. PLoS ONE 2014, 9, e91841. [Google Scholar] [CrossRef] [PubMed]
- Mora, C.; Sale, P.F. Ongoing global biodiversity loss and the need to move beyond protected areas: A review of the technical and practical shortcomings of protected areas on land and sea. Mar. Ecol. Progr. Ser. 2011, 434, 251–266. [Google Scholar] [CrossRef]
- Guidetti, P.; Sala, E. Community-wide effects of marine reserves in the Mediterranean Sea. Mar. Ecol. Progr. Ser. 2007, 335, 43–56. [Google Scholar] [CrossRef]
- Selig, E.R.; Bruno, J.F. A global analysis of the effectiveness of marine protected areas in preventing coral loss. PLoS ONE 2010, 5, e9278. [Google Scholar] [CrossRef]
- Olds, A.D.; Pitt, K.A.; Maxwell, P.S.; Babcock, R.C.; Rissis, D.; Connolly, R.M. Marine reserves help coastal ecosystems cope with extreme weather. Glob. Chang. Biol. 2014, 20, 3050–3058. [Google Scholar] [CrossRef]
- Sanabria-Fernandez, J.A.; Alday, J.G. Marine protection enhances the resilience of biological communities on temperate rocky reefs. Acquat. Conserv. Mar. Freshw. Ecosyst. 2024, 34, e4101. [Google Scholar] [CrossRef]
- Soto, C.G. The potential impacts of global climate change on marine protected areas. Rev. Fish Biol. Fish. 2002, 11, 181–195. [Google Scholar] [CrossRef]
- Bruno, J.F.; Bates, A.E.; Cacciapaglia, C.; Pike, E.P.; Amstrup, S.C.; Van Hooidonk, R.; Henson, S.A.; Aronson, R.B. Climate change threatens the world’s marine protected areas. Nat. Clim. Chang. 2018, 8, 499–503. [Google Scholar] [CrossRef]
- Graham, N.A.J.; McClanahan, T.R.; MacNeil, M.A.; Wilson, S.K.; Polunin, N.V.C.; Jennings, S.; Chabanet, P.; Clark, S.; Spalding, M.D.; Letourneur, Y.; et al. Climate warming, marine protected areas and the ocean-scale integrity of coral reef ecosystems. PLoS ONE 2008, 3, e3039. [Google Scholar] [CrossRef]
- Selig, E.R.; Casey, K.S.; Bruno, J.F. Temperature-driven coral decline: The role of marine protected areas. Glob. Chang. Biol. 2012, 18, 1561–1570. [Google Scholar] [CrossRef]
- Montero-Serra, I.; Garrabou, J.; Doak, D.F.; Ledoux, J.B.; Linares, C. Marine protected areas enhance structural complexity but do not buffer the consequences of ocean warming for an overexploited precious coral. J. Appl. Ecol. 2019, 56, 1063–1074. [Google Scholar] [CrossRef]
- Bates, A.E.; Cooke, R.S.; Duncan, M.I.; Edgar, G.J.; Bruno, J.F.; Benedetti-Cecchi, L.; Côté, I.M.; Lefcheck, J.S.; Costello, M.J.; Barrett, N.; et al. Climate resilience in marine protected areas and the ‘Protection Paradox’. Biol. Conserv. 2019, 236, 305–314. [Google Scholar] [CrossRef]
- Johnson, J.V.; Dick, J.T.; Pincheira-Donoso, D. Marine protected areas do not buffer corals from bleaching under global warming. BMC Ecol. Evol. 2022, 22, 58. [Google Scholar]
- O’Regan, S.M.; Archer, S.K.; Friesen, S.K.; Hunter, K.L. A global assessment of climate change adaptation in marine protected area management plans. Front. Mar. Sci. 2021, 8, 711085. [Google Scholar] [CrossRef]
- Craig, R.K. Marine biodiversity, climate change, and governance of the oceans. Diversity 2012, 4, 224–238. [Google Scholar] [CrossRef]
- Olenin, S.; Elliott, M.; Minchin, D.; Katsanevakis, S. Marine ecosystem health and biological pollution: Reconsidering the paradigm. Mar. Pollut. Bull. 2024, 200, 116054. [Google Scholar] [CrossRef] [PubMed]
- Scianna, C.; Niccolini, F.; Bianchi, C.N.; Guidetti, P. Applying organization science to assess the management performance of Marine Protected Areas: An exploratory study. J. Environ. Manag. 2018, 223, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Scianna, C.; Niccolini, F.; Giakoumi, S.; Di Franco, A.; Gaines, S.D.; Bianchi, C.N.; Scaccia, L.; Bava, S.; Cappanera, V.; Charbonnel, E.; et al. Organization Science improves management effectiveness of Marine Protected Areas. J. Environ. Manag. 2019, 240, 285–292. [Google Scholar] [CrossRef] [PubMed]
- McLeod, E.; Salm, R.; Green, A.; Almany, J. Designing Marine Protected Area networks to address the impacts of climate change. Front. Ecol. Environ. 1999, 7, 362–370. [Google Scholar] [CrossRef]
- Álvarez-Romero, J.G.; Munguía-Vega, A.; Beger, M.; Mancha-Cisneros, M.D.M.; Suárez-Castillo, A.N.; Gurney, G.G.; Pressey, R.L.; Gerber, L.R.; Morzaria-Luna, H.N.; Reyes-Bonilla, H.; et al. Designing connected marine reserves in the face of global warming. Glob. Chang. Biol. 2018, 24, e671–e691. [Google Scholar] [CrossRef] [PubMed]
- Rossi, V.; Pipitone, C.; Yates, K.L.; Badalamenti, F.; D’Anna, G.; Pita, C.; Alves, F.L.; Argente-García, J.E.; Basta, J.; Claudet, J.; et al. Poor online information on European marine protected areas impairs public participation under the Aarhus Convention. Mar. Policy 2024, 161, 106012. [Google Scholar] [CrossRef]
- Popova, E.; Yool, A.; Byfield, V.; Cochrane, K.; Coward, A.C.; Salim, S.S.; Gasalla, M.A.; Henson, S.A.; Hobday, A.J.; Pecl, G.T.; et al. From global to regional and back again: Common climate stressors of marine ecosystems relevant for adaptation across five ocean warming hotspots. Glob. Chang. Biol. 2016, 22, 2038–2053. [Google Scholar] [CrossRef] [PubMed]
- Tortonese, E. Natura e naturalisti in Liguria. Atti Accad. Ligure Sci. Lett. 1971, 28, 1–16. [Google Scholar]
- Semeria, V.; Tucci, S. Aspetti Oceanografici dell’Inquinamento Marino nell’Alto Tirreno (Progetto R.I.M.A.T.); F.C. 1056; Istituto Idrografico della Marina: Genova, Italy, 1974; pp. 1–53. [Google Scholar]
- Bianchi, C.N.; Morri, C.; Peirano, A.; Romeo, G.; Tunesi, L. Bibliografia Ecotipologica sul Mar Ligure; Collana di Studi Ambientali; ENEA: Rome, Italy, 1987; pp. 1–90. [Google Scholar]
- Bavestrello, G.; Betti, F.; Bianchi, C.N.; Bo, M.; Cappanera, V.; Corradi, N.; Montefalcone, M.; Morri, C.; Relini, G. Il promontorio di Portofino: 150 anni di storia di biologia marina. Notiz. Soc. Ital. Biol. Mar. 2022, 81, 53–114. [Google Scholar]
- Albertelli, G.; Cattaneo, M.; Drago, N. Macrobenthos du plateau continental ligure et de l’Archipel Toscan: Aperçus zoogeographiques. Rapp. Comm. Int. Mer Médit. 1981, 27, 127–128. [Google Scholar]
- Tunesi, L.; Peirano, A. Lineamenti biogeografici del Mar Ligure centro-orientale: Invertebrati megabentici dei fondi mobili. Oebalia 1990, 16, 349–356. [Google Scholar]
- Roberts, C.M.; Hawkins, J.P. Extinction risk in the sea. Trends Ecol. Evol. 1999, 14, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Estaque, T.; Richaume, J.; Bianchimani, O.; Schull, Q.; Mérigot, B.; Bensoussan, N.; Bonhomme, P.; Vouriot, P.; Sartoretto, S.; Monfort, T.; et al. Marine heatwaves on the rise: One of the strongest ever observed mass mortality event in temperate gorgonians. Glob. Chang. Biol. 2023, 29, 6159–6162. [Google Scholar] [CrossRef] [PubMed]
- Prioux, C.; Tignat-Perrier, R.; Gervais, O.; Estaque, T.; Schull, Q.; Reynaud, S.; Béraud, E.; Mérigot, B.; Beauvieux, A.; Marcus, M.I.; et al. Unveiling microbiome changes in Mediterranean octocorals during the 2022 marine heatwaves: Quantifying key bacterial symbionts and potential pathogens. Microbiome 2023, 11, 271. [Google Scholar] [CrossRef]
- Bo, M.; Tazioli, S.; Spanò, N.; Bavestrello, G. Antipathella subpinnata (Antipatharia, Myriopathidae) in Italian seas. Ital. J. Zool. 2008, 75, 185–195. [Google Scholar] [CrossRef]
- Enrichetti, F.; Bavestrello, G.; Cappanera, V.; Mariotti, M.; Massa, F.; Merotto, L.; Povero, P.; Rigo, I.; Toma, M.; Tunesi, L.; et al. High megabenthic complexity and vulnerability of a mesophotic rocky shoal support its inclusion in a Mediterranean MPA. Diversity 2023, 15, 933. [Google Scholar] [CrossRef]
- Grossi, F.; Lagasio, M.; Napoli, A.; Provenzale, A.; Tepsich, P. Phytoplankton spring bloom in the NW Mediterranean Sea under climate change. Sci. Total Environ. 2024, 914, 169884. [Google Scholar] [CrossRef]
- Azzola, A.; Bianchi, C.N.; Mangraviti, S.; Redoano, C.; Varenne, A.; Morri, C.; Oprandi, A.; Montefalcone, M. A novel driver of change for benthic communities: The mucilaginous event of summer 2018 at Portofino (Ligurian Sea). Biol. Mar. Medit. 2023, 27, 57–60. [Google Scholar]
- Azzola, A.; Picchio, V.; Asnaghi, V.; Bianchi, C.N.; Morri, C.; Oprandi, A.; Montefalcone, M. Troubles never come alone: Outcome of multiple pressures on a temperate rocky reef. Water 2023, 15, 825. [Google Scholar] [CrossRef]
- Danovaro, R. Climate change impacts on the Mediterranean Sea ecosystems. Atti Accad. Ligure Sci. Lett. 2023, 69, 69–75. [Google Scholar]
- Betti, F.; Venturini, S.; Merotto, L.; Cappanera, V.; Ferrando, S.; Aicardi, S.; Mandich, A.; Castellano, M.; Povero, P. Population trends of the fan mussel Pinna nobilis from Portofino MPA (Ligurian Sea, Western Mediterranean Sea) before and after a mass mortality event and a catastrophic storm. Eur. Zool. J. 2021, 88, 18–25. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Carella, F.; Çinar, M.E.; Čižmek, H.; Jimenez, C.; Kersting, D.K.; Moreno, D.; Rabaoui, L.; Vicente, N. The fan mussel Pinna nobilis on the brink of extinction in the Mediterranean. In Imperiled: The Encyclopedia of Conservation; Della Sala, D.A., Goldstein, M.I., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 2, pp. 700–709. [Google Scholar]
- Oprandi, A.; Aicardi, S.; Azzola, A.; Benelli, F.; Bianchi, C.N.; Chiantore, M.C.; Ferranti, M.P.; Mancini, I.; Molinari, A.; Morri, C.; et al. A tale of two sisters: The southerner Pinna rudis is getting north after the extinction of the congeneric P. nobilis (Mollusca: Bivalvia). Diversity 2024, 16, 120. [Google Scholar] [CrossRef]
- Ben Rais Lasram, F.; Mouillot, D. Increasing southern invasion enhances congruence between endemic and exotic Mediterranean fish fauna. Biol. Invasions 2009, 11, 697–711. [Google Scholar] [CrossRef]
- Chevaldonné, P.; Lejeusne, C. Regional warming induced species shift in NW Mediterranean marine caves. Ecol. Lett. 2003, 6, 371–379. [Google Scholar] [CrossRef]
- Noël, P. Les crustacés du Parc National de Port-Cros et de la région des îles d’Hyères (Méditerranée), France. État actuel des connaissances. Sci. Rep. Port-Cros Nat. Park 2003, 19, 135–306. [Google Scholar]
- Morri, C.; Montefalcone, M.; Gatti, G.; Vassallo, P.; Paoli, C.; Bianchi, C.N. An alien invader is the cause of homogenization in the recipient ecosystem: A simulation-like approach. Diversity 2019, 11, 146. [Google Scholar] [CrossRef]
- Anton, A.; Geraldi, N.R.; Lovelock, C.E.; Apostolaki, E.T.; Bennett, S.; Cebrian, J.; Krause-Jensen, D.; Marbà, N.; Martinetto, P.; Pandolfi, J.M.; et al. Global ecological impacts of marine exotic species. Nat. Ecol. Evol. 2019, 3, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Pessarrodona, A.; Foggo, A.; Smale, D.A. Can ecosystem functioning be maintained despite climate-driven shifts in species composition? Insights from novel marine forests. J. Ecol. 2019, 107, 91–104. [Google Scholar] [CrossRef]
- Wesselmann, M.; Apostolaki, E.T.; Anton, A. Species range shifts, biological invasions and ocean warming. Mar. Ecol. Progr. Ser. 2024, 728, 81–83. [Google Scholar] [CrossRef]
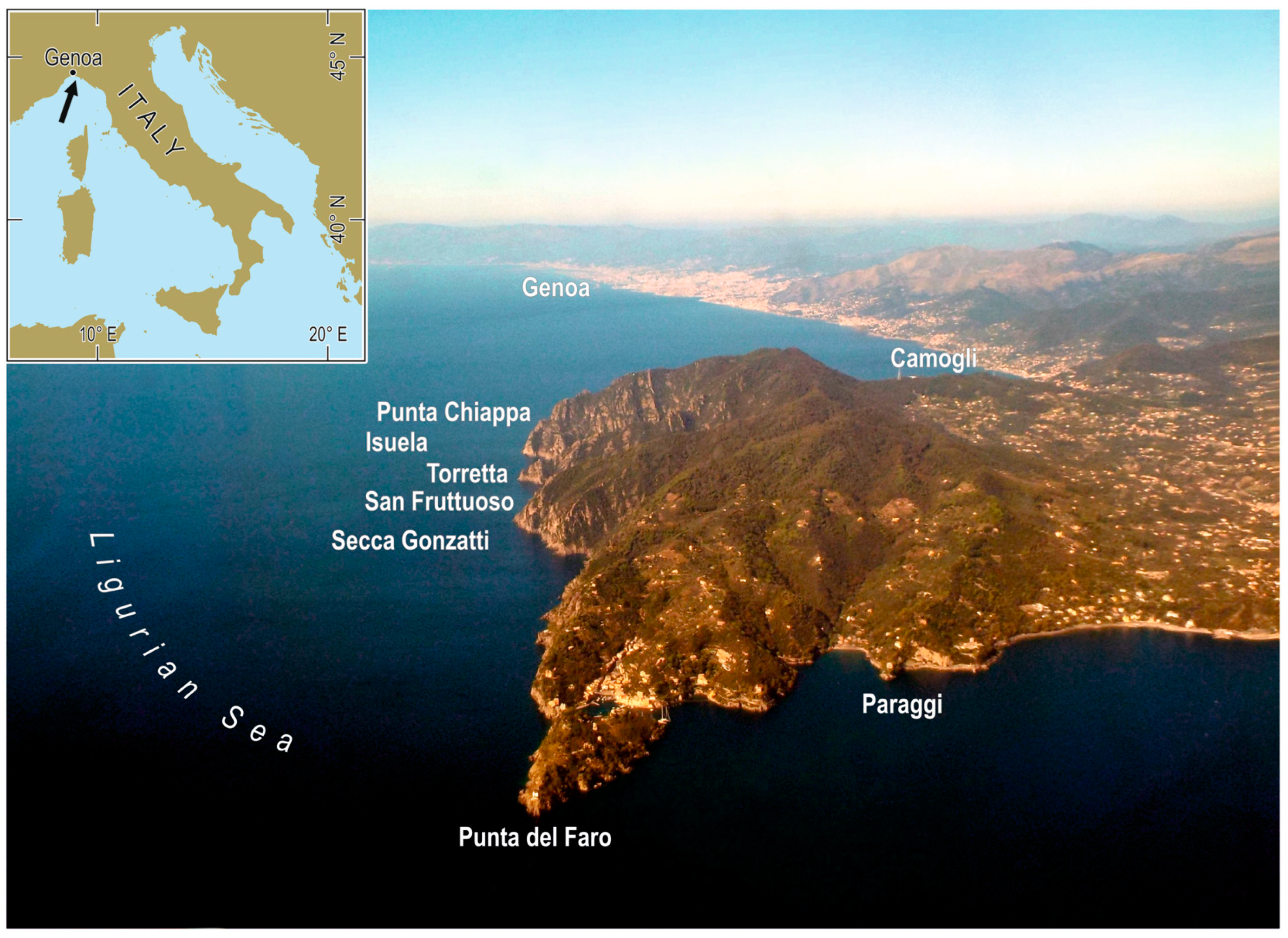


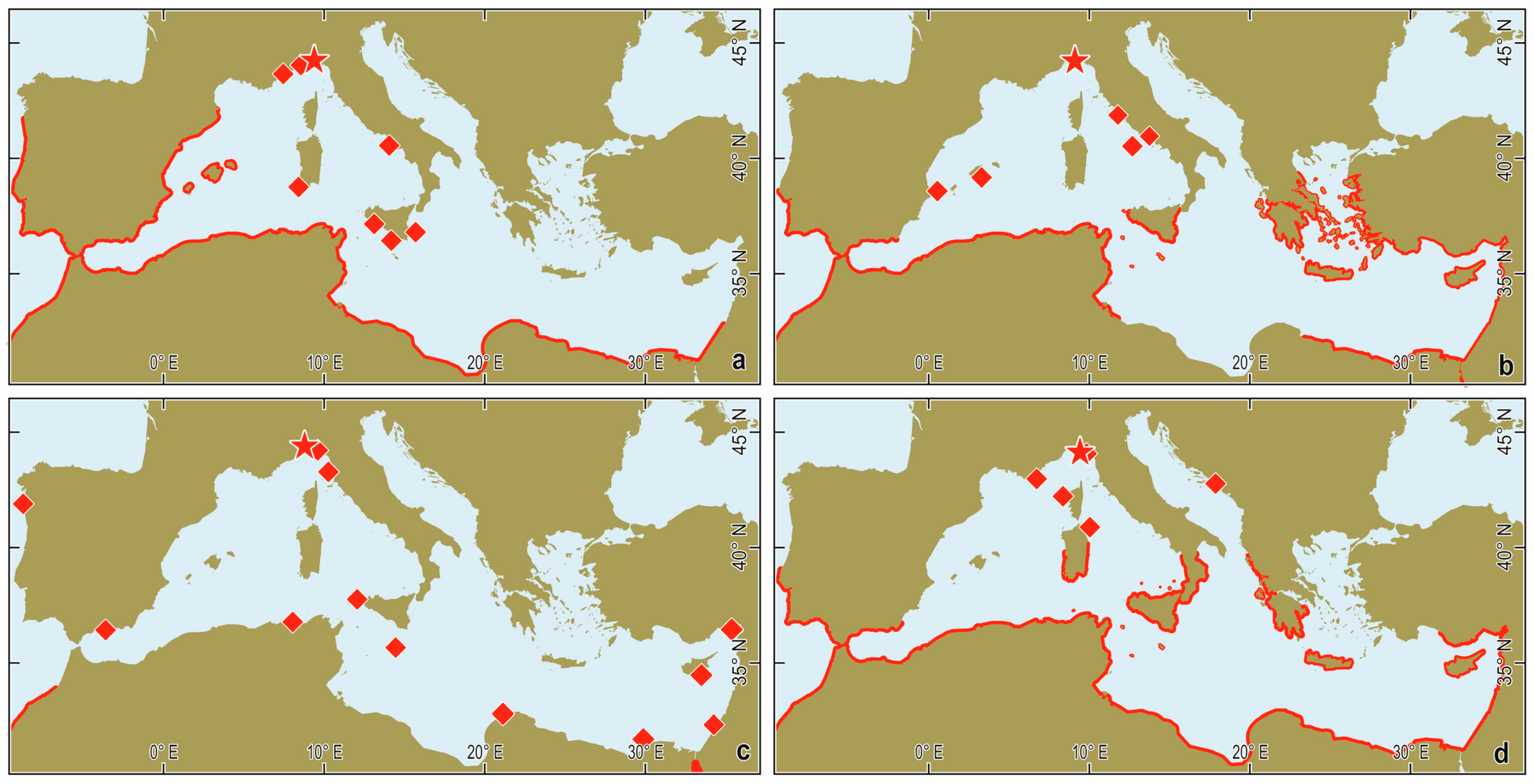

| Date | Site | Depth (m) | Number of Individuals |
|---|---|---|---|
| 3 August 2016 | Secca Gonzatti | 15 | 1 |
| 29 July 2020 | Secca Gonzatti | 8 | 1 |
| 6 June 2020 | Isuela | 15 | 1 |
| 9 September 2020 | Isuela | 20 | 1 |
| 12 June 2021 | Secca Gonzatti | 5 | 1 |
| 15 June 2021 | Punta del Faro | 10 | 1 |
| 19 June 2021 | Secca Gonzatti | 5 | 5 |
| 24 June 2021 | Isuela | 15 | 1 |
| 11 July 2021 | Secca Gonzatti | 5 | 1 |
| 21 July 2021 | Secca Gonzatti | 10 | 2 |
| 17 June 2023 | Secca Gonzatti | 7 | 1 |
| 30 July 2023 | Isuela | 15 | 1 |
| 17 September 2023 | Punta del Faro | 5 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azzola, A.; Bianchi, C.N.; Merotto, L.; Nota, A.; Tiralongo, F.; Morri, C.; Oprandi, A. The Changing Biogeography of the Ligurian Sea: Seawater Warming and Further Records of Southern Species. Diversity 2024, 16, 159. https://doi.org/10.3390/d16030159
Azzola A, Bianchi CN, Merotto L, Nota A, Tiralongo F, Morri C, Oprandi A. The Changing Biogeography of the Ligurian Sea: Seawater Warming and Further Records of Southern Species. Diversity. 2024; 16(3):159. https://doi.org/10.3390/d16030159
Chicago/Turabian StyleAzzola, Annalisa, Carlo Nike Bianchi, Lorenzo Merotto, Alessandro Nota, Francesco Tiralongo, Carla Morri, and Alice Oprandi. 2024. "The Changing Biogeography of the Ligurian Sea: Seawater Warming and Further Records of Southern Species" Diversity 16, no. 3: 159. https://doi.org/10.3390/d16030159
APA StyleAzzola, A., Bianchi, C. N., Merotto, L., Nota, A., Tiralongo, F., Morri, C., & Oprandi, A. (2024). The Changing Biogeography of the Ligurian Sea: Seawater Warming and Further Records of Southern Species. Diversity, 16(3), 159. https://doi.org/10.3390/d16030159








