Age Difference, Not Food Scarcity or Sibling Interactions, May Drive Brood Reduction in Wild Scarlet Macaws in Southeastern Peru
Abstract
1. Introduction
1.1. Siblicide
1.2. Filial Infanticide
2. Materials and Methods
2.1. Study Site
2.2. Background Methodology
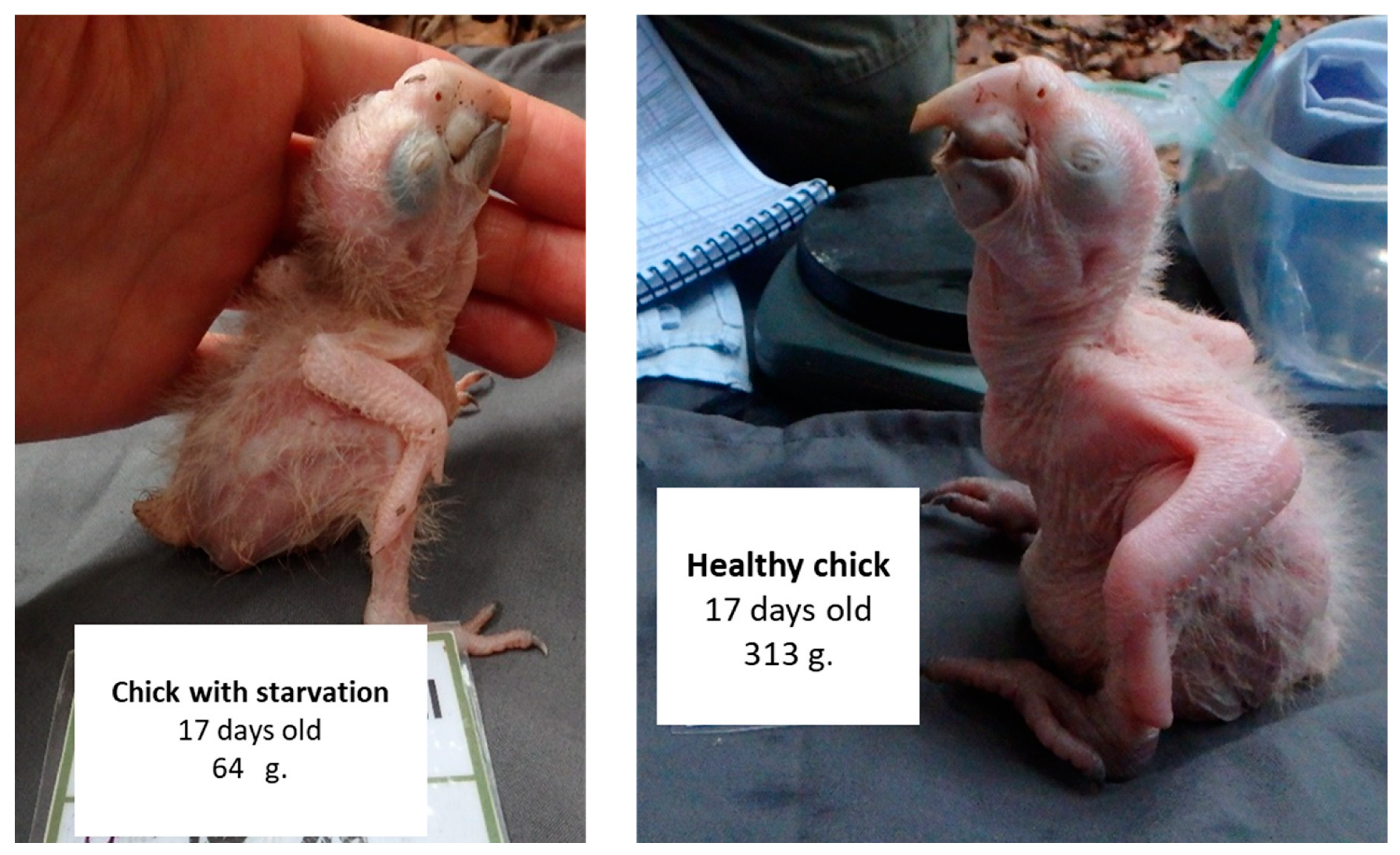
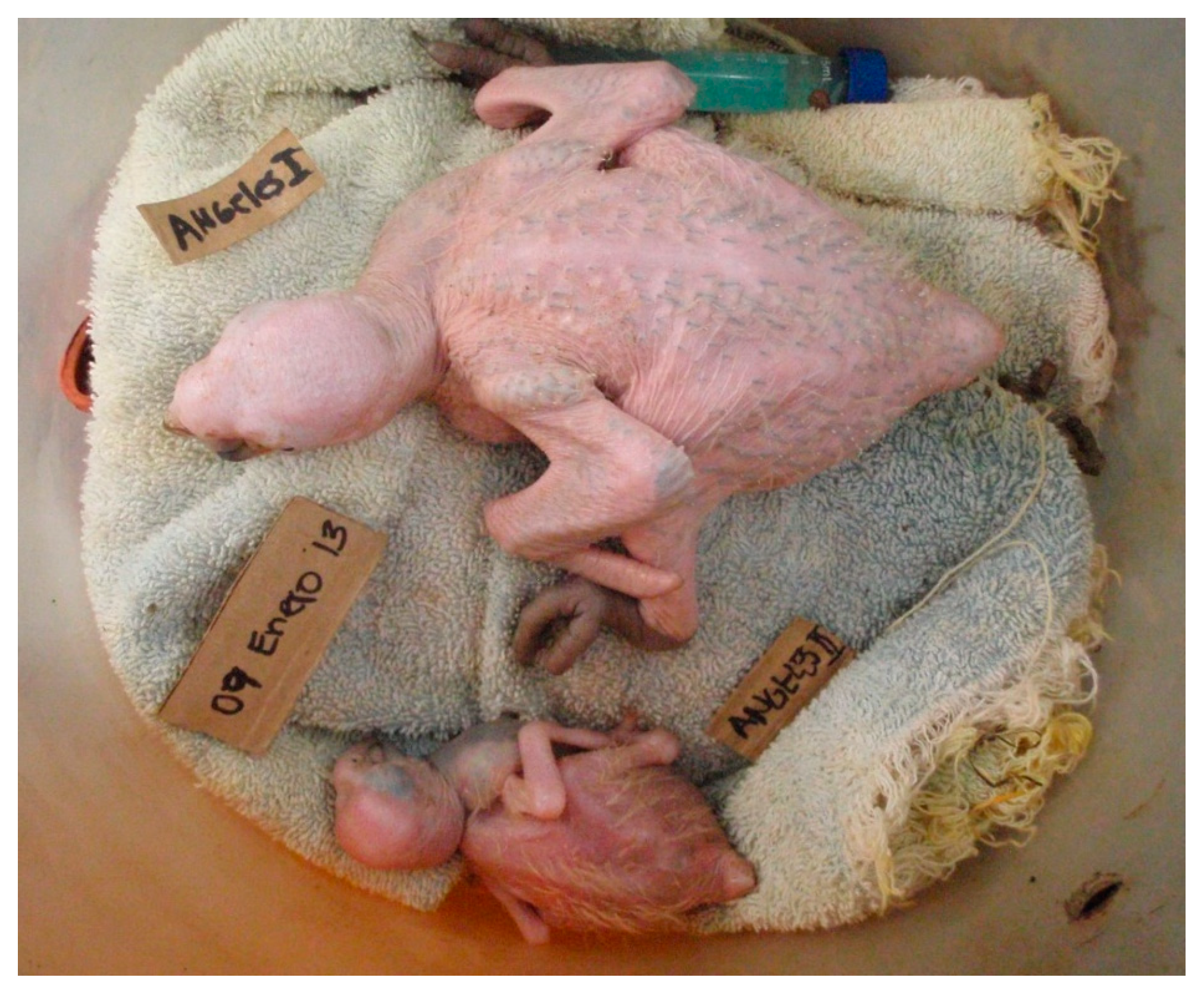
2.3. Section 1: General Description of Macaw Chick Starvation
2.3.1. Weight at Hatch
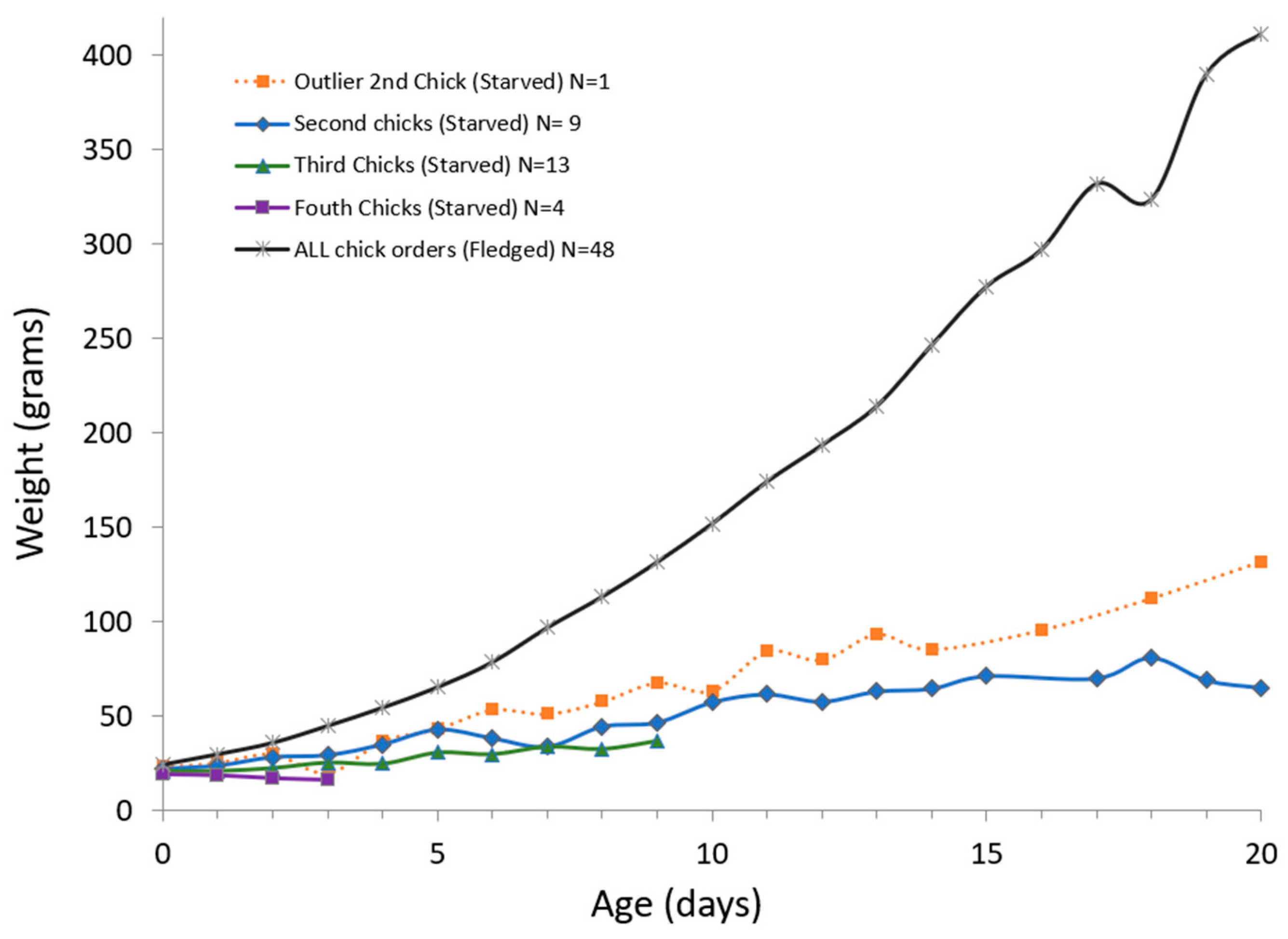
2.3.2. Growth Description of Chicks That Starved
2.4. Section 2: Drivers of Second Chick Starvation
2.4.1. Chick–Chick Interaction Analysis
2.4.2. Parent–Environment Interaction Analysis
2.4.3. Parent–Chick Interaction Analysis
3. Results
3.1. Section 1: General Description of Macaw Chick Starvation
3.2. Section 2: Drivers of Second Chick Starvation
3.2.1. Chick–Chick Interaction
3.2.2. Parent–Environment Interaction
3.2.3. Parent–Chick Interaction
4. Discussion
4.1. Section 1: General Description of Macaw Chick Starvation
4.2. Section 2: Drivers of Second Chick Starvation
4.2.1. Chick–Chick Interaction Analysis: Siblicide
4.2.2. Parent–Environment Interaction Analysis
4.2.3. Parent–Chick Interaction Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| English Name | Scientific Name | Location Reported | Source |
|---|---|---|---|
| Hyacinth Macaw | Anodorhunchus hyacinthinus | Brazil | [73] |
| Green-wing Macaw | Ara chloropterus | Peru | [74] |
| Scarlet Macaw | Ara macao | Mexico | [75] |
| Guatemala | [35] | ||
| Costa Rica | [34,76] | ||
| Peru | This study, [37,39] | ||
| Blue-and-yellow Macaw | Ara ararauna | Peru | [77,78] |
| Yellow-headed Amazon | Amazona oratrix | Mexico | [12] |
| Red-crowned Amazon | Amazona autumnales | Mexico | [12] |
| Red-lored Amazon | Amazona viridiginales | Mexico | [12] |
| Black-billed Amazon | Amazona agilis | Jamaica | [14] |
| Yellow-billed Amazon | Amazona collaria | Jamaica | [14] |
| Puerto Rican Amazon | Amazona vittata | Puerto Rico | [64] |
| Yellow-Shouldered Amazon | Amazona barbadensis | Venezuela | [79] |
| Red-spectacled Amazon | Amazona petrei | Brazil | [80] |
| Red-tailed Amazon | Amazona brasilensis | Brazil | [80] |
| Blue-fronted Amazon | Amazona aestiva | Brazil | [81] |
| Monk Parakeet | Miopsitta monachhus | Argentina | [82] |
| Burrowing Parrot | Cyaniliseus patagonus | Argentina | [23] |
| Green-rumped Parrotlet | Forpus passerinus | Venezuela | [13,69] |
| Black-cockatoo | Zanda sp | Australia | [22] |
| Red-tailed black cockatoo | Calyptorhynchus magnificus | Australia | [83] |
| Long-billed Corella | Cacatua pastinator | Australia | [84] |
| Galah | Cacatua roseicapilla | Australia | [83,85] |
| Crimson Rosella | Platycercus elegans | Australia | [16] |
| Ouvea Parakeet | Eunymphicus cornutus uvaeensis | New Caledonia | [86] |
References
- Stanning, M.J. Hatching asynchrony, brood reduction and other rapidly reproducing hypotheses. Trends Ecol. Evol. 1996, 11, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Lack, D. The significance of clutch-size. IBIS 1947, 89, 302–352. [Google Scholar] [CrossRef]
- Stoleson, S.; Beissinger, S. Hatching asynchrony and the onset of incubation in birds, revisited. In Current Ornithology; Power, D.M., Ed.; Springer Science + Business Media: New York, NY, USA, 1995; pp. 191–271. [Google Scholar]
- Clark, A.B.; Wilson, D.S. Avian Breeding Adaptations: Hatching Asynchrony, Brood Reduction, and Nest Failure. Q. Rev. Biol. 1981, 56, 253–277. [Google Scholar] [CrossRef]
- Lack, D. The Natural Regulation of Animal Numbers; Clarendon: Oxford, UK, 1954. [Google Scholar]
- Ricklefs, R. Brood reduction in the Curve-billed Thrasher. Condor 1965, 67, 505–510. [Google Scholar] [CrossRef]
- Marc, B. Food suply and the occurence of Brood Reduction in Swainson’s Hawk. Wilson Bull. 1983, 95, 233–242. [Google Scholar]
- Drummond, H.; Havelas, C.G. Food shortage influences sibling aggresion in the blue-footed booby. Anim. Behav. 1989, 37, 806–819. [Google Scholar] [CrossRef]
- Drummond, H. A review of parent-offspring conflict and brood reduction in the Pelicaniformes. Colon. Waterbirds 1987, 10, 1–15. [Google Scholar] [CrossRef]
- Browne, T.; Lalas, C.; Matterns, T.; Vann Heezik, Y. Chick starvation in yellow-eyed penguins: Evidence for poor diet quality and selective provisioning of chicks from conventional diet analysis and stable isotopes. Austral Ecol. 2011, 36, 99–108. [Google Scholar] [CrossRef]
- Harper, R.G.; Julianho, S.A.; Thompson, C. Hatching asynchrony in the house wren. Behav. Ecol. Sociobiol. 1992, 3, 76–83. [Google Scholar] [CrossRef]
- Enkerlin-Hoeflich, E.C. Comparitive Ecology and Reproductive Biology of Three Species of Amazona parrots in Northeastern Mexico; Texas A&M University: College Station, TX, USA, 1995. [Google Scholar]
- Stoleson, S.H.; Beissinger, S.R. Hatching asynchrony, brood reduction, and food limitation in a Neotropical parrot. Ecol. Monogr. 1997, 76, 131–154. [Google Scholar] [CrossRef]
- Koenig, S.E. The breeding biology of Black-billed Parrot Amazona agilis and Yellow-billed Parrot Amazona collaria in Cockpit Country, Jamaica. Bird Conserv. Int. 2001, 11, 205–225. [Google Scholar] [CrossRef]
- Krebs, E. Breeding biology of crimson rosellas (Platycercus elegans) on Black Mountain Australian Capital Territory. Aust. J. Zool. 1998, 46, 119–136. [Google Scholar] [CrossRef]
- Krebs, E.A. Last but not least: Nestling growth and survival in asynchronously hatching Crimson Rosellas. J. Anim. Ecol. 1999, 68, 266–281. [Google Scholar] [CrossRef]
- Viñuela, J. Opposing selectie pressures on hatching asynchronoy: Egg viability, brood reduction and nestling growth. Behav. Ecol. Sociobiol. 2000, 48, 333–343. [Google Scholar]
- Simmons, R.E. Offspring quality and the evolution of cainism. Ibis 1988, 130, 339–357. [Google Scholar] [CrossRef]
- Moreno, J. Parental Infanticide in birds through early eviction from the nest: Rare or under-reported? J. Anim. Ecol. 2012, 43, 43–49. [Google Scholar] [CrossRef]
- Tortosa, F.S.; Redondo, T. Motives for Parental Infanticide in White Storks Ciconia ciconia. Ornis Scand. (Scand. J. Ornithol.) 1992, 23, 185–189. [Google Scholar] [CrossRef]
- Heinsohn, R.; Langmore, N.; Cockburn, A.; Kokko, H. Adaptative secondary sex ratio adjustments via sex-specific infanticidae in birds. Curr. Biol. 2011, 21, 1744–1747. [Google Scholar] [CrossRef]
- Saunders, D.A. The breeding behaviour and biology of the short-billed form of the White-tailed Black Cockatoo Calyptorhynchus funereus. Ibis 1982, 124, 422–455. [Google Scholar] [CrossRef]
- Masello, J.F.; Quillfeldt, P. Chick growth and breeding success of the burrowing parrot. Condor 2002, 104, 574–586. [Google Scholar] [CrossRef]
- Mock, D.W. Parent-offspring conflict, an unequal parental investment by egrets and herons. Behav. Ecol. Sociobiol. 1987, 20, 247–256. [Google Scholar] [CrossRef]
- Morandini, V.; Ferrer, M. Sibling agression and brood reduction: A review. Ethol. Ecol. Evol. 2015, 27, 2–16. [Google Scholar] [CrossRef]
- Krebs, E. Sibling competition and parental control: Patterns of begging in parrots. In The Evolution of Begging; Wright, J., Leonard, M.L., Eds.; Kluwer Academic Publishers: New York, NY, USA, 2002; pp. 319–336. [Google Scholar]
- Alastair, F.; Galipeau, P.; Nikolaiczuk, L. Brood reduction by Infanticidae in Peregrine Falcon. Artic 2013, 66, 226–229. [Google Scholar]
- Beissinger, S. Long term studies of the Green Rumped Parrotlet. Hatching asynchrony, social system and population structure. Ornitol. Neotrop. 2008, 73, 73–83. [Google Scholar]
- Braun, B.; Hunt, G.L. Brood Reduction in Black-Legged Kittiwakes. Auk 1983, 100, 469–476. [Google Scholar] [CrossRef]
- Krist, M. Egg size and offspring quality: A meta-analysis in birds. Biol. Rev. 2011, 86, 692–716. [Google Scholar] [CrossRef]
- Voren, H.; Jordan, R. Maintenance of Nestlings (Brooding). In Parrots: Hand Feeding & Nursery Management; Silvio Mattacchione, & Company: Port Perry, ON, Canada, 1992. [Google Scholar]
- Clubb, S.L.; Clubb, K.J.; Skidmore, D.; Wolf, S.; Phillips, A. Psittacine neonatal care and hand-feeding. In Psittacine Aviculture: Perspectives, Techniques and Research; Shubot, R.M., Clubb, K.J., Clubb, S.L., Eds.; Avicultural Breeding and Research Center: Loxahatchee, FL, USA, 1992. [Google Scholar]
- Vigo, G.; Williams, M.; Brightsmith, D.J. Growth of Scarlet Macaw (Ara macao) chicks in southeastern Peru. Neotrop. Ornithol. 2011, 22, 143–153. [Google Scholar]
- Vaughan, C. Conservacion de la lapa roja (Ara macao) con manejo in situ en el Pacifico Central de Costa Rica. Rev. Cienc. Ambient. 2019, 53, 166–188. [Google Scholar] [CrossRef]
- Boyd, J.; McNab, R.B. The Scarlet Macaw in Guatemala and El Salvador: 2008 Status and Future Possibilities. Findings and Recommendations from a Species Recovery Workshop 9–15 March 2008; Wildlife Conservation Society—Guatemala Program: Guatemala City and Flores, Peten, Guatemala, 2008; p. 178. [Google Scholar]
- Britt, C.; Anleu, R.G.; Desmond, M.J. Nest survival of a long=lived psittacid: Scarlet Macaws (Ara macao cyanoptera) in the Maya Biosphere Reserve of Guatemala and Chiquibul Forest of Belize. Condor Ornithol. Appl. 2014, 116, 265–274. [Google Scholar] [CrossRef]
- Nycander, E.; Blanco, D.H.; Holle, K.M.; Campo, A.D.; Munn, C.A.; Moscoso, J.I. Manu and Tambopata: Nesting success and techniques for increasing reproduction in wild macaws in southeastern Peru. In The Large Macaws: Their Care, Breeding and Conservation; Abramson, J., Spear, B.L., Thomsen, J.B., Eds.; Raintree Publications: Fort Bragg, CA, USA, 1995; pp. 423–443. [Google Scholar]
- Olah, G.; Vigo, G.; Heinsohn, R.; Brightsmith, D.J. Nest site selection and efficacy of artificial nests for breeding success of Scarlet Macaws (Ara macao) in lowland Peru. J. Nat. Conserv. 2014, 22, 176–185. [Google Scholar] [CrossRef]
- Vigo Trauco, G. Crecimiento de Pichones de Guacamayo Escarlata, Ara macao (Linneus: 1758) en la Reserva Nacional Tambopata—Madre de Dios—Perú. Professional. Licensure Thesis, Universidad Nacional Agraria La Molina, Lima, Peru, 2007. [Google Scholar]
- Vigo, G. Scarlet Macaw Nesting Ecology and Behavior: Implications for Conservation Management. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 2020. [Google Scholar]
- Tosi, J.A. Zonas de Vida Natural en el Perú. Memoria Explicativa Sobre el Mapa Ecológico del Perú; Instituto Interamericano de las Ciencias Agricolas de la Organización de los Estados Americanos: Lima, Peru, 1960; p. 271. [Google Scholar]
- Brightsmith, D.J. Effects of weather on avian geophagy in Tambopata, Peru. Wilson Bull. 2004, 116, 134–145. [Google Scholar] [CrossRef]
- Perry, D.R. A method of access into the crowns of emergent and canopy trees. Biotropica 1978, 10, 155–157. [Google Scholar] [CrossRef]
- Perry, D.R.; Williams, J. The tropical rain forest canopy: A method providing total access. Biotropica 1981, 13, 283–285. [Google Scholar] [CrossRef]
- Purcell, K.L.; Verner, J.; Oring, L.W. A Comparison of the Breeding Ecology of Birds Nesting in Boxes and Tree Cavities. Auk 1997, 114, 646–656. [Google Scholar] [CrossRef]
- Sudyka, J.; Di Lecce, I.; Szulkin, M. Microclimate shifts in nest-boxes and natural cavities throughout reproduction. J. Avian Biol. 2023, 2023, e03000. [Google Scholar] [CrossRef]
- Stamps, J.; Clark, A.B.; Arrowood, P.; Kus, B. Parent-offspring conflict in Budgerigars. Behav. Ecol. Sociobiol. 1984, 94, 40. [Google Scholar] [CrossRef]
- Lee, A.T.K.; Brightsmith, D.J.; Vargas, M.P.N.; Leon, K.Q.; Mejia, A.J.R.; Huanca, L.Y.Q.; Lee, A.K.; Marsden, S.J. Diet and geophagy across a western Amazonian parrot assemblage. Biotropica 2014, 46, 322–330. [Google Scholar] [CrossRef]
- Vaughan, C.; Nemeth, N.; Marineros, L. Scarlet macaw, Ara macao, (Psittaciformes: Psittacidae) diet in Central Pacific Costa Rica. Rev. Biol. Trop. 2006, 54, 919–926. [Google Scholar] [CrossRef]
- Brightsmith, D.J.; McDonald, D.; Matsufuji, D.; Matuzak, G. Diets of Wild Psittacines in Tambopata, Peru. A Progress Report to South Lakes Wildlife Animal Park; Duke University Department of Biology: Durham, NC, USA, 2006. [Google Scholar]
- Brightsmith, D.J.; Hobson, E.A.; Martinez, G. Food availability and breeding season as predictors of geophagy in Amazonian parrots. Ibis 2018, 160, 112–129. [Google Scholar] [CrossRef]
- Gilardi, J.D. Ecology of Parrots in the Peruvian Amazon: Habitat Use, Nutrition, and Geophagy. Ph.D. Dissertation, UC Davis, Davis, CA, USA, 1996. [Google Scholar]
- Gilardi, J.D.; Toft, C.A. Parrots Eat Nutritious Foods despite Toxins. PLoS ONE 2012, 7, e38293. [Google Scholar] [CrossRef]
- Brightsmith, D.J. Unpublished Database of Observations of Parrots Foraging in Southeastern Peru; Texas A&M University: College Station, TX, USA, 2000–2020. [Google Scholar]
- SAS Institute. JMP, Version 16.0.0; SAS Institute Inc.: Cary, NC, USA, 2021. [Google Scholar]
- Abramson, J.; Spear, B.L.; Thomsen, J.B. The Large Macaws: Their Care, Breeding and Conservation; Raintree Publications: Ft. Bragg, CA, USA, 1995. [Google Scholar]
- Clubb, S.L.; Wolf, S.; Phillips, A. Psittacine pediatric medicine. In Psittacine Aviculture: Perspectives, Techniques and Research; Shubot, R.M., Clubb, K.J., Clubb, S.L., Eds.; Avicultural Breeding and Research Center: Loxahatchee, FL, USA, 1992. [Google Scholar]
- Voren, H.; Jordan, R. Potential health problems in the nursery. In Parrots: Hand Feeding & Nursery Management; Silvio Mattacchione, & Company: Port Perry, ON, Canada, 1992. [Google Scholar]
- Speer, B.L. Stunting in the large macaws. Semin. Avian Exot. Pet Med. 1995, 4, 9–14. [Google Scholar] [CrossRef]
- Beissinger, S.; Stoleson, S. Nestling mortality patterns in relation to brood size and hatchingasynchrony in Green-rumped Parrotlets. In Proceedings of the Acta XX Congressus Internationalis Ornithologici, Christchurch, New Zealand, 2–9 December 1990. [Google Scholar]
- Vigo-Trauco, G. Unpublished Database of Observations of Parental Feeding Behavior by Macaw Parents in Southeastern Peru; Texas A&M University: College Station, TX, USA, 2018–2019. [Google Scholar]
- Evans, M.R.; Lank, D.B.; Boyd, W.S.; Cooke, F. A Comparison of the Characteristics and Fate of Barrow’s Goldeneye and Bufflehead Nests in Nest Boxes and Natural Cavities. Condor 2002, 104, 610–619. [Google Scholar] [CrossRef]
- Miller, K.E. Nesting Success of the Great Crested Flycatcher in Nest Boxes and in Tree Cavities: Are Nest Boxes Safer from Nest Predation? Wilson Bull. 2002, 114, 179–185. [Google Scholar] [CrossRef]
- Snyder, N.; Wiley, J.; Kepler, C. Reproductive Biology. In The parrots of Luquillo: Natural History and Conservation of the Puerto Rican Parrot; Morrison, M., Ed.; The Western Foundation of Vertebrate Zoology: Los Angeles, CA, USA, 1987. [Google Scholar]
- Brightsmith, D.; Boyd, J.; Hobson, E.; Randel, C. Satellite telemetry reveals complex migratory movement patterns of two large macaw species in the western Amazon basin. Avian Conserv. Ecol. 2021, 16, 14. [Google Scholar] [CrossRef]
- Krebs, E.A.; Cunningham, R.B.; Donnelly, C.F. Complex patterns of food allocation in asynchronously hatching broods of Crimson Rosellas. Anim. Behav. 1999, 57, 753–763. [Google Scholar] [CrossRef]
- Budden, A.E.; Beissinger, S. Resouce allocation varies with parental sex and brood size in the asynchronously hatching green-rumped parrotlet. Behav. Ecol. Sociobiol. 2009, 63, 637–647. [Google Scholar] [CrossRef][Green Version]
- Krebs, E.; Margrath, R. Food allocation in Crimson Rosella broods: Parent differ in their responses to chick hunger. Anim. Behav. 2000, 59, 739–751. [Google Scholar] [CrossRef][Green Version]
- Beissinger, S.R.; Waltman, J.R. Extraordinary clutch size and hatching asynchrony of a Neotropical parrot. Auk 1991, 108, 863–871. [Google Scholar]
- GRANT, M.C. Relationships between egg size, chick size at hatching, and chick survival in the Whimbrel Numenius phaeopus. Ibis 1991, 133, 127–133. [Google Scholar] [CrossRef]
- Williams, T.D. Intraspecific variation in egg size and egg composition in birds: Effects on offspring fitness. Biol. Rev. Camb. Philos. Soc. 1994, 69, 35–59. [Google Scholar] [CrossRef] [PubMed]
- Vigo-Trauco, G.; Garcia-Anleu, R.; Brightsmith, D.J. Increasing Survival of Wild Macaw Chicks Using Foster Parents and Supplemental Feeding. Diversity 2021, 13, 121. [Google Scholar] [CrossRef]
- Guedes, N.M.R.; Harper, L.H. Hyacinth Macaws in the Pantanal. In The Large Macaws: Their Care, Breeding and Conservation; Abramson, J., Spear, B.L., Thomsen, J.B., Eds.; Raintree Publications: Fort Bragg, CA, USA, 1995; pp. 395–421. [Google Scholar]
- Vigo-Trauco, G.; Brightsmith, D.J. Unpublished Data from Red-and-Green Macaw Nest Monitoring in Southeastern Peru; Texas A&M University: College Station, TX, USA, 2000–2020. [Google Scholar]
- Iñigo-Elias, E.E. Ecology and Breeding Biology of the Scarlet Macaw (Ara macao) in the Usumacinta Drainage Basin of Mexico and Guatemala. Ph.D. Dissertation, University of Florida, Gainesville, FL, USA, 1996. [Google Scholar]
- Vaughan, C.; Bremmer, M.; Dear, F. Scarlet Macaw (Ara macao) Parental nest visitation in Costa Rica. Rev. Biol. Trop. 2008, 57, 6. [Google Scholar]
- Brightsmith, D.J.; Bravo, A. Ecology and management of nesting Blue-and-yellow Macaws (Ara ararauna) in Mauritia palm swamps. Biodivers. Conserv. 2006, 15, 4271–4287. [Google Scholar] [CrossRef]
- Brightsmith, D.J. Unpublished Data from Blue-and-Yellow Macaw Nest Monitoring in Southeastern Peru; Texas A&M University: College Station, TX, USA, 1999–2004. [Google Scholar]
- Sanz, V.; Rodríquez-Ferraro, A. Reproductive parameters and productivity of the Yellow-shouldered Parrot on Margarita Isalnd, Venezuela: A long-term study. Condor 2006, 108, 178–192. [Google Scholar] [CrossRef]
- Sipinski, E.A.B. Unpublished Data from Monitoring Nests of Red-Spectacled and Red-Tailed Amazon Parrots; Sociedade de Pesquisa em Vida Selvagem e Educação Ambiental (SPVS): Curitiba, Brazil, 2002. [Google Scholar]
- Fernandes Seixas, G.H.; Mourão, G.d.M. Nesting success and hatching survival of the Blue-fronted Amazon (Amazona aestiva) in the Pantanal of Mato Grosso do Sul, Brazil. J. Field Ornithol. 2002, 73, 399–409. [Google Scholar] [CrossRef]
- Navarro, J.L.; Martella, M.B.; Bucher, E. Breeding season and productivity of monk parakeets in Cordoba, Argentina. Wilson Bull. 1992, 104, 413–424. [Google Scholar]
- Smith, G.T.; Saunders, D.A. Clutch size and productivity in three sympatric species of cockatoo (Psittaciformes) in the south-west of Western Australia. Aust. Wildl. Res. 1986, 13, 275–285. [Google Scholar] [CrossRef]
- Smith, G.T. Breeding ecology of the Western Long-billed Corella, Cacatua pastinator pastinator. Wildl. Res. 1991, 18, 91–110. [Google Scholar] [CrossRef]
- Rowley, I. The Behavioral Ecology of the Galah Eolophus roseicapilus in the Wheatbelt of Western Australia; Surrey Beatty & Sons: Chipping Norton, NSW, Australia, 1990. [Google Scholar]
- Robinet, O.; Salas, M. Reproductive biology of the endangered Ouvea Parakeet Eunymphicus cornutus uvaeensis. IBIS 1999, 141, 660–669. [Google Scholar] [CrossRef]

| Growth Information | Hatch Order | |||||
|---|---|---|---|---|---|---|
| Second Chick (N = 8) | Third Chick (N = 13) | Fourth Chick (N = 4) | ||||
| Mean ± St. Dev. | Range | Mean ± St. Dev. | Range | Mean ± St. Dev. | Range | |
| Weight at hatch | 21.7 ± 4.7 | 15.6–29.7 | 20.8 ± 2.6 | 18–27 | 18.9 ± 1.4 | 17.2–20.5 |
| Max weight | 47.9 ± 23.6 | 19.5–81 | 28.3 ± 13.7 | 18–68 | 18.9 ± 1.4 | 17.2–20.5 |
| Age at max weight | 7 ± 6 | 0–18 | 2 ± 2 | 0–6 | 1 ± 1 | 0–1 |
| Weight at death | 37.1 ± 19.1 | 17–65 | 21.9 ± 9.1 | 15–49 | 16.9 ± 2.9 | 13.6–20.5 |
| Age at death | 10 ± 6 | 1–17 | 6 ± 4 | 2–14 | 3 ± 1 | 2–4 |
| Average crop size | 0.81 ± 0.78 | 0–2.1 | 0.84 ± 0.78 | 0–2.0 | 0.69 ± 0.85 | 0–1.8 |
| Hatch ± 7 Days | 15 Days Post-Hatch | 36 Days Post-Hatch | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | N | Mean | SD | N | Mean | SD | N | |
| Starved | 35.1 | 2.0 | 5 | 35.4 | 1.1 | 8 | 35.4 | 1.1 | 8 |
| Not Starved | 35.0 | 1.9 | 9 | 35.9 | 2.8 | 9 | 36.3 | 2.9 | 8 |
| p-value | 0.90 | 0.59 | 0.39 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vigo-Trauco, G.; Martínez-Sovero, G.; Brightsmith, D.J. Age Difference, Not Food Scarcity or Sibling Interactions, May Drive Brood Reduction in Wild Scarlet Macaws in Southeastern Peru. Diversity 2024, 16, 657. https://doi.org/10.3390/d16110657
Vigo-Trauco G, Martínez-Sovero G, Brightsmith DJ. Age Difference, Not Food Scarcity or Sibling Interactions, May Drive Brood Reduction in Wild Scarlet Macaws in Southeastern Peru. Diversity. 2024; 16(11):657. https://doi.org/10.3390/d16110657
Chicago/Turabian StyleVigo-Trauco, Gabriela, Gustavo Martínez-Sovero, and Donald J. Brightsmith. 2024. "Age Difference, Not Food Scarcity or Sibling Interactions, May Drive Brood Reduction in Wild Scarlet Macaws in Southeastern Peru" Diversity 16, no. 11: 657. https://doi.org/10.3390/d16110657
APA StyleVigo-Trauco, G., Martínez-Sovero, G., & Brightsmith, D. J. (2024). Age Difference, Not Food Scarcity or Sibling Interactions, May Drive Brood Reduction in Wild Scarlet Macaws in Southeastern Peru. Diversity, 16(11), 657. https://doi.org/10.3390/d16110657






