Abstract
Coffee (Coffea arabica), produced and marketed in Ecuador and worldwide, can be organoleptically improved by means of microorganisms such as well-characterized yeasts. This study aimed to isolate and characterize yeasts from three postharvest fermentation processes (i.e., Natural aerobic at room temperature; Carbonic maceration with a CO2 atmosphere at room temperature; and Carbonic refrigerated maceration with a CO2 atmosphere to 10 °C) of coffee fruits in Ecuador. Phenotypic and molecular analyses were conducted on 329 yeast isolates obtained from coffee farms in Loja, Olmedo, and Gonzanamá. Three universal media were used for yeast isolation diversity, and phenotypic characterization included morphology, sugar fermentation, salt tolerance, and ethanol resistance. Molecular characterization involved DNA analysis. The isolated diversity was classified into 12 morphotypes, nine distinct biochemical groups and nine genetic species. Only six species (i.e., Kurtzmaniella quercitrusa, Hanseniaspora opuntiae, Pichia. kluyveri, Torulaspora delbrueckii, T. quercuum, and Wickerhamomyces anomalus) identified phylogenetically corresponded to the designated morphotypes. But surprisingly, nine genetic species matched with the nine biochemical groups determined phenotypically analyzed using principal component analysis (PCA). Most of this diversity was found in the coffee plantation located in Gonzanamá, in contrast to Olmedo and Loja, without statistical significance (p value: 0.08295). On the other hand, the richness is not similar statistically (p value: 0.02991) between postharvest fermentation treatments. The findings suggest that the application of biochemical tests is useful for species determination, although morphological data may be ambiguous. Notably, Pichia kluyveri, detected in this study, holds potential for biotechnological evaluation in coffee fermentation processes.
1. Introduction
Coffee is currently one of the most traded products worldwide, as it is one of the main agro-export products in Ecuador [1]. In total, 20 of the country’s 24 provinces cultivate commercial coffee species: 135,466.2 ha are occupied by arabica varieties (Coffea arabica L.) and 63,748.8 ha are occupied by robusta (Coffea canephora Pierre.) [2]. The Ecuadorian region produces quality coffee, but its chemical and organoleptic improvements are sought through fermentative processes (beneficiation) prior to drying and milling [3].
In beneficiation processes, microorganisms such as yeasts play important roles as facilitators in speed and conversion of organic compounds, helping coffee beans to improve their organoleptic properties (taste and aroma) [4]. Fermentation involves several catabolic processes where organic substances are oxidized, transforming sugars into energy and other simple compounds such as ethanol, acetic acid, lactic acid, and butyric acid [5]. Usually, the farmers apply a typical or natural fermentation method after the harvest where the coffee fruits are dried after being cleaned [4], and other farmers employ carbonic maceration, an analogous technique to the wine fermentation process, where the coffee grains undergo fermentation within a medium saturated with carbon dioxide (CO2), generally maintained at room temperature or below 10 °C [5,6].
Microorganisms such as yeasts, a group of unicellular fungi with or without the presence of hyphae or pseudohyphae, act strongly in that fermentation processes [7]. Ecologically, the yeasts are present in a diverse range of habitats, including soil, aquatic environments, plant surfaces, foods, and skin and mucosal surfaces of animal hosts, but the soil environments represent the major ecological niche for fungi including yeast [8], helping in metabolism and facilitating carbon assimilation of fruits [9].
However, the diversity of microorganisms, especially yeasts, has been reported using molecular techniques [10], especially next-generation sequencing methods (i.e., metagenomics), useful mainly for diversity estimates using operational taxonomic units [11]. Likewise, the nuclear ribosomal DNA region ITS-5.8S is one of the most widely used regions as a DNA barcode [12], as well as the D1/D2 region of the 26S gene [13]. Also, yeast species can be determined by biochemical tests (mandatory to describe new species) through their fermentative capacity, as well as resistance to ethanol, NaCl, or germ tube generation tests [9]. The diversity of yeasts in coffee still requires exploration and adequate taxonomic characterization, as well as their ecological or biotechnological functionality in fermentative processes of fruits such as coffee [1,14,15].
Worldwide, the use of yeasts to improve organoleptic properties is gaining popularity, modifying the flavor and potential of the coffee bean with the use of species such as Saccharomyces cerevisiae and Pichia kluyveri [16,17]. However, in Ecuador and in the province of Loja renowned for its quality coffee [18], studies on improvements in coffee varieties and coffee pathogenic microorganisms are recorded [19]. However, in other localities, biotransformation of coffee flavor is proposed with the use of yeasts such as Saccharomyces cerevisiae and Pichia kluyveri through inoculations [6].
Now, very little is known about the biodiversity of yeasts associated with coffee fermentation processes on Ecuador, with only the study by Jumbo and Martínez [20], which evaluates the capacity of the Saccharomyces cerevisiae species to improve the chemical and organoleptic properties of coffee.
Due to the lack of information on the culturable diversity of yeasts from coffee in this region, the present research sought to isolate, phenotypically and molecularly characterize yeasts from three fermentation processes of coffee fruits (i.e., Natural aerobic at room temperature; Carbonic maceration with CO2 atmosphere at room temperature; and Carbonic refrigerated maceration with CO2 atmosphere to 10 °C) in three different farms in the province of Loja applied by local farmers to improve the sensory properties of coffee. In addition, the conservation of pure strains can be evaluated in the future in controlled fermentation processes of coffee, and the effect on the chemical and sensory properties of the final beverage can be determined.
2. Materials and Methods
2.1. Sampling
Nine samples of coffee beans were randomly collected from coffee farms located in the cantons (Figure 1, Table 1): Loja, Olmedo and Gonzanamá, collecting beans of the Geisha variety of Coffea arabica, characterized mainly by its slender trunk, elongated leaves and branches pointing towards the sky [21]. It is a variety of coffee growing around the Andes Mountain range known as shade coffee, and it is considered to be the best-quality coffee in the region [18].
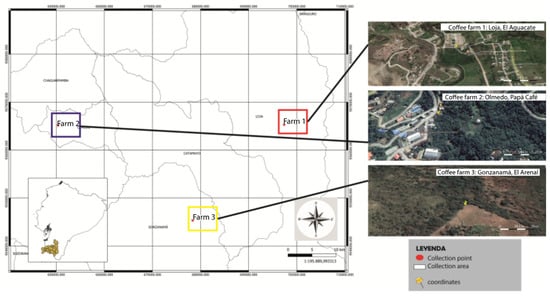
Figure 1.
Geographical location of the coffee farms sampled in the province of Loja.

Table 1.
Reference information of the samples evaluated for each treatment and farm.
2.2. Postharvest Fermentation Process
Coffee fruits (Coffea arabica L.) of the Geisha variety were collected in three farms at different altitudes (1230, 1500, 2010 m a.s.l) located in Loja province of Ecuador. The harvest was conducted by hand, and only ripe fruits were used. The samples were cleaned under continuous flow of tap water [3]. Then, the coffee fruits were placed in 4 L polyethylene bottles and fermented for 144 h under three different conditions: (a) Natural or “blank sample” aerobic at room temperature (N); (b) Carbonic maceration with a CO2 atmosphere at room temperature (CM); and (c) Carbonic refrigerated maceration with a CO2 atmosphere up to 10 °C (CRM). The atmospheric air in the bottles was constantly monitored by a gas analyzer (Oxybaby M+, Dortmund, Germany). Immediately after the fermentation process was completed, the microbiological analyses were carried out [5].
2.3. Yeast Isolation
In total, 10 g of each sample was inoculated into a sterilized stock solution (200 mL) of Yeast Peptone Dextrose Broth (YPD-DIFCO) and incubated at 27 °C for 24 h with continuous motion at 50 rpm [22].
After the incubation, a volume of 30 µL of the stock culture was seeded by a depletion technique on three solid media: Yeast Mold Agar (YMA-DIFCO), Sabouroad Dextrose Agar (SDA-DIFCO) and Yeast Extract Glucose agar (YGC) using Floran (FAVETEX) as a broad-spectrum antibiotic. The cultures were also incubated at 27 °C for 1–2 days. Subsequently, a random number (between 10 and 20 colonies) was selected for replication and purification on Potato dextrose Agar (PDA-DIFCO) solid medium plus Floran antibiotic at a final concentration of 1%. This process was repeated at 48 and 72 h [23].
2.4. Phenotypic Characterization of Pure Strain Sampling
Morphology: Strains were classified by morphotypes (MT) according to their growth form based on outline, size, coloration (British Standard Specification for colors) and aroma [20]. In addition, yeast shape and size were microscopically checked using an Olympus CX41 optical microscope and a 100× magnification. Preparations were performed under direct staining with 1% Phloxine B and 10% KOH.
Biochemistry: Biochemical tests correspond to (a) Sugar fermentation (differential with positive red and negative yellow staining) on a YPD-DIFCO culture medium (5 mL of medium plus 1.6 mL of methyl red prepared at 100 ppm) [21]; (b) Tolerance to NaCl at 10 and 15% (positive when there is colony growth) in a YMA medium under depletion stress and incubation at 30 °C (overnight); (c) Resistance to ethanol in a YPD medium, two tubes for each strain, one with 4.5 mL plus 0.5 mL of 96% ethanol and another 4.25 mL plus 0.75 mL of 96% ethanol, incubated at 30 °C with continuous movement at 60 rpm [24].
Additionally, a yeast germ tube test was performed to determine whether any yeast species corresponded to Candida albicans. All pure strains were tested by inoculating them in human serum and incubating at 37 °C for 2 h [25].
2.5. Statistical Analysis of Ordination and Analysis of Isolates by Farms
To determine differences between yeast species richness by location and treatment, we used one-way analysis of variance (ANOVA). We tested the normality of distributions of richness using the Shapiro–Wilk test (p value > 0.05).
Principal component analysis was performed based on the presence of each yeast population recovered to visualize the grouping of biochemical characteristics by species according to the characteristics of fermentation tests based on the Jaccard similarity index. Principal component analysis was performed using freely available statistical analysis PAST software version 4.10 [26]. We also computed a non-metric MDS (multidimensional scaling) ordination from the species to reveal the degree of similarity among treatments. We used the Euclidean distance as a metric for species similarity.
2.6. DNA Extraction, Amplification and Sequencing
A total of 36 strains were selected to be molecularly worked up due to similar characteristics found in the phenotypic analysis of the strains. Three strains from the same morphotypes, MT, were chosen. One colony for each selected strain was used for DNA extraction and amplification using the Phire Plant Direct PCR Master Mix commercial kit (Thermo Scientific, Vilna, Lithuania) according to the manufacturer’s specifications. The ITS-5.8S region of DNArn and partial LSU (D1/D2) was amplified with the following primers: ITS1F 5′ CTGGTCATTTAGAGGAAGTAA 3′ [27] and NL4 5′ GGTCCGTGTTTCAAGACGG 3′ [28]. PCR conditions were as follows: an initial denaturation at 98 °C for 5 min, followed by 45 cycles of denaturation at 98 °C for 10 s; banding at 55 °C for 10 s and extension at 72 °C for 30 s, and a final extension at 72 °C for 5 min.
PCR results were evaluated by 1% agarose gel electrophoresis and 1X GelRED staining. Positive products were purified using the PureLinkTM Quick PCR Purification Kit (Invitrogen, Vilna, Lithuania) and sequenced with the same set of PCR primers at Macrogen (Seoul, Republic of Korea).
2.7. Phylogenetic Analysis
The sequences obtained were visualized and edited in CodonCode Aligner 9.0.2 software (CodonCode Corporation, Centerville, MA, USA). The concatenated sequences (forward and reverse) were compared in GenBank Blast (https://www.ncbi.nlm.nih.gov/genbank/ accessed on 22 June 2023) to download the most similar sequences, preferably with taxonomic value (assigned species names). All sequences (37 new and 35 from GenBank) were aligned in MAFFT Version 7.489 using the G-INS-i strategy [29].
Two phylogenetic trees were performed under Maximum Likelihood algorithms [30], Kimura-2 parameter model and G + I nucleotide substitution rate model, followed by 1000 Bootstrap replicates employing MEGA 11 software [31].
2.8. Strain Preservation
The pure strains obtained were inoculated in a solution of 1.64 mL YPD medium plus 20% glycerol (permeable cryoprotectant) and allowed to grow for 1 h at 27 °C. Subsequently, they were frozen at −80 °C in cryovials. Additionally, yeast suspensions were made in 2 mL of triple-sterilized distilled water and 2 mL of 100% sterile mineral oil, which were kept refrigerated at 4 °C [32,33].
3. Results
3.1. Phenotyping and Diversity
A total of 329 pure strains were obtained and classified into 12 morphotypes (MT) (Figure 2). Several MT correlated by shape: rounded (MT1, MT3 and MT5) differentiated by attachments such as protrusions (MT5), visualization of vacuoles (MT1) and visualization of cell walls and membranes typical of the genera Torulaspora and Wickerhamomyces. On the other hand, oval-type morphology was found in MT2, MT6 and MT10, typical of the genera Kurztmaniella, Hanseniaspora and Pichia, respectively. Furthermore, elongated morphotypes (MT4, MT7, MT8, MT9 and MT11) were classified as recurrent in the genus Pichia; finally, protrusions attached to a rectangular oval MT (MT12) were found. Sporulation of MT2 strain BMF4L2 was recorded (Figure 2), and four internal segments were differentiated in the yeast.
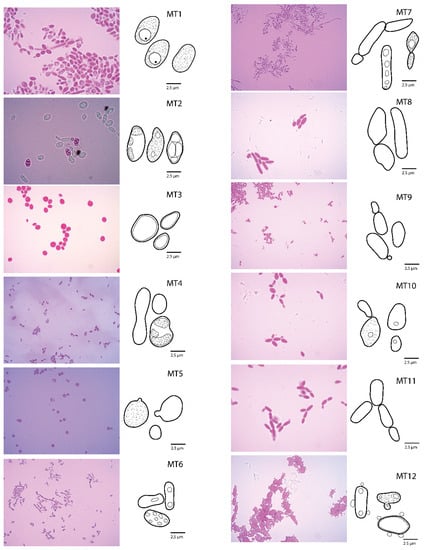
Figure 2.
Morphotypes determined for the yeast isolates in this study. Photographs indicate reddish staining by Phloxine 1%. Illustrations represent in detail the yeast forms.
Most of the strains isolated and macroscopically checked showed similar characteristics in terms of colony shape and odor.
The morphotype designated for Kurtzmaniella quercitrusa clustered with similar biochemical properties to those of Pichia fermentans, Hanseniaspora opuntiae and Torulaspora delbrueckii (Figure 3), standing out from the others by the presence of a germ tube, as well as growth in lactose and 15% NaCl. P. kluyveri, H. uvarum and Wickerhamomyces anomalus showed fermentation affinity in glucose and maintained a higher resistance in 10% NaCl.
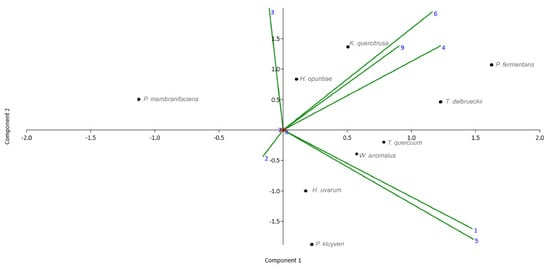
Figure 3.
PCA for the nine species determined biochemically, according to the fermentation tests on (1) malt, (2) sucrose, (3) glucose, (4) lactose. Tolerance to (5) 10% NaCl, (6) 15% NaCl, (7) 10% ethanol, (8) 15% ethanol. (9) Germ Tube Test.
Most species diversity was found in the coffee plantation located in Gonzanamá (Figure 4), in contrast to Olmedo and Loja, but statistically not significant (p value: 0.08295) (Table 2, Figure 4). In the isolates from Olmedo and Loja, the species Hanseniaspora opontuiae was not identified (Table 3). On the other hand, the isolates from Loja “El aguacate” (Table 1) presented a lower number of species that did not include Kurtzmaniella quercitrusa and Torulaspora delbrueckii, but presented a higher abundance for the genus Pichia (Table 2, Figure 4).
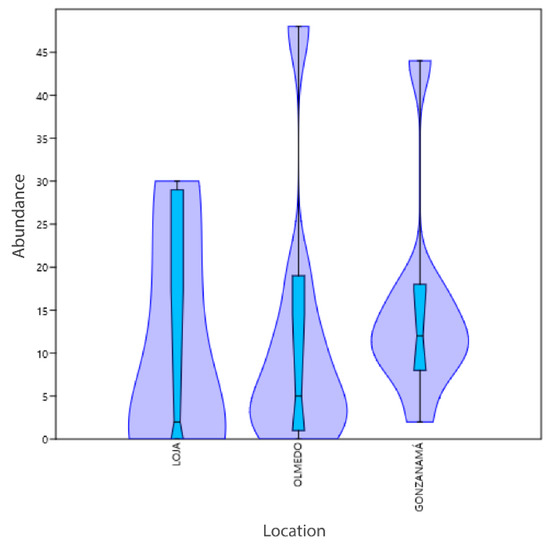
Figure 4.
Diversity analysis by sampling site. The numbers on abundance axis represents isolates by each of the nine species.

Table 2.
ANOVA of the richness of yeasts found according to the fermentation treatment used and the origin of the sample.

Table 3.
Strains obtained and their BLAST reference information.
The richness was not similar between treatments (p value: 0.02991), which was statistically different from the natural fermentation, with a dispersion like the mean F: 4.077 (Figure 5A,B).
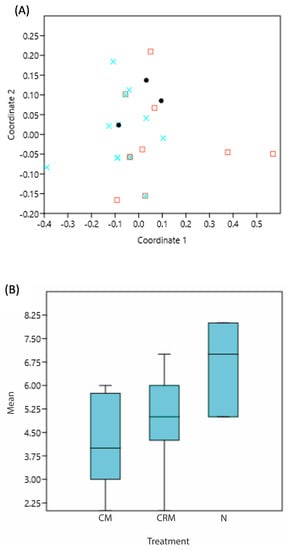
Figure 5.
(A) NMDS of the species found according to the treatment: x = Natural or “blank sample” aerobic at room temperature (N); □ = Carbonic maceration with CO2 atmosphere at room temperature (CM); and • = Carbonic refrigerated maceration with CO2 atmosphere to 10 °C (CRM), (B) boxplot of the species according to the treatments.
Biochemically from 329 strains, nine groups corresponding to the species Hanseniaspora opuntiae, H. uvarum, Kurtzmaniella quercitrusa, Pichia fermentans, P. kluyveri, P. membranifaciens, Torulaspora delbrueckii, T. quercuum and Wickerhamomyces anomalus were determined based on the description of Kurtzman et al. (2011).
Kurtzmaniella quercitrusa, Hamniospora opuntiae, P. fermentans and Torulaspora delbrueckii showed higher fermentation in glucose and lactose. In addition, they grew on media with NaCl up to 15% and 15% ethanol; this differed from P. kluyveri, which maintained similar tolerance characteristics up to 10% of each reagent.
3.2. Molecular Species Definition
From 36 molecularly worked-up strains, 60 (ITS-5.8S plus D1/D2 partial LSU) sequences (forward and reverse) were obtained. Twelve sequences were discarded due to multiple peaks in the chromatograms. All concatenated sequences (37 in total, Table 3) corresponded to the Ascomycota division (Table 3). Nine genetic species were determined (Table 3, Figure 6). The species Pichia membranifaciens was considered the most recurrent with 15 sequences and strains clustered in one clade (Figure 6).
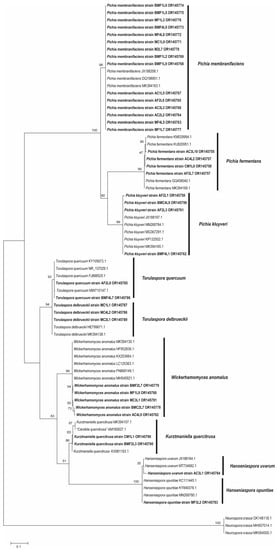
Figure 6.
Phylogenetic placement of ITS-5.8S plus D1/D2 LSU partial of yeast sequences. Maximum likelihood phylogenetic analysis, with Bootstrap values greater than 50, are presented above the nodes.
The nine genetic species (Figure 6) corresponded to the nine biochemically determined phenotypes (i.e., Hanseniaspora opuntiae, H. uvarum, Kurtzmaniella quercitrusa, Pichia fermentans, P. kluyveri, P. membranifaciens, Torulaspora delbrueckii, T. quercuum and Wickerhamomyces anomalus) (Figure 2 and Figure 3). On the other hand, the nine genetic species only corresponded to six MTs where MT1 matched with Torulaspora delbrueckii; MT2 to Kurtzmaniela quercitrusa; MT3 to Wickerhamomyces anomalus; MT5 to Torulaspora quercuum; MT9 to Hanseniaspora opuntiae and MT10 matched with Pichia kluyveri.
4. Discussion
The diversity of microorganisms in different ecosystems is high and still unknown [1]. Also, not all microorganisms are culturable. However, 329 strains were isolated in this study from coffee fruits corresponding to 12 morphotypes, suggesting 12 morphospecies. Moreover, nine biochemical groups corresponding to the species Hanseniaspora opuntiae, H. uvarum, Kurtzmaniella quercitrusa, Pichia fermentans, P. kluyveri, P. membranifaciens, Torulaspora delbrueckii, T. quercuum, and Wickerhamomyces anomalus (Figure 3 and Figure 5) were determined.
Surprisingly, all these species identified biochemically are of the same genetic species determined phylogenetically. Nonetheless, only six of the designated morphospecies (Kurtzmaniella quercitrusa, Hanseniaspora opuntiae, Pichia. kluyveri, Torulaspora delbrueckii, T. quercuum, and Wickerhamomyces anomalus) corresponded to the genetic species. This is an indication that morphological characters are not entirely conclusive for defining yeast species [48] or other fungal species [24]. This is due to the variability that microorganisms can generate depending on the environment in which they develop [50]. In this regard, rapid and accurate identification of yeasts can be more effective using molecular tools [11], such as metagenomics [10].
On the other hand, it is well known that biochemical tests are an essential tool for the description of microorganisms [51] and are widely used in the description of yeast species such as within the genus Candida [52]. Likewise, De Melo Pereira et al. [53] indicate that fermentative capacity are unique characteristics to define species within different genera like Saccharomyces into Saccharomycotina. Biochemically and morphologically, the MT2 was determined as the species Candida quercitrusa, actually synonymized with Krutzmaniella quercitrusa [34]. The genetic species determined as Pichia membranifaciens (98% Bootstrap) contains several sequences from strains M2L7, MF4L8, BMF1L6, AC1L5, BMF1L2, BMF1L5, BMF4L9, AC3L3, MF1L3, MF1L7, AC2L2, AF2L6, MC1L9, BMF1L9 and MF4L5, which were preliminarily classified into two morphotypes, MT7 and MT12, respectively (Figure 2). It is likely that the morphological variation corresponds to an adaptation of each strain to the culture medium, as suggested for other yeasts [54].
Of the species isolated in this study, the species Torulaspora delbruekii and T. quercuum have been previously reported in studies of fungal diversity associated with coffee in the species Coffea arabica L. in different locations of the planet such as USA, Europe and Asia, but no records of these species were found in Ecuador [54,55]. These species are considered to be sisters, as they are grouped within the same phylogenetic clade [15], accompanied by morphological similarity as indicated in this study (Figure 2 (MT1 and MT5)). Additionally, similar results in NaCl, ethanol resistance, malt and lactose fermentation were found for Torulaspora delbruekii and T. quercuum, contributing to the evidence of their taxonomic closeness.
Similarly, the species Krutzmaniella quercitrusa, Hanseniaspora opuntiae, Pichia kluyveri, and Torulaspora delbrueckii [56,57] have been reported from coffee fruits in nearby countries such as Brazil, Colombia and Chile. In addition, isolation of yeasts from coffee (Coffea arabica) belonging to the genera Pichia, Candida and Saccharomycopsis has been reported [58,59]. For the southern region of Ecuador, the nine genetic species described in this study and species such as Candida albicans and Saccharomyces cerevisiae have been reported in a study on the microbiome in the beverage called colada morada [60]. It is known that the diversity of microorganisms can vary due to environmental factors [55] such as fermentation process in closed tanks with airlocks used by the farmers to generate new sensory profiles.
Local farmers apply similar fermentation processes as evaluated here: natural fermentation or controlled atmosphere with a CO2-rich environment at room temperature and refrigeration to improve sensory profiles mainly conducted by development of different microorganisms like yeasts [56,57]. Biotechnologically, yeasts such as those determined in this study, for example, Pichia membranifaciens, are applicable in fermentation processes of fruits such as coffee [58,59,61], or as biocontrollers of fungal pathogens (e.g., Botrytis cinerea) [62]. Likewise, species such as Pichia fermentans and P. Kluyveri have been used in the wine and brewing industry including various fruits such as coffee [61]; likewise, Hanseniaspora uvarum and Wickerhamomyces anomalus have been used to date in the wine industry, increasing their organoleptic properties such as aroma and flavors [60].
On the other hand, the species Torulaspora delbrueckii, a yeast with remarkable resistance to osmotic and freezing stress [53], possesses flavor- and aroma-enhancing properties in wine, beer, or bread dough fermentation processes [58]. This yeast is considered a biotechnological model that can be used in food industries [53]. Kurtzmaniella quercitrusa and Hanseniaspora opuntiae species have been used and reported in the fermentation of cocoa beans in Malaysia [14]. In the case of Torulaspora quercuum, it has been reported in cider fermentation in association with other yeasts described in this study [15]. However, its metabolic potential is currently being studied, and it is defined as a potential biotechnological model in the production of ethanol associated with microalgae [63].
Future studies are required to determine the capabilities and behavior of these yeasts in coffee fermentation processes and the effect on the chemical and sensory properties of the final beverage.
5. Conclusions
The diversity of yeast can vary due to environmental factors such as fermentation process, as is evaluated here (i.e., Natural aerobic at room temperature; Carbonic maceration with CO2 atmosphere at room temperature; and Carbonic refrigerated maceration with CO2 atmosphere to 10 °C), allowing the obtention of culturable yeast. This diversity after biochemical and molecular test is represented by nine species, Hanseniaspora opuntiae, H. uvarum, Kurtzmaniella quercitrusa, Pichia fermentans, P. kluyveri, P. membranifaciens, Torulaspora delbrueckii, T. quercuum, and Wickerhamomyces anomalus. These nine species determined biochemically and molecularly have congruent correspondence, contrary to morphological characters that can be ambiguous due to overlapping between species as, for example, within the genus Pichia.
The nine species determined here are considered new Saccharomycotina records for southern Ecuador according to the revised literature.
Author Contributions
D.C., D.A., Á.B. and J.M.F. contributed to the conceptualization and methodology of this study. J.M.F. and J.G.F. carried out sampling, and critical revision on manuscript, K.E., contributed with laboratory work and general comments on manuscript. All authors contributed with their experience to improve the whole manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded with five thousand American dollars (USD 5000) into the internal project PROY_INV_QUI_2021_2837 by the Vicerrectorado de Investigación of the Universidad Técnica Particular de Loja.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not Applicable.
Acknowledgments
The authors would like to thank some students at the Biology career for help during the laboratory work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barrezueta-Unda, S.; Moreira Blacio, W.; Quezada Abad, C. Análisis del cacao y café ecuatoriano desde su cadena de valor en el periodo 2010–2015. Rev. Científica Agroecosistemas 2018, 6, 6–17. [Google Scholar]
- Mercedes, M.S.; Otiniano, A.J.; Borjas Ventura, R. Sustentabilidad de fincas productoras de café en Jipijapa (Manabí, Ecuador). Saber Hacer-Rev. Fac. Ing. USIL 2016, 3, 23–25. [Google Scholar]
- Duicela, G.L.A.; Andrade, M.J.; Farfán, T.D.S.; Velásquez, C.S.D. Calidad organoléptica, métodos de beneficio y cultivares de café robusta (Coffea canephora Pierre ex Froehner) en la amazonía del Ecuador. Rev. Iberoam. Tecnol. Postcosecha 2018, 19, 2. [Google Scholar]
- Elhalis, H.; Cox, J.; Frank, D.; Zhao, J. The crucial role of yeasts in the wet fermentation of coffee beans and quality. Int. J. Food Microbiol. 2020, 333, 108796. [Google Scholar] [CrossRef] [PubMed]
- Guzzon, R.; Malacarne, M.; Larcher, R.; Franciosi, E.; Toffanin, A. The impact of grape processing and carbonic maceration on the microbiota of early stages of winemaking. J. Appl. Microbiol. 2020, 128, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sun, J.; Lassabliere, B.; Yu, B.; Liu, S.Q. Coffee flavour modification through controlled fermentations of green coffee beans by Saccharomyces cerevisiae and Pichia kluyveri: Part I. Effects from individual yeasts. Food Res. Int. 2020, 136, 109588. [Google Scholar] [CrossRef]
- Arastehfar, A.; Fang, W.; Pan, W.; Lackner, M.; Liao, W.; Badiee, P.; Boekhout, T. YEAST PANEL multiplex PCR for identification of clinically important yeast species: Stepwise diagnostic strategy, useful for developing countries. Diagn. Microbiol. Infect. Dis. 2019, 93, 112–119. [Google Scholar] [CrossRef]
- Yurkov, A.M.; Röhl, O.; Carvalho, C.; Maldonado, C.; Sampaio, J.P. Local climatic conditions constrain soil yeast diversity patterns in mediterranean forests, woodlands and scrubbiome. FEMS Yeast Res. 2015, 16, fov103. [Google Scholar] [CrossRef]
- Toro, M.; Oro, N.; Vega, A.; Maturano, Y.; Nally, M.; Fernández, E.; Pucheta, E.; Vázquez, F. Diversidad de levaduras en canopias y suelos asociados con Bulnesia retama y Larrea divaricata. Rev. Argent. De Microbiol. 2005, 37, 209–213. [Google Scholar]
- Kurtzman, C.; Robnett, C. Phylogenetic relationships among yeasts of the ‘Saccharomyces complex’ determined from multigene sequence analyses. FEMS Yeast Res. 2003, 3, 417–432. [Google Scholar] [CrossRef]
- Vásquez, C.J.A.; Ramirez, C.M.; Monsalve, F.Z.I. Actualización en caracterización molecular de Levaduras de Interés Industrial. Rev. Colomb. Biotecnol. 2016, 18, 129. [Google Scholar] [CrossRef]
- Nerva, L.; Turina, M.; Zanzotto, A.; Gardiman, M.; Gaiotti, F.; Gambino, G.; Chitarra, W. Isolation, molecular characterization and virome analysis of culturable wood fungal endophytes in esca symptomatic and asymptomatic grapevine plants. Environ. Microbiol. 2019, 21, 2886–2904. [Google Scholar] [CrossRef] [PubMed]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef] [PubMed]
- Segura, G.L.E.; Kirchmayr, M.R.; Flores, B.E.P.; Gschaedler, M.A.C. PCR-RFLP de las regiones ITS-5.8S como herramienta de identificación de levaduras: Ventajas y desventajas. e-Gnosis 2010, 8, 1–12. [Google Scholar]
- Ooi, T.S.; Ting, A.S.Y.; Siow, L.F. Influence of selected native yeast starter cultures on the antioxidant activities, fermentation index and total soluble solids of Malaysia cocoa beans: A simulation study. LWT 2020, 122, 108977. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, Y.; Qiu, Y.; Guo, H.; Ju, H.; Wang, Y.; Yuan, Y.; Yue, T. Chemical composition, sensorial properties, and aroma-active compounds of ciders fermented with Hanseniaspora osmophila and Torulaspora quercuum in co- and sequential fermentations. Food Chem. 2020, 306, 12–56. [Google Scholar] [CrossRef]
- Evangelista, S.R.; Silva, C.F.; da Cruz Miguel, M.G.P.; de Souza, C.C.; Pinheiro, A.C.M.; Duarte, W.F.; Schwan, R.F. Improvement of coffee beverage quality by using selected yeasts strains during the fermentation in dry process. Food Res. Int. 2020, 61, 183–195. [Google Scholar] [CrossRef]
- Santos, D.K.F.; Resende, A.H.M.; de Almeida, D.G.; Soares da Silva, R.C.F.; Rufino, R.D.; Luna, J.M.; Banat, I.M.; Sarubbo, L.A. Candida lipolytica UCP0988 Biosurfactant: Potential as a Bioremediation Agent and in Formulating a Commercial Related Product. Front. Microbiol. 2017, 8, 767. [Google Scholar] [CrossRef]
- Sepúlveda, W.S.; Ureta, I.; Sepulveda-Sepúlveda, A. Profile and preference of Ecuadorian consumers for quality attributes in coffee production. Coffee Sci. 2016, 11, 298–307. [Google Scholar]
- Aguirre, L.A.; Rodríguez, Z.; Boucourt, R.; Saca, V.; Salazar, R.; Jiménez, M. Effect of whey on solid state fermentation of coffee (Coffea arabica L.) pulp for feeding ruminants. Rev. Cuba. De Cienc. Agrícola 2018, 52, 303–312. [Google Scholar]
- Jumbo, N.; Martínez, M. Efecto del Uso de Saccharomyces Cerevisiae Bajo Condiciones Fermentativas en la Calidad de Taza del Café (Coffea arabica L.) en el Cantón Loja; Trabajo de titulación, Universidad Nacional de Loja: Loja, Ecuador, 2021. [Google Scholar]
- Arcila Pulgarín, J. Crecimiento y Desarrollo de la Planta de Café. Sistemas de Producción de Café en Colombia; CENICAFE: Chinchiná, Colombia, 2007; pp. 21–60. [Google Scholar]
- Delgado-Ospina, J.; Triboletti, S.; Alessandria, V.; Serio, A.; Sergi, M.; Paparella, A.; Rantsiou, K.; Chaves-López, C. Functional Biodiversity of Yeasts Isolated from Colombian Fermented and Dry Cocoa Beans. Microorganisms 2020, 8, 1086. [Google Scholar] [CrossRef]
- Versalovic, J. (Ed.) Manual of Clinical Microbiology; American Society for Microbiology Press: Washington, DC, USA, 2011; Volume 1. [Google Scholar]
- Rodrigues, C.B.; Barabasz, R.F.; Silva, R.H.D.; Sustakowski, M.C.; Kuhn, O.J.; Carvalho, J.C.; Stangarlin, J.R. Yeast Potential for the Biological Control of Colletotrichum musae. J. Agric. Sci. 2020, 12, 301. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Leaw, S.N.; Chang, H.C.; Sun, H.F.; Barton, R.; Bouchara, J.P.; Chang, T.C. Identification of medically important yeast species by sequence analysis of the internal transcribed spacer regions. J. Clin. Microbiol. 2006, 44, 693–699. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics, 1st ed.; Oxford University Press: New York, NY, USA, 2000; pp. 154–196. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. Mega X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Ocares, P.; Yocelyn; Castro, F.; Jean, F. Preservación de microorganismos por congelación. Inst. Investig. Agropecu. 2020, 428, 119–134. [Google Scholar]
- Wang, F.; Cui, M.; Liu, H.; Li, X.; Yu, J.; Huang, Y.; Liu, Y. Characterization and identification of a fraction from silver carp (Hypophthalmichthys molitrix) muscle hydrolysates with cryoprotective effects on yeast. LWT 2021, 137, 110388. [Google Scholar] [CrossRef]
- Xiao, M.; Wang, H.; Lu, J.; Chen, S.C.; Kong, F.; Ma, X.J.; Xu, Y.C. Three clustered cases of candidemia caused by Candida quercitrusa and mycological characteristics of this novel species. J. Clin. Microbiol. 2014, 52, 3044–3048. [Google Scholar] [CrossRef]
- Eddouzi, J.; Lohberger, A.; Vogne, C.; Manai, M.; Sanglard, D. Identification and antifungal susceptibility of a large collection of yeast strains isolated in Tunisian hospitals. Med. Mycol. 2013, 51, 737–746. [Google Scholar] [CrossRef][Green Version]
- Ben Taheur, F.; Mansour, C.; Ben Jeddou, K.; Machreki, Y.; Kouidhi, B.; Abdulhakim, J.A.; Chaieb, K. Aflatoxin B1 degradation by microorganisms isolated from kombucha culture. Toxicon 2020, 179, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.S.; Arruda, L.M.; Xavier, P.L.; Ramírez, M.X.; da Silveira, F.A.; Santana, W.C.; da Silva, P.H.; Fietto, L.G.; Eller, M.R. Selection of yeasts from bee products for alcoholic beverage production. Braz. J. Microbiol. 2020, 51, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Bourret, T.B.; Grove, G.G.; Vandemark, G.J.; Henick-Kling, T.; Glawe, D.A. Diversity and molecular determination of wild yeasts in a central Washington State vineyard. N. Am. Fungi 2013, 8, 1–32. [Google Scholar] [CrossRef]
- Zabukovec, P.; Čadež, N.; Čuš, F. Isolation and Identification of Indigenous Wine Yeasts and Their Use in Alcoholic Fermentation. Food Technol. Biotechnol. 2020, 58, 337–347. [Google Scholar] [CrossRef]
- Stavrou, A.A.; Lackner, M.; Lass-Flörl, C.; Boekhout, T. The changing spectrum of saccharomycotina yeasts causing candidemia: Phylogeny mirrors antifungal susceptibility patterns for azole drugs and amphothericin B. FEMS Yeast Res. 2019, 19, foz037. [Google Scholar] [CrossRef]
- Li, D.; Han, T.; Liao, J.; Hu, X.; Xu, S.; Tian, K.; Gu, X.; Cheng, K.; Li, Z.; Hua, H.; et al. Oridonin, a promising ent-kaurane diterpenoid lead compound. Int. J. Mol. Sci. 2016, 17, 1395. [Google Scholar] [CrossRef] [PubMed]
- Arteau, M.; Labrie, S.; Roy, D. Terminal-restriction fragment length polymorphism and automated ribosomal intergenic spacer analysis profiling of fungal communities in Camembert cheese. Int. Dairy J. 2010, 20, 545–554. [Google Scholar] [CrossRef]
- Vasques, D.T.; Ebihara, A.; Hirai, R.Y.; Prado, J.; Motomi, I. Phylogeny of Hymenophyllum subg. Mecodium (Hymenophyllaceae), with special focus on the diversity of the Hymenophyllum polyanthos species complex. Plant Syst. Evol. 2019, 305, 811–825. [Google Scholar] [CrossRef]
- Irinyi, L.; Serena, C.; Garcia-Hermoso, D.; Arabatzis, M.; Desnos-Ollivier, M.; Vu, D.; Meyer, W. International Society of Human and Animal Mycology (ISHAM)-ITS reference DNA barcoding database—The quality controlled standard tool for routine identification of human and animal pathogenic fungi. Med. Mycol. 2015, 53, 313–337. [Google Scholar] [CrossRef]
- Wu, Z.W.; Robert, V.; Bai, F.Y. Genetic diversity of the Pichia membranifaciens strains revealed from rRNA gene sequencing and electrophoretic karyotyping, and the proposal of Candida californica comb. nov. FEMS Yeast Res. 2006, 6, 305–311. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Golić, N.; Čadež, N.; Terzić-Vidojević, A.; Šuranská, H.; Beganović, J.; Lozo, J.; Topisirović, L. Evaluation of lactic acid bacteria and yeast diversity in traditional white pickled and fresh soft cheeses from the mountain regions of Serbia and lowland regions of Croatia. Int. J. Food Microbiol. 2013, 166, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Vu, D.; Groenewald, M.; Szöke, S.; Cardinali, G.; Eberhardt, U.; Stielow, B.; Robert, V. DNA barcoding analysis of more than 9 000 yeast isolates contributes to quantitative thresholds for yeast species and genera delimitation. Stud. Mycol. 2016, 85, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.M.; Xu, J.; Wang, H.; Li, J.; Bai, F.Y. Torulaspora quercuum sp. nov. and Candida pseudohumilis sp. nov., novel yeasts from human and forest habitats. FEMS Yeast Res. 2009, 9, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Zha, Y.; Hossain, A.H.; Tobola, F.; Sedee, N.; Havekes, M.; Punt, P.J. Pichia anomala 29X: A resistant strain for lignocellulosic biomass hydrolysate fermentation. FEMS Yeast Res. 2013, 13, 609–617. [Google Scholar] [CrossRef]
- Tanahashi, M.; Hawes, C. The presence of a mycangium in European Sinodendron cylindricum (Coleoptera: Lucanidae) and the associated yeast symbionts. J. Insect Sci. 2016, 16, 76. [Google Scholar] [CrossRef] [PubMed]
- Glushakova, A.M.; Maximova, I.A.; Kachalkin, A.V.; Yurkov, A.M. Ogataea cecidiorum sp. nov., a methanol-assimilating yeast isolated from galls on willow leaves. Antonie Van Leeuwenhoek 2010, 98, 93–101. [Google Scholar] [CrossRef]
- Knop, M. Yeast cell morphology and sexual reproduction–A short overview and some considerations. Comptes Rendus Biol. 2011, 334, 599–606. [Google Scholar] [CrossRef]
- De Melo Pereira, G.V.; Soccol, V.T.; Pandey, A.; Medeiros, A.B.P.; Lara, J.M.R.A.; Gollo, A.L.; Soccol, C.R. Isolation, selection and evaluation of yeasts for use in fermentation of coffee beans by the wet process. Int. J. Food Microbiol. 2014, 188, 60–66. [Google Scholar] [CrossRef]
- Lopes, M.R.; Santos, A.R.O.; Moreira, J.D.; Santa-Brígida, R.; Martins, M.B.; Pinto, F.O.; Valente, P.; Morais, P.B.; Jacques, N.; Grondin, C.; et al. Kurtzmaniella hittingeri f.a., sp. nov., isolated from rotting wood and fruits, and transfer of three Candida species to the genus Kurtzmaniella as new combinations. Int. J. Syst. Evol. Microbiol. 2019, 69, 1504–1508. [Google Scholar] [CrossRef]
- De Carvalho Neto, D.P.; De Melo Pereira, G.V.; Tanobe, V.O.; Thomaz, S.V.G.; Da Silva, B.J.; Rodrigues, C.; Soccol, C.R. Yeast diversity and physicochemical characteristics associated with coffee bean fermentation from the Brazilian Cerrado Mineiro region. Fermentation 2017, 3, 11. [Google Scholar] [CrossRef]
- Kurtzman, C.; Fell, J.W.; Boekhout, T. The Yeasts a Taxonomic Study, 5th ed.; Elsevier Science: Madrid, Spain, 2011; pp. 543–562. [Google Scholar]
- Rodrigues, J.D.O.; Höfling, J.F.; Tavares, F.C.A.; Duarte, K.M.R.; Gonçalves, R.B.; Azevedo, R.A.D. Evaluation of biochemical and serological methods to identify and clustering yeast cells of oral Candida species by CHROMagar test, SDS-PAGE and ELISA. Braz. J. Biol. 2004, 64, 317–326. [Google Scholar] [CrossRef]
- Martins, P.M.M.; Ribeiro, L.S.; Miguel, M.G.D.C.P.; Evangelista, S.R.; Schwan, R.F. Production of coffee (Coffea arabica) inoculated with yeasts: Impact on quality. J. Sci. Food Agric. 2019, 99, 5638–5645. [Google Scholar] [CrossRef] [PubMed]
- Op De Beeck, M.; Lievens, B.; Busschaert, P.; Declerck, S.; Vangronsveld, J.; Colpaert, J.V. Comparison and validation of some ITS primer pairs useful for fungal metabarcoding studies. PLoS ONE 2014, 9, 6–29. [Google Scholar] [CrossRef]
- Armijos, C.; Valarezo, E.; Cartuche, L.; Zaragoza, T.; Finzi, P.V.; Mellerio, G.G.; Vidari, G. Chemical composition and antimicrobial activity of Myrcianthes fragrans essential oil, a natural aromatizer of the traditional Ecuadorian beverage colada morada. J. Ethnopharmacol. 2018, 225, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Shapaval, V.; Brandenburg, J.; Blomqvist, J.; Tafintseva, V.; Passoth, V.; Sandgren, M.; Kohler. Biochemical profiling, prediction of total lipid content and fatty acid profile in oleaginous yeasts by FTIR spectroscopy. Biotechnol. Biofuels 2019, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhou, Y.; Zeng, K. Effect of Pichia membranaefaciens on ROS metabolism and postharvest disease control in citrus fruit. Crop Prot. 2013, 53, 96–102. [Google Scholar] [CrossRef]
- Vicente, J.; Calderón, F.; Santos, A.; Marquina, D.; Benito, S. High potential of Pichia kluyveri and other Pichia species in wine technology. Int. J. Mol. Sci. 2021, 22, 1196. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).