Hot Is Rich—An Enormous Diversity of Simple Trichal Cyanobacteria from Yellowstone Hot Springs
Abstract
1. Introduction
2. Materials and Methods
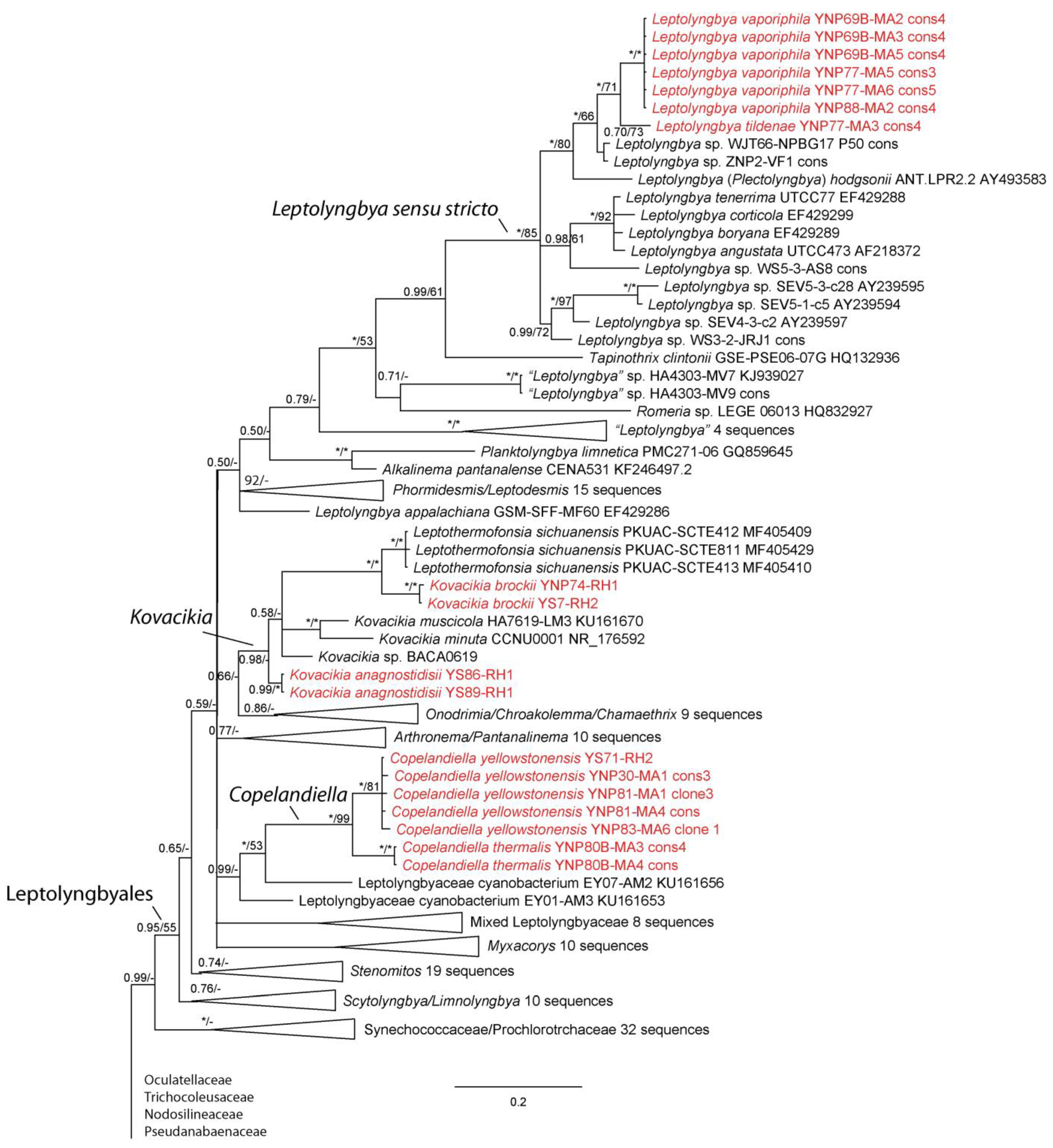
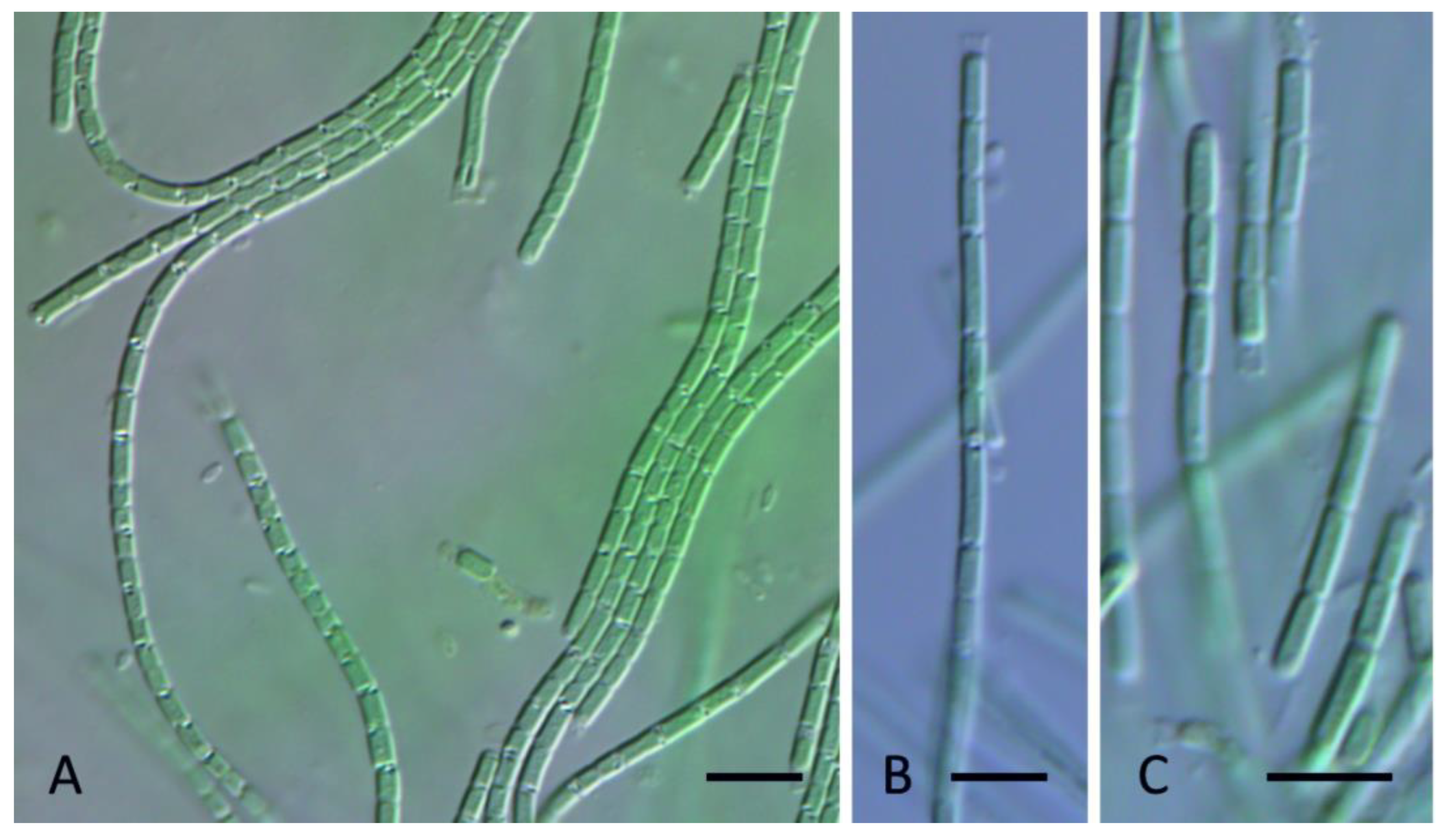
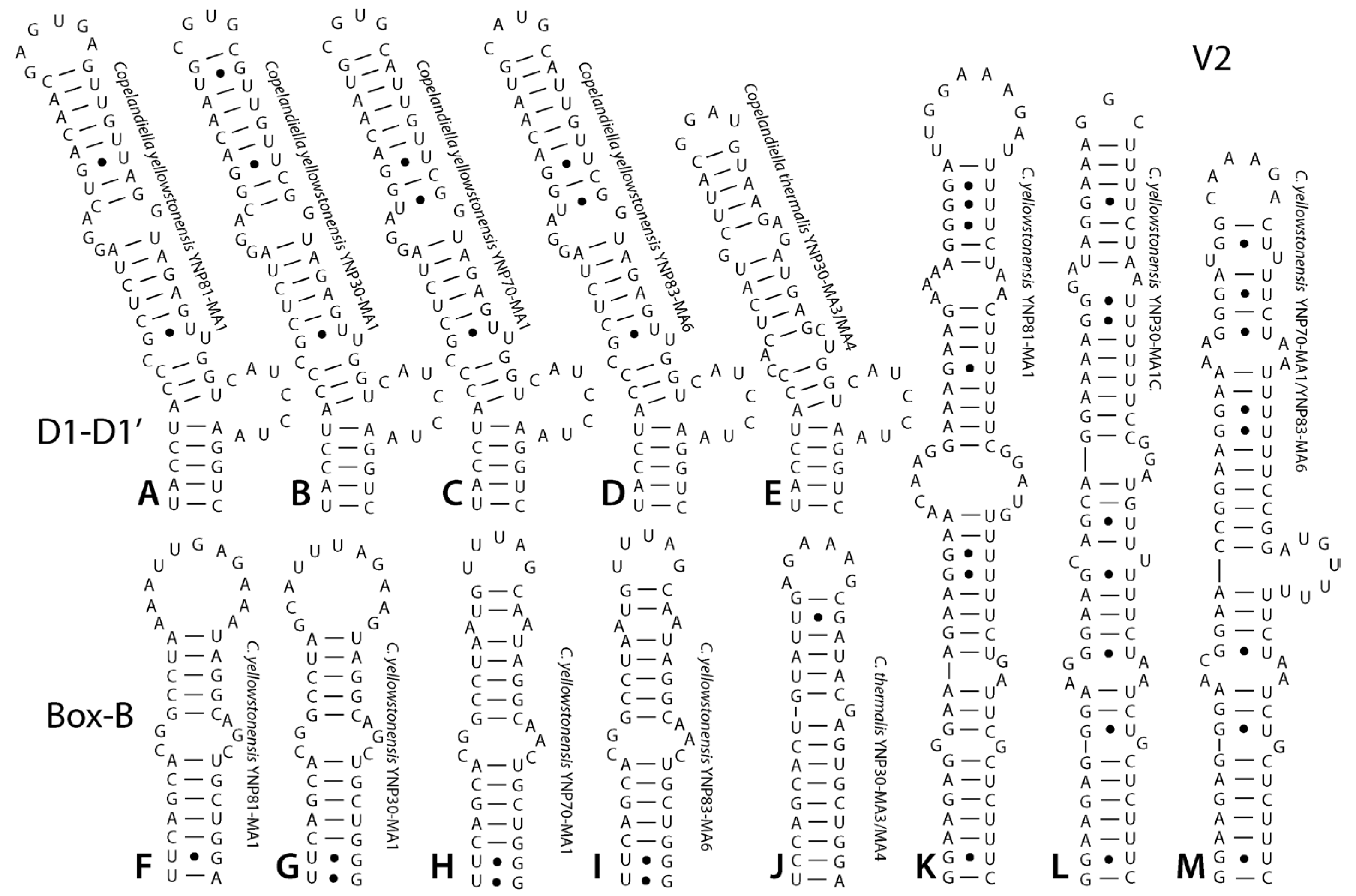
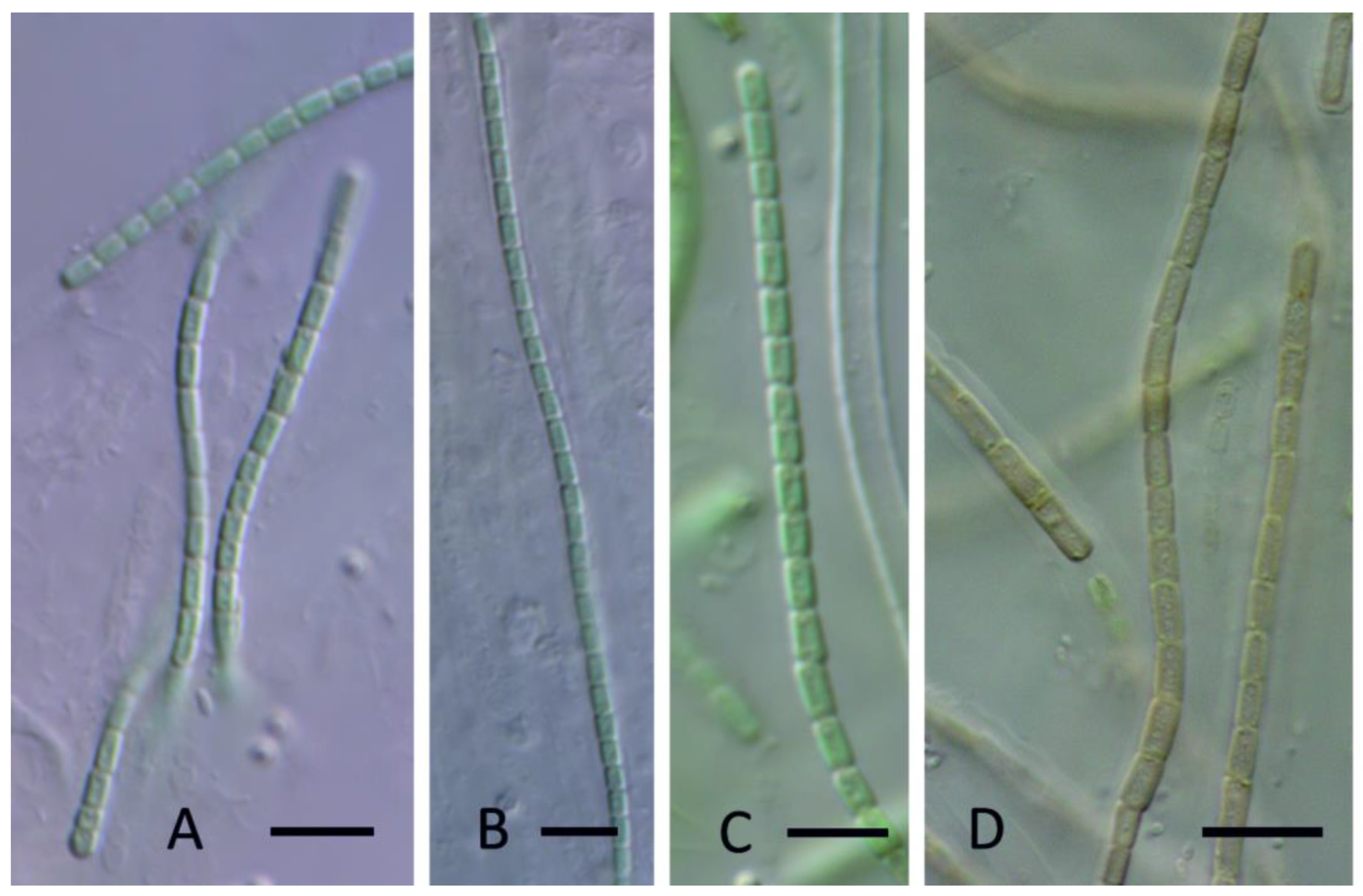
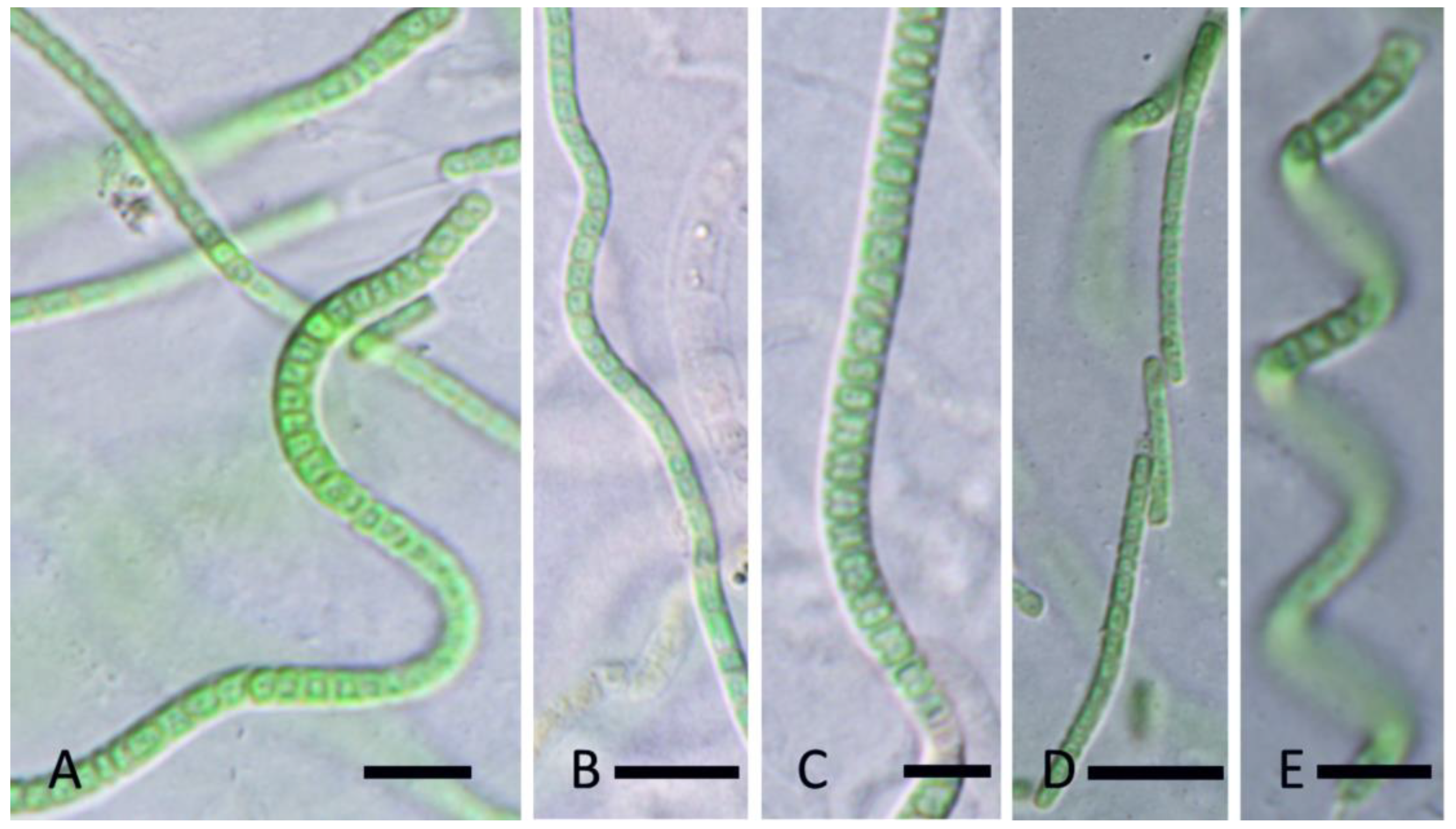
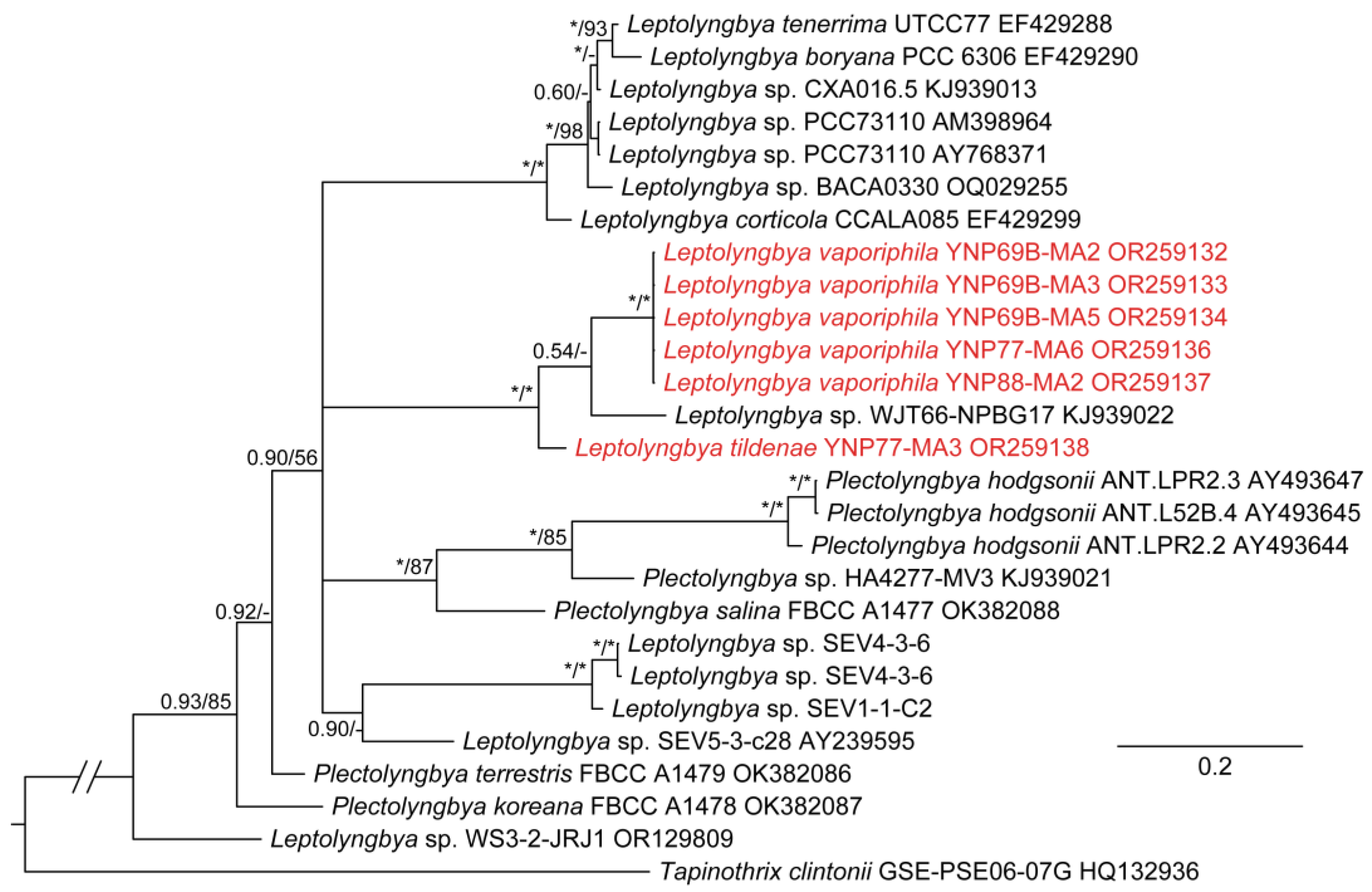
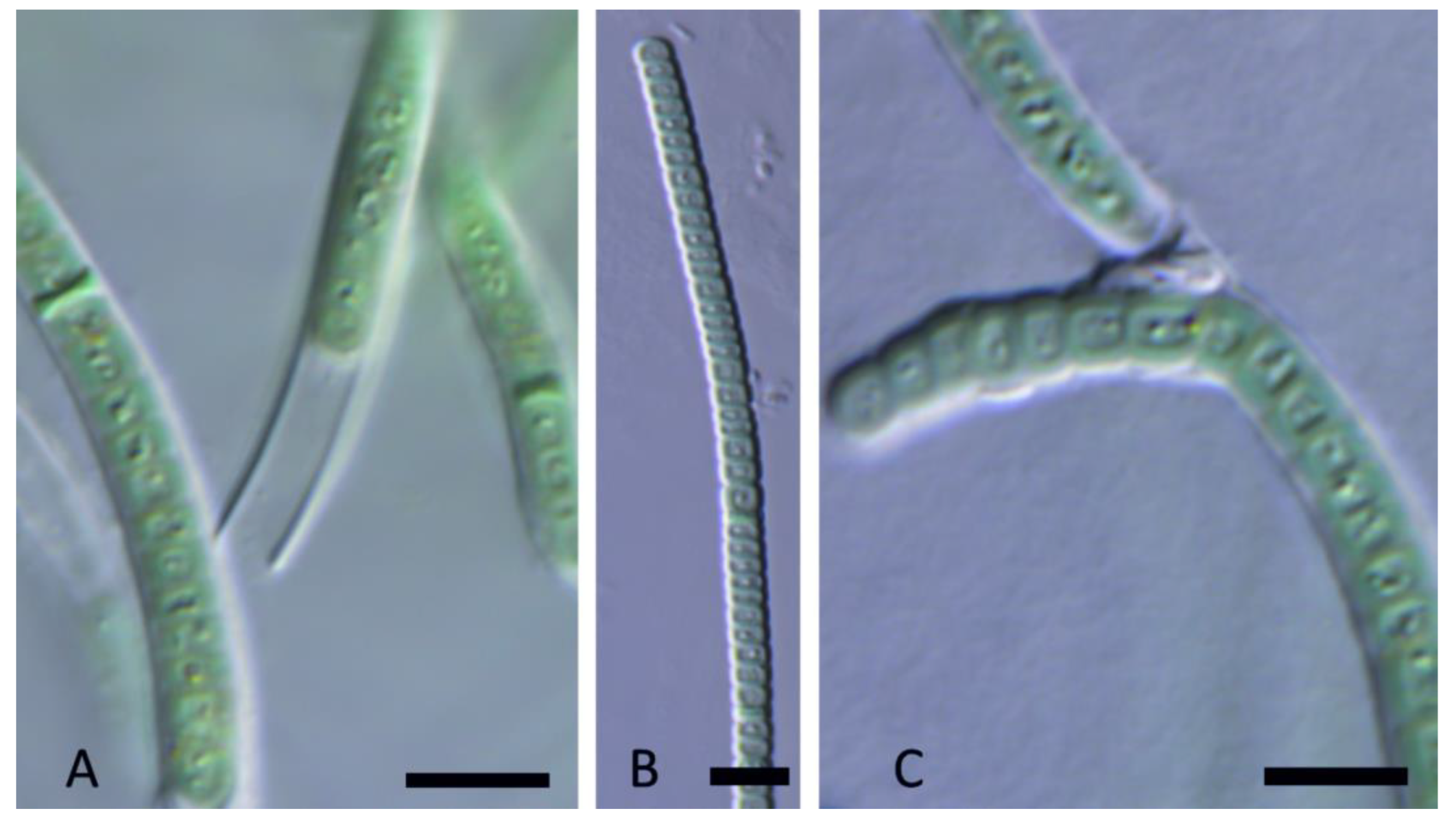
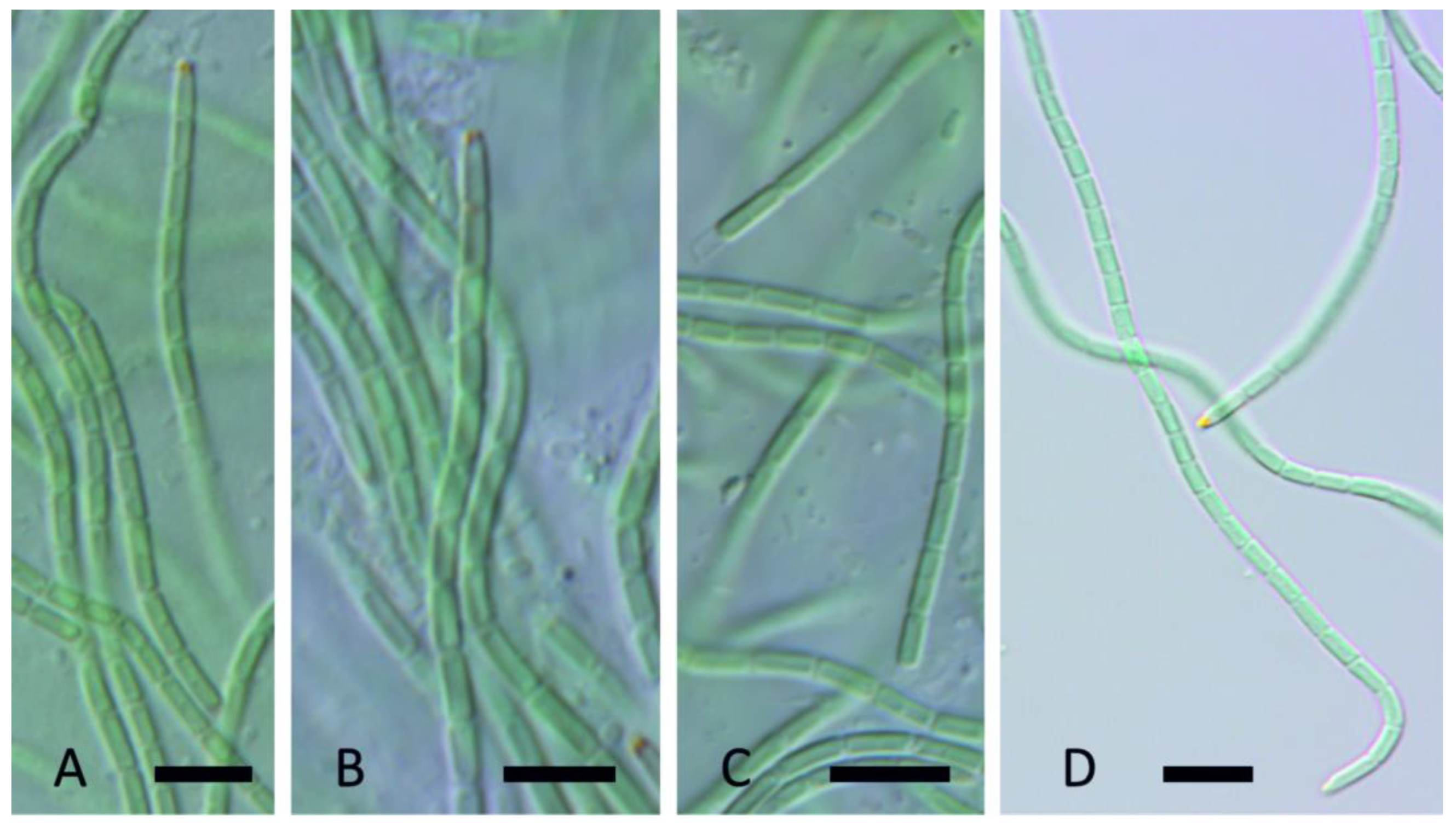
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cohn, F. Über die Algen des Karlsbader Sprudels, mit Rücksicht auf die Bildung des Sprudelsinsters. Abh. Schlesis Gesellsch Vaterländ Kult. 1862, 5, 37–55. [Google Scholar]
- Copeland, J.J. Yellowstone thermal Myxophyceae. Ann. N. Y. Acad. Sci. 1936, 36, 1–232. [Google Scholar] [CrossRef]
- Dyer, D.L.; Gafford, R.D. Some characteristics of a thermophilic blue-green alga. Science 1961, 134, 616–617. [Google Scholar] [CrossRef] [PubMed]
- Brock, T.D. Life at high temperatures. Science 1967, 158, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Castenholz, R.W. Thermophilic blue-green algae and the thermal environment. Bacteriol. Rev. 1969, 33, 476–504. [Google Scholar] [CrossRef]
- Mullis, K.B.; Ferré, F.; Gibbs, R.A. The Polymerase Chain Reaction; Birkhäuser: Boston, MA, USA, 1994; p. 458. [Google Scholar]
- Brock, T.D.; Freeze, H. Thermus aquaticus gen. n. and sp. n., a nonsporulating extreme thermophile. J. Bacteriol. 1969, 98, 289–297. [Google Scholar] [CrossRef]
- Bunbury, F.; Rivas, C.; Calatrava, V.; Shelton, A.N.; Grossman, A.; Bhayaa, D. Differential Phototactic Behavior of Closely Related Cyanobacterial Isolates from Yellowstone Hot Spring Biofilms. Appl. Environ. Microbiol. 2022, 88, e0019622. [Google Scholar] [CrossRef]
- Momper, L.; Hu, E.; Moore, K.R.; Skoog, E.J.; Tyler, M.; Evans, A.J.; Bosak, T. Metabolic versatility in a modern lineage of cyanobacteria from terrestrial hot springs. Free Radic. Biol. Med. 2019, 140, 224–232. [Google Scholar] [CrossRef]
- Becraft, E.D.; Cohan, F.M.; Kühl, M.; Jensen, S.I.; Ward, D.M. Fine-Scale Distribution Patterns of Synechococcus Ecological Diversity in Microbial Mats of Mushroom Spring, Yellowstone National Park. Appl. Environ. Microbiol. 2011, 77, 7689–7697. [Google Scholar] [CrossRef][Green Version]
- Bennett, A.C.; Murugapiran, S.K.; Hamilton, T.L. Temperature impacts community structure and function of phototrophic Chloroflexi and Cyanobacteria in two alkaline hot springs in Yellowstone National Park. Environ. Microbiol. Rep. 2020, 12, 503–513. [Google Scholar] [CrossRef]
- Bosak, T.; Liang, B.; Wu, T.-D.; Templer, S.P.; Evans, A.; Vali, H.; Guerquin-Kern, J.-L.; Klepac-Ceraj, V.; Sim, M.S.; Mui, J. Cyanobacterial diversity and activity in modern conical microbialites. Geobiology 2012, 10, 384–401. [Google Scholar] [CrossRef] [PubMed]
- Fecteau, K.M.; Boyd, E.S.; Lindsay, M.R.; Amenabar, M.J.; Robinson, K.J.; Debes, R.V., II; Shock, E.L. Cyanobacteria and algae meet at the limits of their habitat ranges in moderately acidic hot springs. J. Geophysic. Res. Biogeosci. 2022, 127, e2021JG006446. [Google Scholar] [CrossRef]
- Kees, E.D.; Murugapiran, S.K.; Bennett, A.C.; Hamilton, T.L. Distribution and Genomic Variation of Thermophilic Cyanobacteria in Diverse Microbial Mats at the Upper Temperature Limits of Photosynthesis. Environ. Microbiol. 2022, 7, e00317-22. [Google Scholar] [CrossRef] [PubMed]
- Becraft, E.D.; Wood, J.M.; Cohan, F.M.; Ward, D.M. Biogeography of American Northwest Hot Spring A/B′-Lineage Synechococcus Populations. Front. Microbiol. 2020, 11, 77. [Google Scholar] [CrossRef]
- Hamilton, T.L.; Haviga, J. Meet Me in the Middle: Median Temperatures Impact Cyanobacteria and Photoautotrophy in Eruptive Yellowstone Hot Springs. Environ. Microbiol. 2022, 7, e01450-21. [Google Scholar] [CrossRef] [PubMed]
- Reyes, K.; Gonzalez, N.I., III; Stewart, J.; Ospino, F.; Nguyen, D.; Cho, D.T.; Ghahremani, N.; Spear, J.R.; Johnson, H.A. Surface orientation affects the direction of cone growth by Leptolyngbya sp. strain C1, a likely architect of coniform structures Octopus Spring (Yellowstone National Park). Appl. Environ. Microbiol. 2013, 79, 1302–1308. [Google Scholar] [CrossRef]
- Fournier, R.O. Geochemistry and dynamics of the Yellowstone National Park hydrothermal system. Ann. Rev. Earth Planet. Sci. 1989, 17, 13–53. [Google Scholar] [CrossRef]
- Carmichael, W.W. Isolation, culture, and toxicity testing of toxic freshwater cyanobacteria (blue-green algae). In Fundamental Research in Homogenous Catalysis; Shilov, V., Ed.; Gordon & Breach: New York, NY, USA, 1986; Volume 3, pp. 1249–1262. [Google Scholar]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (Order Chroococcales). Bacter. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef] [PubMed]
- Komárek, J.; Kaštovský, J.; Mareš, J.; Johansen, J.R. Taxonomic classification of cyanoprokaryotes (cyanobacterial genera) 2014 using a polyphasic approach. Preslia 2014, 86, 235–295. [Google Scholar]
- Strunecký, O.; Ivanova, A.P.; Mareš, J. An updated classification of cyanobacterial orders and families based on phylogenomic and polyphasic analysis (Review). J. Phycol. 2023, 59, 12–51. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase; World-Wide Electronic Publication, National University of Ireland: Galway, Ireland, 2009; Available online: https://www.algaebase.org (accessed on 28 May 2023).
- Burke, D.J.; Kretzer, A.M.; Rygiewicz, P.T.; Topa, A.M. Soil bacterial diversity in a loblolly pine plantation: Influence of ecotomycorrhizas and fertilization. FEMS Microbial. Ecol. 2006, 57, 409–419. [Google Scholar] [CrossRef]
- Wilmotte, A.; Van Der Auwera, G.; Dewachter, R. Structure of the 16-S ribosomal RNA of the thermophilic cyanobacterium Chlorogloeopsis HTF (Mastigocladus laminosus HTF) strain PCC 7518, and phylogenetical analysis. FEBS Lett. 1993, 317, 96–100. [Google Scholar] [CrossRef]
- Boyer, S.L.; Flechner, V.R.; Johansen, J.R. Is the 16S–23S rRNA internal transcribed spacer region a good tool for use in molecular systematics and population genetics? A case study in cyanobacteria. J. Mol. Biol. Evol. 2001, 18, 1057–1069. [Google Scholar] [CrossRef]
- Nübel, U.; Garcia-Pichel, F.; Muyzer, G. PCR primers to amplify 16S rRNA genes from cyanobacteria. Appl. Environ. Microbiol. 1997, 63, 3327–3332. [Google Scholar] [CrossRef]
- Řeháková, K.; Johansen, J.R.; Bowen, M.B.; Martin, M.P.; Sheil, C.A. Variation in secondary structure of the 16S rRNA molecule in cyanobacteria with implications for phylogenetic analysis. Fottea 2014, 14, 161–178. [Google Scholar] [CrossRef]
- Taton, A.; Grubisic, S.; Brambilla, E.; De Wit, R.; Wilmotte, A. Cyanobacterial diversity in natural and artificial microbial mats of Lake Fryxell (McMurdo dry valleys, Antarctica): A morphological and molecular approach. Appl. Environ. Microbiol. 2003, 69, 5157–5169. [Google Scholar] [CrossRef]
- Miller, M.; Schwartz, T.; Pickett, B.; He, S.; Klem, E.; Scheuermann, R.H.; Passarotti, M.; Kaufman, S.; O’Leary, M.A. A RESTful API for Access to Phylogenetic Tools via theCIPRES Science Gateway. Evol. Bioinform. 2015, 11, 43–48. [Google Scholar] [CrossRef]
- Drummond, A.J.; Ho, S.Y.W.; Philips, M.J.; Rambaut, A. Relaxed phylogenetics and dating with confidence. PLoS Biol. 2006, 4, e88. [Google Scholar] [CrossRef]
- Gelman, A.; Rubin, D.B. Inference from iterative simulation using multiple sequences. Stat. Sci. 1992, 7, 157–511. [Google Scholar] [CrossRef]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef]
- Bevilacqua, P.C.; Blose, J.M. Structures, kinetics, thermodynamics and biological functions of RNA hairpins. Ann. Rev. Physic. Chem. 2008, 59, 79–103. [Google Scholar] [CrossRef]
- Miscoe, L.H.; Johansen, J.R.; Vaccarino, M.A.; Pietrasiak, N.; Sherwood, A.R. The diatom flora and cyanobacteria from caves on Kauai, Hawaii. II. Novel cyanobacteria from caves on Kauai, Hawaii. Biblioth. Phycol. 2016, 123, 75–152. [Google Scholar]
- Anagnostidis, K.; Komárek, J. Modern approach to the classification system of cyanophytes. 3. Oscillatoriales. Arch. Hydrobiol. Suppl. 1988, 80, 327–472. [Google Scholar]
- Zammit, G. Systematics and biogeography of sciophilous cyanobacteria; an ecological and molecular description of Albertania skiophila (Leptolyngbyaceae) gen. & sp. nov. Phycologia 2018, 57, 481–491. [Google Scholar]
- Zammit, G.; Billi, D.; Albertano, P. The subaerophytic cyanobacterium Oculatella subterranea (Oscillatoriales, Cyanophyceae) gen. et sp. nov.: A cytomorphological and molecular description. Eur. J. Phycol. 2012, 47, 341–354. [Google Scholar] [CrossRef]
- Perkerson, R.B.; Johansen, J.R.; Kovácik, L.; Brand, J.; Kaštovský, J.; Casamatta, D.A. A unique pseudanabaenalean (cyanobacteria) genus Nodosilinea gen. nov. based on morphological and molecular data. J. Phycol. 2011, 47, 1397–1412. [Google Scholar] [CrossRef]
- Yarza, P.; Yilmaz, P.; Pruesse, E.; Glöckner, F.O.; Ludwig, W.; Schleifer, K.H.; Whitman, W.B.; Euzéby, J.; Amann, R.; Rosselló-Móra, R. Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences. Nat. Rev. Microbiol. 2014, 12, 635–645. [Google Scholar] [CrossRef]
- Erwin, P.M.; Thacker, R.W. Cryptic diversity of the symbiotic cyanobacterium Synechococcus spongiarum among sponge hosts. Mol. Ecol. 2008, 17, 2937–2947. [Google Scholar] [CrossRef]
- Osorio-Santos, K.; Pietrasiak, N.; Bohunická, M.; Miscoe, L.H.; Kováčik, L.; Martin, M.P.; Johansen, J.R. Seven new species of Oculatella (Pseudanabaenales, Cyanobacteria): Taxonomically recognizing cryptic diversification. Eur. J. Phycol. 2014, 49, 450–470. [Google Scholar] [CrossRef]
- Tang, J.; Shah, M.R.; Yao, D.; Jiang, Y.; Du, L.; Zhao, K.; Li, L.; Li, M.; Waleron, M.M.; Waleron, M.; et al. Polyphasic Identification and Genomic Insights of Leptothermofonsia sichuanensis gen. sp. nov., a Novel Thermophilic Cyanobacteria Within Leptolyngbyaceae. Front. Microbiol. 2022, 13, 765105. [Google Scholar] [CrossRef]
- Shen, L.Q.; Zhang, Z.-C.; Shang, J.L.; Li, Z.-K. Kovacikia minuta sp. nov. (Leptolyngbyaceae, Cyanobacteria), a new freshwater chlorophyll f-producing cyanobacterium. J. Phycol. 2022, 58, 424–435. [Google Scholar] [CrossRef]
- Anagnostidis, K. Untersuchen über die Cyanophycen einiger Thermen in Griechenland. Inst. Syst. Bot. Pflanzengeogr. Univ. Thessalon. 1961, 7, 1–322. [Google Scholar]
- Bohunická, M.; Johansen, J.R.; Fučíková, K. Tapinothrix clintonii sp. nov. (Pseudanabaenaceae, Cyanobacteria), a new species at the nexus of five genera. Fottea 2011, 11, 127–140. [Google Scholar] [CrossRef]
- Tilden, J. Observations on some west American thermal algae. Bot. Gaz. 1898, 26, 89–105. [Google Scholar] [CrossRef]
- Strunecký, O.; Raabová, L.; Bernardová, A.; Ivanova, A.P.; Semanova, A.; Crossley, J.; Kaftan, D. Diversity of cyanobacteria at the Alaska North Slope with description of two new genera: Gibliniella and Shackletoniella. FEMS Microbiol. Ecol. 2022, 96, fiz189. [Google Scholar] [CrossRef] [PubMed]
- Prát, S. Studie o Biolithogenesi; Česká Akademie věd a Umění: Praha, Czech Republic, 1929; 187p. [Google Scholar]
- Kaštovský, J. Welcome to the jungle!—An overview of modern taxonomy of Cyanobacteria. Hydrobiologia, 2023; in press. [Google Scholar]
- Rigonato, J.; Sant’Anna, C.L.; Giani, A.; Azevedo, M.T.P.; Gama, W.A.; Viana, W.L.F.; Fiore, M.F.; Werner, V.R. Sphaerocavum: A coccoid morphogenus identical to Microcystis in terms of 16S rDNA and ITS sequence phylogenies. Hydrobiologia 2003, 811, 35–48. [Google Scholar] [CrossRef]
- Aguilera, A.; Berrendero, E.G.; Kaštovský, J.; Echenique, R.; Salerno, G.L. The polyphasic analysis of two native Raphidiopsis isolates supports the unification of the genera Raphidiopsis and Cylindrospermopsis (Nostocales, Cyanobacteria). Phycologia 2018, 57, 130–146. [Google Scholar] [CrossRef]
- Taton, A.; Wilmotte, A.; Šmarda, J.; Elster, J.; Komárek, J. Plectolyngbya hodgsonii: A novel filamentous cyanobacterium from Antarctic lakes. Polar Biol. 2011, 34, 181–191. [Google Scholar] [CrossRef]
- Turland, N.J.; Wiersema, J.H.; Barrie, F.R.; Greuter, W.; Hawksworth, D.L.; Herendeen, P.S.; Knapp, S.; Kusber, W.-H.; Li, D.-Z.; Marhold, K.; et al. (Eds.) International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. In Regnum Vegetabile; Koeltz Botanical Books: Glashütten, Germany, 2018; Volume 159. [Google Scholar]
- Casamatta, D.A.; Johansen, J.R.; Vis, M.L.; Broadwater, S.T. Molecular and morphological characterization of ten polar and near-polar strains within the Oscillatoriales (Cyanobacteria). J. Phycol. 2005, 41, 421–438. [Google Scholar] [CrossRef]
- Johansen, J.R.; Kováčik, L.; Casamatta, D.A.; Fučíková, K.; Kaštovský, J. Utility of 16S–23S ITS sequence and secondary structure for recognition of intrageneric and intergeneric limits within cyanobacterial taxa: Leptolyngbya corticola sp. nov. (Pseudanabaenaceae, Cyanobacteria). Nova Hedwig. 2011, 92, 283–302. [Google Scholar] [CrossRef]
- Anagnostidis, K. Nomenclatural changes in cyanoprokaryotic order Oscillatoriales. Preslia 2001, 73, 359–375. [Google Scholar]
- Kim, D.-H.; Lee, N.-J.; Lee, J.-H.; Yang, E.-C.; Lee, O.-M. Three new Plectolyngbya species (Leptolyngbyaceae, Cyanobacteria) isolated from rocks and saltern of the Republic of Korea. Diversity 2022, 14, 1013. [Google Scholar] [CrossRef]
- Gomont, M. Monographie des Oscillariées (Nostocacées Homocystées). Deuxième partie—Lyngbyées. Ann. Sci. Natur. Bot. Série 7 1893, 16, 91–264. [Google Scholar]
- Gomont, M. Monographie des Oscillariées (Nostocacées homocystées). Ann. Sci. Natur. Bot. Série 7 1892, 15, 263–368. [Google Scholar]
- Mai, T.; Johansen, J.R.; Pietrasiak, N.; Bohunická, M.; Martin, M.P. Revision of the Synechococcales (Cyanobacteria) through recognition of four families including Oculatellaceae fam. nov. and Trichocoleaceae fam. nov. and six new genera containing 14 species. Phytotaxa 2018, 365, 1–59. [Google Scholar] [CrossRef]
- Phillipson, J. Some algae of Victorian soils. Proc. R. Soc. Vic. 1935, 47, 262–287. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota. 2. Teil: Oscillatoriales. In Süsswasserflora von Mitteleuropa; Büdel, B., Krienitz, L., Gärtner, G., Schagerl, M., Eds.; Elsevier Spektrum Akademischer Verlag: München, Germany, 2005; Volume 19/2, pp. 1–759. [Google Scholar]
- Pietrasiak, N.; Reeve, S.; Osorio-Santos, K.; Lipson, D.A.; Johansen, J.R. Trichotorquatus gen. nov.—A new genus of soil cyanobacteria discovered from American drylands. J. Phycol. 2021, 57, 886–902. [Google Scholar] [CrossRef]







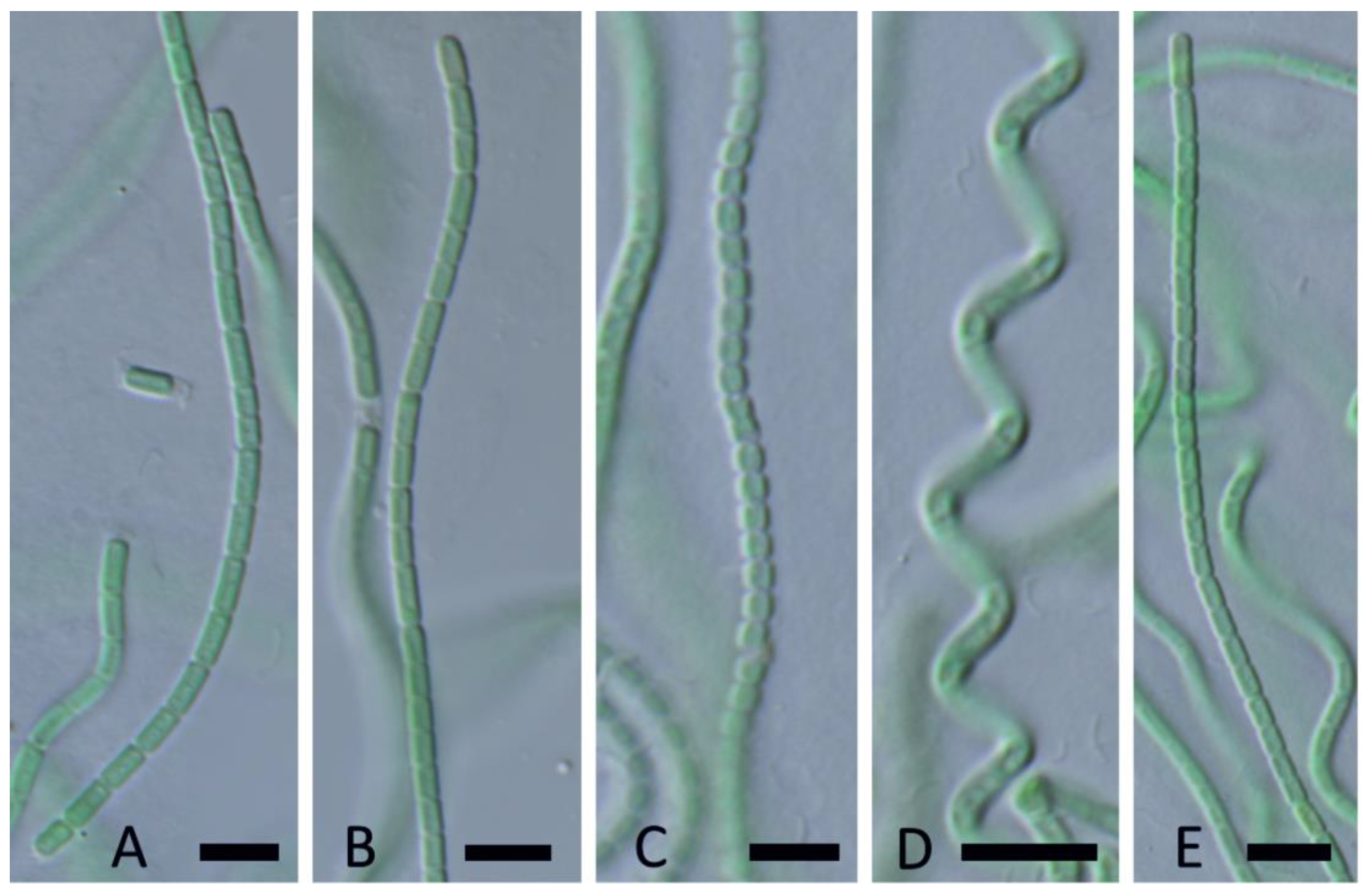
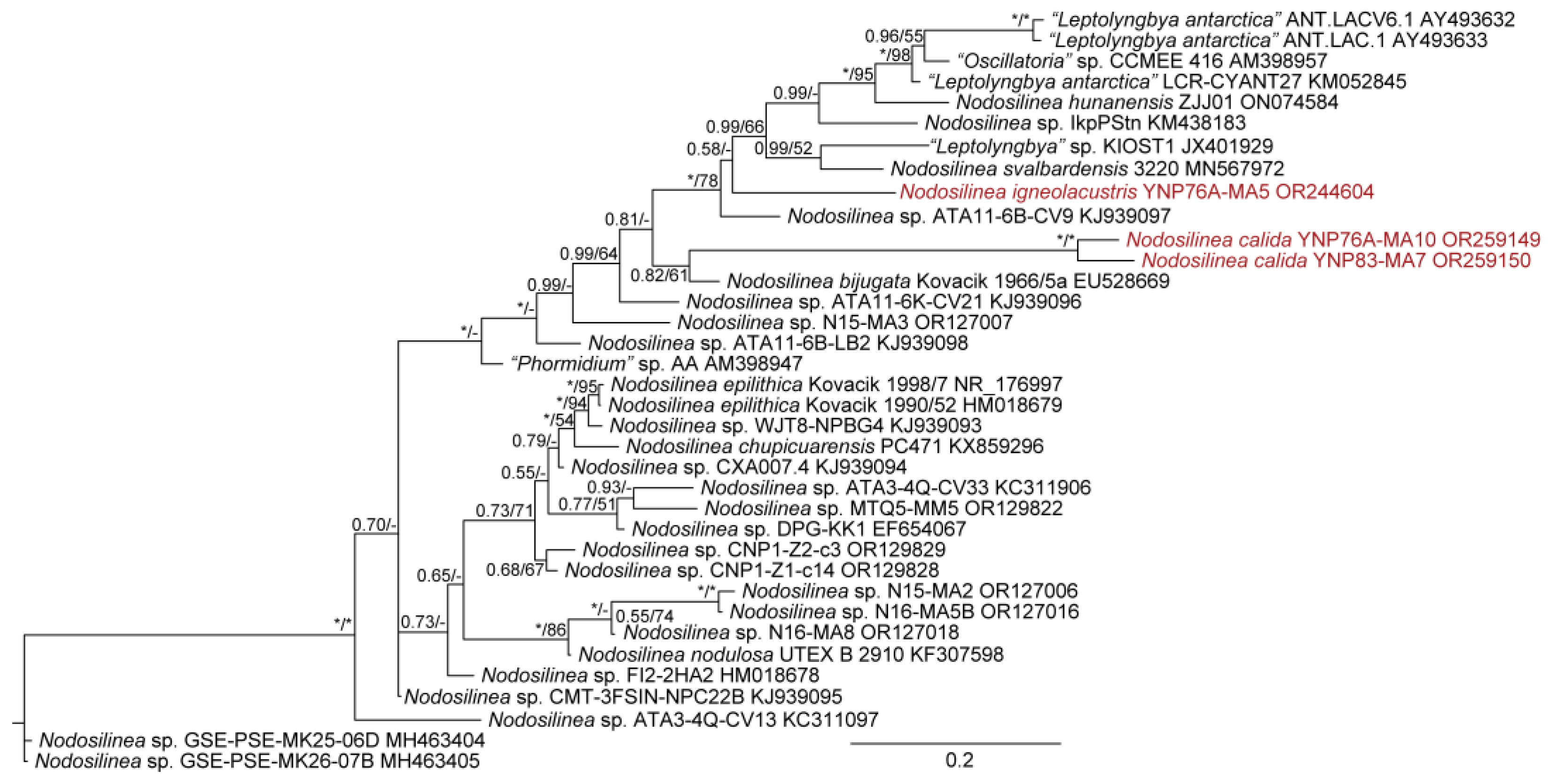
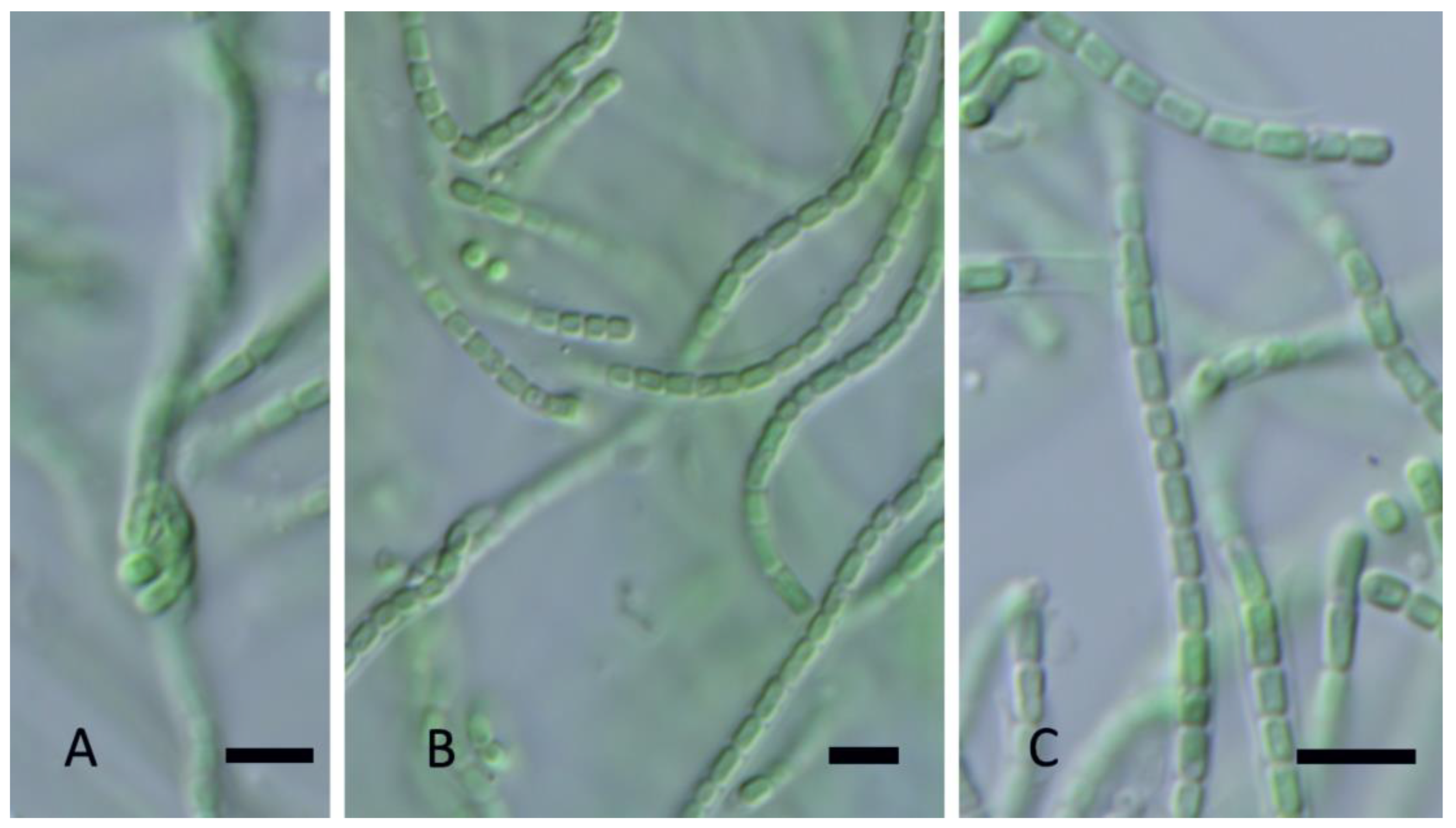
| YNP30-MA1 | YS71-RH2 | YNP81-MA3 | YNP81-MA4 | YNP83-MA6 | YN80B-MA3 | YN80B-MA4 | EY07 AM2 | |
|---|---|---|---|---|---|---|---|---|
| Copelandiella yellowstonensis YNP30-MA1 | ||||||||
| Copelandiella yellowstonensis YS71-RH2 | 99.8 | |||||||
| Copelandiella yellowstonensis YNP81-MA3 | 99.7 | 99.8 | ||||||
| Copelandiella yellowstonensis YNP81-MA4 | 99.7 | 99.8 | 99.8 | |||||
| Copelandiella yellowstonensis YNP83-MA6 | 99.6 | 99.7 | 99.6 | 99.7 | ||||
| Copelandiella thermalis YN80B-MA3 | 97.8 | 97.8 | 97.8 | 97.8 | 97.7 | |||
| Copelandiella thermalis YN80B-MA4 | 97.8 | 97.8 | 97.8 | 97.8 | 97.7 | 100.0 | ||
| Leptolyngbyaceae cynobacterium EY07 AM2 | 91.5 | 91.5 | 91.5 | 91.5 | 91.4 | 90.9 | 90.9 | |
| Leptolyngbyaceae cynobacterium EY01 AM3 | 95.2 | 95.2 | 95.1 | 95.3 | 95.1 | 94.9 | 94.9 | 92.1 |
| YNP81-MA1 | YNP81-MA4 | YNP70-MA1 | YNP83-MA6 | YNP30-MA1 | YNP80B-MA3 | |
|---|---|---|---|---|---|---|
| Copelandiella yellowstonensis YNP81-MA1 | ||||||
| Copelandiella yellowstonensis YNP81-MA4 | 0.0 | |||||
| Copelandiella yellowstonensis YNP70-MA1 | 6.9 | 6.9 | ||||
| Copelandiella yellowstonensis YNP83-MA6 | 7.6 | 7.6 | 1.1 | |||
| Copelandiella yellowstonensis YNP30-MA1 | 7.5 | 7.5 | 4.4 | 3.6 | ||
| Copelandiella thermalis YNP80B-MA3 | 20.2 | 20.2 | 20.3 | 20.1 | 20.8 | |
| Copelandiella thermalis YNP80B-MA4 | 20.2 | 20.2 | 20.3 | 20.1 | 20.8 | 0.0 |
| Leptothermofonsia sichuanensis | Leptolyngbyaceae CENA37 | Leptolyngbyaceae CENA37 | Leptolyngbyaceae CENA35 | Leptolyngbya Greenland 10 | Kovacikia brockii YS7-RH2 | Kovacikia brockii YNP74-RH1 | Kovacikia muscicola HA7619-LM3 | Kovacikia anagnostidisii YS86-RH1 | Kovacikia anagnostidisii YS89-RH1 | Kovacikia minuta CCNU0001 | Kovacikia atmophytica BACA0619 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leptothermofonsia sichuanensis | ||||||||||||
| Leptolyngbyaceae CENA37 | 95.4 | |||||||||||
| Leptolyngbyaceae CENA37 | 95.5 | 99.9 | ||||||||||
| Leptolyngbyaceae CENA35 | 95.3 | 99.7 | 99.8 | |||||||||
| Leptolyngbya Greenland 10 | 96.4 | 96.6 | 96.7 | 96.7 | ||||||||
| Kovacikia brockii YS7-RH2 | 98.0 | 95.5 | 95.6 | 95.4 | 96.2 | |||||||
| Kovacikia brockii YNP74-RH1 | 97.9 | 95.5 | 95.6 | 95.4 | 96.2 | 99.9 | ||||||
| Kovacikia muscicola HA7619-LM3 | 95.2 | 98.8 | 98.9 | 98.7 | 95.8 | 95.4 | 95.4 | |||||
| Kovacikia anagnostidisii YS86-RH1 | 96.1 | 96.8 | 96.9 | 96.7 | 94.8 | 96.0 | 96.0 | 97.3 | ||||
| Kovacikia anagnostidisii YS89-RH1 | 96.0 | 96.8 | 96.9 | 96.7 | 94.8 | 96.0 | 96.0 | 97.3 | 100 | |||
| Kovacikia minuta CCNU0001 | 95.1 | 97.8 | 97.9 | 97.9 | 95.9 | 95.1 | 95.1 | 97.6 | 96.8 | 96.8 | ||
| Kovacikia atmophytica BACA0619 | 95.9 | 96.8 | 96.9 | 96.7 | 95.6 | 95.4 | 95.4 | 97.1 | 98.2 | 98.2 | 97.1 | |
| Leptolyngbyaceae MTQ1-MM2 | 94.3 | 94.3 | 94.4 | 94.3 | 93.7 | 94.6 | 94.6 | 94.8 | 96.1 | 96.1 | 94.0 | 95.3 |
| Leptothermofonsia sichuanensis | Kovacikia brockii YNP74-RH1 | Kovacikia brockii YS7-RH2 | Kovacikia muscicola HA7619-LM3 | Kovacikia anagnostidisii YS86-RH1 | Kovacikia anagnostidisii YS89-RH1 | Kovacikia atmophytica BACA0619 | |
|---|---|---|---|---|---|---|---|
| Leptothermofonsia sichuanensis | |||||||
| Kovacikia brockii YNP74-RH1 | 18.8 | ||||||
| Kovacikia brockii YS7-RH2 | 18.8 | 0.0 | |||||
| Kovacikia muscicola HA7619-LM3 | 23.9 | 20.6 | 20.6 | ||||
| Kovacikia anagnostidisii YS86-RH1 | 28.5 | 24.0 | 24.0 | 18.5 | |||
| Kovacikia anagnostidisii YS89-RH1 | 28.5 | 24.0 | 24.0 | 18.5 | 0.0 | ||
| Kovacikia atmophytica BACA0619 | 26.5 | 21.2 | 21.2 | 12.0 | 18.2 | 18.2 | |
| Kovacikia minuta CCNUW1 | 26.1 | 21.2 | 21.2 | 17.0 | 24.1 | 24.1 | 17.3 |
| A. prattii YNP74-MA3.1 | A. prattii YNP74-MA3.2 | A. skiophila SA373 | A. alaskaensis L1 | Albertania N14-MA1 | Albertania N14-MA3 | Albertania EcFYyy-200 | Albertania CT103 | Albertania CT115 | Albertania CT305 | |
|---|---|---|---|---|---|---|---|---|---|---|
| A. prattii YNP74-MA3.1 | ||||||||||
| A. prattii YNP74-MA3.2 | 99.4 | |||||||||
| A. skiophila SA373 | 96.6 | 96.6 | ||||||||
| A. alaskaensis L1 | 98.1 | 98.0 | 96.6 | |||||||
| Albertania N14-MA1 | 98.0 | 97.9 | 98.1 | 97.9 | ||||||
| Albertania N14-MA3 | 96.6 | 96.5 | 96.9 | 97.8 | 97.7 | |||||
| Albertania EcFYyy-200 | 97.5 | 97.4 | 97.2 | 98.8 | 97.9 | 98.2 | ||||
| Albertania CT103 | 97.8 | 97.7 | 96.9 | 97.1 | 97.7 | 96.1 | 97.0 | |||
| Albertania CT115 | 95.1 | 95.0 | 93.6 | 95.2 | 95.2 | 94.8 | 94.8 | 95.0 | ||
| Albertania CT305 | 96.5 | 96.4 | 97.0 | 97.8 | 97.4 | 98.6 | 98.4 | 96.6 | 94.7 | |
| Albertania CT315 | 96.2 | 96.1 | 96.1 | 97.0 | 96.6 | 97.6 | 97.5 | 95.9 | 95.8 | 97.5 |
| A. prattii YNP74-MA3.1 | A. prattii YNP74-MA3.2 | A. skiophila SA373 | A. alaskaensis KV23 | Albertania sp. N14-MA1 | Albertania sp. N14-MA3 | Albertania sp. CT103 | Albertania sp. CT305 | Albertania sp. CT315 | |
|---|---|---|---|---|---|---|---|---|---|
| Albertania prattii YNP74-MA3.1 | |||||||||
| Albertania prattii YNP74-MA3.2 | 16.7 | ||||||||
| Albertania skiophila SA373 | 13.1 | 22.5 | |||||||
| Albertania alaskaensis KV23 | 15.2 | 18.7 | 19.4 | ||||||
| Albertania sp. N14-MA1 | 13.7 | 16.3 | 15.0 | 15.3 | |||||
| Albertania sp. N14-MA3 | 14.0 | 16.7 | 12.5 | 17.5 | 8.0 | ||||
| Albertania sp. CT103 | 14.7 | 16.1 | 13.8 | 17.3 | 7.8 | 8.1 | |||
| Albertania sp. CT305 | 11.5 | 17.2 | 10.6 | 13.5 | 9.8 | 12.0 | 9.6 | ||
| Albertania sp. CT315 | 14.3 | 19.6 | 15.3 | 20.8 | 14.5 | 11.8 | 12.9 | 11.2 | |
| Albertania sp. CT115 | 11.2 | 26.3 | 20.6 | 14.8 | 15.8 | 13.2 | 13.8 | 16.2 | 23.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaštovský, J.; Johansen, J.R.; Hauerová, R.; Akagha, M.U. Hot Is Rich—An Enormous Diversity of Simple Trichal Cyanobacteria from Yellowstone Hot Springs. Diversity 2023, 15, 975. https://doi.org/10.3390/d15090975
Kaštovský J, Johansen JR, Hauerová R, Akagha MU. Hot Is Rich—An Enormous Diversity of Simple Trichal Cyanobacteria from Yellowstone Hot Springs. Diversity. 2023; 15(9):975. https://doi.org/10.3390/d15090975
Chicago/Turabian StyleKaštovský, Jan, Jeffrey R. Johansen, Radka Hauerová, and Mildred U. Akagha. 2023. "Hot Is Rich—An Enormous Diversity of Simple Trichal Cyanobacteria from Yellowstone Hot Springs" Diversity 15, no. 9: 975. https://doi.org/10.3390/d15090975
APA StyleKaštovský, J., Johansen, J. R., Hauerová, R., & Akagha, M. U. (2023). Hot Is Rich—An Enormous Diversity of Simple Trichal Cyanobacteria from Yellowstone Hot Springs. Diversity, 15(9), 975. https://doi.org/10.3390/d15090975






