Diversity and Composition of Belowground Fungal Communities Associated with Picea abies L. (H.) Karst. and Larix sp. Mill.: A Comparative Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Sampling
2.2. DNA Procedure
2.3. Bioinformatics
2.4. Statistical Analysis
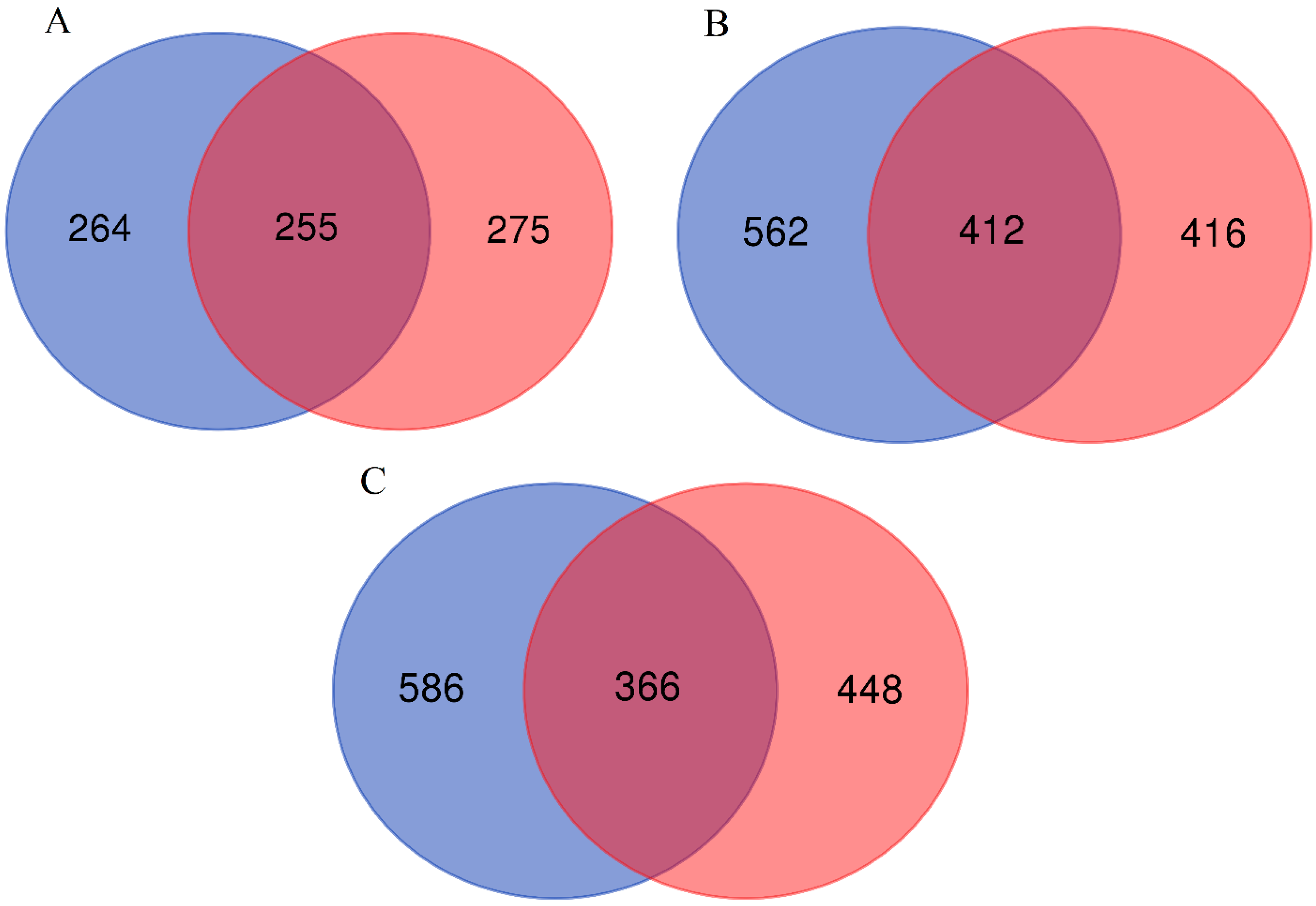
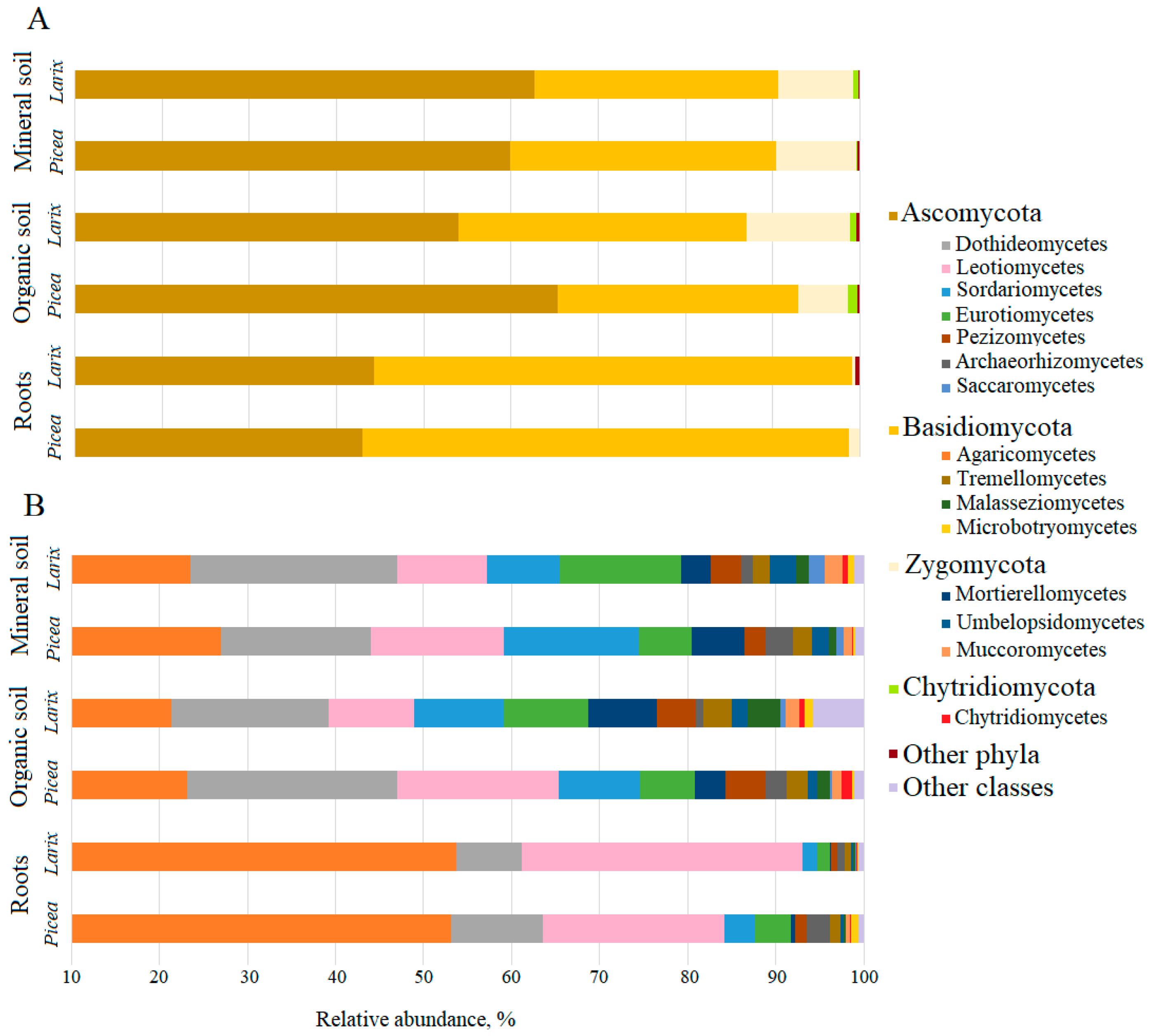
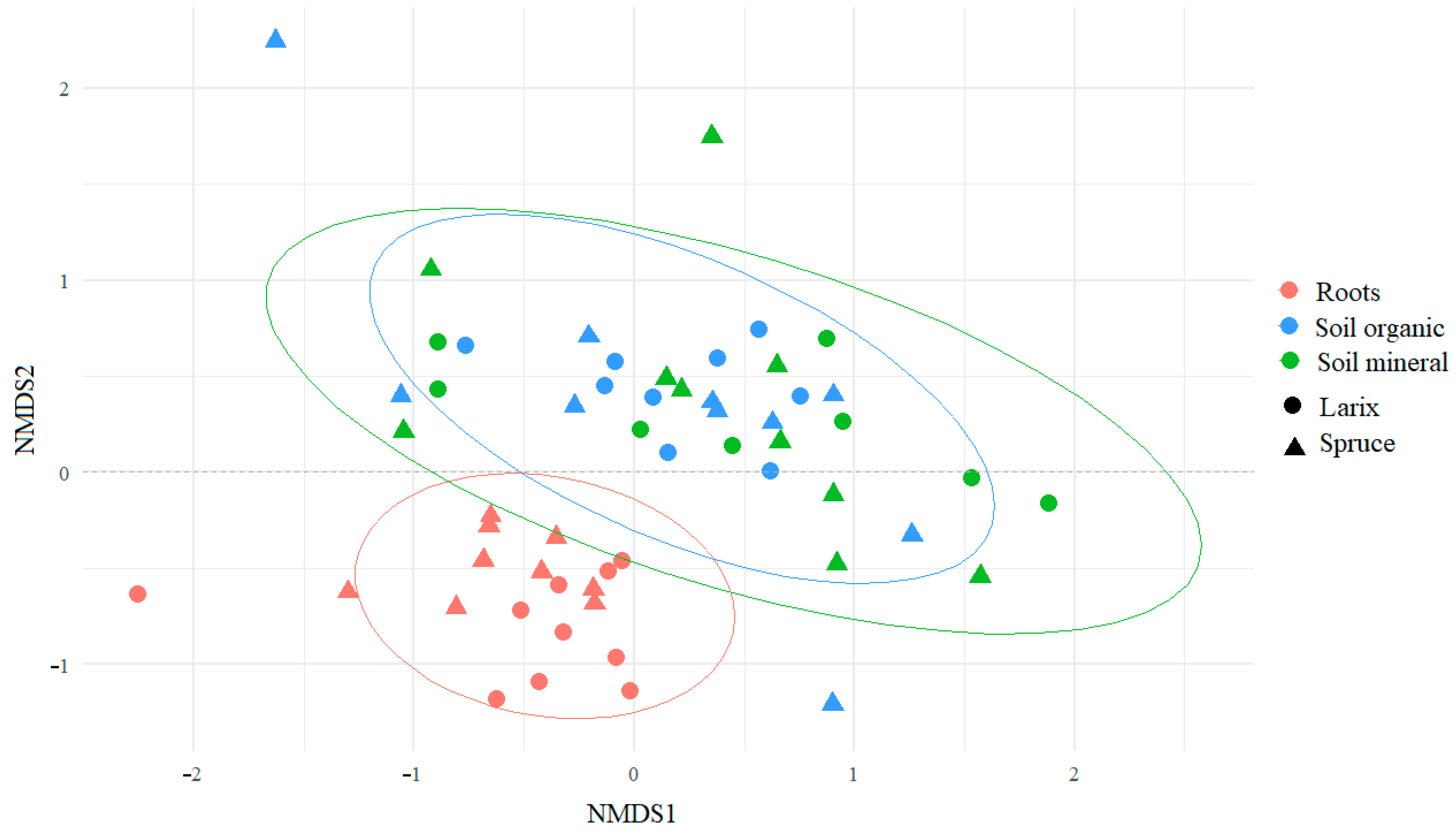
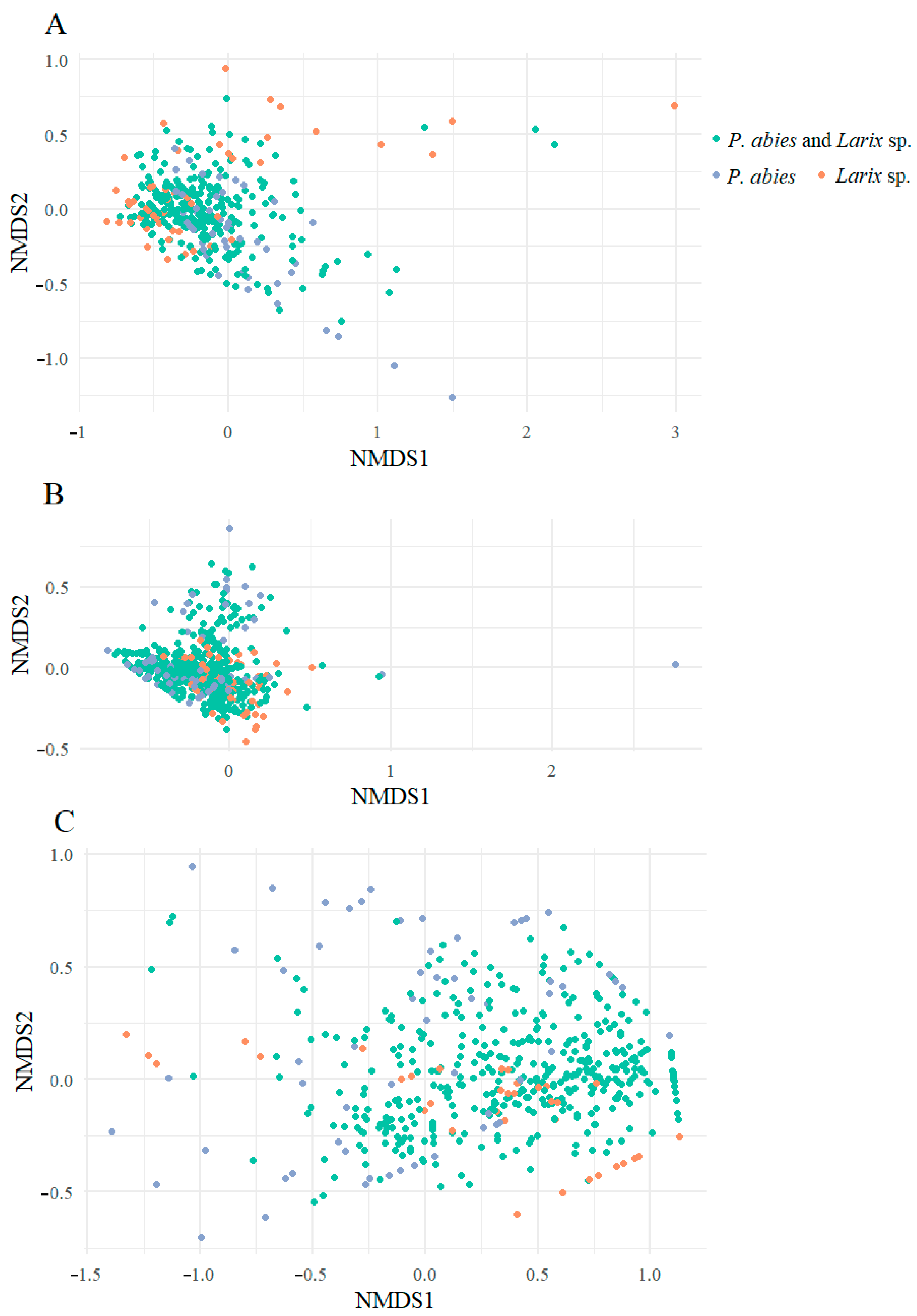
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Terhonen, E.; Blumenstein, K.; Kovalchuk, A.; Asiegbu, F.O. Forest tree microbiomes and associated fungal endophytes: Functional roles and impact on forest health. Forests 2019, 10, 42. [Google Scholar] [CrossRef]
- Turner, T.R.; James, E.K.; Poole, P.S. The plant microbiome. Genome Biol. 2013, 14, 209. [Google Scholar] [CrossRef]
- Treseder, K.K.; Lennon, J.T. Fungal traits that drive ecosystem dynamics on land. Microbiol. Mol. Biol. Rev. 2015, 79, 243–262. [Google Scholar] [CrossRef]
- Brzostek, E.R.; Greco, A.; Drake, J.E.; Finzi, A.C. Root carbon inputs to the rhizosphere stimulate extracellular enzyme activity and increase nitrogen availability in temperate forest soils. Biogeochemistry 2013, 115, 65–76. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, M.; Peng, M.; Sui, X.; Li, W.; Sun, G. Variations in soil functional fungal community structure associated with pure and mixed plantations in typical temperate forests of China. Front. Microbiol. 2019, 10, 1636. [Google Scholar] [CrossRef]
- Tataranni, G.; Dichio, B.; Xyloyannis, C. Soil fungi-plant interaction. In Advances in Selected Plant Physiology Aspects; Montanaro, G., Dichio, B.C., Eds.; InTech: Shah Alam, Malaysia, 2012. [Google Scholar]
- Cline, L.C.; Donald, R.Z. Soil microbial communities are shaped by plant-driven changes in resource availability during secondary succession. Ecology 2015, 96, 3374–3385. [Google Scholar] [CrossRef]
- Rosling, A. Responses of Ectomycorrhizal Fungi to Mineral Substrates. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2003. [Google Scholar]
- Baldrian, P.; Kolařík, M.; Stursová, M.; Kopecký, J.; Valášková, V.; Větrovský, T.; Zifčáková, L.; Snajdr, J.; Rídl, J.; Vlček, C.; et al. Active and total microbial communities in forest soil are largely different and highly stratified during decomposition. ISME J. 2012, 6, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Fitter, A.H.; Garbaye, J. Interactions between mycorrhizal fungi and other soil organisms. Plant Soil 1994, 159, 123–132. [Google Scholar] [CrossRef]
- Marčiulynas, A.; Marčiulynienė, D.; Mishcherikova, V.; Franić, I.; Lynikienė, J.; Gedminas, A.; Menkis, A. High Variability of Fungal Communities Associated with the Functional Tissues and Rhizosphere Soil of Picea abies in the Southern Baltics. Forests 2022, 13, 1103. [Google Scholar] [CrossRef]
- Fang, X.M.; Chen, F.S.; Wan, S.Z.; Yang, Q.P.; Shi, J.M. Topsoil and deep soil organic carbon concentration and stability vary with aggregate size and vegetation type in subtropical China. PLoS ONE 2015, 10, e0139380. [Google Scholar] [CrossRef]
- Aponte, C.; García, L.V.; Marañón, T.; Gardes, M. Indirect host effect on ectomycorrhizal fungi: Leaf fall and litter quality explain changes in fungal communities on the roots of co-occurring Mediterranean oaks. Soil Biol. Biochem. 2010, 42, 788–796. [Google Scholar] [CrossRef]
- Molina, R.; Horton, T.R. Mycorrhiza specificity: Its role in the development and function of common mycelial networks. In Mycorrhizal. Networks, Ecological Studies; Horton, T.R., Ed.; Springer Science+Business Media: Dordrech, The Netherlands, 2015; Volume 224, pp. 1–39. [Google Scholar]
- Heilmann-Clausen, J.; Maruyama, P.K.; Bruun, H.H.; Dimitrov, D.; Læssøe, T.; Frøslev, T.G.; Dalsgaard, B. Citizen science data reveal ecological, historical and evolutionary factors shaping interactions between woody hosts and wood-inhabiting fungi. New Phytol. 2016, 212, 1072–1082. [Google Scholar] [CrossRef]
- Antonovics, J.; Boots, M.; Ebert, D.; Koskella, B.; Poss, M.; Sadd, B.M. The origin of specificity by means of natural selection: Evolved and nonhost resistance in host–pathogen interactions. Evolution 2013, 67, 1–9. [Google Scholar] [CrossRef]
- Sieber, T.N. Endophytic fungi in forest trees: Are they mutualists? Fungal Biol. Rev. 2007, 21, 75–89. [Google Scholar] [CrossRef]
- Lang, C.; Seven, J.; Polle, A. Host preferences and differential contributions of deciduous tree species shape mycorrhizal species richness in a mixed central European forest. Mycorrhiza 2011, 21, 297–308. [Google Scholar] [CrossRef]
- Porras-Alfaro, A.; Bayman, P. Hidden fungi, emergent properties: Endophytes and microbiomes. Annu. Rev. Phytopathol. 2011, 49, 291–315. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jiang, Y.; Deane, D.C.; He, F.; Shu, W.; Liu, Y. Effects of host phylogeny, habitat and spatial proximity on host specificity and diversity of pathogenic and mycorrhizal fungi in a subtropical forest. New Phytol. 2019, 223, 462–474. [Google Scholar] [CrossRef]
- Kohler, M.; Sohn, J.; Nägele, G.; Bauhus, J. Can drought tolerance of Norway spruce (Picea abies) be increased through thinning? Eur. J. For. Res. 2010, 129, 1109–1118. [Google Scholar] [CrossRef]
- Lévesque, M.; Saurer, M.; Siegwolf, R.; Eilmann, B.; Brang, P.; Bugmann, H.; Rigling, A. Drought response of five conifer species under contrasting water availability suggests high vulnerability of Norway spruce and European larch. Glob. Change Biol. 2013, 19, 3184–3199. [Google Scholar] [CrossRef] [PubMed]
- Rehschuh, R.; Mette, T.; Menzel, A.; Buras, A. Soil properties affect the drought susceptibility of Norway spruce. Dendrochronologia 2017, 45, 81–89. [Google Scholar] [CrossRef]
- Honkaniemi, J.; Rammer, W.; Seidl, R. Norway spruce at the trailing edge: The effect of landscape configuration and composition on climate resilience. Landsc. Ecol. 2020, 35, 591–606. [Google Scholar] [CrossRef]
- Johann, E.; Agnoletti, M.; Axelsson, A.L.; Bürgi, M.; Östlund, L.; Rochel, X.; Schmidt, U.E.; Schuler, A.; Skovsgaard, J.P.; Winiwater, V. History of secondary Norway Spruce forests in Europe. In Norway Spruce Conversion Options and Consequences; Hansen, J., Klimo, E., Spiecker, H., Eds.; Brill: Leiden, The Netherlands; Boston, MA, USA, 2004; pp. 25–62. [Google Scholar]
- Schlyter, P.; Stjernquist, I.; Bärring, L.; Jönsson, A.M.; Nilsson, C. Assessment of the impacts of climate change and weather extremes on boreal forests in northern Europe, focusing on Norway spruce. Clim. Res. 2006, 31, 75–84. [Google Scholar] [CrossRef]
- Zeng, H.; Garcia-Gonzalo, J.; Peltola, H.; Kellomäki, S. The effects of forest structure on the risk of wind damage at a landscape level in a boreal forest ecosystem. Ann. For. Sci. 2010, 67, 111. [Google Scholar] [CrossRef]
- Zang, C.; Hartl-Meier, C.; Dittmar, C.; Rothe, A.; Menzel, A. Patterns of drought tolerance in major European temperate forest trees: Climatic drivers and levels of variability. Glob. Change Biol. 2014, 20, 3767–3779. [Google Scholar] [CrossRef]
- Jacob, D.; Petersen, J.; Eggert, B.; Alias, A.; Christensen, O.B.; Bouwer, L.M.; Braun, A.; Colette, A.; De·que, M.; Georgievski, G.; et al. EURO-CORDEX: New high-resolution climate change projections for European impact research. Reg. Environ. Change 2014, 14, 563–578. [Google Scholar] [CrossRef]
- Praeg, N.; Illmer, P. Microbial community composition in the rhizosphere of Larix decidua under different light regimes with additional focus on methane cycling microorganisms. Sci. Rep. 2020, 10, 22324. [Google Scholar] [CrossRef] [PubMed]
- Da Ronch, F.; Caudullo, G.; Tinner, W.; de Rigo, D. Larix decidua and other larches in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the EU: Luxembourg, 2016; pp. 108–110. Available online: https://boris.unibe.ch/80793/1/Larix_decidua.pdf (accessed on 10 March 2023).
- Lepage, B.; Basinger, J. The evolutionary history of the genus Larix (Pinaceae). In General Technical Report—Intermountain Research Station, USDA Forest Service, Proceedings of the Ecology and Management of Larix Forests: A Look Ahead, Whitefish, MT, USA, 5–9 October 1992; Schmidt, W.C., McDonald, K.J., Eds.; No.INT-GTR-319; US Department of Agriculture: Washington, DC, USA, 1995; Volume 5, pp. 19–29. [Google Scholar]
- Franić, I.; Allan, E.; Prospero, S.; Adamson, K.; Attorre, F.; Auger-Rozenberg, M.A.; Augustin, S.; Avtzis, D.; Baert, W.; Barta, M.; et al. Climate, host and geography shape insect and fungal communities of trees. Sci. Report 2023, 13, 11570. [Google Scholar] [CrossRef]
- Müller, M.M.; Hallaksela, A.M. Fungal diversity in Norway spruce: A case study. Mycol. Res. 2000, 104, 1139–1145. [Google Scholar] [CrossRef]
- Rajala, T.; Velmala, S.M.; Tuomivirta, T.; Haapanen, M.; Müller, M.; Pennanen, T. Endophyte communities vary in the needles of Norway spruce clones. Fungal Biol. 2013, 117, 182–190. [Google Scholar] [CrossRef]
- Mishcherikova, V.; Lynikienė, J.; Marčiulynas, A.; Gedminas, A.; Prylutskyi, O.; Marčiulynienė, D.; Menkis, A. Biogeography of Fungal Communities Associated with Pinus sylvestris L. and Picea abies (L.) H. Karst. along the Latitudinal Gradient in Europe. J. Fungi 2023, 9, 829. [Google Scholar] [CrossRef] [PubMed]
- Uroz, S.; Oger, P.; Tisserand, E.; Cébron, A.; Turpault, M.P.; Buée, M.; de Boer, W.; Leveau, J.H.J.; Frey-Klett, P. Specific impacts of beech and Norway spruce on the structure and diversity of the rhizosphere and soil microbial communities. Sci. Report 2016, 6, 27756. [Google Scholar] [CrossRef]
- Schön, M.E.; Nieselt, K.; Garnica, S. Belowground fungal community diversity and composition associated with Norway spruce along an altitudinal gradient. PLoS ONE 2018, 13, e0208493. [Google Scholar] [CrossRef] [PubMed]
- Leski, T.; Rudawska, M. Ectomycorrhizal fungal community of naturally regenerated European larch (Larix decidua) seedlings. Symbiosis 2012, 56, 45–53. [Google Scholar] [CrossRef]
- Margit, B.; Margit, Z.; Ursula, P. Ectomycorrhizal status of Larix decidua-, Picea abies- and Pinus cembra-nursery plants in South Tyrol. For. Obs. 2010, 5, 3–30. [Google Scholar]
- Grayston, S.J.; Campbell, C.D. Functional biodiversity of microbial communities in the rhizospheres of hybrid larch (Larix eurolepis) and Sitka spruce (Picea sitchensis). Tree Physiol. 1996, 16, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Lynikienė, J.; Gedminas, A.; Marčiulynas, A.; Marčiulynienė, D.; Menkis, A. Can Larix sp. Mill. Provide Suitable Habitats for Insects and Lichens Associated with Stems of Picea abies (L.) H. Karst. in Northern Europe? Diversity 2022, 14, 729. [Google Scholar] [CrossRef]
- Baldarian, P.; Větrovský, T.; Lepinay, C.; Kohout, P. High-throughput sequencing view on the magnitude of global fungal diversity. Fungal Divers. 2022, 114, 539–547. [Google Scholar] [CrossRef]
- Scheepers, D.; Eloy, M.C.; Briquet, M. Identification of larch species (Larix decidua, Larix kaempferi and Larix X eurolepis) and estimation of hybrid fraction in seed lots by RAPD fingerprints. Theor. Appl. Genet. 2000, 100, 71–74. [Google Scholar] [CrossRef]
- Navasaitis, M. Dendrologija [Dendrology], 2nd ed.; Margi Raštai: Vilnius, Lithuania, 2008; pp. 169–183. (In Lithuanian) [Google Scholar]
- Vaičys, M. Miško dirvožemių klasifikacija. In Lietuvos Dirvožemiai; Mokslas: Vilnius, Lithuania, 2001; pp. 1040–1043. (In Lithuanian) [Google Scholar]
- Karazija, S. Miško Tipologija. In Miško Ekologija; Padaiga, V., Stravinskienė, V., Eds.; Enciklopedija: Vilnius, Lithuania, 2008; pp. 220–254. (In Lithuanian) [Google Scholar]
- Marčiulynienė, D.; Marčiulynas, A.; Mishcherikova, V.; Lynikienė, J.; Gedminas, A.; Franic, I.; Menkis, A. Principal Drivers of Fungal Communities Associated with Needles, Shoots, Roots and Adjacent Soil of Pinus Sylvestris. J. Fungi 2022, 8, 1112. [Google Scholar] [CrossRef]
- Ihrmark, K.; Bödeker, I.T.M.; Cruz-Martinez, K.; Friberg, H.; Kubartova, A.; Schenck, J.; Strid, Y.; Stenlid, J.; Brandström-Durling, M.; Clemmensen, K.E.; et al. New primers to amplify the fungal ITS2 region—Evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol. Ecol. 2012, 82, 666–677. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal Rna Genes for Phylogenetics. PCR Protoc. 1990, 18, 315–322. [Google Scholar]
- Clemmensen, K.E.; Ihrmark, K.; Brandström-Durling, M.; Lindahl, B.D. Sample preparation for fungal community analysis by high-throughput sequencing of barcode amplicons. In Microbial Environmental Genomics (MEG); Methods in Molecular, Biology; Martin, F., Uroz, S., Eds.; Humana Press: New York, NY, USA, 2016; Volume 1399, pp. 61–88. [Google Scholar]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef] [PubMed]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Magurran, A.E. Ecological Diversity and Its Measurement; Princeton University Press: Princeton, NJ, USA, 1988; 192p. [Google Scholar]
- Hawksworth, D.L. The fungal dimension of biodiversity: Magnitude, significance, and conservation. Mycol. Res. 1991, 95, 641–655. [Google Scholar] [CrossRef]
- Finlay, R.D. Ecological aspects of mycorrhizal symbiosis: With special emphasis on the functional diversity of interactions involving the extraradical mycelium. J. Exp. Bot. 2008, 59, 1115–1126. [Google Scholar] [CrossRef]
- Hartmann, M.; Frey, B.; Mayer, J.; Mäder, P.; Widmer, F. Distinct soil microbial diversity under long-term organic and conventional farming. ISME J. 2015, 9, 1177–1194. [Google Scholar] [CrossRef]
- Ponge, J.F. The soil as an ecosystem. Biol. Fertil. Soils 2015, 51, 645–648. [Google Scholar] [CrossRef]
- Van der Heijden, M.G.; Wagg, C. Soil microbial diversity and agro-ecosystem functioning. Plant Soil 2013, 363, 1–5. [Google Scholar] [CrossRef]
- Kivlin, S.N.; Smith, A.P.; Sulman, B.N.; Buscardo, E. Forest Rhizosphere Interactions: Cascading Consequences for Ecosystem-Level Carbon and Nutrient Cycling. Front. For. Glob. Change 2021, 4, 33. [Google Scholar] [CrossRef]
- Kembel, S.W.; O’Connor, T.K.; Arnold, H.K.; Hubbell, S.P.; Wright, S.J.; Green, J.L. Relationships between phyllosphere bacterial communities and plant functional traits in a neotropical forest. Proc. Natl. Acad. Sci. USA 2014, 111, 13715–13720. [Google Scholar] [CrossRef]
- Bowles, T.M.; Acosta-Martínez, V.; Calderón, F.; Jackson, L.E. Soil enzyme activities, microbial communities, and carbon and nitrogen availability in organic agroecosystems across an intensively-managed agricultural landscape. Soil Biol. Biochem. 2014, 68, 252–262. [Google Scholar] [CrossRef]
- Jacquemyn, H.; Merckx, V.; Brys, R.; Tyteca, D.; Cammue, B.; Honnay, O. Analysis of network architecture reveals phylogenetic constraints on mycorrhizal specificity in the genus Orchis (Orchidaceae). New Phytol. 2011, 192, 518–528. [Google Scholar] [CrossRef]
- Põlme, S.; Bahram, M.; Yamanaka, T.; Nara, K.; Dai, Y.C.; Grebenc, T. Biogeography of ectomycorrhizal fungi associated with alders (Alnus spp.) in relation to biotic and abiotic variables at the global scale. New Phytol. 2013, 198, 1239–1249. [Google Scholar] [CrossRef]
- Tedersoo, L.; Mett, M.; Ishida, T.A.; Bahram, M. Phylogenetic relationships among host plants explain differences in fungal species richness and community composition in ectomycorrhizal symbiosis. New Phytol. 2013, 199, 822–831. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Williams, L.J.; Vincent, J.B.; Stefanski, A.; Cavender-Bares, J.; Messier, C.; Paquette, A.; Gravel, D.; Reich, P.B.; Kennedy, P.G. Ectomycorrhizal fungal diversity and saprotrophic fungal diversity are linked to different tree community attributes in a field-based tree experiment. Mol. Ecol. 2016, 25, 4032–4046. [Google Scholar] [CrossRef] [PubMed]
- Molina, R.; Massicotte, H.; Trappe, J.M. Specificity phenomena in mycorrhizal symbioses: Community-ecological consequences and practical implications. In Mycorrhizal Functioning: An Integrative Plant–Fungal Process; Allen, M.F., Ed.; Chapman and Hall: New York, NY, USA, 1992; pp. 357–423. [Google Scholar]
- Põlme, S.; Bahram, M.; Jacquemyn, H.; Kennedy, P.; Kohout, P.; Moora, M.; Tedersoo, L. Host preference and network properties in biotrophic plant–fungal associations. New Phytol. 2018, 217, 1230–1239. [Google Scholar] [CrossRef]
- Jiang, Z.; Fu, Y.; Zhou, L.; He, Y.; Zhou, G.; Dietrich, P.; Zhou, X. Plant growth strategy determines the magnitude and direction of drought-induced changes in root exudates in subtropical forests. Glob. Change Biol. 2023, 29, 3476–3488. [Google Scholar] [CrossRef] [PubMed]
- Harder, C.B.; Hesling, E.; Botnen, S.S.; Lorberau, K.E.; Dima, B.; von Bonsdorff-Salminen, T.; Kauserud, H. Mycena species can be opportunist-generalist plant root invaders. Environ. Microbiol. 2023, 25, 1875–1893. [Google Scholar] [CrossRef] [PubMed]
- Thoen, E.; Harder, C.B.; Kauserud, H.; Botnen, S.S.; Vik, U.; Taylor, A.F.S.; Menkis, A.; Skrede, I. In vitro evidence of root colonization suggests ecological versatility in the genus Mycena. New Phytol. 2020, 227, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Boberg, J.; Finlay, R.; Stenlid, J.; Nasholm, T.; Lindahl, B. Glucose and ammonium additions affect needle decomposition and carbon allocation by the litter degrading fungus Mycena epipterygia. Soil Biol. Biochem. 2008, 40, 995–999. [Google Scholar] [CrossRef]
- Kyaschenko, J.; Clemmensen, K.E.; Hagenbo, A.; Karltun, E.; Lindahl, B.D. Shift in fungal communities and associated enzyme activities along an age gradient of managed Pinus sylvestris stands. ISME J. 2017, 11, 863–874. [Google Scholar] [CrossRef]
- Smith, G.R.; Finlay, R.D.; Stenlid, J.; Vasaitis, R.; Menkis, A. Growing evidence for facultative biotrophy in saprotrophic fungi: Data from microcosm tests with 201 species of wood-decay basidiomycetes. New Phytol. 2017, 215, 747–755. [Google Scholar] [CrossRef]
- Cortés-Pérez, A.; Guzmán-Dávalos, L.; Ramírez-Cruz, V.; Villalobos-Arámbula, A.R.; Ruiz-Sanchez, E.; Ramírez-Guillén, F. New Species of Bioluminescent Mycena Sect. Calodontes (Agaricales, Mycenaceae) from Mexico. J. Fungi 2023, 9, 902. [Google Scholar] [CrossRef]
- Leski, T.; Rudawska, M.; Aučina, A. The ectomycorrhizal status of European larch (Larix decidua Mill.) seedlings from bare-root forest nurseries. For. Ecol. Manag. 2008, 256, 2136–2144. [Google Scholar] [CrossRef]
- Kernaghan, G.; Widden, P.; Bergeron, Y.; Légaré, S.; Paré, D. Biotic and abiotic factors affecting ectomycorrhizal diversity in boreal mixed-woods. Oikos 2003, 102, 497–504. [Google Scholar] [CrossRef]
- Menkis, A.; Vasiliauskas, R.; Taylor, A.F.; Stenlid, J.; Finlay, R. Fungal communities in mycorrhizal roots of conifer seedlings in forest nurseries under different cultivation systems, assessed by morphotyping, direct sequencing and mycelial isolation. Mycorrhiza 2005, 16, 33–41. [Google Scholar] [CrossRef]
- Iwański, M.; Rudawska, M.; Leski, T. Mycorrhizal associations of nursery grown Scots pine (Pinus sylvestris L.) seedlings in Poland. Ann. For. Sci. 2006, 63, 715–723. [Google Scholar] [CrossRef]
- Rudawska, M.; Leski, T.; Trocha, L.K.; Gornowicz, R. Ectomycorrhizal status of Norway spruce seedlings from bare-root forest nurseries. For. Ecol. Manag. 2006, 236, 375–384. [Google Scholar] [CrossRef]
- Menkis, A.; Bakys, R.; Lygis, V.; Vasaitis, R. Mycorrhization, Establishment and Growth of Outplanted Picea abies Seedlings Produced under Different Cultivation Systems. Silva Fenn. 2011, 45, 283–289. [Google Scholar]
- Heinonsalo, J.; Sun, H.; Santalahti, M.; Bäcklund, K.; Hari, P.; Pumpanen, J. Evidences on the ability of mycorrhizal genus Piloderma to use organic nitrogen and deliver it to Scots pine. PLoS ONE 2015, 10, e0131561. [Google Scholar] [CrossRef]
- Pérez-Pazos, E.; Certano, A.; Gagne, J.; Lebeuf, R.; Siegel, N.; Nguyen, N.; Kennedy, P.G. The slippery nature of ectomycorrhizal host specificity: Suillus fungi associated with novel pinoid (Picea) and abietoid (Abies) hosts. Mycologia 2021, 113, 891–901. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, Y.; Mi, F.; Tang, X.; He, X.; Cao, Y.; Liu, C.; Yang, D.; Dong, J.; Zhang, K.; et al. Recent advances in population genetics of ectomycorrhizal mushrooms Russula spp. Mycology 2015, 6, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Rishbeth, J. Species of Armillaria in southern England. Plant Pathol. 1982, 31, 9–17. [Google Scholar] [CrossRef]
- Guillaumin, J.J.; Lung, B.; Romagnesi, H.; Marxmuller, H.; Lamoure, D.; Durrieu, G.; Mohammed, C. Sustématique des Armilaires du groupe Mellea. Conséquenmces phytopathologiques. Eur. J. For. Pathol. 1985, 15, 268–277. [Google Scholar] [CrossRef]
- Möykkynen, T.; Pukkala, T. Optimizing the management of Norway spruce and Scots pine mixtures on a site infected by Heterobasidion coll. Scand. J. For. Res. 2010, 25, 127–137. [Google Scholar] [CrossRef]


| Site | Tree Species | Relative Abundance (%) | Richness (%) | Shannon H | Sørensen Cs | Relative Abundance (%) | Richness (%) | Shannon H | Sørensen Cs | Relative Abundance (%) | Richness (%) | Shannon H | Sørensen Cs | Relative Abundance (%) | Richness (%) | Shannon H | Sørensen Cs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Roots | Soil | ||||||||||||||||
| Organic Layer | Mineral Layer | TOTAL SOIL | |||||||||||||||
| S1/L1 | Picea | 2.2 | 11.9 | 3.37 | 0.31 | 7.2 | 13.4 | 3.90 | 0.31 | 1.0 | 6.4 | 3.29 | 0.33 | 3.0 | 11.2 | 3.90 | 0.35 |
| Larix | 5.3 | 15.2 | 2.34 | 6.3 | 13.5 | 3.90 | 3.1 | 13.3 | 4.12 | 4.1 | 15.4 | 4.31 | |||||
| S2/L2 | Picea | 4.5 | 13.8 | 3.06 | 0.34 | 0.1 | 1.2 | 2.71 | 0.10 | 2.6 | 9.7 | 3.50 | 0.25 | 1.8 | 7.3 | 3.53 | 0.32 |
| Larix | 11.6 | 16.2 | 3.05 | 1.4 | 8.7 | 4.12 | 0.7 | 4.5 | 2.26 | 0.9 | 8.3 | 3.70 | |||||
| S3/L3 | Picea | 2.4 | 12.9 | 3.71 | 0.21 | 3.2 | 11.4 | 3.84 | 0.22 | 1.0 | 5.9 | 3.14 | - | 1.7 | 10.3 | 3.81 | 0.27 |
| Larix | 7.2 | 10.2 | 1.71 | 5.5 | 22.1 | 4.55 | - | - | - | 1.8 | 15.5 | 4.55 | |||||
| S4/L4 | Picea | 2.6 | 12.8 | 3.55 | 0.36 | 7.6 | 15.8 | 4.07 | 0.24 | 0.3 | 8.1 | 4.26 | 0.17 | 2.7 | 15.1 | 4.31 | 0.29 |
| Larix | 7.0 | 16.6 | 2.90 | 0.6 | 6.1 | 3.99 | 1.1 | 8.0 | 3.74 | 1.0 | 8.7 | 4.14 | |||||
| S5/L5 | Picea | 1.8 | 13.2 | 3.80 | 0.10 | 5.3 | 14.7 | 4.13 | 0.23 | 4.3 | 10.6 | 3.76 | 0.23 | 4.6 | 14.1 | 4.18 | 0.25 |
| Larix | 0.3 | 4.0 | 3.04 | 1.7 | 10.0 | 4.11 | 3.2 | 12.1 | 3.78 | 2.7 | 12.2 | 4.04 | |||||
| S6/L6 | Picea | 1.1 | 7.2 | 3.12 | 0.18 | 17.4 | 17.9 | 3.50 | - | 3.9 | 15.3 | 4.14 | 0.23 | 8.3 | 18.3 | 4.08 | 0.28 |
| Larix | 4.6 | 11.2 | 2.47 | - | - | - | 12.5 | 22.6 | 3.75 | 8.4 | 15.9 | 3.75 | |||||
| S7/L7 | Picea | 5.8 | 16.2 | 3.29 | 0.27 | 25.1 | 20.3 | 4.15 | 0.23 | 26.9 | 29.8 | 4.12 | 0.29 | 26.3 | 27.6 | 4.33 | 0.32 |
| Larix | 4.8 | 12.6 | 2.92 | 2.4 | 12.3 | 3.92 | 25.9 | 15.4 | 3.31 | 18.3 | 16.4 | 3.41 | |||||
| S8/L8 | Picea | 3.6 | 10.9 | 1.99 | 0.32 | 2.3 | 5.5 | 3.19 | 0.21 | 3.0 | 5.5 | 0.80 | 0.20 | 2.8 | 6.7 | 1.89 | 0.34 |
| Larix | 12.4 | 14.2 | 2.65 | 3.4 | 9.9 | 3.78 | 0.2 | 4.3 | 3.36 | 1.2 | 9.0 | 4.04 | |||||
| S9/L9 | Picea | 4.1 | 13.7 | 3.29 | 0.31 | 1.2 | 9.1 | 4.16 | 0.28 | 0.3 | 3.6 | 2.71 | 0.16 | 0.6 | 7.8 | 4.11 | 0.27 |
| Larix | 7.8 | 12.6 | 2.96 | 1.4 | 7.7 | 3.60 | 5.0 | 10.0 | 3.72 | 3.8 | 10.7 | 3.92 | |||||
| S10/L10 | Picea | 5.2 | 15.3 | 3.06 | 0.25 | 2.1 | 10.3 | 3.75 | 0.21 | 5.1 | 15.3 | 3.12 | - | 4.1 | 16.1 | 3.57 | 0.24 |
| Larix | 5.6 | 12.4 | 2.75 | 5.9 | 7.9 | 3.41 | - | - | - | 1.9 | 5.5 | 3.41 | |||||
| All sites * | Picea | 33.3 (7034) | 66.4 (519) | 4.26 | 0.49 | 71.3 (27,028) | 70.1 (974) | 5.12 | 0.47 | 48.4 (38,128) | 68.0 (952) | 4.71 | 0.44 | 55.8 (65,156) | 72.1 (1429) | 5.13 | 0.53 |
| Larix | 66.7 (14,066) | 67.8 (530) | 3.82 | 28.7 (10,881) | 59.6 (828) | 5.13 | 51.6 (40,690) | 58.1 (814) | 4.29 | 44.2 (51,571) | 63.9 (1267) | 4.67 | |||||
| Total | 100.0 (21,100) | 100.0 (782) | 4.20 | 100.0 (37,909) | 100.0 (1389) | 5.43 | 100.0 (78,818) | 100.0 (1401) | 4.79 | 100.0 (116,727) | 100.0 (1983) | 5.22 | |||||
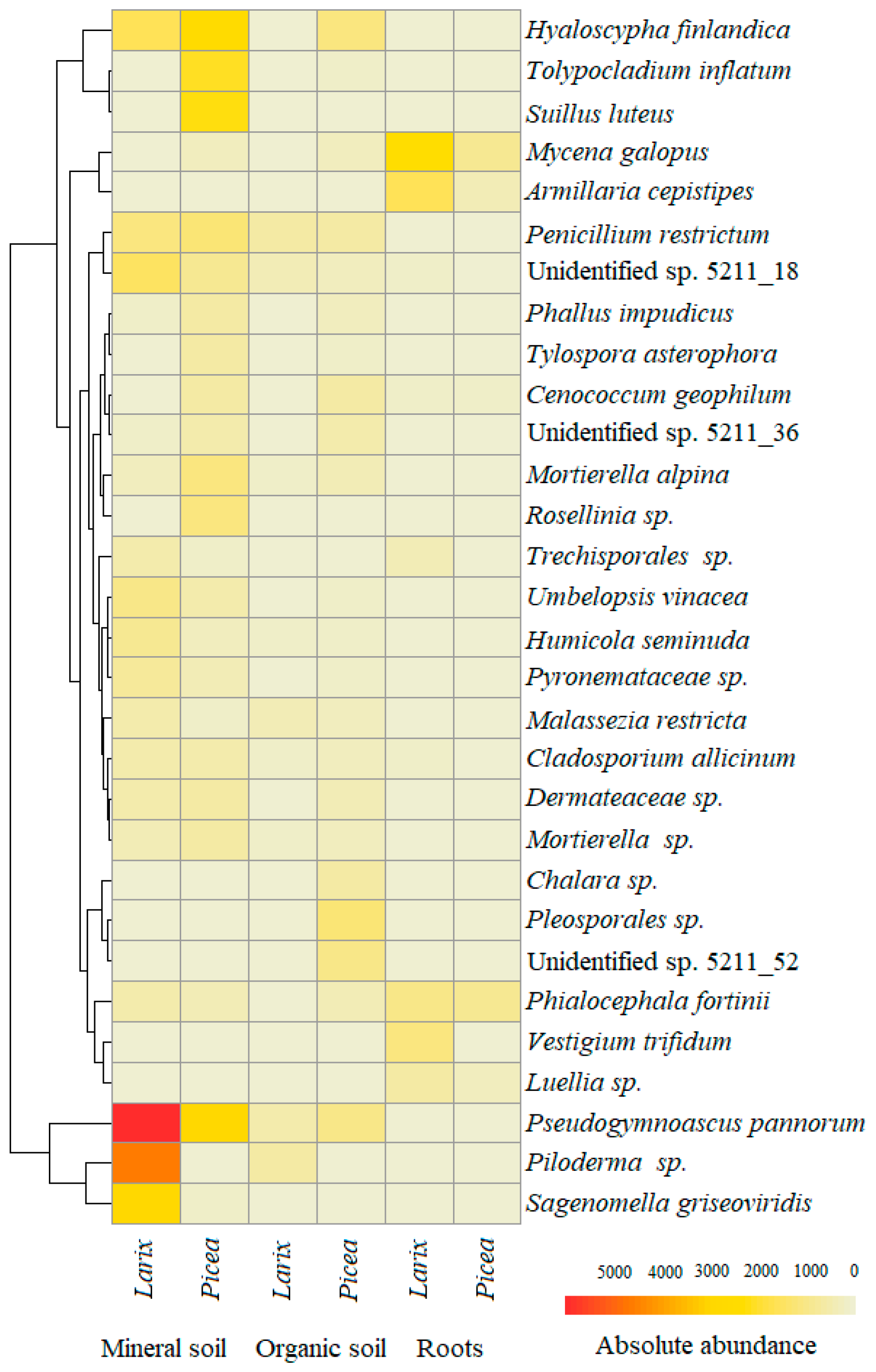
| Phyla * | Fungal OTU | Genbank Reference | Similarity, bp (%) | Roots | Organic Soil | Mineral Soil | All | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P. abies | Larix sp. | Both | P. abies | Larix sp. | Both | P. abies | Larix sp. | Both | |||||
| A | Pseudogymnoascus pannorum | MN879385 | 241/241 (100) | 0.4 | 0.5 | 0.4 | 3.8 | 4.7 | 4.1 | 7.5 | 14.6 | 11.2 | 7.6 |
| A | Hyaloscypha finlandica | MK965770 | 232/236 (98) | 1.4 | 0.4 | 0.7 | 4.3 | 0.7 | 3.2 | 6.7 | 4.0 | 5.3 | 4.0 |
| B | Piloderma sp. | MK838260 | 289/289 (100) | - | 0.6 | 0.4 | 0.01 | 5.6 | 1.6 | - | 11.2 | 5.8 | 3.8 |
| B | Mycena galopus | MK795846 | 305/305 (100) | 13.0 | 16.5 | 15.3 | 1.0 | 0.1 | 0.8 | 0.8 | 0.1 | 0.5 | 2.8 |
| A | Penicillium restrictum | MT090009 | 257/257 (100) | 1.6 | 0.5 | 0.9 | 2.3 | 5.6 | 3.2 | 3.2 | 2.9 | 3.0 | 2.8 |
| A | Unidentified sp. 5211_18 | MZ442004 | 234/235 (99) | 0.7 | 1.3 | 1.1 | 1.2 | 3.9 | 2.0 | 2.4 | 3.7 | 3.1 | 2.5 |
| A | Phialocephala fortinii | MT028045 | 238/238 (100) | 12.9 | 6.8 | 8.9 | 1.5 | 0.5 | 1.2 | 1.2 | 1.2 | 1.2 | 2.4 |
| A | Sagenomella griseoviridis | LC177648 | 249/249 (100) | 0.0 | 0.0 | 0.0 | 0.2 | 0.3 | 0.2 | 0.6 | 7.0 | 3.9 | 2.3 |
| A | Tolypocladium inflatum | MT294423 | 246/246 (100) | 0.0 | - | 0.0 | 0.5 | 0.2 | 0.4 | 5.6 | 0.1 | 2.8 | 1.7 |
| B | Suillus luteus | KU059580 | 331/331 (100) | 0.03 | 0.02 | 0.02 | 0.3 | 0.01 | 0.2 | 5.9 | - | 2.8 | 1.7 |
| B | Armillaria cepistipes | OK324330 | 477/477 (100) | 6.8 | 11.9 | 10.2 | - | - | - | - | 0.04 | 0.02 | 1.6 |
| A | Cladosporium allicinum | OP965390 | 243/243 (100) | 1.1 | 1.3 | 1.2 | 1.1 | 1.3 | 1.2 | 1.3 | 1.3 | 1.3 | 1.2 |
| Z | Mortierella alpina | MT529891 | 345/345 (100) | 0.1 | 0.0 | 0.0 | 1.5 | 1.6 | 1.6 | 2.8 | 0.8 | 1.8 | 1.4 |
| Z | Umbelopsis vinacea | KU727816 | 294/294 (100) | 0.1 | 0.1 | 0.1 | 0.8 | 1.0 | 0.9 | 1.3 | 2.6 | 2.0 | 1.4 |
| A | Dermateaceae sp. | MK965746 | 237/237 (100) | 0.2 | 0.2 | 0.2 | 1.4 | 0.7 | 1.2 | 1.7 | 1.4 | 1.5 | 1.2 |
| A | Cenococcum geophilum | MN450580 | 241/241 (100) | 1.9 | 1.0 | 1.3 | 2.2 | 0.6 | 1.8 | 1.6 | 0.3 | 0.9 | 1.2 |
| A | Humicola seminuda | LT993594 | 249/249 (100) | 0.0 | - | 0.0 | 0.8 | 1.8 | 1.1 | 0.8 | 2.2 | 1.5 | 1.2 |
| Z | Mortierella sp. | KP311419 | 344/344 (100) | 0.1 | 0.1 | 0.1 | 1.3 | 1.6 | 1.4 | 1.6 | 1.0 | 1.3 | 1.1 |
| A | Pyronemataceae sp. | LR603942 | 256/256 (100) | 0.5 | 0.1 | 0.2 | 0.8 | 0.7 | 0.8 | 1.2 | 1.8 | 1.5 | 1.1 |
| B | Malassezia restricta | LT854697 | 369/369 (100) | 0.2 | 0.2 | 0.2 | 1.0 | 3.5 | 1.8 | 0.6 | 1.3 | 1.0 | 1.1 |
| A | Pleosporales sp. | KT269193 | 238/245 (97) | 0.9 | 0.0 | 0.3 | 4.8 | 0.0 | 3.4 | 0.0 | - | 0.0 | 1.0 |
| A | Unidentified sp. | MN902437 | 216/216(100) | 1.3 | 0.0 | 0.5 | 1.9 | 0.1 | 1.4 | 1.5 | 0.3 | 0.9 | 0.9 |
| B | Trechisporales sp. | JF519283 | 297/297 (100) | 1.2 | 3.4 | 2.6 | 0.0 | 0.1 | 0.1 | 0.4 | 1.3 | 0.8 | 0.9 |
| A | Vestigium trifidum | KP783486 | 236/239 (99) | 0.1 | 7.9 | 5.3 | 0.0 | 0.5 | 0.2 | - | 0.0 | 0.0 | 0.9 |
| B | Phallus impudicus | OP603024 | 290/290 (100) | 0.0 | - | 0.0 | 1.0 | 0.8 | 0.9 | 1.7 | 0.4 | 1.0 | 0.8 |
| A | Rosellinia sp. | KT264658 | 257/258 (99) | 0.0 | - | 0.0 | - | - | - | 2.9 | 0.0 | 1.4 | 0.8 |
| B | Tylospora asterophora | HM190017 | 288/288 (100) | 0.6 | 0.1 | 0.3 | 0.7 | 0.5 | 0.7 | 1.8 | 0.0 | 0.9 | 0.7 |
| A | Unidentified sp. 5211_52 | KT196588 | 201/226 (89) | 0.0 | - | 0.0 | 3.7 | - | 2.6 | 0.0 | - | 0.0 | 0.7 |
| B | Luellia sp. | LS447499 | 319/320 (99) | 4.3 | 4.4 | 4.3 | 0.0 | 0.2 | 0.1 | 0.0 | 0.0 | 0.0 | 0.7 |
| A | Chalara sp. | MK965775 | 238/238 (100) | 0.3 | 0.3 | 0.3 | 2.4 | 0.7 | 1.9 | 0.2 | 0.0 | 0.1 | 0.7 |
| Total of 30 fungal OTUs | 49.7 | 57.6 | 55.0 | 40.8 | 37.5 | 39.8 | 55.2 | 59.6 | 57.5 | 52.2 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lynikienė, J.; Marčiulynas, A.; Marčiulynienė, D.; Gedminas, A.; Mishcherikova, V.; Menkis, A. Diversity and Composition of Belowground Fungal Communities Associated with Picea abies L. (H.) Karst. and Larix sp. Mill.: A Comparative Study. Diversity 2024, 16, 160. https://doi.org/10.3390/d16030160
Lynikienė J, Marčiulynas A, Marčiulynienė D, Gedminas A, Mishcherikova V, Menkis A. Diversity and Composition of Belowground Fungal Communities Associated with Picea abies L. (H.) Karst. and Larix sp. Mill.: A Comparative Study. Diversity. 2024; 16(3):160. https://doi.org/10.3390/d16030160
Chicago/Turabian StyleLynikienė, Jūratė, Adas Marčiulynas, Diana Marčiulynienė, Artūras Gedminas, Valeriia Mishcherikova, and Audrius Menkis. 2024. "Diversity and Composition of Belowground Fungal Communities Associated with Picea abies L. (H.) Karst. and Larix sp. Mill.: A Comparative Study" Diversity 16, no. 3: 160. https://doi.org/10.3390/d16030160
APA StyleLynikienė, J., Marčiulynas, A., Marčiulynienė, D., Gedminas, A., Mishcherikova, V., & Menkis, A. (2024). Diversity and Composition of Belowground Fungal Communities Associated with Picea abies L. (H.) Karst. and Larix sp. Mill.: A Comparative Study. Diversity, 16(3), 160. https://doi.org/10.3390/d16030160






