Tricoma (Tricoma) disparseta sp. nov. (Nematoda: Desmoscolecidae), a New Free-Living Marine Nematode from a Seamount in the Northwest Pacific Ocean, with a New Record of T. (T.) longirostris (Southern, 1914) †
Abstract
1. Introduction
2. Material and Methods
2.1. Field Sampling and Sample Processing
2.2. Laboratory Processing and Microscopic Analysis
2.3. Scanning Electron Microscopy (SEM) Analysis
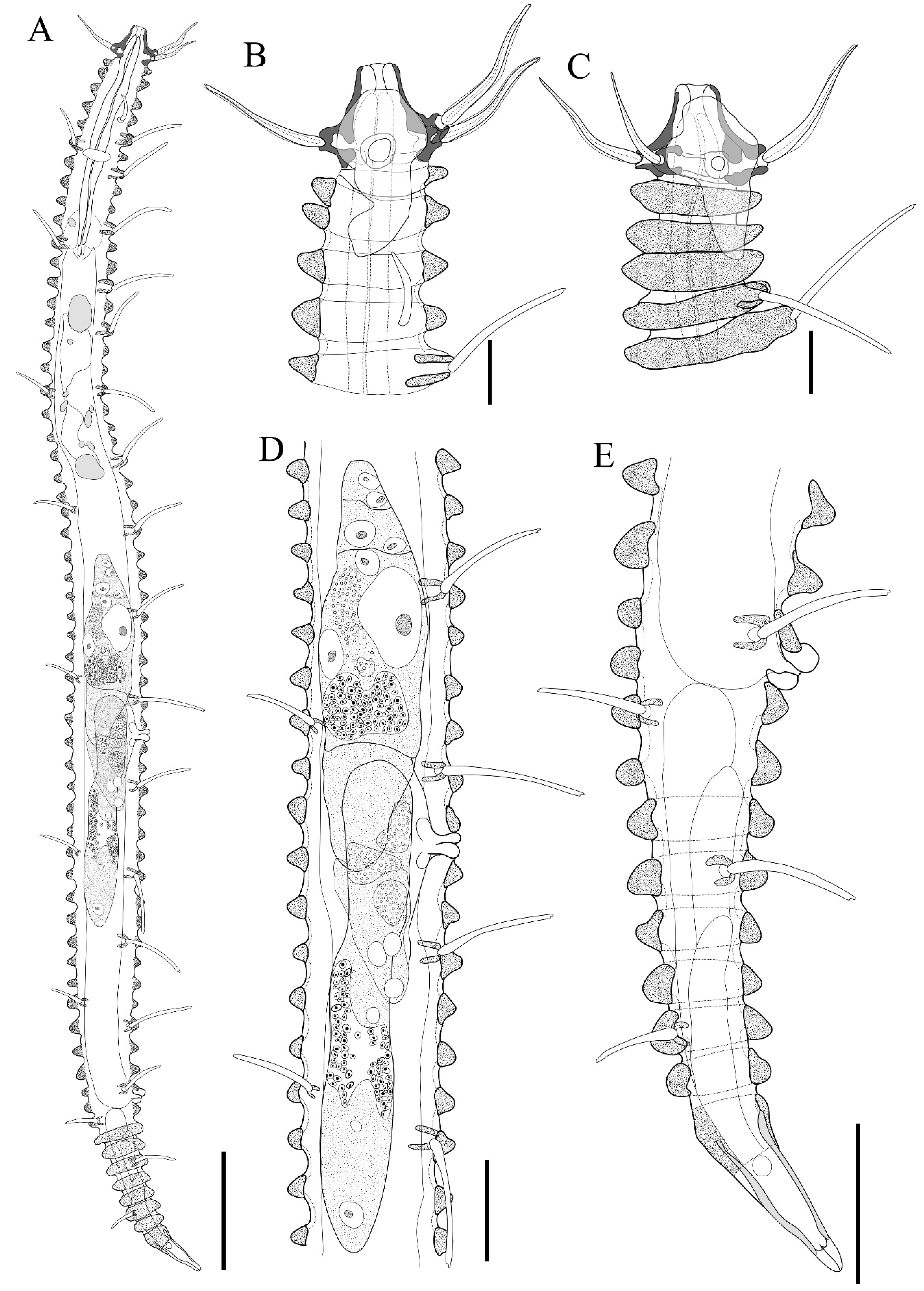
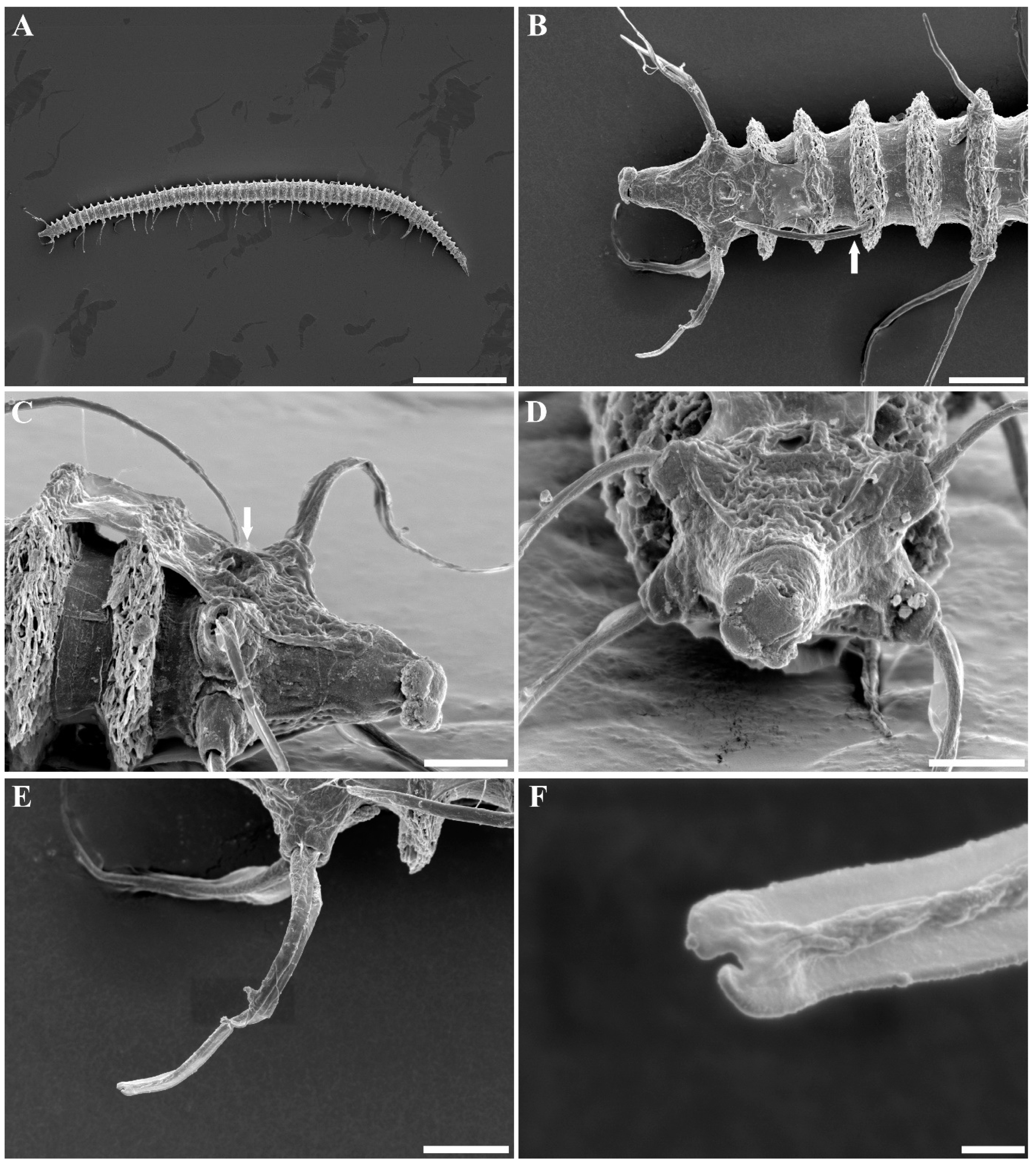
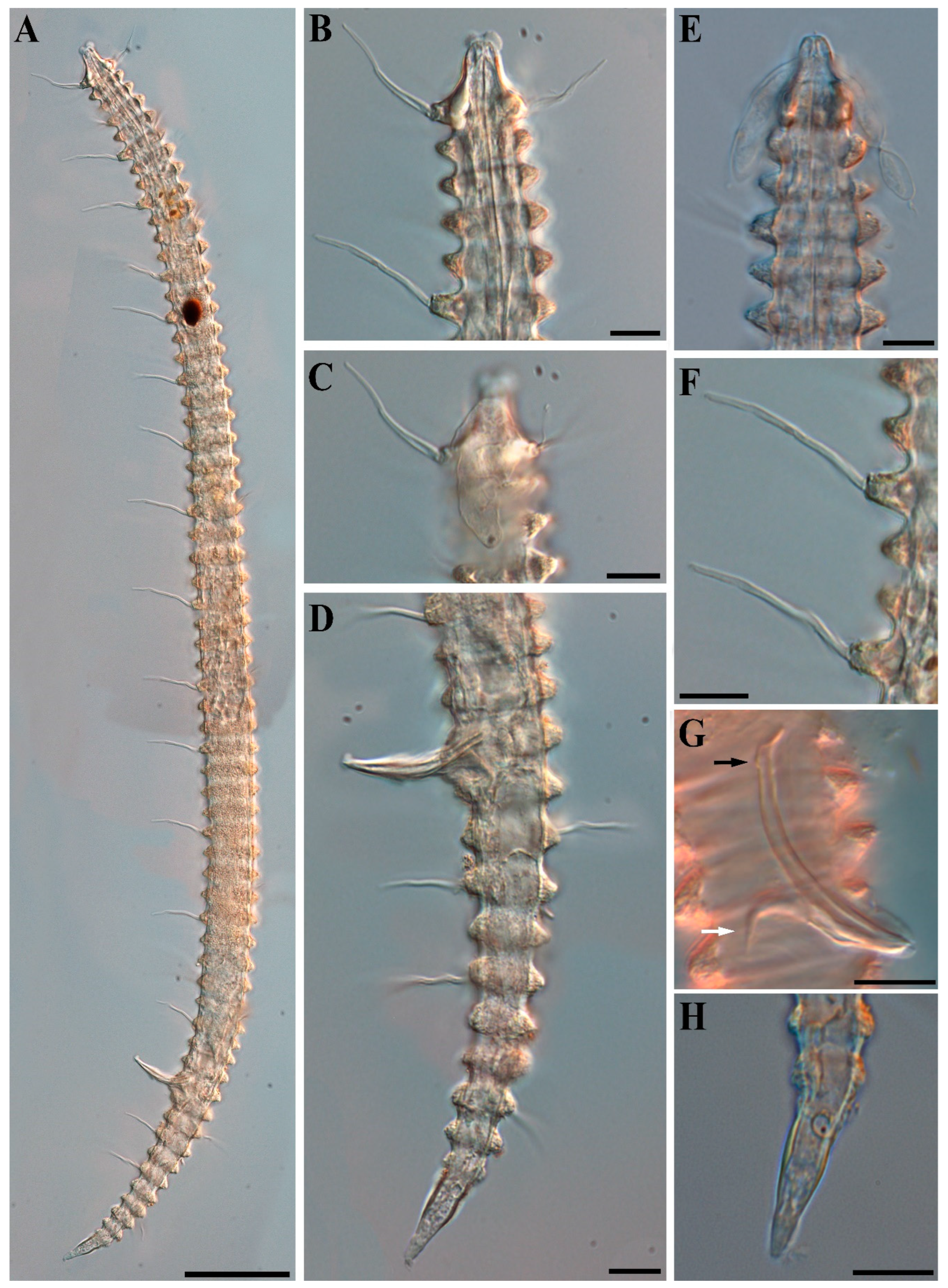
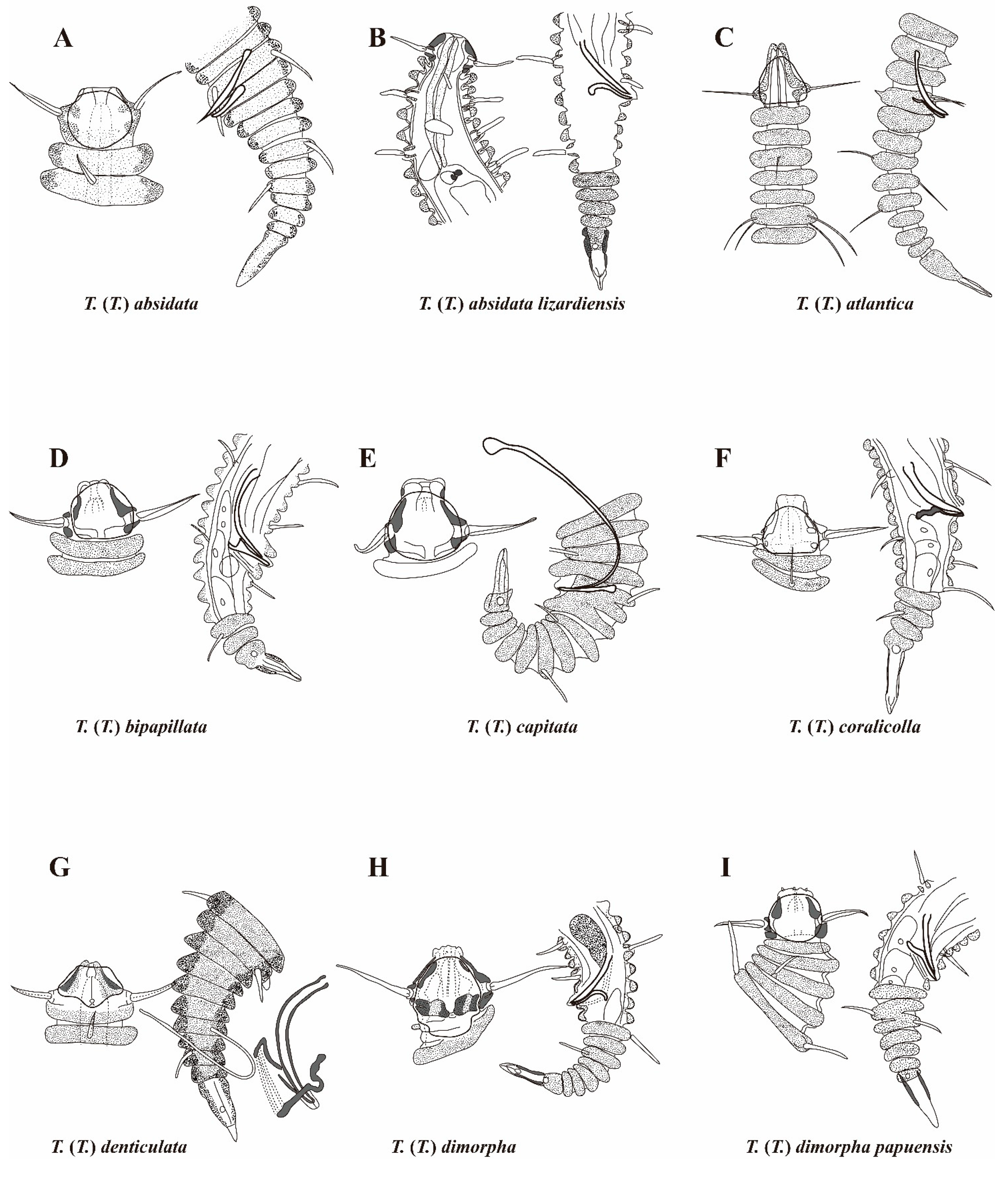
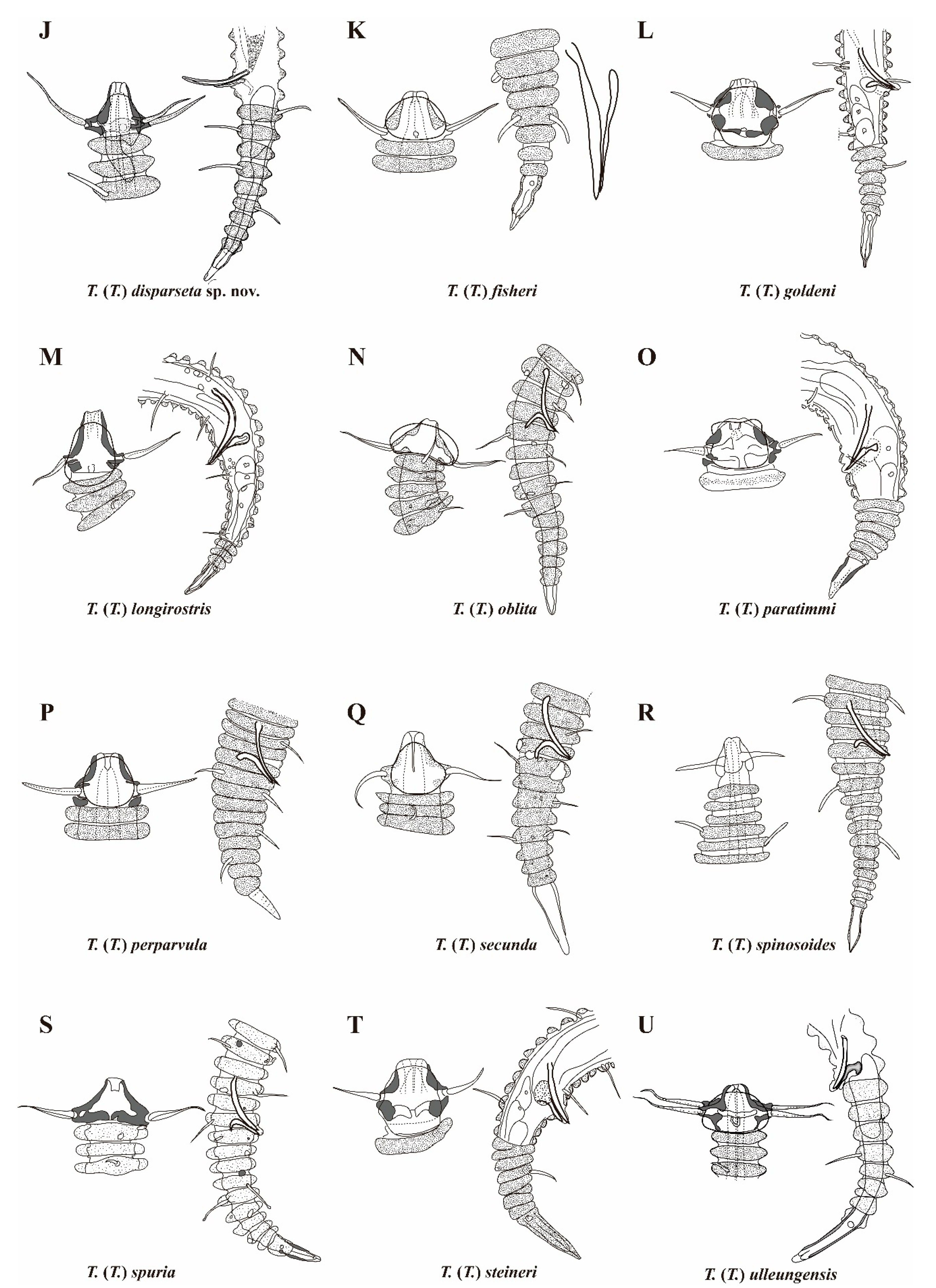
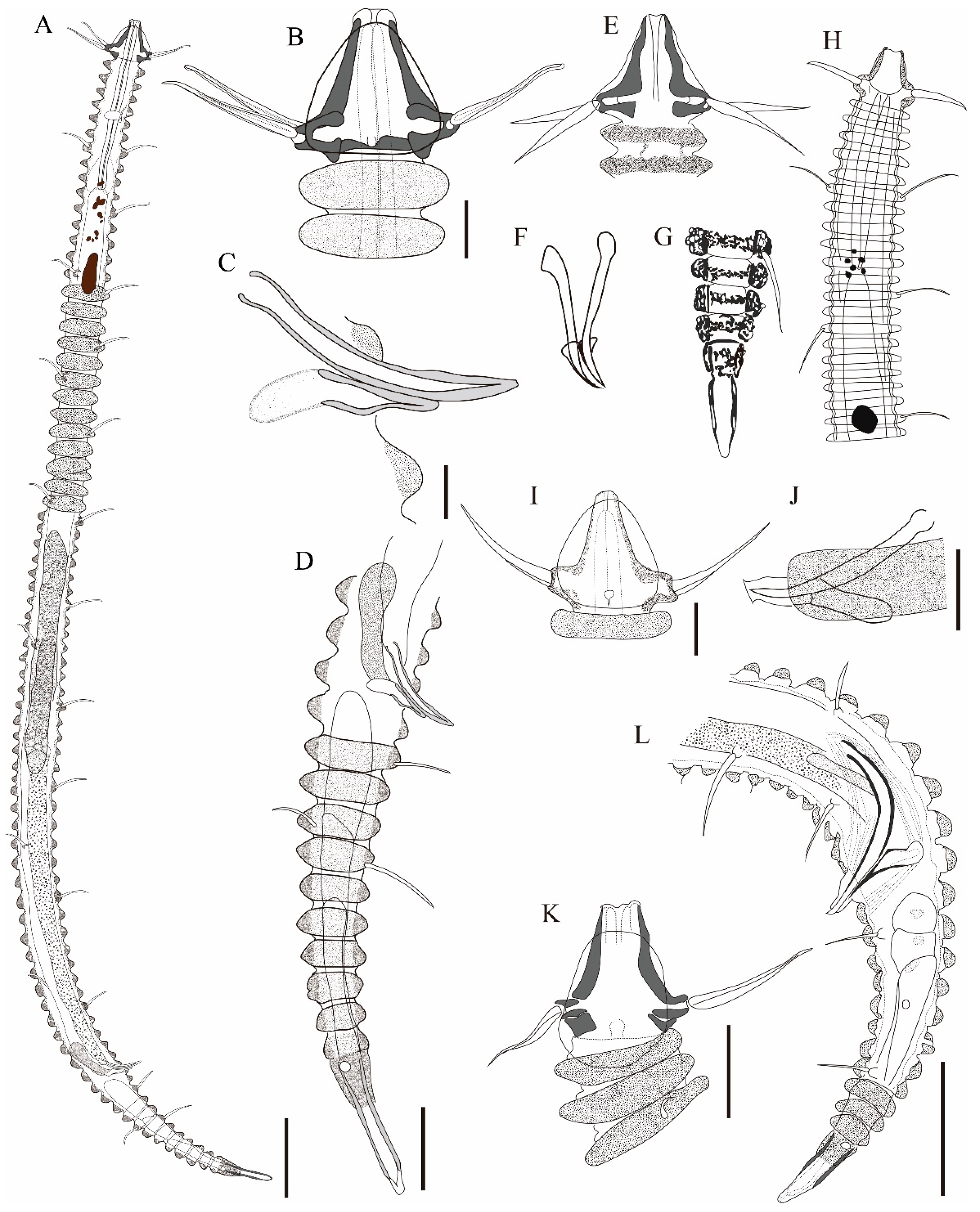
3. Results and Discussion
| Subdorsal | Left side: | 5,10,17,23,31,41,47,53,58 | = 9 |
| Right side: | 6,10,15,19,23,33,39,46,53,59 | = 10 | |
| Subventral | Left side: | 3,5,8,12,14,17,20,23,27,31,34,38,42,45,47,53,55 | = 17 |
| Right side: | 3,5,8,11,14,16,20,23,26,29,32,36,39,42,45,47,52,55 | = 18 |
| Subdorsal | Left side: | 5(4),9(10),17,24(22,23),32(31),41(39),48(45,46,47),52(51,53),58(57) | = 9 |
| Right side: | 5,9(10),18(17),23(25),31(30),39(38),48(46),53(51,52),58(57) | = 9 | |
| Subventral | Left side: | 3,5,8(7),11(9),13(12),16(14),18(17,19),21(20,22),24(23,25,26),28(27),32(30,31),36(33,34,35),40(38,39),43(41,42),46(45),48,53(52),55(54) | = 18(16,17) |
| Right side: | 3,5,8(7),11,14(13),17(16),20(19),24(22,23),27(26,28),32(29,30),35(34),39(37,38),42(41),45(44),48,53(52),55 | = 17(14,16) |
| Subdorsal | Left side: | 6,11,18,23,32,42,47,53,59 | = 9 |
| Right side: | 6,11,18,24,32,40,47,53,59 | = 9 | |
| Subventral | Left side: | 4,5,8,10,12,15,19,22,25,29,33,37,41,45,49,52,55 | = 17 |
| Right side: | 4,5,7,10,13,15,18,22,25,29,33,37,41,44,48,51,55 | = 17 |
| Subdorsal | Left side: | 7(6),11,17(18),24(23),32,40(42),47(48),53,59(58) | = 9 |
| Right side: | 6,11,18(19),23(24,26),32(33),40,48(47),54(53),59(58) | = 9 | |
| Subventral | Left side: | 4(3),5,8,11(12),14(15),16,19(20),22(23),25(26,27),29(30,31),33(34,35),37(38,39),41(42),44(45),49(48),55(51,53),57(56) | = 17(16) |
| Right side: | 4(3),5,7(8),9(10,11),12(13),14(15),16(18),20(21),22,25(24),29(28),34(33),38(37),42(41),45,49(48),52(51),57(56) | = 18(16,17) |
| Subdorsal | Left side: | 7,14,20,28,40,50,57,67 | = 8 |
| Right side: | 7,15,21,28,38,51,59,70 | = 8 | |
| Subventral | Left side: | 4,7,12,17,22,29,36,43,49,54,62,68,72 | = 13 |
| Right side: | 5,7,11,16,19,24,30,36,43,48,54,61,68,71 | = 14 |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Danovaro, R.; Snelgrove, P.V.; Tyler, P. Challenging the paradigms of deep-sea ecology. Trends Ecol. Evol. 2014, 29, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Dell’Anno, A.; Carugati, L.; Corinaldesi, C.; Riccioni, G.; Danovaro, R. Unveiling the biodiversity of deep-sea nematodes through metabarcoding: Are we ready to bypass the classical taxonomy? PLoS ONE 2015, 10, e0144928. [Google Scholar] [CrossRef]
- Armenteros, M.; Quintanar-Retama, O.; Gracia, A. Depth-related patterns and regional diversity of free-living nematodes in the deep-sea Southwestern Gulf of Mexico. Front. Mar. Sci. 2022, 9, 1023996. [Google Scholar] [CrossRef]
- Trebukhova, Y.A.; Miljutin, D.; Pavlyuk, O.; Mar’yash, A.; Brenke, N. Changes in deep-sea metazoan meiobenthic communities and nematode assemblages along a depth gradient (North-western Sea of Japan, Pacific). Deep Sea Res. Part II Top. Stud. Oceanogr. 2013, 86, 56–65. [Google Scholar] [CrossRef]
- dos Santos, G.A.; Silva, A.C.; Esteves, A.M.; Ribeiro-Ferreira, V.P.; Neres, P.F.; Valdes, Y.; Ingels, J. Testing bathymetric and regional patterns in the southwest Atlantic deep sea using infaunal diversity, structure, and function. Diversity 2020, 12, 485. [Google Scholar] [CrossRef]
- Udalov, A.; Azovsky, A.; Mokievsky, V. Depth-related pattern in nematode size: What does the depth itself really mean? Prog. Oceanogr. 2005, 67, 1–23. [Google Scholar] [CrossRef]
- Danovaro, R.; Gambi, C. Cosmopolitism, rareness and endemism in deep-sea marine nematodes. Eur. Zool. J. 2022, 89, 653–665. [Google Scholar] [CrossRef]
- Bezerra, T.N.; Pape, E.; Hauquier, F.; Vanreusel, A. Description and distribution of Erebussau nom. nov. pro Erebus Bussau, 1993 nec Erebus Latreille, 1810 with description of a new species, and of Odetenema gesarae gen. nov., sp. nov.(Nematoda: Desmoscolecida) from nodule-bearing abyssal sediments in the Pacific. Zootaxa 2021, 4903, 542–562. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, H.; Rho, H.S. Six species of Tricoma (Nematoda, Desmoscolecida, Desmoscolecidae) from the East Sea, Korea, with a bibliographic catalog and geographic information. Korean J. Environ. Biol. 2023, 41, 570–607. [Google Scholar] [CrossRef]
- Decraemer, W.; Rho, H.S. Order Desmoscolecida. In Handbook of Zoology: Gastrotricha, Cycloneuralia and Gnathifera, Vol. 2: Nematoda; De Gruyter: Berlin, Germany; Boston, MA, USA, 2013; pp. 351–372. [Google Scholar]
- Rogers, A. The biology of seamounts. In Advances in Marine Biology; Academic Press: Cambridge, MA, USA, 1994; Volume 30, pp. 305–350. [Google Scholar]
- Clark, M.R.; Rowden, A.A.; Schlacher, T.; Williams, A.; Consalvey, M.; Stocks, K.I.; Rogers, A.D.; O’Hara, T.D.; White, M.; Shank, T.M. The ecology of seamounts: Structure, function, and human impacts. Annu. Rev. Mar. Sci. 2010, 2, 253–278. [Google Scholar] [CrossRef]
- Piepenburg, D.; Müller, B. Distribution of epibenthic communities on the Great Meteor Seamount (North-east Atlantic) mirrors pelagic processes. Arch. Fish. Mar. Res. 2004, 51, 55–70. [Google Scholar]
- White, M.; Bashmachnikov, I.; Arístegui, J.; Martins, A. Physical Processes and Seamount Productivity; Blackwell Publishing: Oxford, UK, 2007; pp. 65–84. [Google Scholar]
- Zeppilli, D.; Bongiorni, L.; Cattaneo, A.; Danovaro, R.; Santos, R.S. Meiofauna assemblages of the Condor Seamount (North-East Atlantic Ocean) and adjacent deep-sea sediments. Deep Sea Res. Part II Top. Stud. Oceanogr. 2013, 98, 87–100. [Google Scholar] [CrossRef]
- Rowden, A.A.; Schlacher, T.A.; Williams, A.; Clark, M.R.; Stewart, R.; Althaus, F.; Bowden, D.A.; Consalvey, M.; Robinson, W.; Dowdney, J. A test of the seamount oasis hypothesis: Seamounts support higher epibenthic megafaunal biomass than adjacent slopes. Mar. Ecol. 2010, 31, 95–106. [Google Scholar] [CrossRef]
- Decraemer, W. Morphological and taxonomic study of the genus Tricoma Cobb (Nematoda: Desmoscolecida), with the description of new species from the Great Barrier Reef of Australia. Aust. J. Zool. 1978, 26, 1–121. [Google Scholar] [CrossRef]
- Decraemer, W. Desmoscolecids from sublittoral finesand of Pierre Noire (West Channel) (Nematoda, Desmoscolecida). Bull. Muséum Natl. D’histoire Nat. 1979, 1, 299–321. [Google Scholar] [CrossRef]
- Decraemer, W. Morphology of Tricoma absidata lizardiensis subsp. nov.(Nematoda, Desmoscolecida) with a note on its ontogeny. Biol. Jaarb. Dodonaea 1979, 46, 101–114. [Google Scholar]
- Decraemer, W. Tricominae (Nematoda-Desmoscolecida) from the northern part of Moçambique Channel, with five new species and one new genus. Bull. L’institut R. Sci. Nat. Belg. Biol. 1983, 55, 1–34. [Google Scholar]
- Decraemer, W. Tricominae (Nematoda: Desmoscolecida) from Laing Island, Papua New Guinea, with Descriptions of New Species. Invertebr. Syst. 1987, 1, 231–256. [Google Scholar] [CrossRef]
- Decraemer, W. Descriptions of two new species of Tricoma (Nematoda: Desmoscolecidae) and comments on the taxonomic status of T.(T.) tertia Blome, 1982 and T.(T.) brevirostris (Southern, 1914) Steiner, 1916. Russ. J. Nematol. 1996, 4, 107–114. [Google Scholar]
- Decraemer, W.; Tchesunov, A.V. Some Desmoscolecids from the White Sea. Russ. J. Nematol. 1996, 4, 15–130. [Google Scholar]
- Soetaert, K.; Decraemer, W. Eight new Tricoma species (Nematoda, Desmoscolecidae) from a deep-sea transect off Calvi (Corsica, Mediterranean). Hydrobiologia 1989, 183, 223–247. [Google Scholar] [CrossRef]
- Timm, R.W. A revision of the nematode order Desmoscolecida Filipjev, 1929. Univ. Calif. Publ. Zool. 1970, 93, 1–115. [Google Scholar]
- Timm, R.W. Marine nematodes of the order Desmoscolecida from McMurdo Sound, Antarctica. Biol. Antarct. Seas 1978, 26, 225–236. [Google Scholar]
- Freudenhammer, I. Desmoscolecida aus der Iberischen Tiefsee, zugleich eine Revision dieser Nematoden-ordnung. Meteor Forschungsergebnisse Reihe D Biol. 1975, 20, 1–65. [Google Scholar]
- Southern, R. Clare Island Survey. Nemathelmia, Kinorhyncha and Chaetognatha. In Proceedings of the Royal Irish Academy, Dublin, Ireland, 30 December 1914; pp. 1–80. [Google Scholar]
- Steiner, G. Neue und wenig bekannte nematoden von der Westküste Afrikas I. Zool. Anz. 1916, 47, 337–351. [Google Scholar]
- Ansari, K.; Lyla, P.; Ajmal Khan, S. New distributional records of free-living marine nematodes from Indian waters I. Chromadorids. Indian J. Geo-Mar. Sci. 2015, 44, 756–765. [Google Scholar]
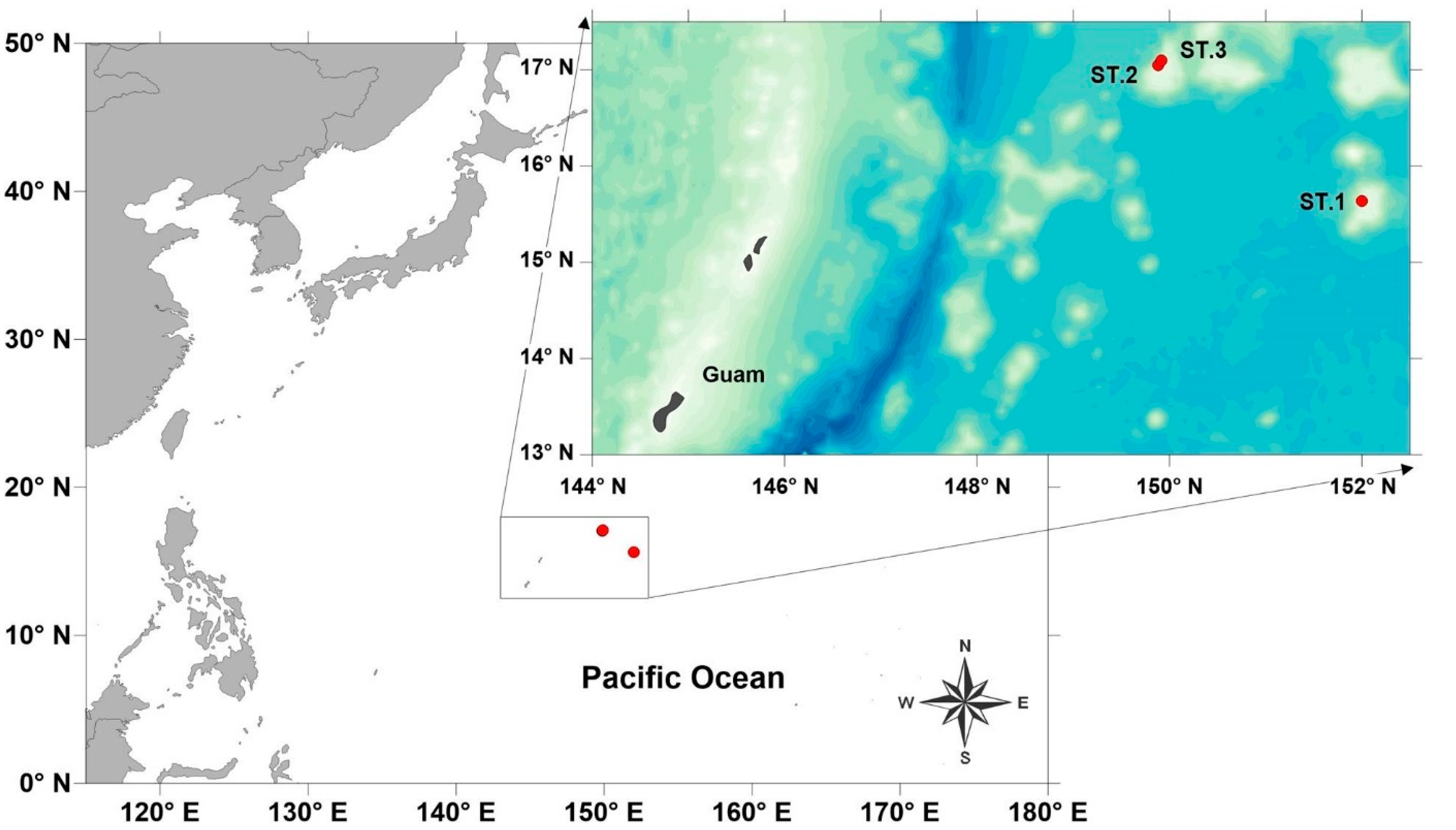




| Stations | Date | Latitude (DMS) | Longitude (DMS) | Depth(m) | Remarks | Specimens | |
|---|---|---|---|---|---|---|---|
| St.1 | BKC90103 | 1 September 2023 | 15°38′15.16″ S | 151°59′50.35″ E | 1425.52 | Starfish habitat | HSV0114 |
| St.2 | BKC80403 | 6 September 2023 | 17°02′47.346″ S | 149°52′50.484″ E | 1366.6 | Starfish habitat | HSV0113_#4, HSV0113_#5, HSV0113_#6, HSV0113_#7, HSV0113_#9, HSV0113_#10 |
| St.3 | BKC80502 | 7 September 2023 | 17°05′45.41″ S | 149°55′07.67″ E | 1510.1 | Sponge | HSV0113_#1, HSV0113_#2, HSV0113_#3, HSV0113_#8 |
| Males | Females | |||
|---|---|---|---|---|
| Holotype | Paratypes (n = 4) Mean ± sd (Range) | Allotype | Paratypes (n = 3) Mean ± sd (Range) | |
| Total body length | 571 | 562 ± 31.5 (527–613) | 543 | 536 ± 35 (490–575) |
| Number of body rings | v:60 d:61 | 60 ± 0.6 (59–61) | v:60 d:61 | 61 ± 0.8 (60–62) |
| a | 19 | 16.8 ± 1.0 (15.4–17.8) | 16 | 14.6 ± 2.5 (11.7–17.8) |
| b | 6 | 5.9 ± 0.2 (5.7–6.3) | 6 | 6.5 ± 2.5 (6.3–6.7) |
| c | 6 | 6.1 ± 0.2 (6.0–6.5) | 7 | 6.9 ± 0.3 (6.5–7.3) |
| Head length | 16 | 16.5 ± 1.1 (14.7–17.8) | 16 | 15.5 ± 0.8 (14.6–16.6) |
| Head diameter at the level of cephalic setae | 21 | 20.1 ± 0.8 (19.1–21.2) | 19 | 20.4 ± 1.1 (18.9–21.4) |
| Body diameter at the level of cardia | 24 | 26.6 ± 1.4 (25.3–28.9) | 28 | 26.6 ± 1.2 (24.9–27.8) |
| Maximum body diameter | 30 | 33.5 ± 1.4 (31.1–34.7) | 35 | 37.6 ± 5.0 (30.5–41.8) |
| Cephalic setae length | 25 | 24.1 ± 2.2 (22.4–27.8) | 22 | 23.4 ± 1.1 (22.3–24.8) |
| Amphideal fovea length | 25 | 25.5 ± 1.6 (22.9–27) | 27 | 25.2 ± 2.5 (21.7–27) |
| Ocelli diameter | 8 | 6.1 ± 1.2 (4.5–7.6) | 10 | 5.7 ± 1.4 (3.7–6.8) |
| Ocelli length | 11 | 7.8 ± 3.2 (4.7–13.1) | 14 | 6.1 ± 2.2 (4.4–9.2) |
| Anterior end to ocelli | 123 | 127.8 ± 25.3 (107.6–171.2) | 119 | 141.6 ± 26.2 (106.9–170.3) |
| Esophagus length | 95 | 94.9 ± 1.6 (92.7–97) | 92 | 82.6 ± 6.7 (73.3–88.3) |
| Number of subventral setae (left) | 17 | 17 ± 0.8 (16–18) | 17 | 17 ± 0.5 (16–17) |
| Number of subventral setae (right) | 18 | 16 ± 1.2(14–17) | 17 | 17 ± 0.8 (16–18) |
| Length of the longest subventral setae | 32 | 33.9 ± 2.6 (29.7–36.4) | 35 | 34.1 ± 2.8 (30.7–37.6) |
| Length of the shortest subventral setae | 17 | 16.8 ± 1.0 (15.3–18.1) | 16 | 20.1 ± 3.5 (15.2–23.1) |
| Number of subdorsal setae (left) | 9 | 9 ± 0 (9–9) | 9 | 9 ± 0 (9–9) |
| Number of subdorsal setae (right) | 10 | 9 ± 0 (9–9) | 9 | 9 ± 0 (9–9) |
| Length of the longest subdorsal setae | 20 | 19.4 ± 1.2 (17.6–20.9) | 19 | 19.6 ± 2.0 (17.5–22.3) |
| Length of the shortest subdorsal setae | 17 | 14.1 ± 1.1(12.7–15.5) | 12 | 14.2 ± 1.7 (12.7–16.6) |
| Spicule length | 31 | 29.9 ± 1.4 (28.5–32.2) | - | - |
| Gubernaculum length | 18 | 17 ± 1.2(15.6–18.5) | - | - |
| Anterior end to vulva | - | - | 305 | 309.2 ± 10.8 (300.9–324.5) |
| Body diameter at the level of the vulva | - | - | 32 | 31.6 ± 4.3 (26.9–37.3) |
| V(%) | - | - | 56 | 57.9 ± 2.6 (55.7–61.4) |
| Anal body diameter | 25 | 26.5 ± 1.6 (24.4–28.8) | 23 | 25.8 ± 0.6 (24.9–26.4) |
| Tail length | 96 | 91.8 ± 2.3 (88.6–95) | 82 | 78.1 ± 5.2 (71.3–83.9) |
| Number of tail’s body ring | 10 | 9.8 ± 0.4 (9–10) | 9 | 8.7 ± 0.5 (8–9) |
| Terminal ring length | 23 | 24.9 ± 1.6 (22.9–26.6) | 26 | 25 ± 0.8 (23.9–25.7) |
| Desmos covering the terminal ring | 7 | 7.3 ± 0.6 (6.5–7.9) | 9 | 8.8 ± 0.7 (8–9.5) |
| Phasmata | 2.5 | 2.6 ± 0.2 (2.5–2.9) | 2.7 | 2.6 ± 0.1 (2.5–2.8) |
| Species | Characters | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Males | Females | Head Diameter | Head Length | Cephalic Setae Length | Spicules Length | Gubernaculum Length | Vulva (Ring) | Number of Tail Ring (Males) | Number of Tail Ring (Females) | |||||
| Body Length | Body Rings | Setae Pattern (sd/sv) | Body Length | Body Rings | Setae Pattern (sd/sv) | |||||||||
| T. (T.) absidata Timm, 1970 | 485–520 | 57–60 | 11,12/19,19 | 510–550 | 59–60 | 11,12/17,19 | 22 | 16 | 18–19 | 42–45 | - | 27 | 9–10 | 9–10 |
| T. (T.) absidata lizardiensis Decraemer, 1979 | 525–710 | 55–57 | 13,13/17,19 | 540–710 | 56–60 | 13,13/21,23 | 25–29 | 16–21 | 21–27 | 42–48 | 24–28 | 24–27 | 9 | 8 |
| T. (T.) atlantica Freudenhammer, 1975 | 450 | 58 | 7,7/11,11 | 490 | 50 | - | 18–20 | 20–22 | 20–22 | 26 | - | 28–29 | 9 | 9 |
| T. (T.) bipapillata Decraemer, 1987 | 185–205 | 48–55 | 9/14–15 | - | - | - | 11–12 | 8–9 | 10–11 | 25–32 | 12–13 | - | 7–9 | - |
| T. (T.) capitata Decraemer, 1987 | 225 | 55–56 | 9/12–13 | 215–260 | 53–57 | 9/11–14 | 11–14 | 9.5–11 | 11–14 | 52 | 12 | 34–35 | 9 | 6–8 |
| T. (T.) coralicolla Decraemer, 1987 | 185 | 56–57 | 9/12 | 220 | 58 | 9/11 | 11–13 | 11 | 12–14 | 14 | 10 | 33 | 8 | 8 |
| T. (T.) denticulata Timm, 1970 | 645–800 | 63–64 | 10/15–17 | 760 | 65–66 | - | 32–39 | 19–26 | 20–22 | 96–104 | 50–56 | 43 | 11–12 | 9 |
| T. (T.) dimorpha Decraemer, 1978 | 305–600 | 52–65 | 12–13/16–17 | 400 | 62–67 | 13/17 | 15–20 | 11–16 | 15–21 | 16–27 | 16–19 | 27–29 | 10–12 | 12–13 |
| T. (T.) dimorpha papuensis Decraemer, 1987 | 175–210 | 48–55 | 9/10–11 | 180–265 | 48–56 | 8–13/11–15 | 9.5–12 | 8–11 | 9–12 | 14–17 | 8.5–10.5 | 26–33 | 8–11 | 5–10 |
| T. (T.) disparseta sp. nov. | 527–613 | 59–61 | 9–10/14–18 | 490–575 | 60–62 | 9/16–18 | 19–22 | 15–18 | 22–28 | 29–32 | 16–19 | 38–40 | 9–10 | 8–9 |
| T. (T.) fisheri Timm, 1970 | 300–390 | 56–61 | 8–9/10–17 | 275–425 | 56–61 | 8–11/13–16 | 16–20 | 10–14 | 13–17 | 25–38 | 13–29 | 31–33 | 8–9 | 7–8 |
| T. (T.) goldeni Decraemer, 1978 | 310–320 | 55–59 | 12/15–16 | - | - | - | 14 | 11 | 12–13 | 21–22 | 13 | - | 11–12 | - |
| T. (T.) longirostris (Southern, 1914) | 250–900 | 63–78 | 8–9/12–15 | 700–1000 | - | - | 15–36 | 12–31 | 21–32 | 19–35 | 14–16 | - | 10–12 | - |
| T. (T.) oblita Blome, 1982 | 477–506 | 60–63 | 8–9/15–16 | 513 | 63 | 10/18 | 25–27 | - | 21–22 | 27 | 11–13 | 37–38 | 9–10 | - |
| T. (T.) paratimmi Decraemer, 1987 | 385–420 | 61 | 11–12/16–17 | 390–450 | 53–56 | 8–13/15–17 | 13–14 | 20–22 | 11–16 | 28–30 | 17–20 | 27–29 | 9 | 5–6 |
| T. (T.) perparvula Timm, 1970 | 275 | 61–62 | 7,9/13,13 | - | - | - | 14 | 11 | 11 | 24 | 9 | - | 9 | - |
| T. (T.) secunda Blome, 1982 | 328 | 56–57 | 9,9/10, 13 | - | - | - | 18 | - | 16 | 22 | 8 | - | 9 | - |
| T. (T.) spinosoides Chitwood, 1951 | 400 | 61 | 10/17 | 380 | - | 10/14 | - | - | - | 26 | 13 | 26 | - | 12 |
| T. (T.) spuria Inglis, 1967 | 710 | 62 | 11/21 | 735 | 62 | 10–11/18–19 | 27–28 | 18–21 | 29 | 46 | 31 | 33 | 10 | 9 |
| T. (T.) steineri de Man, 1922 | 310–408 | 63–55 | 11–13/13–16 | 310–460 | 63–64 | 12/15–16 | 13–15 | 12–13 | 12–17 | 24–27 | 17 | 28–30 | 12 | 11 |
| T. (T.) ulleungensis Lee, Lee & Rho, 2023 | 409–415 | 54–55 | 6–7/10–12 | 462–567 | 55–57 | 6–7/9–10 | 24–26 | 14–16 | 19–22 | 22–24 | 11–13 | 31 | 8–9 | 8–9 |
| Characters | Southern (1914) | Steiner (1916) | Timm (1978) | Decraemer (1983) | Ansari et al. (2015) | This Study |
|---|---|---|---|---|---|---|
| Specimens | 2 males | 1 male | 4 males | 2 males | 16 males, 19 females | 1 male |
| Body length in male | 650 | 250 | 780–847 | 300–420 | 600–900 | 808 |
| Body length in female | - | - | - | - | 700–1000 | - |
| Number of body ring | 70 | 77 | 71–77 | 63–72 | 70–78 | 78 |
| Width of body | 39 | 14 | 32–39 | 19–21 | 44–57 | 30 |
| Length of head | 31 | - | 25 | 12–16 | - | 26 |
| Width of head | 32 | - | 27 | 15–19 | - | 28 |
| Cephalic seta | - | - | 28–32 | 17–21 | 21–23 | 23 |
| Esophagus ring | - | - | - | 11 | - | 10 |
| Spicule length | - | - | 30 | 32–35 | 19–23 | 28 |
| Length of gubernaculum | - | - | - | 14–15 | - | 16 |
| Number of tail rings | - | 10 | 10–11 | 11–12 | - | 12 |
| Tail length | - | 43 | 112–119 | 60–84 | - | 125 |
| Somatic setae pattern (subdorsal/subventral) | - | - | 8–9/12–15 | 9/12–13 | - | 8,8/13,14 |
| Length of the terminal ring | - | - | 22–58 | 16–23 | - | 38 |
| Locality | Clew bay, Atlantic coast of Ireland, 24 fms, bottom of sand and shells | Prampram, Gold coast, the west coast of Africa, 9 m | McMurdo sound, Antarctica, Hut point, 4457 m, Scott Base, 540 m | Mozambique Channel | Bay of Bengal continental shelf, southeast coast of India, 30–176 m, sandy silt sediment | Northeastern of Guam, Pacific Ocean, 1425.52 m, sediment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.J.; Lee, H.; Kihm, J.-H.; Rho, H.S. Tricoma (Tricoma) disparseta sp. nov. (Nematoda: Desmoscolecidae), a New Free-Living Marine Nematode from a Seamount in the Northwest Pacific Ocean, with a New Record of T. (T.) longirostris (Southern, 1914). Diversity 2024, 16, 648. https://doi.org/10.3390/d16100648
Lee HJ, Lee H, Kihm J-H, Rho HS. Tricoma (Tricoma) disparseta sp. nov. (Nematoda: Desmoscolecidae), a New Free-Living Marine Nematode from a Seamount in the Northwest Pacific Ocean, with a New Record of T. (T.) longirostris (Southern, 1914). Diversity. 2024; 16(10):648. https://doi.org/10.3390/d16100648
Chicago/Turabian StyleLee, Hyo Jin, Heegab Lee, Ji-Hoon Kihm, and Hyun Soo Rho. 2024. "Tricoma (Tricoma) disparseta sp. nov. (Nematoda: Desmoscolecidae), a New Free-Living Marine Nematode from a Seamount in the Northwest Pacific Ocean, with a New Record of T. (T.) longirostris (Southern, 1914)" Diversity 16, no. 10: 648. https://doi.org/10.3390/d16100648
APA StyleLee, H. J., Lee, H., Kihm, J.-H., & Rho, H. S. (2024). Tricoma (Tricoma) disparseta sp. nov. (Nematoda: Desmoscolecidae), a New Free-Living Marine Nematode from a Seamount in the Northwest Pacific Ocean, with a New Record of T. (T.) longirostris (Southern, 1914). Diversity, 16(10), 648. https://doi.org/10.3390/d16100648






