Three New Periconia Species Isolated from Wurfbainia villosa in Guangdong, China: A Discussion on the Doubtful Taxa Clustering in this Genus
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection, Observation and Isolation of Samples
2.2. DNA Extraction, PCR Amplification, and Sequencing
2.3. Phylogenetic Analyses
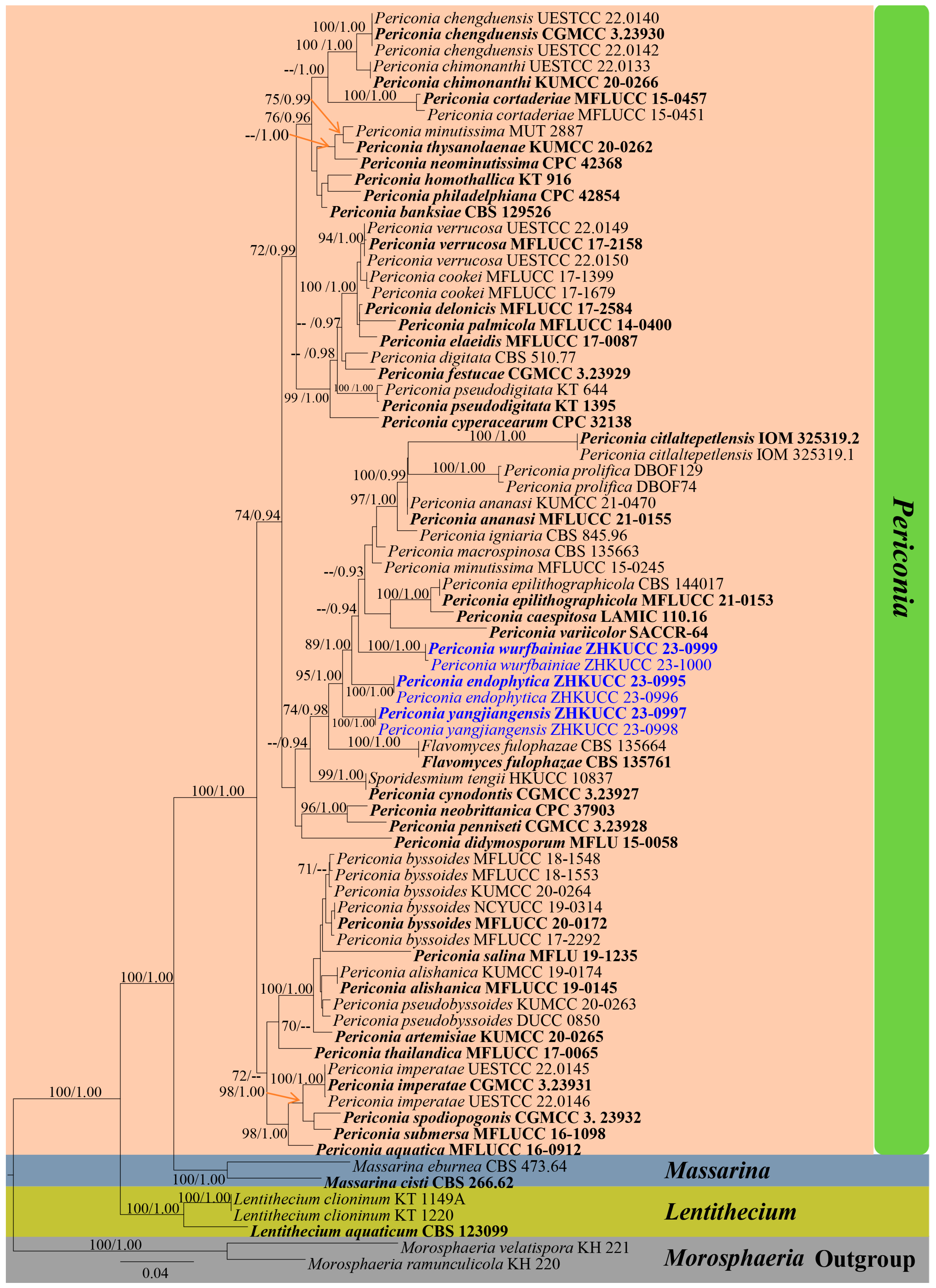
3. Results
3.1. Phylogeny
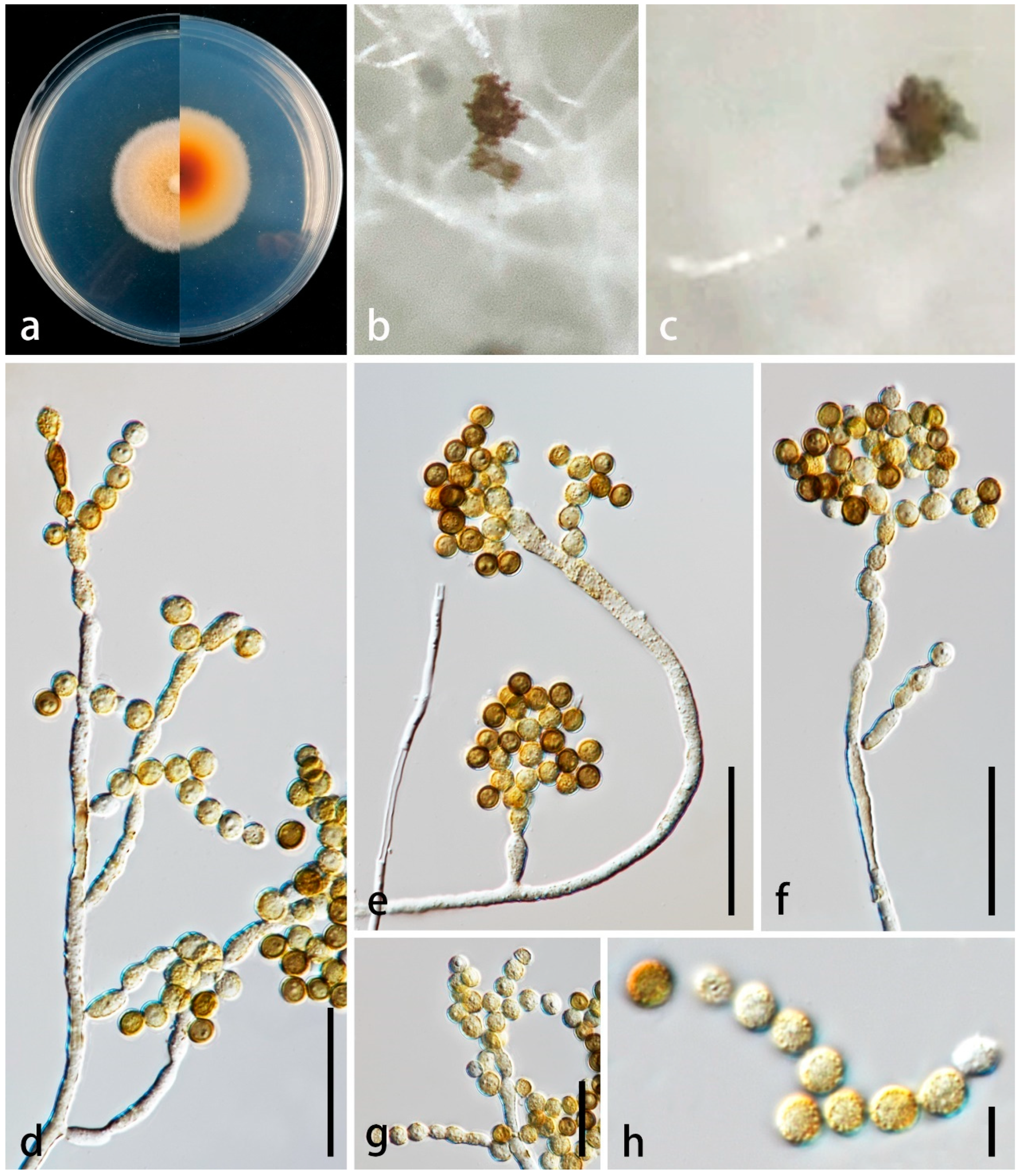
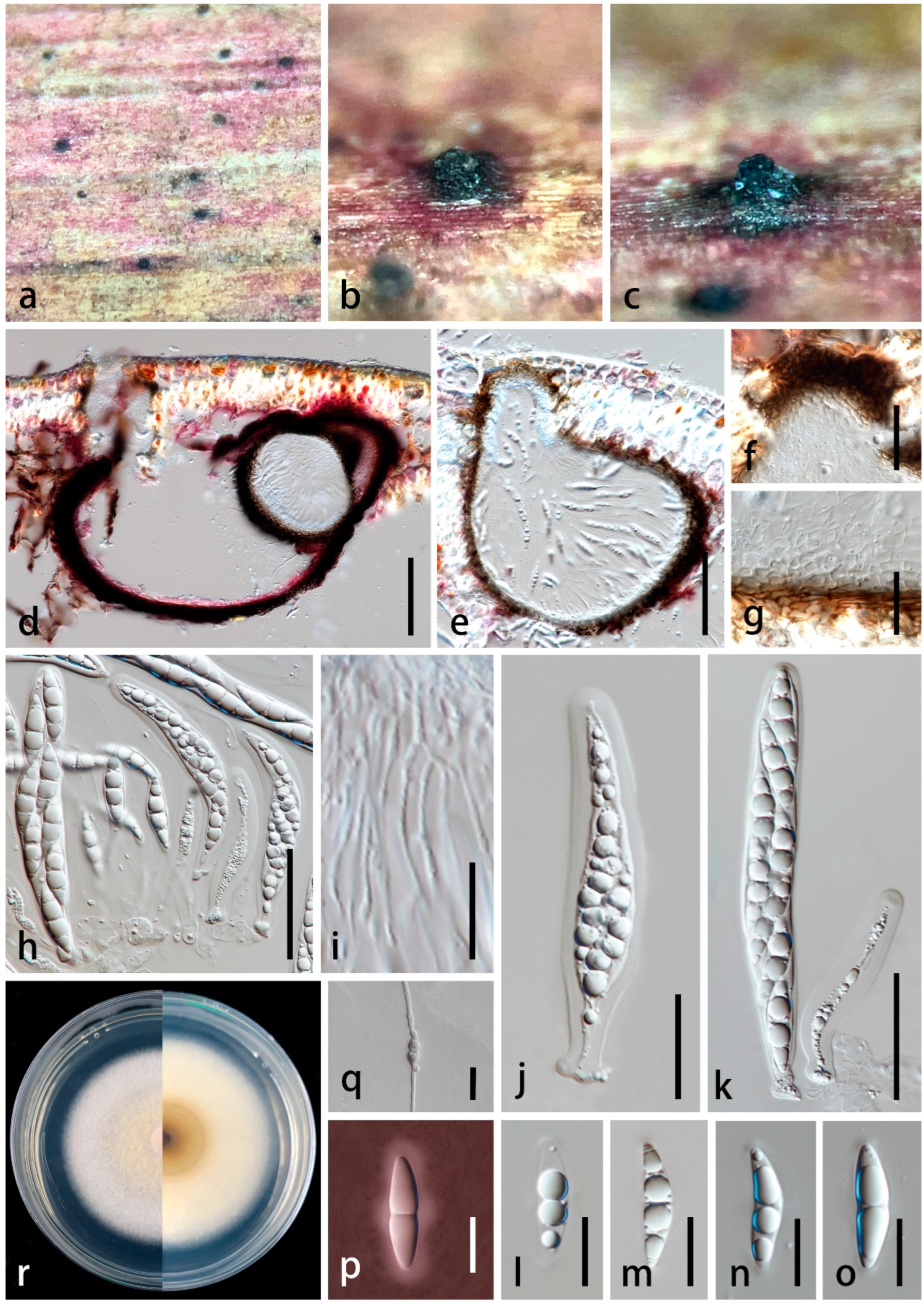
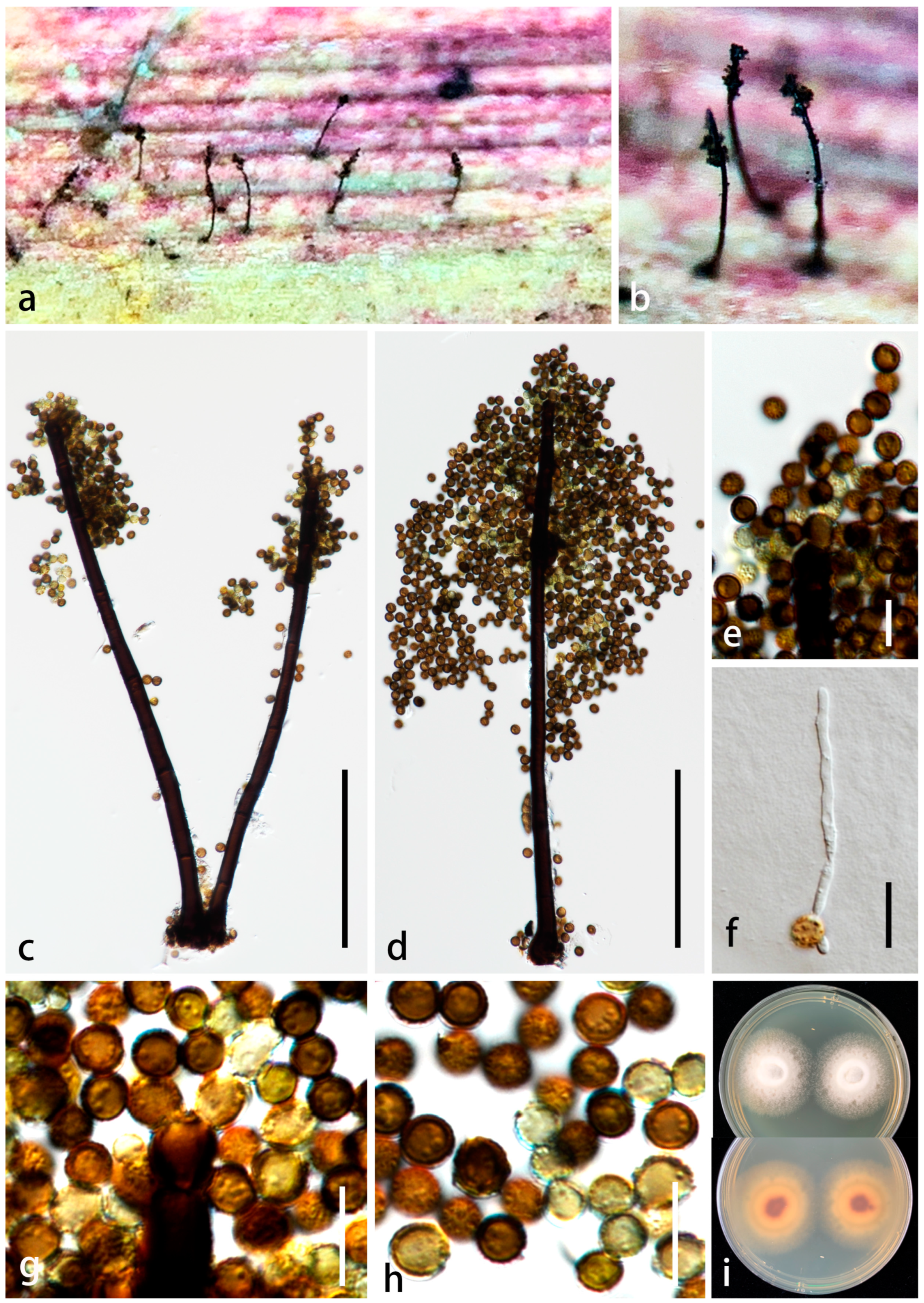
3.2. Taxonomy
| Taxa | Conidiophores | Conidiogenous Cells | Conidia | Reference |
|---|---|---|---|---|
| Periconia caespitosa | Up to 500 µm long, 5–6 µm wide at the base, macronematous, mononematous, septate, unbranched or rarely branched, caespitose, straight to flexuous, setiform and sometimes uncinated at the tip, pale brown at the base, brown towards the apex, minutely roughened at the base and at the apex, as well as in the areas nearest of conidiogenous cells, otherwise smooth | (6–)7.5–9 × 6.5–7.5 µm, poly-blastic, pale brown, finely roughened, intercalary and terminal, globose, subglobose or obpyriform | (6–)6.5–9 µm, globose, aseptate, reddish brown, thick-walled, dry, solitary or in short basipetal chains of 2–4 conidia | Crous et al. [46] |
| P. cambrensis | 150–300 × 3–5 µm, erect, straight or tapering, pale brown, 5–9-septate, cylindrical, bearing a few short branches | 6–7 × 5–6 µm, ovate, hyaline or pale brown, smooth, bearing single or short-chained conidia | 5–8 µm diam., spherical, brown | Ellis [47] |
| P. endophytica | 15–110 × 2.5–5.5 µm ( = 45 × 4 μm, n = 30), micro- to semi-macronematous, mononematous, erect, flexuous or straight, branched, sparsely septate, slightly constricted at septa, rough-walled, verruculose, thin-walled, hyaline, pale brown to yellowish brown in the upper portion | 5–20 × 2.5–5.5 µm ( = 9 × 4 μm, n = 30), holoblastic, mono- and poly-blastic, integrated, determinate, terminal, subcylindrical, obovoid, ellipsoidal with truncate ends, pale brown to yellowish brown, verruculose | 4–6 × 3–5 µm ( = 5 × 4 μm, n = 30), globose to subglobose, aseptate, catenate, with 4–10 conidia in a chain, hyaline when young, becoming pale brown, yellowish brown and reddish brown when mature, verruculose | This study |
| P. epilithographicola | 251.6–270 × 3.6–6.1 µm, macronematous, with creeping hyphae forming stipes straight, branched singly near the base, seven or more septate, grayish to black | (5.1−) 7 × 10 (−11.5) µm, holoblastic, sub-globose to ellipsoid, finely roughened, yellowish to brown | (7.8−) 9.2 (−10.7), globose, golden to brown, echinulated, catenated, sometimes forming long chains | Coronado-Ruiz et al. [48] |
| P. neobrittanica | 100–300 × 10–17 μm, solitary, or in clusters of 2–3, arising from a brown stroma, subcylindrical, straight to flexuous, unbranched, dark brown, smooth, thick-walled, base swollen, 15–25 μm diam. | 10–15 μm long, terminal and intercalary, occurring in an apical chain on primary, or directly on conidiophore | (6–)8–10(–12) μm diam., aseptate, spherical, pale to medium brown, with delicate spines, occurring in branched chains | Crous et al. [49] |
| P. pseudobyssoides | 23–25 μm wide at the base, up to 300–600 μm long, macronematous, arising usually singly, but occasionally 2 or 3(−4) together on stromata, often verruculose, with brown to reddish brown conidial heads | 10–12 × 6–7.5 μm, discrete, determinate, usually monoblastic, sometimes poly-blastic, ellipsoidal, ovoid to clavate, pale brown to reddish brown, verruculose, arising directly from the swollen mid-brown apical cell cut off by a septum from the stipe apex | (12–)15–17(−20) μm diam., spherical, golden yellow to golden brown or reddish brown, verruculose when young, mature conidia with specific ornamentation consisting of irregular lobate crests, born singly or in acropetal chains of 2–4 conidia | Markovskaja and Kačergius [19] |
| P. variicolor | 20–271 × 3–8 μm, simple, macronematous and micronematous, unbranched, mononematous, initially subhyaline, becoming pale to dark brown with age, smooth, occasional rigid hyphae formed but typical conidiophores with a stipe and swollen apex and base lacking | Discrete, determinate, terminal or lateral on the conidiophore, subglobose, ovoid to clavate, often poly-blastic. Terminal conidiogenous cells often form branched, acropetal chains that produce dense clusters of conidia | 7.5–9.5 μm, globose, one-celled, dark brown and verruculose at maturity | Cantrell et al. [45] |
| P. wurfbainiae | 225–315 × 8–14 µm ( = 265 × 10 μm, n = 20), macronematous, mononematous, sometimes 1–2 cluster together on the stroma, erect, subcylindrical, with robust and swollen base, straight or slightly curved, 5–7-septate, black, unbranched, smooth-walled to slightly rough | 7–11 × 5–9 µm ( = 8 × 6 μm, n = 10), holoblastic, poly-blastic, with several prominent conidiogenous loci at the apex, integrated, determinate, terminal and intercalary, reddish brown to black, subcylindrical, verruculose | 3.5–7 µm diam. ( = 5 μm, n = 15), globose, aseptate, catenate, usually 6–8 conidia in a chain, initially hyaline, becoming yellowish brown and greenish brown, and reddish brown to dark brown at maturity, thick-walled and verruculose | This study |
| P. yangjiangensis | 275–420 × 5–15 µm ( = 355 × 10 μm, n = 20), macronematous, mononematous, erect, subcylindrical, tapering towards the apex, with robust base, straight or slightly flexuous, dark brown to black in the lower portion, brown in the upper portion, with 1–4 times of enteroblastic percurrent proliferating, forming 1–4 swollen, pyriform or dumbbell-shaped, dark brown or paler cells from a collar cell, unbranched, sparsely septate, smooth-walled, thick-walled | 15–25 × 8–10 µm ( = 18 × 9 μm, n = 10), holoblastic, poly-blastic, integrated, determinate, terminal, oblong, with rounded apex, wider than conidiophores, brown, smooth-walled, thin-walled | 6–10 µm diam. ( = 7.5 μm, n = 30), globose, catenate, mostly with 3 conidia in a chain, aseptate, initially hyaline, becoming golden brown at maturity, thick-walled, verruculose | This study |
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, X.; Huang, L.; Liang, H.; Hou, C.; Ling, X.; Chen, Y.; Yang, P.; Wu, Q.; Zhao, H.; Wu, S.; et al. Genome-wide identification and functional characterization of borneol dehydrogenases in Wurfbainia villosa. Planta 2023, 258, 69. [Google Scholar] [CrossRef]
- Tian, Y.Q.; Ding, P.; Yan, X.H.; Hu, W.J. Discussion on quality control of preparations with cortex moutanin volume I pharmacopoeia of People’s Republic of China (2005 edition). Zhongguo Zhong Yao Za Zhi 2008, 33, 339–341. [Google Scholar] [PubMed]
- Zeng, Y.; Ali, M.K.; He, W.; Deng, L.; Yang, X.; Li, X.; Pu, X.; Yang, J. Chemical Constituents of Functional Food Amomum villosum to Combat Human Diseases. Curr. Chin. Sci. 2022, 2, 57–67. [Google Scholar] [CrossRef]
- Wu, Z.; Xue, Q.; Miao, P.; Li, C.; Liu, X.; Cheng, Y.; Miao, K.; Yu, Y.; Li, Z. Deep Learning Network of Amomum villosum Quality Classification and Origin Identification Based on X-ray Technology. Foods 2023, 12, 1775. [Google Scholar] [CrossRef]
- Lai, Y.F.; Chen, L.X.; Chen, Y.N.; Zhao, J.; Leong, F.; Li, X.W.; Yang, Q.; Li, P.; Hu, H. Sustainable development of Amomum villosum: A systematic investigation on three different production modes. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, B.; Li, M.; Li, Z.; Tu, Y.; Tang, L.; He, G. Research on germplasm diversity of Amomum villosum. Lour in genuine producing area. PLoS ONE 2022, 17, e0268246. [Google Scholar] [CrossRef]
- Jiang, Z.D.; Chi, P.K. The identification of the fungi deseases of Amomun villosum. J. South China Agric. 1989, 10, 9–16. [Google Scholar]
- Liao, C.F.; Senanayake, I.C.; Dong, W.; Thilini Chethana, K.W.; Tangtrakulwanich, K.; Zhang, Y.X.; Doilom, M. Taxonomic and phylogenetic updates on Apiospora: Introducing four new species from Wurfbainia villosa and Grasses in China. J. Fungi 2023, 9, 1087. [Google Scholar] [CrossRef]
- Tode, H.J. Fungi Mecklenburgenses Selecti. Fasc. II; Generum Novorum Appendicem; Kessinger Publishing, LLC: Lüneburg, Germany, 1791; Volume 2, pp. 1–67. [Google Scholar]
- Hyde, K.D.; Jones, E.B.G.; Liu, J.K.; Ariyawansa, H.; Boehm, E.; Boonmee, S.; Braun, U.; Chomnunti, P.; Crous, P.W.; Dai, D.Q.; et al. Families of Dothideomycetes. Fungal Divers. 2013, 63, 1–313. [Google Scholar] [CrossRef]
- Tanaka, K.; Hirayama, K.; Yonezawa, H.; Sato, G.; Toriyabe, A.; Kudo, H.; Hashimoto, A.; Matsumura, M.; Harada, Y.; Kurihara, Y.; et al. Revision of the Massarineae (Pleosporales, Dothideomycetes). Stud. Mycol. 2015, 82, 75–136. [Google Scholar] [CrossRef]
- Hyde, K.D.; Norphanphoun, C.; Abreu, V.P.; Bazzicalupo, A.; Thilini Chethana, K.W.; Clericuzio, M.; Dayarathne, M.C.; Dissanayake, A.J.; Ekanayaka, A.H.; He, M.Q.; et al. Fungal diversity notes 603–708, taxonomic and phylogenetic notes on genera and species. Fungal Divers. 2017, 87, 1–235. [Google Scholar] [CrossRef]
- Hyde, K.D.; Chaiwan, N.; Norphanphoun, C.; Boonmee, S.; Camporesi, E.; Chethana, K.W.; Dayarathne, M.C.; De Silva, N.I.; Dissanayake, A.J.; Ekanayaka, A.H.; et al. Mycosphere notes 169–224. Mycosphere 2018, 9, 271–430. [Google Scholar] [CrossRef]
- Hyde, K.D.; Tennakoon, D.S.; Jeewon, R.; Bhat, D.J.; Maharachchikumbura, S.S.N.; Rossi, W.; Leonardi, M.; Lee, H.B.; Mun, H.Y.; Houbraken, J.; et al. Fungal diversity notes 1036–1150: Taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Divers. 2019, 96, 1–242. [Google Scholar] [CrossRef]
- Liu, N.G.; Hongsanan, S.; Yang, J.; Bhat, D.J.; Liu, J.; Jumpathong, J.; Liu, Z. Periconia thailandica (Periconiaceae), a new species from Thailand. Phytotaxa 2017, 323, 253–263. [Google Scholar] [CrossRef]
- Yang, E.F.; Phookamsak, R.; Jiang, H.B.; Tibpromma, S.; Bhat, D.J.; Karunarathna, S.C.; Dai, D.Q.; Xu, J.C.; Promputtha, I. Taxonomic reappraisal of Periconiaceae with the description of three new Periconia species from China. J. Fungi 2022, 8, 243. [Google Scholar] [CrossRef]
- Su, P.; Lu, Z.; Tian, W.; Chen, Y.; Maharachchikumbura, S.S.N. Six additions to the genus Periconia (Dothideomycetes: Periconiaceae) from Graminaceous plants in China. J. Fungi 2023, 9, 300. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.B. Dematiaceous Hyphomycetes; Commonwealth Mycological Institute: Kew, UK, 1971; pp. 344–353. [Google Scholar]
- Markovskaja, S.; Kačergius, A. Morphological and molecular characterization of Periconia pseudobyssoides sp. nov. and closely related P. byssoides. Mycol. Prog. 2014, 13, 291–302. [Google Scholar] [CrossRef]
- Calvillo-Medina, R.P.; Cobos-Villagran, A.; Raymundo, T. Periconia citlaltepetlensis sp. nov. (Periconiaceae, Pleosporales): A psychrotolerant fungus from high elevation volcanic glacier (Mexico). Phytotaxa 2020, 459, 235–247. [Google Scholar] [CrossRef]
- Hongsanan, S.; Hyde, K.D.; Phookamsak, R.; Wanasinghe, D.N.; McKenzie, E.H.; Sarma, V.V.; Lücking, R.; Boonmee, S.; Bhat, D.J.; Liu, N.G.; et al. Refined families of Dothideomycetes: Orders and families incertae sedis in Dothideomycetes. Fungal Divers. 2020, 105, 17–318. [Google Scholar] [CrossRef]
- Booth, C. Didymosphaeria igniaria sp. nov., the perfect state of Periconia igniaria. Trans. Br. Mycol. Soc. 1968, 51, 803–805. [Google Scholar] [CrossRef]
- Kohlmeyer, J. Marine fungi of Hawaii including the new genus Helicascus. Can. J. Bot. 1969, 47, 1469–1487. [Google Scholar] [CrossRef]
- Goga, N. Periconia circinata—A new pathogen of roots and stem base of wheat in northwestern Romania. Analele Institutului de Cercetări pentru Cereale și Plante Tehnice Fundulea 2000, 67, 205–214. [Google Scholar]
- Teles, H.L.; Sordi, R.; Silva, G.H.; Castro-Gamboa, I.; da Silva Bolzani, V.; Pfenning, L.H.; de Abreu, L.M.; Costa-Neto, C.M.; Young, M.C.; Araújo, Â.R. Aromatic compounds produced by Periconia atropurpurea, an endophytic fungus associated with Xylopia aromatica. Phytochemistry 2006, 67, 2686–2690. [Google Scholar] [CrossRef]
- Phookamsak, R.; Hyde, K.D.; Jeewon, R.; Bhat, D.J.; Jones, E.B.G.; Maharachchikumbura, S.S.N.; Raspé, O.; Karunarathna, S.C.; Wanasinghe, D.N.; Hongsanan, S.; et al. Fungal diversity notes 929–1035: Taxonomic and phylogenetic contributions on genera and species of fungi. Fungal Divers. 2019, 95, 1–273. [Google Scholar] [CrossRef]
- Gunasekaran, R.; Janakiraman, D.; Rajapandian, S.G.; Appavu, S.P.; Venkatesh, P.N.; Prajna, L. Periconia species-An unusual fungal pathogen causing mycotic keratitis. Indian J. Med. Microbiol. 2021, 39, 36–40. [Google Scholar] [CrossRef]
- Numata, A.; Iritani, M.; Yamada, T.; Minoura, K.; Matsumura, E.; Yamori, T.; Tsuruo, T. Novel antitumour metabolites produced by a fungal strain from a sea hare. Tetrahedron Lett. 1997, 38, 8215–8218. [Google Scholar] [CrossRef]
- Kolomiets, T.; Pankratova, L.; Mukhina, Z.; Kassanelly, D.; Matveeva, T.; Bogomaz, D.; Berner, D. First report of leaf spot caused by Periconia igniaria on yellow starthistle in Russia. Plant Dis. 2008, 92, 983. [Google Scholar] [CrossRef]
- Sarkar, T.; Chakraborty, P.; Karmakar, A.; Saha, A.; Saha, D. First report of Periconia macrospinosa causing leaf necrosis of pointed gourd in India. J. Plant Pathol. 2019, 101, 1281. [Google Scholar] [CrossRef]
- Azhari, A.; Supratman, U. The chemistry and pharmacology of fungal genus Periconia: A review. Sci. Pharm. 2021, 89, 34. [Google Scholar] [CrossRef]
- Wu, Y.H.; Chen, G.D.; He, R.R.; Wang, C.X.; Hu, D.; Wang, G.Q.; Guo, L.D.; Yao, X.S.; Gao, H. Pericolactines A–C, a new class of diterpenoid alkaloids with unusual tetracyclic skeleton. Sci. Rep. 2015, 5, 17082. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Chen, G.D.; Wang, C.X.; Hu, D.; Li, X.X.; Lian, Y.Y.; Lin, F.; Guo, L.D.; Gao, H. Pericoterpenoid A, a new bioactive cadinane-type sesquiterpene from Periconia sp. J. Asian Nat. Prod. Res. 2015, 17, 671–675. [Google Scholar] [CrossRef]
- Wu, Y.H.; Xiao, G.K.; Chen, G.D.; Wang, C.X.; Hu, D.; Lian, Y.Y.; Lin, F.; Guo, L.D.; Yao, X.S.; Gao, H. Pericocins A–D, new bioactive compounds from Periconia sp. Nat. Prod. Commun. 2015, 10, 2127–2130. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, M.; Chen, R.; Xie, K.; Chen, D.; Si, S.; Dai, J. Three new compounds from endophytic fungus Periconia sp. F-31. J. Chin. Pharm. Sci. 2020, 29, 244–251. [Google Scholar]
- Kim, S.; Shin, D.S.; Lee, T.; Oh, K.B. Periconicins, two new fusicoccane diterpenes produced by an endophytic fungus Periconia sp. with antibacterial activity. J. Nat. Prod. 2004, 67, 448–450. [Google Scholar] [CrossRef]
- Senanayake, I.C.; Rathnayaka, A.R.; Marasinghe, D.S.; Calabon, M.S.; Gentekaki, E.; Lee, H.B.; Hurdeal, V.G.; Pem, D.; Dissanayake, L.S.; Wijesinghe, S.N.; et al. Morphological approaches in studying fungi: Collection, examination, isolation, sporulation and preservation. Mycosphere 2020, 11, 2678–2754. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: Cambridge, MA, USA, 1990; Volume 18, pp. 315–322. [Google Scholar]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- Rehner, S.A. Primers for Elongation Factor 1-Alpha (EF1-Alpha). 2001. Available online: http://ocid.NACSE.ORG/research/ (accessed on 30 December 2023).
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef]
- Darriba, D.; Posada, D. jModelTest 2.0 Manual v0. 1.1. 2014. Available online: https://github.com/ddarriba/jmodeltest2 (accessed on 30 December 2023).
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; IEEE: New Orleans, LA, USA, 2010; pp. 1–8. [Google Scholar]
- Rambaut, A. FigTree v1.4: Tree Figure Drawing Tool; Institute of Evolutionary Biology, University of Edinburgh: Edinburgh, UK, 2012; Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 30 December 2023).
- Cantrell, S.A.; Hanlin, R.T.; Emiliano, A. Periconia variicolor sp. nov., a new species from Puerto rico. Mycologia 2007, 99, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Luangsa-Ard, J.J.; Wingfield, M.J.; Carnegie, A.J.; Hernández-Restrepo, M.; Lombard, L.; Roux, J.; Barreto, R.W.; Baseia, I.G.; Cano-Lira, J.F.; et al. Fungal Planet description sheets: 785–867. Persoonia 2018, 41, 238–417. [Google Scholar] [CrossRef]
- Ellis, M.B. More Dematiaceous Hyphomycetes; Commonwealth Mycological Institute: Kew, UK, 1976; p. 28. [Google Scholar]
- Coronado-Ruiz, C.; Avendaño, R.; Escudero-Leyva, E.; Conejo-Barboza, G.; Chaverri, P.; Chavarría, M. Two new cellulolytic fungal species isolated from a 19th-century art collection. Sci. Rep. 2018, 8, 7492. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfield, M.J.; Lombard, L.; Roets, F.; Swart, W.J.; Alvarado, P.; Carnegie, A.J.; Moreno, G.; Luangsaard, J.; Thangavel, R.; et al. Fungal Planet description sheets: 951–1041. Persoonia 2019, 43, 223–425. [Google Scholar] [CrossRef] [PubMed]
- Knapp, D.G.; Kovács, G.M.; Zajta, E.; Groenewald, J.Z.; Crous, P.W. Dark septate endophytic pleosporalean genera from semiarid areas. Persoonia 2015, 35, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Wijayawardene, N.N.; Hyde, K.D.; Dai, D.Q.; Sanchez-Garcia, M.; Goto, B.T.; Magurno, F. Outline of Fungi and fungus-like taxa–2021. Mycosphere 2022, 13, 53–453. [Google Scholar] [CrossRef]
- Wu, W.P.; Zhuang, W.Y. Sporidesmium, Endophragmiella and related genera from China. Fungal Divers. 2005, 15, 1–351. [Google Scholar]
- Shenoy, B.D.; Jeewon, R.; Wu, W.P.; Bhat, D.J.; Hyde, K.D. Ribosomal and RPB2 DNA sequence analyses suggest that Sporidesmium and morphologically similar genera are polyphyletic. Mycol. Res. 2006, 110, 916–928. [Google Scholar] [CrossRef]

| Species Name | Strain Number | GenBank Accession Number | |||
|---|---|---|---|---|---|
| ITS | LSU | SSU | tef1-α | ||
| Flavomyces fulophazae | CBS 135664 | KP184000 | KP184039 | KP184081 | – |
| F. fulophazae T | CBS 135761 | NR_137960 | NG_058131 | NG_061191 | – |
| Lentithecium aquaticum T | CBS 123099 | NR_160229 | NG_064211 | NG_016507 | GU349068 |
| L. clioninum T | KT 1149A | LC014566 | AB807540 | AB797250 | AB808515 |
| L. clioninum | KT 1220 | LC014567 | AB807541 | AB797251 | AB808516 |
| Massarina cisti T | CBS 266.62 | – | AB807539 | AB797249 | AB808514 |
| M. eburnea | CBS 473.64 | – | GU301840 | GU296170 | GU349040 |
| Morosphaeria ramunculicola | KH 220 | – | AB807554 | AB797264 | AB808530 |
| M. velatispora | KH 221 | LC014572 | AB807556 | AB797266 | AB808532 |
| Periconia alishanica | KUMCC 19-0174 | MW063167 | MW063231 | – | MW183792 |
| P. alishanica T | MFLUCC 19-0145 | MW063165 | MW063229 | – | MW183790 |
| P. ananasi T | MFLUCC 21-0155 | OL753685 | OL606153 | OL606142 | OL912946 |
| P. ananasi | KUMCC 21-0470 | OM102539 | OL985955 | OL979226 | OM007977 |
| P. aquatica T | MFLUCC 16-0912 | KY794701 | KY794705 | – | KY814760 |
| P. artemisiae T | KUMCC 20-0265 | MW448657 | MW448571 | MW448658 | MW460898 |
| P. banksiae T | CBS 129526 | – | NG_064279 | – | – |
| P. byssoides | KUMCC 20-0264 | MW444854 | MW444855 | MW444856 | MW460895 |
| P. byssoides | MFLUCC 17-2292 | MK347751 | MK347968 | MK347858 | MK360069 |
| P. byssoides | MFLUCC 18-1548 | MK347794 | MK348013 | MK347902 | MK360070 |
| P. byssoides | MFLUCC 18-1553 | MK347806 | MK348025 | MK347914 | MK360068 |
| P. byssoides T | MFLUCC 20-0172 | MW063162 | MW063226 | – | – |
| P. byssoides | NCYUCC 19-0314 | MW063163 | MW063227 | – | – |
| P. caespitosa T | LAMIC 110 16 | MH051906 | MH051907 | – | – |
| P. chengduensis T | CGMCC 3.23930 | OP955987 | OP956012 | OP956056 | OP961453 |
| P. chengduensis | UESTCC 22.0140 | OP955977 | OP956002 | OP956046 | OP961443 |
| P. chengduensis | UESTCC 22.0142 | OP955978 | OP956003 | OP956047 | OP961444 |
| P. chimonanthi T | KUMCC 20-0266 | NR_176752 | MW448572 | MW448656 | MW460897 |
| P. chimonanthi | UESTCC 22.0133 | OP955964 | OP955989 | OP956033 | OP961430 |
| P. citlaltepetlensis T | IOM 325319 | MH890645 | MT625978 | – | – |
| P. citlaltepetlensis | IOM 325319.2 | MT649221 | MT649216 | – | – |
| P. cookei | MFLUCC 17-1399 | MG333490 | MG333493 | – | MG438279 |
| P. cookei | MFLUCC 17-1679 | – | MG333492 | – | MG438278 |
| P. cortaderiae | MFLUCC 15-0451 | KX965734 | KX954403 | KX986346 | KY429208 |
| P. cortaderiae T | MFLUCC 15-0457 | KX965732 | KX954401 | KX986345 | KY310703 |
| P. cynodontis T | CGMCC 3.23927 | OP909925 | OP909921 | OP909920 | OP961434 |
| P. cyperacearum T | CPC 32138 | NR_160357 | NG_064549 | – | – |
| P. delonicis T | MFLUCC 17-2584 | – | NG_068611 | NG_065770 | MK360071 |
| P. didymosporum T | MFLU 15-0058 | KP761734 | KP761731 | KP761738 | KP761728 |
| P. digitata | CBS 510.77 | LC014584 | AB807561 | AB797271 | AB808537 |
| P. elaeidis T | MFLUCC 17-0087 | MG742713 | MH108552 | MH108551 | – |
| P. endophytica T | ZHKUCC 23-0995 | OR995582 | OR995588 | PP277722 | PP025968 |
| P. endophytica | ZHKUCC 23-0996 | OR995583 | OR995589 | PP277723 | PP025969 |
| P. epilithographicola T | CBS 144017 | NR_157477 | – | – | – |
| P. epilithographicola | MFLUCC 21-0153 | OL753687 | OL606155 | OL606144 | OL912948 |
| P. festucae T | CGMCC 3.23929 | OP955973 | OP955998 | OP956042 | OP961439 |
| P. philadelphiana T | CPC 42854 | OQ628486 | OQ629068 | – | – |
| P. homothallica T | KT 916 | AB809645 | AB807565 | AB797275 | |
| P. igniaria | CBS 845.96 | LC014586 | AB807567 | AB797277 | AB808543 |
| P. imperatae T | CGMCC 3.23931 = UESTCC 22.0129 | OP955984 | OP956009 | OP956053 | OP961450 |
| P. imperatae | UESTCC 22.0145 | OP955979 | OP956004 | OP956048 | OP961445 |
| P. imperatae | UESTCC 22.0146 | OP955983 | OP956008 | OP956052 | OP961449 |
| P. macrospinosa | CBS 135663 | KP183999 | KP184038 | KP184080 | – |
| P. minutissima | MFLUCC 15-0245 | KY794703 | KY794707 | – | – |
| P. minutissima | MUT 2887 | MG813227 | – | – | – |
| P. neobrittanica T | CPC 37903 | NR_166344 | NG_068342 | – | – |
| P. neominutissima T | CPC 42368 | OQ628478 | OQ629060 | – | – |
| P. palmicola T | MFLUCC 14-0400 | – | NG_068917 | MN648319 | MN821070 |
| P. penniseti T | CGMCC 3.23928 | OP955971 | OP955996 | OP956040 | OP961437 |
| P. prolifica | DBOF74 | JQ724435 | – | – | – |
| P. prolifica | DBOF129 | JQ724490 | – | – | – |
| P. pseudobyssoides | DUCC 0850 | MG333491 | MG333494 | – | MG438280 |
| P. pseudobyssoides | KUMCC 20-0263 | MW444851 | MW444852 | MW444853 | MW460894 |
| P. pseudodigitata | KT 644 | LC014589 | AB807562 | AB797272 | AB808538 |
| P. pseudodigitata T | KT 1395 | NR_153490 | NG_059396 | NG_064850 | AB808540 |
| P. spodiopogonis | CGMCC 3.23932 | OP955963 | OP955988 | OP956032 | OP961429 |
| P. salina T | MFLU 19-1235 | MN047086 | MN017846 | MN017912 | – |
| P. submersa T | MFLUCC 16-1098 | KY794702 | KY794706 | – | KY814761 |
| P. thailandica T | MFLUCC 17-0065 | KY753887 | KY753888 | KY753889 | – |
| P. thysanolaenae T | KUMCC 20-0262 | MW442967 | MW444850 | MW448659 | MW460896 |
| P. variicolor T | SACCR-64 | DQ336713 | – | – | – |
| P. verrucosa T | MFLUCC 17-2158 | MT310617 | MT214572 | MT226686 | MT394631 |
| P. verrucosa | UESTCC 22.0149 | OP955976 | OP956001 | OP956045 | OP961442 |
| P. verrucosa | UESTCC 22.0150 | OP955980 | OP956005 | OP956049 | OP961446 |
| P. wurfbainiae T | ZHKUCC 23-0999 | OR995586 | OR995592 | PP277726 | PP025972 |
| P. wurfbainiae | ZHKUCC 23-1000 | OR995587 | OR995593 | PP277727 | PP025973 |
| P. yangjiangensis T | ZHKUCC 23-0997 | OR995584 | OR995590 | PP277724 | PP025970 |
| P. yangjiangensis | ZHKUCC 23-0998 | OR995585 | OR995591 | PP277725 | PP025971 |
| Sporidesmium tengii | HKUCC 10837 | – | DQ408559 | – | – |
| Taxa | Ascomata | Peridium | Asci | Ascospores | |
|---|---|---|---|---|---|
| Periconia homothallica | 140–190 μm high, 160–180 μm diam., scattered, immersed to erumpent, globose, with an ostiole | Longitudinal section uniformly 11–15 μm thick, composed of 4–6 layers of polygonal, thin-walled, 3–15 × 2–5 μm, pale brown cells | 85–119.5 × 13–17.5 μm ( 96.5 × 15.3 μm, n = 20), fissitunicate, cylindrical to lageniform, with a shallow ocular chamber, short-stalked (3.5–6 μm long), with 8 biseriate ascospores | 22–31 × 7–10 μm ( 26.3 × 8.7 μm, n = 60), l/w 2.6–3.7 ( 3.0, n = 60), broadly fusiform, with a nearly median septum (0.48–0.53; 0.51, n = 38), hyaline, smooth, with an entire sheath; sheath gelatinous, up to 10 μm wide when fresh, later 1–2 μm wide | Tanaka et al. [11] |
| P. pseudodigitata | 160–200 μm high, 130–250 μm diam., numerous, scattered or 2–3 grouped, immersed to erumpent, globose | Longitudinal section 8–13 μm thick at side, 5–8 μm thick at the base, composed of 3–5 layers of thin-walled, 6–13 × 2–5 μm, brown cells | 70–110 × 10.5–15.5 μm ( 88.4 × 12.2 μm, n = 33), fissitunicate, cylindrical, rounded at the apex and with an apical chamber, short-stalked (5–15 μm long), with 8 irregularly biseriate ascospores | 19.5–27(–32) × 5–7 μm ( 22.5 × 6.1 μm, n = 134), l/w 2.9–4.5 ( 3.7, n = 134), broadly fusiform with rounded ends, straight or slightly curved, with almost median septum (0.48–0.55, 5.1, n = 36), slightly constricted at the septum, hyaline, with or without guttules, smooth, with an entire sheath; sheath gelatinous, 1–2 μm wide at side and 2–4 μm wide at both ends in fresh, becoming delimited sheath in dry condition; senescent spores brown, echinulate, 1-septate | Tanaka et al. [11] |
| P. wurfbainiae | 220–300 × 150–220 µm, solitary to scattered, immersed to semi-immersed, pyriform, black, papillate stain the substrate purple | 13–30 µm thick ( = 19 μm, n = 20), composed of 6–12 layers, hyaline to brown cells of textura angularis | 65–100 × 9.5–15 µm ( = 81 × 12 μm, n = 20), 8-spored, bitunicate, cylindrical, with rounded apex and narrower, short pedicellate, with an obscured ocular chamber | 20–26 × 5–8 µm ( = 23.5 × 7 μm, n = 30), overlapping uniseriate, biseriate in the middle portion, fusiform, slightly curved, 1-septate, slightly constricted at the septum, narrowly rounded at both ends, hyaline, guttulate, smooth-walled, surrounded by a mucilaginous sheath | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, C.; Hyde, K.D.; Thilini Chethana, K.W.; Dong, W.; Yang, Y.; Doilom, M. Three New Periconia Species Isolated from Wurfbainia villosa in Guangdong, China: A Discussion on the Doubtful Taxa Clustering in this Genus. Diversity 2024, 16, 141. https://doi.org/10.3390/d16030141
Liao C, Hyde KD, Thilini Chethana KW, Dong W, Yang Y, Doilom M. Three New Periconia Species Isolated from Wurfbainia villosa in Guangdong, China: A Discussion on the Doubtful Taxa Clustering in this Genus. Diversity. 2024; 16(3):141. https://doi.org/10.3390/d16030141
Chicago/Turabian StyleLiao, Chunfang, Kevin D. Hyde, Kandawatte Wedaralalage Thilini Chethana, Wei Dong, Yunhui Yang, and Mingkwan Doilom. 2024. "Three New Periconia Species Isolated from Wurfbainia villosa in Guangdong, China: A Discussion on the Doubtful Taxa Clustering in this Genus" Diversity 16, no. 3: 141. https://doi.org/10.3390/d16030141
APA StyleLiao, C., Hyde, K. D., Thilini Chethana, K. W., Dong, W., Yang, Y., & Doilom, M. (2024). Three New Periconia Species Isolated from Wurfbainia villosa in Guangdong, China: A Discussion on the Doubtful Taxa Clustering in this Genus. Diversity, 16(3), 141. https://doi.org/10.3390/d16030141






