Abstract
Compared to single damming, the impact of cascade damming on nitrogen-related microorganisms in river ecosystems exhibits greater complexity. However, there is still a lack of research on the response of denitrifiers to the construction of cascade reservoirs. A study was conducted on 10 cascade reservoirs in the upper reaches of the Yellow River to investigate the impact of cascade reservoir construction on nirS-type denitrifying bacteria in sediments. Sediment samples were collected in May (dry season) and August (wet season) of 2023. The spatiotemporal characteristics of the nirS-type denitrifying bacterial community and gene abundance were analyzed using Illumina high-throughput sequencing and real-time fluorescence quantification PCR (qPCR). Redundancy analysis (RDA) and variation partitioning (VP) were utilized to assess the impact of environmental factors on these communities. The results showed the following: (1) Proteobacteria was the predominant phylum of nirS-type denitrifying bacteria in cascade reservoir sediments. At the genus level, unclassified Proteobacteria (69.51–95.64%) showed the highest relative abundance, followed by Paracoccus, Rhodanobacter, and Pseudomonas, indicating that unclassified Proteobacteria may dominate denitrification in these reservoir sediments. (2) The α and β diversity indices of nirS-type denitrifying bacteria were higher in the dry season than in the wet season, and also higher in young reservoirs compared to old reservoirs (p < 0.05). (3) Temporally, the abundance of the nirS gene was significantly higher in the wet season (12.71 × 107 copies/g dry sediment) compared to the dry season (66.35 × 105 copies/g dry sediment). Spatially, the abundance of the nirS gene was higher in the central region, while relatively lower at both ends. (4) RDA and VP analysis indicated that the community structure and abundance of nirS-type denitrifying bacteria were significantly influenced by the total nitrogen in sediments (19.31%) and water temperature (14.13%). Spearman correlation analysis showed that organic carbon significantly affected the diversity of nirS-type denitrifying bacteria (p < 0.05). The results contribute to a better understanding of the nitrogen-related microbial community in cascade reservoir sediments of the Yellow River, providing a scientific basis for reservoir management.
1. Introduction
Nitrous oxide (N2O) is a potent greenhouse gas, and although its concentration in the atmosphere is much lower than that of carbon dioxide (CO2), its warming potential on the centennial scale is 273 times that of CO2 [1]. N2O has a long residence time in the atmosphere, where it can participate in various photochemical reactions. Additionally, it can be transported to the stratosphere, where it contributes to ozone layer depletion, posing a significant threat to global ecological balance. [2]. The concentration of N2O in the atmosphere has increased by 124% compared to pre-industrial levels (before 1750), reaching 335.8 ± 0.1 ppb (2022) [3]. The pathways of N2O production can be divided into biologically driven and non-biologically driven processes. Non-biologically driven processes involve the chemical reaction between high concentrations of hydroxylamine and nitrite to generate N2O [4]. In freshwater ecosystems, N2O is primarily produced through microbial processes, including nitrification and denitrification [4,5]. The microbial denitrification process is an important source of N2O emissions in terrestrial and aquatic ecosystems, whereby microorganisms reduce nitrate or nitrite to NO, N2O, and N2 [6]. Therefore, changes in the denitrifying microbial community can directly or indirectly affect N2O emissions [7]. Nitrite reductase (nir) is the most critical enzyme in the denitrification process, which is usually catalyzed by nirS and nirK genes with different structures but the same functions. Although the enzymes encoded by two genes are similar in function and physiology, the former has more advantages and is more widely distributed in freshwater ecosystems [8], making it a more efficient option for detecting denitrifying communities [9]. Furthermore, research has indicated that compared to nirK-type denitrifying microorganisms, nirS-type denitrifying bacteria make a more significant contribution to N2O emissions [10].
Reservoirs are one of the hotspots for N2O emissions from inland waters. Considering that the number of constructed, under construction, and planned reservoirs around the world exceeds one million, the N2O emissions from reservoirs cannot be ignored [11]. The construction of dams can lead to the accumulation of sediment and nutrients, resulting in a significant reduction in the transport of terrestrial organic matter to the ocean, and there is even a cumulative effect on ecosystems as a result of cascade damming [12]. Reservoirs typically receive more organic matter inputs from surrounding watersheds than freshwater lakes due to their higher ratio of watershed area to surface area. These factors promote the accumulation of nutrients in sediments, which in turn may stimulate the activity of nitrogen-related microorganisms in reservoirs [13]. As the country with the largest number of reservoirs in the world, China has built nearly 100,000 reservoirs with a total storage capacity of more than 979.62 km3. Many of these reservoirs are located on large rivers in the southwest (such as the Yangtze River, the Yellow River, and the Lancang River), forming a unique cascade landscape [14]. Previous studies have shown that the construction of cascade reservoirs significantly increases the spatiotemporal heterogeneity of N2O emissions [15]. Abiotic factors, such as reservoir properties, regional climate, and the physical and chemical characteristics of water and sediment, have been extensively studied in research on the influencing factors of N2O emission from cascade reservoirs [1]. However, N2O emissions from reservoirs are a complex biochemical process regulated by microbial dynamics related to nitrogen cycling. Currently, there are few reports on the microbiological mechanisms of N2O production in cascade reservoirs, with most focusing on ponds, paddy fields, and freshwater lakes, etc. [16], indicating a need for further investigation into the denitrifying microbial communities in cascade reservoir sediments.
Under the background of global warming, the Qinghai Plateau is developing in the direction of warming and humidification [17], which has a notable impact on regional nitrogen cycling [18]. Global warming accelerates the melting of glaciers and permafrost, resulting in the release of significant amounts of N2O. Concurrently, thawed dissolved nitrogen enters aquatic ecosystems through runoff, further enhancing N2O emissions by influencing associated microbial processes [19]. The Qinghai Plateau has numerous reservoirs, with enormous potential for N2O emissions. However, the spatiotemporal distribution of nirS-type denitrifying bacteria communities and functional gene abundance in the sediment of regional cascade reservoirs have not been fully studied. This study aims to investigate the spatial (Ten cascade reservoirs) and temporal (dry and wet seasons) characteristics of nirS-type denitrifying bacteria in the cascade reservoir sediments of the upper Yellow River during the non-glacial period in 2023. The specific objectives are as follows: (1) to investigate the community characteristics of nirS-type denitrifying bacteria in cascade reservoir sediments; (2) to elucidate the spatiotemporal variations in the abundance of nirS gene in cascade reservoir sediments; (3) to reveal the influencing factors on the community composition of nirS-type denitrifying bacteria in cascade reservoir sediments. Through this research, we hope to better understand the role of nirS-type denitrifying bacteria in the nitrogen cycling within reservoir ecosystems.
2. Materials and Methods
2.1. Study Area
The research is conducted in the upper reaches of the Yellow River (100°53′41″–102°45′16″ E, 35°50′15″–37°12′31″ N). The Yellow River, known as the mother river of China, originates from the northern foot of the Bayan Har Mountains on the Qinghai Plateau and eventually flows into the Bohai Sea. It spans a total length of 5465 km and covers a basin area of 795,000 km2. This region belongs to a plateau continental climate with low and concentrated precipitation in summer, characterized by an annual average rainfall of only 577.9 mm and an annual average evaporation of 927 mm, resulting in significant alternation between the dry and wet seasons in hydrology. Since 1974, a series of hydropower reservoirs have been constructed along the upper reaches of the Yellow River. In this study, ten reservoirs, namely Longyangxia (LYX), Ninna (NN), Lijiaxia (LJX), Zigonglaka (ZGLK), Kangyang (KY), Gongboxia (GBX), Suzhi (SZ), Huangfeng (HF), Jishixia (JXS), and Dahejia (DHJ), were selected from upstream to downstream (Figure 1). The reservoirs were classified into old reservoirs (LYX, LJX, GBX, NN) and young reservoirs (DHJ, JSX, HF, SZ, ZGLK, KY) based on the years since their construction. LYX is the largest reservoir in terms of water storage area and also has the longest construction history among all the studied reservoirs. DHJ is located at the lowest reach and is also the youngest among them. The average elevation in this region is around 3000 m with a fall of approximately 800 m from upstream to downstream. The main features of these cascade reservoirs can be found in Table S1. In 2023, we conducted field sampling of ten cascade reservoirs in the upper reaches of the Yellow River in both the dry (May) and wet seasons (August). Six sampling points were set up at two large reservoirs (LYX and LJX), while three sampling points were arranged at each of the remaining eight reservoirs, totaling thirty-six sampling points. It should be noted that due to road collapse in the area of the GBX reservoir in May and the LJX reservoir in August, relevant sampling data for two seasons were missing.
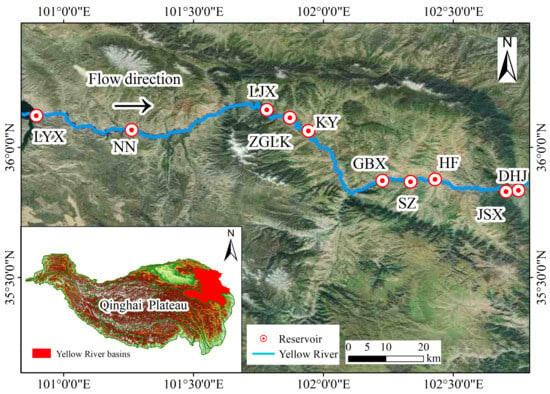
Figure 1.
Distribution of cascade reservoirs in the upper reaches of the Yellow River. Longyangxia (LYX), Ninna (NN), Lijiaxia (LJX), Zigonglaka (ZGLK), Kangyang (KY), Gongboxia (GBX), Suzhi (SZ), Huangfeng (HF), Jishixia (JXS), and Dahejia (DHJ).
2.2. Sediment and Surface Water Sampling and Analysis
Surface sediment samples (0–5 cm) were collected from the reservoirs using a Peterson grab sampler. All sediment samples collected from the same reservoir were mixed and divided into two parts. One part was packed in sterile sealed bags for physicochemical property determination, while the other part was packed in 20 mL sterile centrifuge tubes and preserved with liquid nitrogen for transportation back to the laboratory and storage at −80 °C for nirS-type denitrifying bacterial community characteristics and gene abundance analysis. The abbreviations starting with “w” represent the physicochemical parameters of surface water, while those starting with “s” refer to the parameters in sediments. After acidification and freeze-drying, the organic carbon (sOC) and total nitrogen (sTN) contents of sediments were determined using a vario MACRO cube elemental analyzer (Elementar Inc., Langenselbold, Germany) [20]. The content of total phosphorus (sTP) in sediments was determined by molybdenum-antimony resistance colorimetry [21]. Sediment pH (spH) was measured using a portable pH meter, while sediment temperature (sTemp) was measured using a portable soil thermometer. Surface water (0.5 m below the water surface) pH (wpH) and temperature (wTemp) were measured on site using a multi-parameter water quality meter HQ40d (Hach, Loveland, CO, USA). Total organic carbon (wTOC), total phosphorus (wTP), and total nitrogen (wTN) of water were determined according to Chinese national standards [22].
2.3. Extraction of Sediment DNA, Illumina Sequencing and Quantification PCR
Reservoir sediment DNA was extracted using the FastDNA SPIN Kit for soil (MP Biomedicals, Santa Ana, CA, USA) following the manufacturer’s instructions. The nirS gene was amplified using primer set cd3AF (5′-GTSAACGTSAAGGARACSGG-3′) and R3cd (5′-GASTTCGGRTGSGTCTTGA-3′) on the T100 Thermal Cycler (Bio-Rad, Hercules, CA, USA), with a product length of approximately 426 bp [23]. High-throughput sequencing was conducted on the Illumina MiSeq platform (Illumina Inc., San Diego, CA, USA) according to the standard protocol of Majorbio Bio-Pharm Technology Co; Ltd. (Shanghai, China) [24]. Raw sequences of nirS gene reads have been deposited in the NCBI Sequence Read Archive under the BioProject number PRJNA1126413. The abundance of nirS gene was quantified using quantitative PCR (qPCR) on the ABI 7300 Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). The reaction mixture had a total volume of 10 μL, consisting of 5 μL ChamQ SYBR Color qPCR Master Mix (Vazyme, Nanjing, China), 0.4 μL each of forward and reverse primers (5 μM), 0.2 μL ROX Reference Dye 1, 1 μL DNA template, and 3 μL double-distilled H2O. For nirS gene, the qPCR program began at a first denaturation at 95 °C (5 min), followed by 40 cycles consisting of melting at 95 °C (5 s), annealing at 58 °C (30 s), and extending at 72 °C (1 min). The standard curve for nirS is y = −3.3647x + 39.486, and R2 = 0.9984. The amplification efficiencies for nirS are 98.25%.
2.4. Data Analysis
Statistical analysis of all data in this study was conducted using R 4.3.2 software, with a significance level set at p < 0.05. A Venn diagram of the Operational Taxonomic Units (OTUs) was created using the VennDiagram package (version 1.7.3). The alpha diversity of nirS-type denitrifying bacterial communities was calculated using the vegan package (version 2.6-6.1), and differences in alpha diversity between two seasons were tested using a one-way analysis of variance (ANOVA). Principal Co-ordinates Analysis (PCoA) based on Bray–Curtis dissimilarity was performed for beta diversity of nirS-type denitrifying bacterial communities using the vegan package. The linear discriminant analysis effect size (LEfSe) was performed using Galaxy online software (version 1.0, https://huttenhower.sph.harvard.edu/galaxy, accessed on 15 July 2024) to identify bacterial taxa that significantly contribute to group differentiation. Redundancy analysis (RDA) and variation partitioning were used to explore the relationship between nirS-type denitrifying bacterial communities and environmental factors [25]. Spearman correlation analysis was employed to examine the relationship between nirS-type denitrifying bacterial diversity and environmental factors.
3. Results
3.1. Composition of nirS-Type Denitrifying Bacterial Community
The composition of OTUs in sediments of the nirS-type denitrifying bacterial community varied significantly among different reservoirs (Figure 2). A total of 56 common OTUs were generated in the young group (Figure 2a). Only two common OTUs were generated in the old group (Figure 2b).
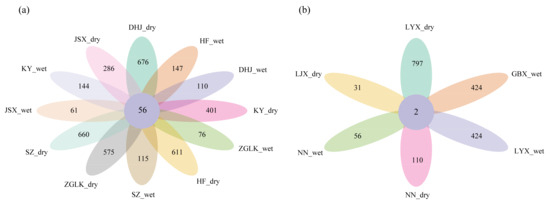
Figure 2.
Venn diagram of OTUs of reservoirs in the young reservoirs (a) and old reservoirs (b). The first part of the abbreviation represents the reservoir name, while the second part represents the sampling seasons.
The nirS-type denitrifying bacterial community in sediments of the cascade reservoirs in the upper reaches of the Yellow River was predominantly composed of Proteobacteria (17.07–74.27%) (Figure 3a). Figure 3b showed the top eight dominant bacterial genera with relative abundance. The highest relative abundance was unclassified Proteobacteria (69.51–95.64%), followed by Paracoccus, Rhodanobacter, and Pseudomonas.
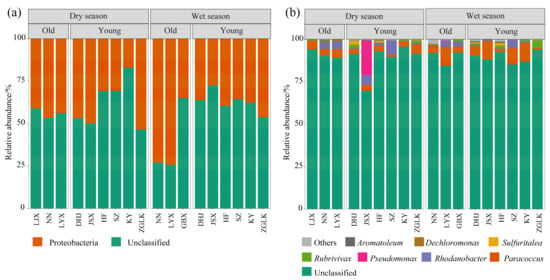
Figure 3.
Relative abundance of nirS-type denitrifying bacterial communities in sediment at phylum (a) and genus levels (b) with reservoir abbreviations as described in Section 2.1.
3.2. Differences in the Composition of nirS-Type Denitrifying Bacterial Community
The results of the LEfSe analysis between different seasons are shown in Figure 4. When the LDA score exceeded 4, there were eight indicator nirS-type denitrifying bacterial groups that exhibit differences between two seasons. Vogesella, Neisseriales, Vogesella_sp__EB, and Chromobacteriaceae had essential roles in the nirS-type denitrifying bacterial groups of the dry season. In the wet season, four indicator taxonomies were observed as follows: Rhodobacteraceae, Rhodobacterales, Paracoccus, and unclassified nirS-type denitrifying bacteria.
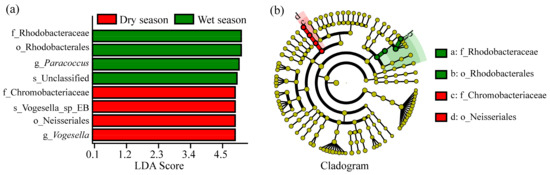
Figure 4.
LEfSe analysis of nirS-type denitrifying bacteria in different seasons. LDA value histogram (a) and Cladogram diagram (b).
3.3. Diversity of nirS-Type Denitrifying Bacterial Community
The alpha diversity of nirS-type denitrifying bacteria in sediments of cascade reservoirs is shown in Figure 5. In terms of time, the average values of Chao1 and Observed indices were higher in the dry season compared to the wet season (p < 0.05). Similarly, the variation trend of Simpson’s and Shannon’s indices was consistent with that of Chao1 and Observed indices (Figure 5a). Among reservoirs of different ages, Chao1, Observed, Shannon’s, and Simpson’s indices showed no significant differences. On the whole, the young reservoirs scored higher than the old ones (Figure 5b).
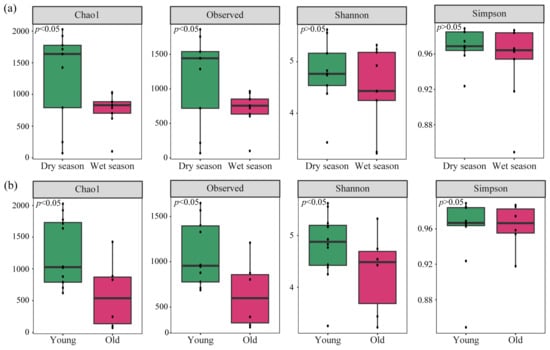
Figure 5.
Alpha diversity of nirS-type denitrifying bacteria in sediments between different seasons (a) and reservoir ages (b).
Principal Coordinate Analysis (PCoA) was conducted to analyze the community composition of nirS-type denitrifying bacteria in sediment samples. The results are shown in Figure 6. PCoA 1 and PCoA 2 together explained 33.7% of the variation in the composition of the nirS-type denitrifying bacterial communities. The samples from different groups were distributed across distinct quadrants, indicating variation in the composition of the nirS-type denitrifying bacterial communities at the OTU level. Overall, the seasonal differences in these bacterial communities were less pronounced compared to the variations observed across different reservoir ages.
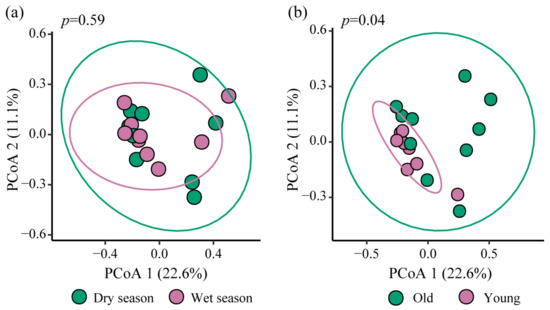
Figure 6.
PCoA analysis of sediment nirS-type denitrifying bacteria between different seasons (a) and reservoir ages (b).
3.4. Abundance of nirS Gene
The gene abundance of denitrifying microorganisms in various habitats is an important indicator reflecting their ecological functions, and has been widely studied. This study found differences in the abundance of nirS-type denitrifying bacteria in the sediment of the cascade reservoirs in the upper reaches of the Yellow River between different seasons (Figure 7). In the dry season, the range of nirS gene abundance was 4.44 × 105 copies/g dry sediment to 274 × 105 copies/g dry sediment, which was significantly lower than that in the wet season (0.62 × 107–30.2 × 107 copies/g dry sediment) (p < 0.05). The highest nirS gene abundance in sediments in the dry season was found in KY at 263 × 107 copies/g dry sediment, while the lowest was found in DHJ at 5.39 × 107 copies/g dry sediment (Figure 7). In the wet season, ZGLK and JSX had the highest nirS gene abundance (29.77 × 107 copies/g dry sediment and 27.27 × 107 copies/g dry sediment, respectively), while DHJ and NN had the lowest (1.14 × 107 copies/g dry sediment and 0.69 × 107 copies/g dry sediment, respectively). In addition, the abundance of nirS-type denitrifying bacteria in sediments of young reservoirs was significantly higher than that of old reservoirs.
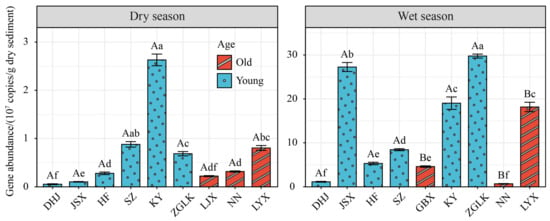
Figure 7.
Abundance of nirS gene in different seasons and reservoir age. Uppercase and lowercase letters denote statistically significant differences (p < 0.05) in gene abundance between young and old reservoirs, and among 10 cascade reservoirs within the same season, respectively. Error bars represent standard deviations.
3.5. Relationship Between nirS-Type Denitrifying Bacteria and Environmental Factors
Redundancy analysis (RDA) of the community structure of nirS-type denitrifying bacteria in sediments is shown in Figure 8. The cumulative explanation of RDA 1 and RDA 2 is 75.9%, effectively explaining the relationship between environmental factors and the community structure (Figure 8a). Temperature and TN significantly influenced the community structure of nirS-type denitrifying bacteria (p < 0.05). The relative importance of environmental factors to the nirS-type denitrifying bacterial community is shown in Figure 8b. Among all environmental factors, sTN and wTemp had the highest contributions to nirS-type denitrifying bacteria at 19.31% and 14.13%, respectively, followed by sTP (12.77%), wpH (10.56%), wTN (9.46%), wTOC (7.97%), spH (7.03%), wTP (6.95%), sTemp (6.62%), and sOC (5.17%).
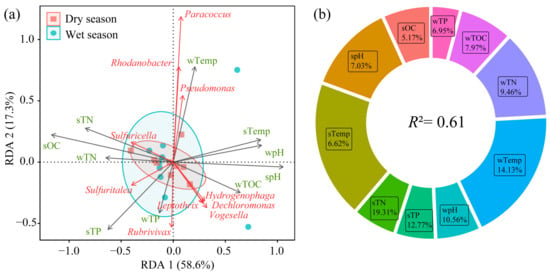
Figure 8.
Redundancy analysis (a) and hierarchical partitioning (b) of environmental factors and nirS-type denitrifying bacteria communities. Red arrows indicate dominant bacterial genera, while gray arrows represent environmental factors. Blue and red circles denote the 95% confidence intervals for reservoir ordination in the wet and dry seasons, respectively.
The relationship between the dominant genus, diversity, and abundance of nirS-type denitrifying bacteria and environmental factors is illustrated in Figure 9. It was found that sTN showed a significant positive correlation with Dechloromonas (p < 0.05), while Azoarcus exhibited a significant negative correlation with wpH and wTN (p < 0.05). Furthermore, wTN was significantly negatively correlated with Vogesella (p < 0.05). The alpha diversity of nirS-type denitrifying bacteria demonstrated a significant negative correlation with wTP, but a positive correlation with wTOC (p < 0.05). Additionally, the abundance of the nirS gene was significantly negatively correlated with pH, wTN, and wTP, while it was positively correlated with wTOC, sOC, and sTemp (p < 0.05).
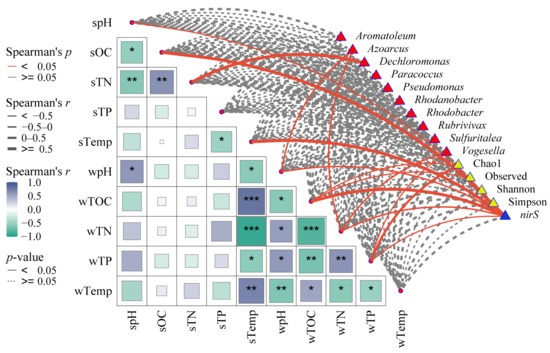
Figure 9.
Correlation heatmap of the dominant genus, diversity, and abundance of nirS-type denitrifying bacteria and environmental factors. The size of the boxes in the lower left corner represents the size of the correlation between the environment factors, and the color represents the positive or negative correlation. The color of lines represents the magnitude of significance, and the thickness represents the magnitude of correlation with environmental factors. “*”, “**”, and “***” indicate statistically significant differences at the significance levels of p < 0.05, 0.01, and 0.001, respectively.
4. Discussion
4.1. Composition and Diversity of nirS-Type Denitrifying Bacterial Community
Denitrifying bacteria in the sediment of reservoirs play a crucial role in the ecosystem. Their community composition and diversity are closely related to the environmental conditions of the sediment and water, effectively reflecting the health status of the reservoir ecosystem [26]. In this study, it was found that the dominant phylum in the cascade reservoir sediments of the upper reaches of the Yellow River is mainly Proteobacteria. This result is consistent with the common pattern that Proteobacteria are usually the dominant phylum in reservoirs and river sediments [27]. Proteobacteria are commonly found in aquatic ecosystems worldwide, showing high abundance in reservoirs. This may be attributed to the diverse nutritional types of bacteria within this phylum. Proteobacteria, the largest phylum of bacteria, contains a significant number of nitrogen-fixing bacteria. These bacteria exhibit robust survival abilities in polluted environments and play a critical role in pollutant degradation. They are essential for the self-purification process of water and sediments [28]. Paracoccus, Rhodanobacter, and Pseudomonas were dominant genera of nirS-type denitrifying bacteria found in all sediment samples. These nirS-type denitrifying bacteria play a key role in sediments from cascade reservoirs in the upper Yellow River. This is consistent with the results of Ming et al. [29], indicating the widespread presence of these two genera among sediment samples. Due to its high altitude and low temperature, the metabolic activity and community composition of denitrifying bacteria were significantly affected by the extreme environment of the Qinghai Plateau. Studies have shown that nirS-type denitrifying bacteria in the plateau environment usually have a stronger cold tolerance and a lower metabolic rate, reflecting the adaptability to the extreme environment of the plateau. These bacteria were able to perform effective denitrification at low temperatures, in contrast to the warm, humid conditions at lower altitudes [30].
The results showed that the abundance of nirS-type denitrifying bacteria in reservoir sediment in the wet season was lower than that in the dry season, consistent with previous studies [31]. This phenomenon may be attributed to the wet season coinciding with the rainy season, resulting in an increase in rainfall and a subsequent rise in water level and flow velocity of the Yellow River. These environmental changes may have had an adverse effect on nirS-type denitrifying bacteria in sediment, resulting in a decrease in their abundance [32]. In the dry season, the diversity of nirS-type denitrifying bacteria is higher than that in the wet season. This trend is similar to the number of species but differs from previous research results [33]. Although previous studies have demonstrated the significance of temperature as a key environmental factor influencing microbial diversity, with higher microbial diversity generally observed in the wet season compared to the dry season, our study found that there was no significant correlation between nirS-type denitrifying bacteria diversity and temperature (Figure 9). Instead, we observed a significant correlation with organic carbon and total phosphorus (p < 0.05). It is speculated that there are three reasons, as follows: First, high nitrogen and phosphorus levels in the dry season promote the growth of nirS-type denitrifying bacteria in sediment, while rain dilution during the wet season inhibits their metabolism [34]. Second, as the study area is a plateau with minimal temperature variation during sampling, temperature may not significantly impact the community structure and activity of nirS-type denitrifying bacteria. Third, due to numerous environmental factors considered in this study, the direct correlation between temperature, total nitrogen, and nirS-type denitrifying bacteria might be obscured.
4.2. Temporal and Spatial Characteristics of nirS Gene Abundance
The abundance of nirS gene is an important indicator of the potential denitrification capacity of the ecosystem. In the sediment of the cascade reservoirs in the upper reaches of the Yellow River, this gene abundance significantly increased in the wet season, with an average value reaching 12.71 × 107 copies/g dry sediment; this is far higher than the values of 66.35 × 105 copies/g dry sediment in the dry season, indicating that seasons have a significant impact on the abundance of the nirS gene. This phenomenon may be closely related to temperature, as temperature is a key factor driving bacterial migration and growth in freshwater ecosystems such as rivers, lakes, and reservoirs [35]. Specifically, the higher temperature in the wet season may promote the growth of nirS-type denitrifying bacteria, leading to a significant increase in their gene abundance [36]. Spatially, there was significant heterogeneity in the abundance of nirS gene. For instance, in the DHJ reservoir, the abundance of nirS gene was relatively low in both the dry and wet seasons (1.14 × 105 copies/g dry sediment and 5.39 × 105 copies/g dry sediment, respectively. This indicates that the physicochemical environmental characteristics of reservoirs have a substantial impact on nirS-type denitrifying bacteria, which is consistent with previous research [37]. Moreover, the abundance of the nirS gene exhibits significant variation across different environments. Compared to the coastal wetlands of China, the abundance of the nirS gene is notably lower in the sediments of the cascade reservoirs in the upper reaches of the Yellow River [36]. This disparity could be attributed to the higher levels of human activity in coastal wetlands, such as fishing and aquaculture, which indirectly promote the growth and reproduction of nirS-type denitrifying bacteria [38]. In contrast, the sediments in plateau reservoirs are generally nutrient-poor, characterized by low nitrogen and phosphorus concentrations. Coupled with the lower temperatures of the Qinghai Plateau, the degradation of organic matter is slower, further limiting microbial metabolic activities [39].
4.3. Response of nirS-Type Denitrifying Bacteria to Environmental Factors
Denitrification is a biochemical process mediated by denitrifying bacteria. This process relies on organic carbon as the necessary electron donor for the reduction in nitrate or nitrite [40]. The availability of organic carbon plays a crucial role in this process, indirectly creating an anoxic environment suitable for the survival of denitrifying bacteria by enhancing the consumption of oxygen by aerobic microorganisms. Denitrifying bacteria are mostly heterotrophic; they must obtain organic carbon from the external environment as a carbon source. Therefore, the supply of organic carbon has become a core environmental factor regulating the composition and diversity of denitrifying bacterial communities [41]. This study further confirms this point, namely that organic carbon significantly influences the diversity of nirS-type denitrifying bacteria in sediment (Figure 9). This is consistent with previous conclusions [42], which indicate that an increase in organic carbon can promote the diversity of denitrifying bacterial communities and lead to differences in community structure. In addition to organic carbon, environmental factors such as temperature, nitrogen, and phosphorus content have been found to regulate the composition and diversity of denitrifying bacterial communities [43,44]. For example, Wang et al. found that total phosphorus content is a key factor in regulating the relative abundance of Pseudomonas in the sediment from the Taihu Lake, while other environmental factors have limited impact on its dominance position [45]. In the sediment of cascade reservoirs in the upper reaches of the Yellow River, temperature, organic carbon content, sediment pH, as well as the concentration of total nitrogen in water collectively influence the community composition and diversity of nirS-type denitrifying bacteria. This finding is similar to the results of Wolsing et al. [46], which together revealed the complex regulatory mechanism of environmental factors on the community dynamic of denitrifying bacterial communities. Reservoir age significantly impacts denitrifying bacterial communities. As reservoir age increases, the accumulation of nutrients in sediments alters the microbial habitat, affecting denitrification efficiency. In young reservoirs, high nutrient input stimulates more active denitrification, whereas in old reservoirs, the anaerobic conditions created by prolonged nutrient accumulation may inhibit this process [47]. Reservoir age also influences sediment organic carbon content and the structure of denitrifying bacterial communities; while high organic matter provides a carbon source, it exacerbates anaerobic conditions, limiting denitrification efficiency. Additionally, reservoir age alters denitrifying bacterial activity, further affecting the nitrogen cycle [48]. With the increasing number of reservoirs globally, studying the long-term effects of reservoir age on the denitrification process is critical for water resource management and environmental protection.
5. Conclusions
In this study, real-time fluorescence quantification PCR and high-throughput sequencing were used to analyze the community characteristics and gene abundance of nirS-type denitrifying bacteria in the sediment of 10 cascade reservoirs in the upper reaches of the Yellow River in the northeastern Qinghai Plateau. The main conclusions are as follows:
- (1)
- The dominant type of denitrifying bacteria in the upper Yellow River cascade reservoirs is probably unclassified Proteobacteria. LEfSe analysis showed that the genera with the highest LDA scores were Paracoccus and Vogesella.
- (2)
- The abundance of the nirS gene in sediment showed significant differences between different seasons (p < 0.05), with the abundance in the wet season (0.62 × 107–30.2 × 107 copies/g dry sediment) being significantly higher than that in the dry season (4.44 × 105–274 × 105 copies/g dry sediment) (p < 0.05).
- (3)
- The community structure of nirS-type denitrifying bacteria was jointly influenced by multiple environmental factors. Among them, sediment total nitrogen (19.31%) and water temperature (14.13%) had the most significant impact. Reservoir age significantly influences the nirS-type denitrifying bacterial community, suggesting a potential decreasing trend in N2O emissions as reservoir age increases.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/d16110656/s1. Figure S1: Environmental parameters in 10 cascade reservoirs. spH, sOC, sTN, and sTP represent sediment pH, total organic carbon, total nitrogen, and total phosphorus, respectively. wpH, wTOC, wTN, wTP represent water pH, total organic carbon, total nitrogen, and total phosphorus, respectively. (A: DHJ; B: JSX; C: HF; D: SZ; E: GBX; F: KY; G: ZGLK; H: LJX; I: NN; J: LYX); Table S1: Characteristics of cascade reservoirs in the upper reach of the Yellow River.
Author Contributions
Methodology, X.M., H.Y. and H.L.; Data curation, Y.M. (Yuhua Mo), F.X., H.J. and Y.M. (Yuanjie Ma); Writing, Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (52070108).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Acknowledgments
We would like to express our gratitude to Tianjiang Gu, Zebi Liu, Shan Huang, Jintao Zhang, and Nan Zhou for their invaluable assistance during the field sampling. Additionally, we extend our thanks to the reviewers for their constructive comments and suggestions which have greatly improved the quality of this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, Y.; Tian, H.; Yao, Y.; Shi, H.; Bian, Z.; Shi, Y.; Wang, S.; Maavara, T.; Lauerwald, R.; Pan, S. Increased nitrous oxide emissions from global lakes and reservoirs since the pre-industrial era. Nat. Commun. 2024, 15, 942. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.S.; Xiong, R.N.; Zhang, H.H.; Yang, S.Q.; Gao, N. Research progress on microbial-mediated mitigation of nitrous oxide emissions from agricultural soils. Acta Pedol. Sin. 2023, 60, 332–344. [Google Scholar] [CrossRef]
- WMO. The State of Greenhouse Gases in the Atmosphere Based on Global Observations through 2022; WMO: Geneva, Switzerland, 2023. [Google Scholar]
- Soler-Jofra, A.; Stevens, B.; Hoekstra, M.; Picioreanu, C.; Sorokin, D.; van Loosdrecht, M.C.; Pérez, J. Importance of abiotic hydroxylamine conversion on nitrous oxide emissions during nitritation of reject water. Chem. Eng. J. 2016, 287, 720–726. [Google Scholar] [CrossRef]
- Wang, J.; Wu, W.; Zhou, X.; Huang, Y.; Guo, M. Nitrous oxide (N2O) emissions from the high dam reservoir in longitudinal range-gorge regions on the Lancang-Mekong River, southwest China. J. Environ. Manag. 2021, 295, 113027. [Google Scholar] [CrossRef]
- Hong, X.; Hong, Y.W.; Chen, Z.W.; Zhao, C.G.; Yang, S.P. Phylogenetic diversity of nirS-type denitrifying bacteria in Jiulong River estuary. Microbiol. China 2015, 42, 1639–1650. [Google Scholar] [CrossRef]
- Bouwman, A.; Beusen, A.; Griffioen, J.; Van Groenigen, J.; Hefting, M.; Oenema, O.; Van Puijenbroek, P.; Seitzinger, S.; Slomp, C.; Stehfest, E. Global trends and uncertainties in terrestrial denitrification and N2O emissions. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130112. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.R. Distribution Characteristics and Driving Mechanism of nirK and nirS Denitrifiers in Soils: A Case Study from the Mid-Channel Bar. Master’s Thesis, University of South China, Hengyang, China, 2022. [Google Scholar]
- Zhou, S.; Huang, T.; Zhang, C.; Fang, K.; Xia, C.; Bai, S.; Zeng, M.; Qiu, X. Illumina MiSeq sequencing reveals the community composition of nirS-Type and nirK-Type denitrifiers in Zhoucun reservoir—A large shallow eutrophic reservoir in northern China. RSC Adv. 2016, 6, 91517–91528. [Google Scholar] [CrossRef]
- Xing, X.Y.; Tang, Y.F.; Xu, H.F.; Qin, H.L.; Liu, Y.; Zhang, W.Z.; Chen, A.L.; Zhu, B.L. Warming shapes nirS- and nosZ-type denitrifier communities and stimulates N2O emission in acidic paddy soil. Appl. Environ. Microbiol. 2021, 87, e02965-20. [Google Scholar] [CrossRef]
- Wang, J.; Vilmin, L.; Mogollón, J.M.; Beusen, A.H.; van Hoek, W.J.; Liu, X.; Pika, P.A.; Middelburg, J.J.; Bouwman, A.F. Inland waters increasingly produce and emit nitrous oxide. Environ. Sci. Technol. 2023, 57, 13506–13519. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.B.; Li, C.; Guo, J.S.; Xiao, Y.; Lu, L.H. Advances of eco-environmental effects and adaptive management in river cascading development. Adv. Earth Sci. 2018, 33, 675–686. [Google Scholar] [CrossRef]
- Zhou, J.; Leavitt, P.R.; Zhang, Y.; Qin, B. Anthropogenic eutrophication of shallow lakes: Is it occasional? Water Res. 2022, 221, 118728. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Fan, C.; Zhu, J.; Wang, J.; Sheng, Y.; Liu, K.; Chen, T.; Zhan, P.; Luo, S.; Yuan, C.; et al. A comprehensive geospatial database of nearly 10 0000 reservoirs in China. Earth Syst. Sci. Data 2022, 14, 4017–4034. [Google Scholar] [CrossRef]
- Yuan, X.; Fu, K.D.; Tao, Y.C.; Zhang, N.; Yang, L.S. Spatial-temporal distribution and influencing factors of nitrous oxide flux across the water-air interface in Lancang River, China. Ecol. Environ. Sci. 2024, 33, 54–61. [Google Scholar] [CrossRef]
- Wan, Z.; Wang, L.; Huang, G.; Rasul, F.; Awan, M.I.; Cui, H.; Liu, K.; Yu, X.; Tang, H.; Wang, S.; et al. nirS and nosZII bacterial denitrifiers as well as fungal denitrifiers are coupled with N2O emissions in long-term fertilized soils. Sci. Total Environ. 2023, 897, 165426. [Google Scholar] [CrossRef] [PubMed]
- Duan, A.; Xiao, Z.; Wu, G. Characteristics of climate change over the Tibetan Plateau under the global warming during 1979–2024. Clim. Change Res. 2016, 12, 374–381. [Google Scholar]
- Chen, H.; Ju, P.; Zhu, Q.; Xu, X.; Wu, N.; Gao, Y.; Feng, X.; Tian, J.; Niu, S.; Zhang, Y. Carbon and nitrogen cycling on the Qinghai–Tibetan plateau. Nat. Rev. Earth Environ. 2022, 3, 701–716. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.; Xia, X.; Battin, T.J.; Liu, S.; Wang, Q.; Liu, R.; Yang, Z.; Ni, J.; Stanley, E.H. Unexpectedly minor nitrous oxide emissions from fluvial networks draining permafrost catchments of the East Qinghai-Tibet Plateau. Nat. Commun. 2022, 13, 950. [Google Scholar] [CrossRef]
- Zhou, M.Z.; Bao, Y.F.; Wang, Y.C.; Hao, W.L.; Liu, S.; Sun, M.; Wen, j.; Li, S.Z.; Zhao, J.W. Vertical niche differentiation of comammox bacteria and the influencingfactors in sediments of the Lancang River reservoir. Acta Sci. Circumstantiae 2024, 13, 950. [Google Scholar] [CrossRef]
- Tao, M.M.; Li, Y.N.; Song, H.Y.; Li, W.B.; Zhou, X.H.; Xu, X.H. The structural and functional characteristies of the sedimentarybacterial community in the root area of Acorus calamus. Environ. Chem. 2024, 44, 302–310. [Google Scholar] [CrossRef]
- State Environmental Protection Administration. Methods for Water and Wastewater Monitoring and Analysis; China Environmental Science Press: Beijing, China, 2002. [Google Scholar]
- Throbäck, I.N.; Enwall, K.; Jarvis, Å.; Hallin, S. Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiol. Ecol. 2004, 49, 401–417. [Google Scholar] [CrossRef]
- Ren, Y.; Yu, G.; Shi, C.; Liu, L.; Guo, Q.; Han, C.; Zhang, D.; Zhang, L.; Liu, B.; Gao, H.; et al. Majorbio Cloud: A one-stop, comprehensive bioinformatic platform for multiomics analyses. iMeta 2022, 1, e12. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ding, X.Y.; Lin, M.X.; Sun, Y.X. Abundance and structure diversity of denitrifying bacterial community in sediments of Guangzhou Liuxi River. J. South China Agric. Univ. 2018, 38, 65–721. [Google Scholar] [CrossRef]
- LI, B.; Yang, A.J.; Hu, X.; Xu, K.; Ji, L. Bacterial community structure in reservoir sediments under the influence of antimony ore waste water. Microbiol. China 2021, 48, 2956–2971. [Google Scholar] [CrossRef]
- Sorokin, D.Y.; Lücker, S.; Vejmelkova, D.; Kostrikina, N.A.; Kleerebezem, R.; Rijpstra, W.I.C.; Damsté, J.S.S.; Le Paslier, D.; Muyzer, G.; Wagner, M.; et al. Nitrification expanded: Discovery, physiology and genomics of a nitrite-oxidizing bacterium from the phylum Chloroflexi. ISME J. 2012, 6, 2245–2256. [Google Scholar] [CrossRef]
- Liu, X.; Huang, Y.; Wang, Y.; Wang, B.; Wu, B.; Lu, G. Structural Characteristics of Microbial Communities in the Sediments of the Niyang River in Tibet. Environ. Sci. Technol. 2020, 41, 3249–3256. [Google Scholar] [CrossRef]
- Ming, H.X.; Chen, Q.R.; Shi, Y.Y.; Su, J.; Yu, Y.; Fan, J.F. Study on denitrification process of sediment in the Liaohe estuary analysis of the abundance of denitrification functional genes and the community structrure of nirK-type bacteria. Haiy. Xue. 2020, 42, 82–92. [Google Scholar]
- Gu, Y.F.; Liu, T.; Bai, Y.; Xiang, Q.J.; Zhang, X.P.; Chen, Q. Pyrosequencing of nirS gene revealed spatial variation of denitrifying bacterial assemblages in response to wetland desertification at Tibet plateau. J. Mt. Sci. 2019, 16, 1121–1132. [Google Scholar] [CrossRef]
- Watanabe, K.; Komatsu, N.; Ishii, Y.; Negishi, M. Effective isolation of bacterioplankton genus Polynucleobacter from freshwater environments grown on photochemically degraded dissolved organic matter. FEMS Microbiol. Ecol. 2009, 67, 57–68. [Google Scholar] [CrossRef]
- Liu, Y. Simultaneous Treatment of Biogas Desulfurization and Wastewater Denitrification Based on Autotrophic Denitrification. Doctoral Thesis, China University of Geosciences, Beijing, China, 2019. [Google Scholar]
- Qin, Y.; Fan, B.; Mei, L. Comparative of bacterial community composition and diversity in the Wangfeng Lake between wet season and dry season. J. Dalian Ocean. Univ. 2023, 38, 414–422. [Google Scholar] [CrossRef]
- Li, Y.X.; Ding, J.W.; Yan, L.Y.; Yin, W.; Gao, Z.H.; Song, Q.; Wang, L.; Liang, J. Bacterial community diversity in river sediments of Qinghai section of the Makehe River in dry and wet seasons. J. Northwest A F Univ. (Nat. Sci. Ed.) 2024, 52, 1–13. [Google Scholar] [CrossRef]
- McCaulou, D.R.; Bales, R.C.; Arnold, R.G. Effect of Temperature-Controlled Motility on Transport of Bacteria and Microspheres Through Saturated Sediment. Water Resour. Res. 1995, 31, 271–280. [Google Scholar] [CrossRef]
- Gao, J.; Hou, L.; Zheng, Y.; Liu, M.; Yin, G.; Li, X.; Lin, X.; Yu, C.; Wang, R.; Jiang, X. nirS-Encoding denitrifier community composition, distribution, and abundance along the coastal wetlands of China. Appl. Microbiol. Biotechnol. 2016, 100, 8573–8582. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.X.; Chen, H.D.; Li, Z.; Chen, Z.; Liao, M.J. Abundance of aerobic ammonia-oxidizing and nirS type denitrifying microorganisms in sediments of Lancang River. China Environ. Sci. 2023, 43, 3107–3117. [Google Scholar] [CrossRef]
- Xu, C.Y.; Pu, L.J.; Zhu, M. Effect of reclamation activity on coastal ecological environment: Progress and perspectives. Acta Ecol. Sin. 2017, 38, 1148–1162. [Google Scholar] [CrossRef]
- Li, H.; Qiu, Y.; Yao, T.; Han, D.; Gao, Y.; Zhang, J.; Ma, Y.; Zhang, H.; Yang, X. Nutrients available in the soil regulate the changes of soil microbial community alongside degradation of alpine meadows in the northeast of the Qinghai-Tibet Plateau. Sci. Total Environ. 2021, 792, 148363. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, B.; Nan, B.; Hu, P. Diversity of bacterial community and detection of nirS-and nirK-encoding denitrifying bacteria in sandy intertidal sediments along Laizhou Bay of Bohai Sea, China. Mar. Pollut. Bull. 2014, 88, 215–223. [Google Scholar] [CrossRef]
- Xiu, Y.N.; Chen, S.Z.; Li, X.Y.; Jiang, Y.Z.; Tang, B.J.; Ping, X.Y.; Fan, R.L.; Li, N.N.; Quan, W.M. Abundance and structure diversity of denitrifying bacterial community in sediment of the typical habitats of Xiangshan Bay. Chin. J. Ecol. 2021, 40, 84–92. [Google Scholar] [CrossRef]
- Mosier, A.C.; Francis, C.A. Denitrifier abundance and activity across the San Francisco Bay estuary. Environ. Microbiol. Rep. 2010, 2, 667–676. [Google Scholar] [CrossRef]
- Gao, Z.Q.; Zhu, L.; Zhu, W.; Liu, S.F.; Fan, Y.J.; Zhuang, Z.M. Diversity of denitrifying bacteria by encoding nirS gene from surface sediments of the pear river esturary. Oceanol. Limnol. Sin. 2012, 43, 1114–1121. [Google Scholar] [CrossRef]
- Yang, W.H.; Shi, D.J.; Zhang, Y.; Li, W.P.; Jing, S.Y.; Yu, L.H. Community characteristics of denitrifying microorganisms in plateau lake sediments—Taking Nanhaihu lake as example. China Environ. Sci. 2020, 40, 431–438. [Google Scholar] [CrossRef]
- Wang, C.C.; Lee, C.M. Isolation of the ɛ-caprolactam denitrifying bacteria from a wastewater treatment system manufactured with acrylonitrile–butadiene–styrene resin. J. Hazard. Mater. 2007, 145, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Wolsing, M.; Priemé, A. Observation of high seasonal variation in community structure of denitrifying bacteria in arable soil receiving artificial fertilizer and cattle manure by determining T-RFLP of nir gene fragments. FEMS Microbiol. Ecol. 2004, 48, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Wang, F.; Fu, Z.; Tang, Y.; Ma, J.; Qin, Y.; Li, M.; Yang, M.; Chen, X.-P. Differential response of microbial diversity and abundance to hydrological residual time and age in cascade reservoirs. J. Soils Sediments 2021, 21, 1290–1301. [Google Scholar] [CrossRef]
- Hohman, S.P.; Smyth, A.R.; Bean, E.Z.; Reisinger, A.J. Internal nitrogen dynamics in stormwater pond sediments are influenced by pond age and inorganic nitrogen availability. Biogeochemistry 2021, 156, 255–278. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).