The Upemba National Park (Upper Congo Basin, DR Congo): An Updated Checklist Confirming Its Status as an African Fish Biodiversity Hotspot
Abstract
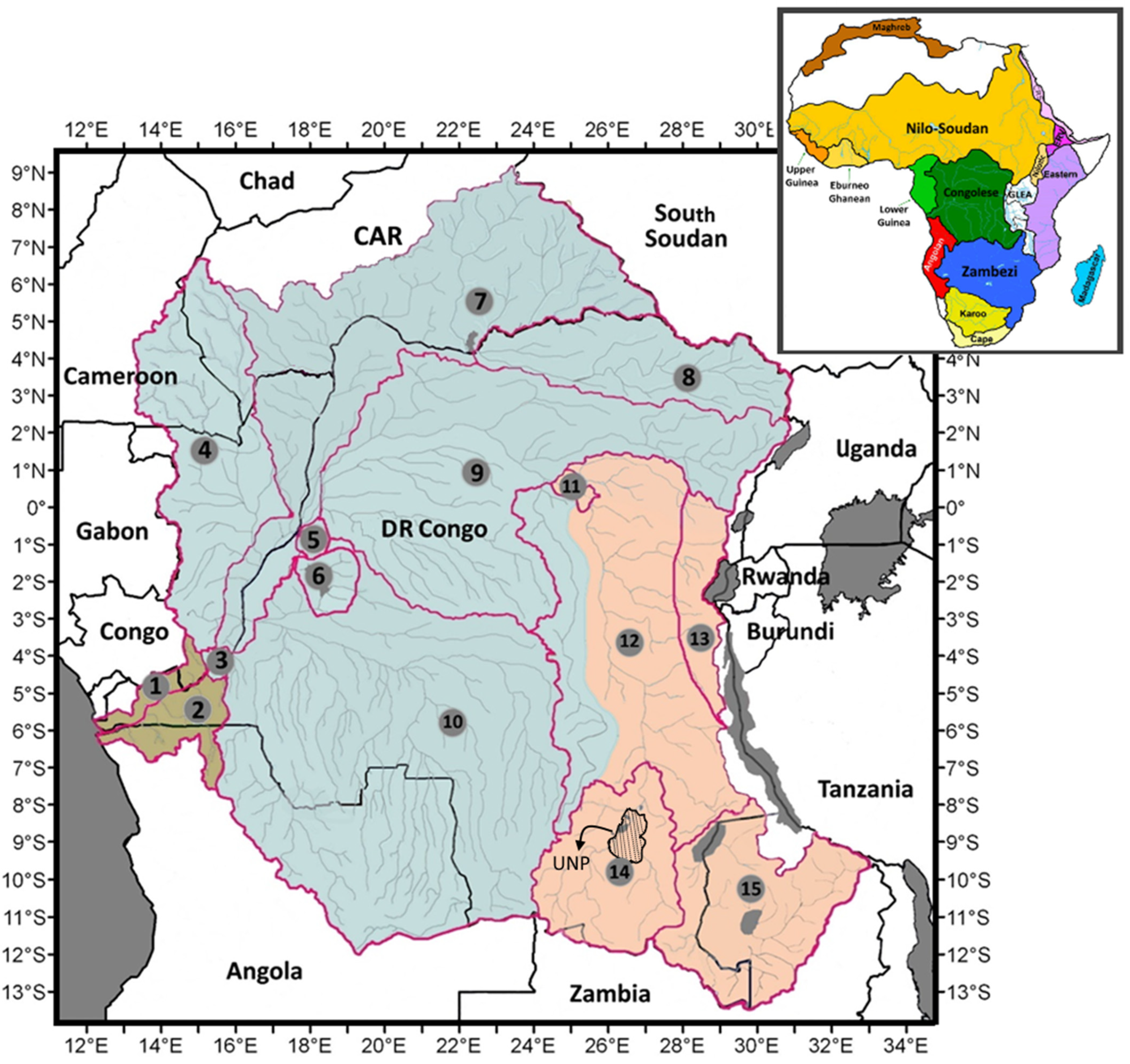
:1. Introduction
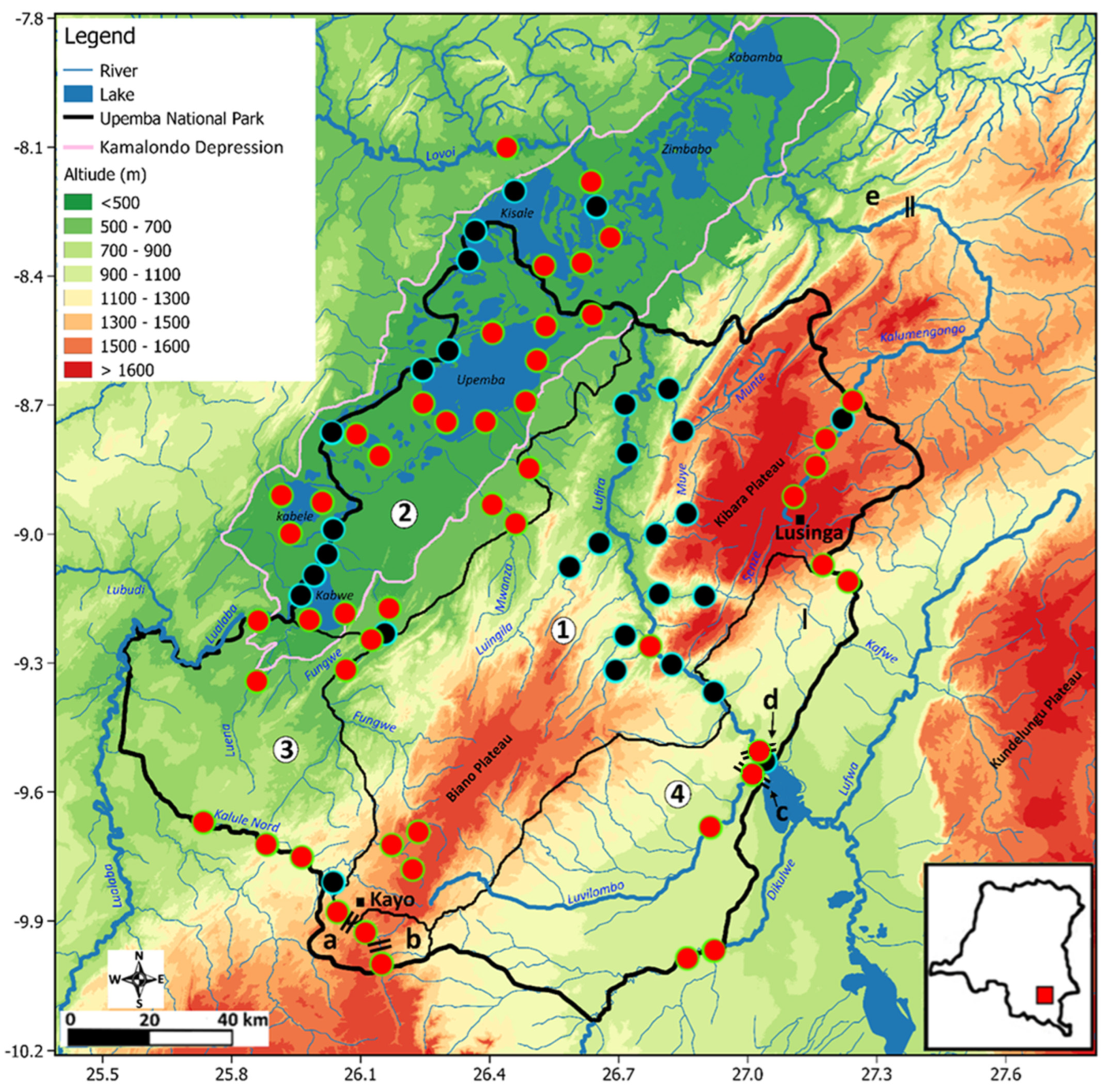
2. Materials and Methods
3. Results
3.1. Fish Diversity
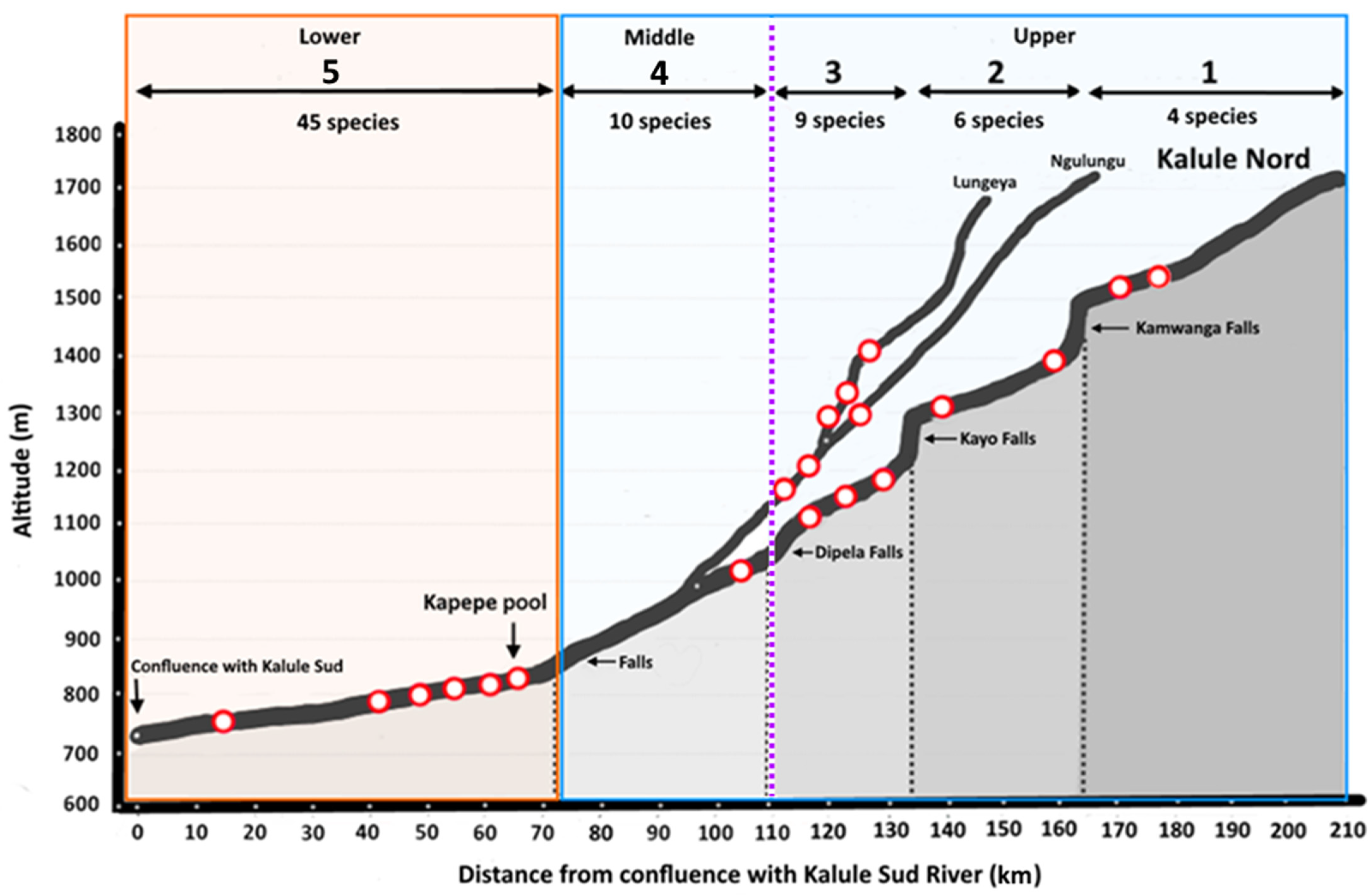
3.2. Longitudinal Distribution of Fishes in the Park: The Case of the Kalule Nord River
3.3. Taxa Unidentified at Species Level
3.3.1. Mormyridae
3.3.2. Kneriidae
3.3.3. Cyprinidae
3.3.4. Distichodontidae
3.3.5. Clariidae
3.3.6. Amphiliidae
3.3.7. Mochokidae
3.3.8. Claroteidae
3.3.9. Mastacembelidae
3.3.10. Cichlidae
4. Discussion
4.1. Contribution to the Ichthyological Knowledge of the UNP
4.2. The Endemic Fishes of the UNP
4.3. Introduced Species
4.4. Longitudinal Patterns in Species Composition
4.5. Ichthyofaunal Affinities with the Neighbouring Ecoregions
4.6. Threats and the Need for More Efficient Protection of the Ichthyofauna of the UNP
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Family (No. of Genera/Species) | Kalule Nord River | ||||
|---|---|---|---|---|---|
| Upper | Middle | Lower | |||
| 1 | 2 | 3 | |||
| Polypteridae (1/1) | |||||
| Polypterus ornatipinnis | ✗ | ||||
| Mormyridae (4/4) | |||||
| Campylomormyrus rhynchophorus | ✗ | ||||
| Cyphomyrus discorhynchus | ✗ | ||||
| Marcusenius macrolepidotus | ✗ | ||||
| Mormyrops anguilloides | ✗ | ||||
| Clupeidae (1/1) | |||||
| Microthrissa congica | ✗ | ||||
| Kneriidae (2/2) | |||||
| Kneria sp. ‘kalule’ | ✗ * | ✗ * | ✗ * | ✗ * | |
| Parakneria sp. ‘kalule’ | ✗ * | ||||
| Cyprinidae (3/15) | |||||
| Enteromius kamolondoensis | ✗ | ||||
| Enteromius kerstenii | ✗ | ||||
| Enteromius cf. mocoensis | ✗ | ✗ | ✗ | ✗ | |
| Enteromius neefi | ✗ | ✗ | ✗ | ✗ | |
| Enteromius thespesios | ✗ | ||||
| Enteromius sp. ‘ngulungu’ | ✗ | ||||
| Labeo cylindricus | ✗ | ||||
| Labeo parvus | ✗ | ||||
| Labeo simpsoni | ✗ | ||||
| Labeo weeksii | ✗ | ||||
| Labeobarbus sp. ‘kapepe’ | ✗ * | ||||
| Labeobarbus sp. ‘kayo’ | ✗ * | ✗ * | ✗ * | ||
| Labeobarbus sp. ‘ngulungu’ | ✗ * | ✗ * | ✗ * | ||
| Labeobarbus sp. ‘nshila’ | ✗ * | ||||
| Labeobarbus sp. ‘thick lip’ | ✗ | ||||
| Danionidae (3/3) | |||||
| Chelaethiops congicus | ✗ | ||||
| Opsaridium ubangiense | ✗ | ||||
| Raiamas marqueti | ✗ | ||||
| Distichodontidae (2/2) | |||||
| Nannocharax hadros | ✗ * | ||||
| Alestidae (4/6) | |||||
| Alestes macrophthalmus | ✗ | ||||
| Brycinus grandisquamis | ✗ | ||||
| Brycinus imberi | ✗ | ||||
| Hydrocynus vittatus | ✗ | ||||
| Micralestes acutidens | ✗ | ||||
| Micralestes stormsi | ✗ | ||||
| Bagridae (1/1) | |||||
| Bagrus ubangensis | ✗ | ||||
| Clariidae (2/3) | |||||
| Clarias buthupogon | ✗ | ||||
| Clarias cf. liocephalus | ✗ | ✗ | |||
| Heterobranchus longifilis | ✗ | ||||
| Amphiliidae (2/4) | |||||
| Amphilius sp. ‘kayo_abave’ | ✗ * | ✗ * | |||
| Amphilius sp. ‘kayo_below’ | ✗ * | ✗ * | |||
| Zaireichthys brevis | ✗ | ✗ | |||
| Zaireichthys sp. ‘upemba’ | ✗ | ||||
| Malapteruridae (1/1) | |||||
| Malapterurus monsembeensis | ✗ | ||||
| Mochokidae (3/5) | |||||
| Chiloglanis sp. ‘kalule’ | ✗ * | ✗ * | |||
| Euchilichthys royauxi | ✗ | ||||
| Synodontis decorus | ✗ | ||||
| Synodontis dorsomaculatus | ✗ | ||||
| Synodontis greshoffi | ✗ | ||||
| Claroteidae (3/3) | |||||
| Auchenoglanis occidentalis | ✗ | ||||
| Parauchenoglanis sp. ‘kalule’ | ✗ | ||||
| Chrysichthys cranchii | ✗ | ||||
| Schilbeidae (1/1) | |||||
| Schilbe intermedius | ✗ | ||||
| Mastacembelidae (1/1) | |||||
| Mastacembelus congicus | ✗ | ||||
| Cichlidae (3/3) | |||||
| Coptodon rendalli | ✗ | ||||
| Orthochromis kimpala | ✗ | ||||
| Tilapia sparrmanii | ✗ | ||||
| Total | 4 (7%) | 6 (11%) | 9 (16%) | 10 (18%) | 45 (80%) |
| UNP | UL | BM | UC | Kas | UZ | |
|---|---|---|---|---|---|---|
| N | 249 | 311 | 159 | 284 | 319 | 158 |
| Shared species | 249 | 82 | 149 | 125 | 43 | |
| Shared species (%) | 100 | 33 | 60 | 50 | 17 | |
| J | 0.77 | 0.25 | 0.39 | 0.29 | 0.12 |


References
- Frechkop, S. Mammifères. In Exploration du Parc National de l’Upemba (1946–1949); Mission, G., de Witte, F., Eds.; Institut des parcs Nationaux du Congo Belge: Brussels, Belgium, 1954; Volume 14, p. 84. [Google Scholar]
- de Witte, G.F. Introduction. In Exploration du Parc National de l’Upemba (1946–1949); Mission, G., de Witte, F., Eds.; Institut des parcs Nationaux du Congo Belge: Brussels, Belgium, 1966; Volume 1, p. 122. [Google Scholar]
- UICN/PACO. Parcs et Réserves de la République Démocratique du Congo: Évaluation de L’efficacité de Gestion des Aires Protégées; UICN/PACO: Ouagadougou, Burkina Faso, 2010; p. 136. [Google Scholar]
- Symoens, J.-J. Le Parc National de L’Upemba. Son histoires—Son intérêt. Publ. De L’université D’elisabethville 1963, 6, 43–55. [Google Scholar]
- Verheyen, R. Contribution à l’étude éthologique des mammifères du Parc National de l’Upemba. In Exploration du Parc National de l’Upemba; Institut des parcs Nationaux du Congo Belge: Brussels, Belgium, 1951; p. 84. [Google Scholar]
- Abell, R.; Thieme, M.L.; Revenga, C.; Bryer, M.; Kottelat, M.; Bogutskaya, N.; Coad, B.; Mandrak, N.; Balderas, S.C.; Bussing, W.; et al. Freshwater ecoregions of the world: A new map of biogeographic units for freshwater biodiversity conservation. BioScience 2008, 58, 403–414. [Google Scholar] [CrossRef]
- Roberts, T.R. Geographical distribution of African freshwater fishes. Zool. J. Linn. Soc. 1975, 57, 249–319. [Google Scholar] [CrossRef]
- Poll, M. Contribution à la faune ichthyologique du Katanga. Ann. Mus. Congo Belg. 1933, 3, 101–152. [Google Scholar]
- Poll, M. Poissons du Katanga (bassin du Congo) récoltés par le Professeur Paul Brien. Rev. Zool. Bot. Afr. 1938, 30, 389–423. [Google Scholar]
- Poll, M. Poissons recueillis au Katanga par H.J. Bredo. Bull. Mus. Roy. Hist. Nat. Belg. 1948, 24, 1–24. [Google Scholar]
- Banister, K.E.; Bailey, R.G. Fishes collected by the Zaïre River expedition 1974–1975. Zool. J. Linn. Soc. 1979, 66, 205–249. [Google Scholar] [CrossRef]
- David, L.; Poll, M. Contribution à la faune ichthyologique du Congo Belge. Ann. Mus. Congo Belg. 1937, 5, 189–296. [Google Scholar]
- Thys van den Audenaerde, D.F.E. Description d’une espèce nouvelle d’Haplochromis (Pisces, Cichlidae) avec observations sur les Haplochromis rhéophiles du Congo oriental. Rev. Zool. Bot. Afr. 1963, 68, 140–151. [Google Scholar]
- Poll, M. Contribution à l’étude des Kneriidae et description d’un nouveau genre, le genre Parakneria (Pisces, Kneriidae). Mém. Acad. Roy. Belg. 1965, 36, 1–28. [Google Scholar]
- Banister, K.E.; Poll, M. Description de trois nouvelles espèces de Varicorhinus provenant de la région du Parc National de l’Upemba (Rép. Zaïre) (Pisces, Cyprinidae). Rev. Zool. Bot. Afr. 1973, 87, 81–95. [Google Scholar]
- Poll, M. Poissons: Exploitation du Parc National de l’Upemba; Fondation pour Favoriser les Recherches Scientifiques en Afrique: Bruxelles, Belgium, 1976; Volume 73, pp. 1–127. [Google Scholar]
- USBGN. Republic of the Congo (Léopoldville): Official Standard Names Approved by the United States Board on Geographic Names; Office of Geography, Department of the Interior, Gazetteer: Washington, DC, USA, 1964; Volume 80, p. 401.
- Poll, M. Révision des Synodontis africains (famille Mochocidae). Ann. Mus. R. Afr. Cent. 1971, 191, 1–497. [Google Scholar]
- Bonou, C.A.; Teugels, G.G. Révision systématique du genre Parachanna Teugels et Daget 1984 (Pisces: Channidae). Rev. Hydrobiol. Trop. 1985, 18, 267–280. [Google Scholar]
- Teugels, G.G. A systematic revision of the African species of the genus Clarias (Pisces, Clariidae). Ann. Mus. R. Afr. Cent. 1986, 247, 1–199. [Google Scholar]
- Stiassny, M.L.J. A taxonomic revision of the African genus Tylochromis (Labroidei, Cichlidae); with notes on the anatomy and relationships of the group. Ann. Mus. R. Afr. Cent. 1989, 258, 1–159. [Google Scholar]
- Teugels, G.G.; Denayer, B.; Legendre, M. A systematic revision of the African catfish genus Heterobranchus Geoffroy-Saint-Hilaire, 1809 (Pisces: Clariidae). Zool. J. Linn. Soc. 1990, 98, 237–257. [Google Scholar] [CrossRef]
- Gourène, G.; Teugels, G.G. Synopsis de la classification et phylogénie des Pellonulidae de l’Afrique Occidentale et Centrale (Teleostei; Clupeidae). J. Afr. Zool. 1994, 108, 77–91. [Google Scholar]
- De Vos, L. A systematic revision of the African Schilbeidae (Teleotei, Siluriformes). Ann. Mus. R. Afr. Cent. 1995, 271, 1–450. [Google Scholar]
- Tshibwabwa, S.M. Systématique des espèces africaines du genre Labeo (Teleostei, Cyprinidae) dans les Régions Ichtyogéographiques de Basse-Guinée et du Congo; Thèse de Doctorat, Faculté des Sciences, Facultés Universitaires Notre-Dame de la Paix de Namur/Belgique; Presses Universitaires de Namur: Namur, Belgium, 1997; Volume I–II, p. 530. [Google Scholar]
- Norris, S.M. A revision of the African electric catfishes, Family Malapteruridae (Teleostei, Siluriformes), with erection of a new genus and descriptions of fourteen new species, and an annotated bibliography. Ann. Mus. Congo Belge Zool. 2002, 2002, 289. [Google Scholar]
- Moritz, T.; Britz, R. Revision of the extant Polypteridae (Actinopterygii: Cladistia). Ichthyol. Explor. Freshw. 2019, 29, 97–192. [Google Scholar] [CrossRef]
- Fricke, R.; Eschmeyer, W.N.; Fong, J.D. (Eds.) Eschmeyer’s Catalog of Fishes: Genera, Species, References. Available online: http://research.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed on 4 July 2023).
- Sigovini, M.; Keppel, E.; Tagliapietra, D. Open nomenclature in the biodiversity era. Methods Ecol. Evol. 2016, 7, 1217–1225. [Google Scholar] [CrossRef]
- Real, R.; Vargas, J.M. The Probabilistic basis of Jaccard’s Index of Similarity. Syst. Biol. 1996, 45, 380–385. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. (Eds.) FishBase. World Wide Web Electronic Publication. 2022. Available online: http://www.fishbase.org (accessed on 11 February 2022).
- Braganca, P.H.; Smith, T.G.; Vreven, E.J.; Chakona, A. Integrative taxonomy reveals hidden diversity in the southern African darters genus Nannocharax Günther 1867 (Characiformes: Distichodontidae). J. Fish Biol. 2020, 97, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Kambikambi, M.J.; Kadye, W.T.; Chakona, A. Allopatric differentiation in the Enteromius anoplus complex in South Africa, with the revalidation of E. cernuus and E. oraniensis, and description of a new species, E. mandelai (Teleostei: Cyprinidae). J. Fish Biol. 2021, 99, 931–954. [Google Scholar] [CrossRef]
- Kashindye, B.B.; Katemo Manda, B.; Friel, J.P.; Chakona, A.; Vreven, E. Chiloglanis msirii, a new species of African suckermouth catfish (Teleostei: Mochokidae), from the Upper Congo Basin. Ichthyol. Explor. Freshw. 2021, 31, 33–46. [Google Scholar] [CrossRef]
- Mbimbi Mayi Munene, J.J.; Stiassny, M.L.; Monsembula Iyaba, R.J.; Liyandja, T.L. Fishes of the Lower Lulua River (Kasai basin, Central Africa): A Continental Hotspot of Ichthyofaunal Diversity under Threat. Diversity 2021, 13, 341. [Google Scholar] [CrossRef]
- Skelton, P.H.; Stauffer, J.R.; Chakona, A.; Wisor, J.M. Two new species of African bubble-nesting Microctenopoma (Teleostei: Anabantidae) from Angola. Ichthyol. Explor. Freshw. 2021, 30, 345–360. [Google Scholar] [CrossRef]
- Sabaj, M.H. Codes for natural history collections in ichthyology and herpetology. Copeia 2020, 108, 593–669. [Google Scholar] [CrossRef]
- Boulenger, G.A. Les Poissons du Bassin du Congo; Publications de l’Etat Indépendant du Congo: Brussels, Belgium, 1901; p. 532. [Google Scholar]
- Daget, J.; Gosse, J.P.; Thys van den Audenaerde, D.F.E. Check-List of the Freshwater Fishes of Africa; Office de la Recherche Scientifique et Technique Outre-Mer and Royal Museum for Central Africa; George Michiels: Tongres, Belgium, 1984; Volume I, p. 410. [Google Scholar]
- Günther, A. Description of a new species of Mormyrus. Proc. Zool. Soc. Lond. 1864, 1, 1–22. [Google Scholar]
- Bigorne, R. Mise en synonymie de Gnathonemus brevicaudatus Pellegrin 1919 avec Gnathonemus petersii Günther, 1862 (Teleostei, Mormyridae). Cybium 1990, 14, 125–129. [Google Scholar]
- Lavoué, S. Petrocephalus Marcusen, 1854 (Osteoglossomorpha: Mormyridae) of the Bangweulu-Mweru ecoregion (Luapula River system, Congo Basin), with the description of a new species. J. Nat. Hist. 2012, 46, 2159–2178. [Google Scholar] [CrossRef]
- Kisekelwa, T.; Snoeks, J.; Decru, E.; Schedel, F.B.; Isumbisho, M.; Vreven, E. A mismatch between morphological and molecular data in lineages of Enteromius (Cypriniformes: Cyprinidae) from the Lowa basin (East Democratic Republic of the Congo: DRC) with the description of a new species. Syst. Biodivers. 2022, 20, 1–22. [Google Scholar] [CrossRef]
- Fischer, J.G. Über einige Afrikanische Reptilien, Amphibien, und Fische des Naturhistorischen Museums. I. Über die von Herrn G. A. Fischer im Massai-Gebiete (Ost-Afrika) auf seiner in Veranlassung der Geographischen Geselschaft in Hamburg unternommenen Expedition gesammelten Reptilen, Amphibien und Fische. Jb. Hamb. Wiss. Anst. 1884, 1, 1–32. [Google Scholar]
- Vreven, E.J.; Musschoot, T.; Snoeks, J.; Schliewen, U.K. The African hexaploid Torini (Cypriniformes: Cyprinidae): Review of a tumultuous history. Zool. J. Linn. Soc. 2016, 177, 231–305. [Google Scholar] [CrossRef]
- Vreven, E.J.; Musschoot, T.; Decru, E.; Wamuini Lunkayilakio, S.; Obiero, K.; Cerwenka, A.F.; Schliewen, U.K. The complex origins of mouth polymorphism in the Labeobarbus (Cypriniformes: Cyprinidae) of the Inkisi River basin (Lower Congo, DRC, Africa): Insights from an integrative approach. Zool. J. Linn. Soc. 2019, 186, 414–482. [Google Scholar] [CrossRef]
- Boulenger, G.A. Additions à la faune ichthyologique de bassin du Congo. Matériaux pour la faune du Congo. Ann. Mus. Congo Belge 1902, 2, 19–57. [Google Scholar]
- Decru, E.; Snoeks, J.; Walanga, A.; Vreven, E.J. Disentangling the Diversity of the Labeobarbus Taxa (Cypriniformes: Cyprinidae) from the Epulu Basin (DR Congo, Africa). Diversity 2022, 14, 1022. [Google Scholar] [CrossRef]
- Boulenger, G.A. Mission Stappers au Tanganika-Moero. Diagnoses de poissons nouveaux. II. Mormyrides, kneriides, characinides, cyprinides, silurides. Rev. Zool. Afric. 1915, 4, 162–171. [Google Scholar] [CrossRef]
- Katemo Manda, B.; Snoeks, J.; Decru, E.; Brecko, J.; Vreven, E.J.W.M.N. Revision of Nannocharax luapulae Boulenger, 1915 (Characiformes: Distichodontidae) from the Upper Congo basin: Evidence for a species pair. J. Fish Biol. 2023, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Devaere, S.; Jansen, G.; Adriaens, D.; Weekers, P. Phylogeny of the African representatives of the catfish family Clariidae (Teleostei, Siluriformes) based on a combined analysis: Independent evolution towards anguilliformity. J. Zool. Syst. Evol. Res. 2007, 45, 214–229. [Google Scholar] [CrossRef]
- Hanssens, M. A review of the Clarias species (Pisces; Siluriformes) from the Lower Congo and the Pool Malebo. A review of the Clarias species (Pisces; Siluriformes) from the Lower Congo and the Pool Malebo. J. Afr. Zool. 2009, 5, 27–40. [Google Scholar]
- Boulenger, G.A. On a second collection of fishes made by Mr. S. L. Hinde in the Kenya District, East Africa. Proc. Zool. Soc. Lond. 1905, 1, 62–64. [Google Scholar] [CrossRef]
- Skelton, P.H. A systematic revision of species of the catfish genus Amphilius (Siluroidei, Amphiliidae) from east and southern Africa. Ann. Cape Prov. Mus. 1984, 16, 41–71. [Google Scholar]
- Thomson, A.W.; Page, L.M. Taxonomic revision of the Amphilius uranoscopus group (Teleostei: Siluriformes) in Kenya, with the description of a new species from the Athi River. Bull. Fla. Mus. Nat. Hist. 2010, 49, 45–66. [Google Scholar]
- Pellegrin, J. Poissons de la région du Kivu adressés par M. Guy Babault. Bull. Soc. Zool. 1933, 58, 169–175. [Google Scholar]
- Seegers, L. The Catfishes of Africa: A Handbook for Identification and Maintenance; Aqualog Verlag: Rodgau, Germany, 2008; p. 407. [Google Scholar]
- Ferraris, C.J., Jr.; Vari, R.P.; Skelton, P.H. A new genus of African loach catfish (Siluriformes: Amphiliidae) from the Congo River basin, the sister-group to all other genera of the Doumeinae, with the description of two new species. Copeia 2011, 2011, 477–489. [Google Scholar] [CrossRef]
- Roberts, T.R. Systematics and osteology of Leptoglaninae, a new subfamily of the African catfish family Amphiliidae, with descriptions of three new genera and six new species. Proc. Calif. Acad. Sci. 2003, 54, 83–132. [Google Scholar]
- Vreven, E.J. A Systematic Revision of the African Spinyeels (Mastacembelidae; Synbranchiformes). Doctoral Thesis, Katholieke Universiteit, Leuven, Belgium, 2001; p. 2312. [Google Scholar]
- Schedel, F.D.B.; Vreven, E.J.W.M.N.; Katemo Manda, B.; Abwe, E.; Schliewen, U.K. Description of five new rheophilic Orthochromis species (Teleostei: Cichlidae) from the Upper Congo drainage in Zambia and the Democratic Republic of the Congo. Zootaxa 2018, 446, 301–349. [Google Scholar] [CrossRef]
- Boulenger, G.A. On a collection of fishes from Lake Bangwelo. Ann. Mag. Nat. Hist. 1905, 16, 642–647. [Google Scholar] [CrossRef]
- Teugels, G.G.; Thieme, L.M. Freshwater Fish Biodiversity in the Congo Basin. In Freshwater Ecoregions of Africa and Madagascar. A Conservation Assessment; Thieme, L.M., Abell, R., Stiassny, M.L.J., Skelton, P., Lehner, B., Teugels, G.G., Dinerstein, E., Kamdem, A.T., et al., Eds.; WWF: Washington, DC, USA, 2005; pp. 51–53. [Google Scholar]
- Stiassny, M.L.J.; Brummett, R.E.; Harrison, I.J.; Monsembula, R.; Mamonekene, V. Chapter 3. The status and distribution of freshwater fishes in central Africa. In The Status and Distribution of Freshwater Biodiversity in Central Africa; Brooks, E.G.E., Allen, D.J., Darwall, W.R.T., Eds.; IUCN: Gland, Switzerland; Cambridge, UK, 2011; pp. 27–46. [Google Scholar]
- Matthes, H. Quelques poissons nouveaux du Congo. Rev. Zool. Bot. Afr. 1965, 81, 177–193. [Google Scholar]
- Holland, R.A.; Darwall, W.R.T.; Smith, K.G. Conservation priorities for freshwater biodiversity: The Key Biodiversity Area approach refined and tested for continental Africa. Biol. Conserv. 2012, 148, 167–179. [Google Scholar] [CrossRef]
- Snoeks, J.; Harrison, I.J.; Stiassny, M.L.J. The status and distribution of freshwater fishes. In The Diversity of Life in African Freshwaters: Under Water, Under Threat. An Analysis of the Status and Distribution of Freshwater Species Throughout Mainland Africa; Darwall, W.R.T., Smith, K.G., Allen, D.J., Holland, R.A., Harrison, I.J., Brooks, E.G.E., Eds.; IUCN: Cambridge, UK, 2011; pp. 42–73. [Google Scholar]
- Poll, M. Zoogéographie ichtyologique du cours supérieur du Lualaba. Publ. Univers. Elisabethv. 1963, 6, 95–106. [Google Scholar]
- Moore, A.E.; Cotterill, F.P.D.; Eckardt, F.D. The evolution and ages of Makgadikgadi palaeo-lakes: Consilient evidence from Kalahari drainage evolution. S. Afr. J. Geol. 2012, 115, 385–413. [Google Scholar] [CrossRef]
- Balon, E.K.; Stewart, D.J. Fish assemblages in a river with unusual gradient (Luongo, Africa—Zaire system), reflections on river zonation, and description of another new species. Environ. Biol. Fishes 1983, 9, 225–252. [Google Scholar] [CrossRef]
- Van Steenberge, M.; Vreven, E.; Snoeks, J. The fishes of the Upper Luapula area (Congo Basin): A fauna of mixed origin. Ichthyol. Explor. Freshw. 2014, 24, 329–345. [Google Scholar]
- Roberts, T.R.; Stewart, D.J. An ecological and systematical survey of fishes in the rapids of the lower Zaire or Congo River. Bull. Mus. Comp. Zool. 1976, 147, 239–317. [Google Scholar]
- Skelton, P.H. A Complete Guide to the Freshwater of Southern Africa; Struit Publishers: Cape Town, South Africa, 2001; p. 395. [Google Scholar]
- Sasanami, M.; Hustedt, J.; Alexander, N.; Horstick, O.; Bowman, L.; Hii, J.; Echaubard, P.; Braack, L.; Overgaard, H.J. Does anthropogenic introduction of guppy fish (Poecilia reticulata) impact faunal species diversity and abundance in natural aquatic habitats? A systematic review protocol. Environ. Evid. 2021, 10, 1–11. [Google Scholar] [CrossRef]
- Burke, J. Historique de la lutte contre la maladie du sommeil au Congo. Ann. Soc. Belg. Méd. Trop. 1971, 51, 465–477. [Google Scholar]
- Moreau, J.; Palomares, M.L.D. Atlas Démographique des Populations de Poissons d’Eau Douce d’Afrique; ICLARM Technical Reports 45; Centre International de Gestion des Ressources Aquatiques Vivantes and I’Agence de Coopération Culturelle et Technique: Manille, Philippines, 1995; p. 135. [Google Scholar]
- Welcomme, R.L. International Introductions of Inland Aquatic Species; United Nations Food and Agricultural Organization: Rome, Italy, 1988; p. 318. [Google Scholar]
- Baloko, J. Le nouveau poisson dans le fleuve Congo. Rev. Inf. Agric. 1968, 7–8, 18–20. [Google Scholar]
- Moreau, J. Exposé synoptique des données biologiques sur Heterotis niloticus (Cuvier, 1829). Food Agric. Organ. Synop. Pêches 1982, 131, 1–45. [Google Scholar]
- Welcomme, R.L. A history of international introductions of inland aquatic species. ICES Mar. Sci. Symp. 1992, 194, 3–14. [Google Scholar]
- Monentcham, S.E.; Kouam, J.; Pouomogne, V.; Kestemont, P. Biology and prospect for aquaculture of African bonytongue, Heterotis niloticus (Cuvier, 1829): A review. Aquaculture 2009, 289, 191–198. [Google Scholar] [CrossRef]
- Kouamélan, E.P.; Teugels, G.G.; N’douba, V.; Bi, G.G.; Koné, T. Fish diversity and its relationships with environmental variables in a West African basin. Hydrobiologia 2003, 505, 139–146. [Google Scholar] [CrossRef]
- Ibala-Zamba, A.; Vreven, E.; Mamonekene, V.; Snoeks, J. Fish community assemblages in relation to environmental variables in the Lefini River, middle Congo River basin (Republic of Congo). Cybium 2019, 43, 83–95. [Google Scholar] [CrossRef]
- Grenouillet, G.; Pont, D.; Hérissé, C. Within-basin fish assemblage structure: The relative influence of habitat versus stream spatial position on local species richness. Can. J. Fish. Aquat. Sci. 2004, 61, 93–102. [Google Scholar] [CrossRef]
- Winemiller, K.O.; Agostinho, A.A.; Pellegrini-Carasmachi, E. Fish ecology in tropical streams. In Tropical Stream Ecology; Dudgeon, D., Ed.; Academic Press: London, UK, 2008; pp. 107–146. [Google Scholar]
- Tejerina-Garro, F.L.; Maldonado, M.; Ibañez, C.; Pont, D.; Roset, N.; Oberdorff, T. Effects of natural and anthropogenic environmental changes on riverine fish assemblages: A framework for ecological assessment of rivers. Braz. Arch. Biol. Technol. 2005, 48, 91–108. [Google Scholar] [CrossRef]
- Daget, J. Les poissons du Fouta Djalon et de la Basse Guinée. Mém. Inst. Franc. Afr. Noire 1962, 65, 1–210. [Google Scholar]
- Malaisse, F. Les faciès d’un cours d’eau tropical: La Luanza (Haut-Katanga, Rép. dém. Congo). Verh. Int. Verein. Limnol. 1969, 17, 936–940. [Google Scholar] [CrossRef]
- Seegers, L. The fishes of the Lake Rukwa Drainage. Ann. Mus. R. Afr. Cent. 1996, 278, 1–407. [Google Scholar]
- Banister, K.E. 6A. Fish of the Zaire system. Ecol. River Syst. 1986, 60, 215–224. [Google Scholar]
- Lévêque, C.; Paugy, D. Geographical distribution and affinities of African freshwater fishes. In The Inland Water Fishes of Africa: Diversity, Ecology and Human Use; IRD Editions: Marseille, France, 2017; pp. 97–114. [Google Scholar]
- Bell-Cross, G. Movement of fish across the Congo-Zambezi watershed in the Mwinilunga District of Northern Rhodesia. Proc. Cent. Afr. Sci. Med. Congr. 1965, 1963, 415–424. [Google Scholar]
- Skelton, P.H. Diversity and distribution of freshwater fishes in East and Southern Africa. Ann. Mus. R. Afr. Cent. 1994, 275, 95–1131. [Google Scholar]
- Flügel, T.J.; Eckardt, F.D.; Cotterill, F.P.D. The present-day drainage patterns of the Congo River system and their neogene evolution. In Geology and Resource Potential of the Congo Basin; de Wit, M.J., Guillocheau, F., de Wit, M.C., Eds.; Regional Geology Reviews; Springer Science & Business Madia: Berlin/Heidelberg, Germany, 2015; pp. 315–337. [Google Scholar] [CrossRef]
- Cotterill, F.P.D. Drainage evolution in south-central Africa and vicariant speciation in swamp-dwelling weaver birds and swamp flycatchers. Honeyguide 2004, 50, 7–25. [Google Scholar]
- Thys van den Audenaerde, D.F.E. Les Tilapia (Pisces, Cichlidae) du Sud-Cameroun et du Gabon. Étude systématique. Ann. Mus. R. Afr. Cent. 1966, 153, 1–98. [Google Scholar]
- Van Steenberge, M.W.; Vanhove, M.P.; Chocha Manda, A.; Larmuseau, M.H.; Swart, B.L.; Khang’Mate, F.; Arndt, A.; Hellemans, B.; Van Houdt, J.; Micha, J.-C.; et al. Unravelling the evolution of Africa’s drainage basins through a widespread freshwater fish, the African sharptooth catfish Clarias gariepinus. J. Fish Biol. 2020, 47, 1739–1754. [Google Scholar] [CrossRef]
- Broadley, D.G.; Cotterill, F.P.D. The reptiles of southeast Katanga, an overlooked ‘hot spot’. Afr. J. Herpetol. 2004, 53, 35–61. [Google Scholar] [CrossRef]
- Barson, M.; Přikrylová, I.; Vanhove, M.P.M.; Huyse, T. Parasite hybridization in African Macrogyrodactylus spp. (Monogenea, Platyhelminthes) signals historical host distribution. Parasitology 2010, 137, 1585–1595. [Google Scholar] [CrossRef]
- Vanhove, M.P.; Van Steenberge, M.; Dessein, S.; Volckaert, F.A.M.; Snoeks, J.; Huyse, T.; Pariselle, A. Biogeographical implications of Zambezian Cichlidogyrus species (Platyhelminthes: Monogenea: Ancyrocephalidae) parasitizing Congolian cichlids. Zootaxa 2013, 3608, 398–400. [Google Scholar] [CrossRef]
- Robert, M. Géologie et Géographie du Katanga: Y Compris L’étude Des Ressources et de la Mises en Valeur; Presses Marcel Hayez: Bruxelles, Belgique, 1956; p. 620. [Google Scholar]
- He, L.M.; Troiano, J.; Wang, A.; Goh, K. Environmental chemistry, ecotoxicity, and fate of lambda-cyhalothrin. Rev. Environ. Contam. Toxicol. 2008, 2008, 71–91. [Google Scholar] [CrossRef]
- Katemo Manda, B.; Colinet, G.; André, L.; Chocha Manda, A.; Marquet, J.-P.; Micha, J.-C. Evaluation de la contamination de la chaîne trophique par les éléments traces (Cu, Co, Zn, Pb, Cd, U, V et As) dans le bassin de la Lufira supérieure (Katanga/RD Congo). Tropicultura 2010, 28, 246–252. [Google Scholar]
- Atibu, E.K.; Devarajan, N.; Thevenon, F.; Mwanamoki, P.M.; Tshibanda, J.B.; Mpiana, P.T.; Prabakar, K.; Mubedi, J.I.; Wildi, W.; Poté, J. Concentration of metals in surface water and sediment of Luilu and Musonoie Rivers, Kolwezi-Katanga, Democratic Republic of Congo. J. Appl. Geochem. 2013, 39, 26–32. [Google Scholar] [CrossRef]
- Mees, F.; Masalehdani, M.N.N.; De Putter, T.; D’Hollander, C.; Van Biezen, E.; Mujinya, B.B.; Potdevin, J.L.; Van Ranst, E. Concentrations and forms of heavy metals around two ore processing sites in Katanga, Democratic Republic of Congo. J. Afr. Earth Sci. 2013, 77, 22–30. [Google Scholar] [CrossRef]
- Rojat, D. Cogestion de la pêche crevettière à Madagascar. Liaison Energ.-Francoph. 2005, 66–67, 111–118. [Google Scholar]

| Family (No. Genera/Species) | Upemba National Park | Neighbouring Ecoregions | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Major (Sub) Basins | Management Area | IUCN Status | ||||||||||||||||||
| KM | Lu | Ng | KD | Mw | Fu | Ln | KN | Di | Lv | Ka | CZ | AZ | BM | LS | UC | BM | Kas | UZ | ||
| Polypteriformes | ||||||||||||||||||||
| Polypteridae (1/4) | ||||||||||||||||||||
| Polypterus bichir (Geoffroy, 1802) | - | - | - | N | - | - | - | - | - | - | - | - | N | - | - | LC | + | - | - | - |
| Polypterus congicus Boulenger, 1898 | - | - | - | + | - | - | - | - | - | - | - | - | + | - | - | LC | + | - | - | - |
| Polypterus ornatipinnis Boulenger, 1902 | - | + | - | + | - | - | - | N | - | N | - | + | + | N | N | LC | + | - | + | - |
| Polypterus senegalus Cuvier, 1829 | - | - | - | + | - | + | - | - | - | - | - | - | + | - | - | LC | + | - | - | - |
| Osteoglossiformes | ||||||||||||||||||||
| Arapaimidae (1/1) | ||||||||||||||||||||
| Heterotis niloticus (Cuvier, 1829) | - | - | - | N I | - | N I | - | - | - | - | - | - | N I | - | - | LC | + | - | + | - |
| Mormyridae (10/26) | ||||||||||||||||||||
| Campylomormyrus elephas (Boulenger, 1898) | - | + | - | - | - | - | - | - | - | - | - | + | - | - | - | LC | + | - | + | - |
| Campylomormyrus rhynchophorus (Boulenger, 1898) | - | + | - | + | - | - | - | N | - | N | - | - | + | N | N | LC | + | - | + | - |
| Campylomormyrus sp. ‘kadia’ | - | - | - | N E | - | - | - | - | - | - | - | - | N E | - | - | NE | - | - | - | - |
| Campylomormyrus tamandua (Günther, 1864) | - | - | - | N | - | - | - | - | - | - | - | - | N | - | - | LC | + | + | + | - |
| Cyphomyrus discorhynchus (Peters, 1852) | - | + | - | N | N | + | - | N | - | - | - | + | N | N | - | LC | + | + | + | + |
| Cyphomyrus lufirae Mukweze et al., 2020 | - | N E | - | - | - | - | - | - | N E | N | - | N E | - | - | N | NE | - | - | - | - |
| Genyomyrus donnyi Boulenger, 1898 | - | - | - | + | - | - | - | - | - | - | - | - | + | - | - | LC | + | - | - | - |
| Gnathonemus sp. ‘kamalondo’ | - | N E | - | N E * | N E | N E | - | - | - | - | - | N E * | - | - | - | NE | - | - | - | - |
| Heteromormyrus tavernei (Poll, 1972) | - | - | - | - | - | - | - | - | - | - | + * | - | - | - | + * | LC | - | - | - | - |
| Heteromormyrus sp. ‘dikulwe’ | - | N E * | - | - | - | - | - | - | N | - | - | N E * | - | - | N | NE | - | - | - | - |
| Marcusenius greshoffi (Schilthius, 1891) | - | + | - | - | - | - | - | - | - | - | - | + | - | - | - | LC | + | - | + | - |
| Marcusenius macrolepidotus (Peters, 1852) | - | N | - | + | N | + | N | N | N | - | - | N | + | N | N | LC | + | + | - | + |
| Marcusenius monteiri (Günther, 1873) | - | - | - | + | - | - | - | - | - | - | - | - | + | - | - | LC | + | + | + | - |
| Marcusenius multisquamatus Kramer and Wink, 2013 | - | N | - | - | - | - | - | - | - | - | - | N | - | - | - | NE | + | + | + | - |
| Marcusenius stanleyanus (Boulenger, 1897) | - | - | - | N | - | - | - | - | - | - | - | - | N | - | - | LC | + | - | + | - |
| Mormyrops anguilloides (Linnaeus, 1758) | - | + | - | N | - | - | - | N | - | N | - | + | N | N | N | LC | + | + | + | + |
| Mormyrus caballus Boulenger, 1898 | - | + | - | - | - | - | - | - | - | N | - | + | - | - | N | LC | + | + | + | - |
| Mormyrus rume Valenciennes, 1847 | - | N | - | + | - | - | - | - | - | - | - | N | + | - | - | NE | + | - | + | - |
| Petrocephalus cf. christyi Boulenger, 1920 | - | - | - | - | - | + | - | - | - | - | - | - | + | - | - | LC | + | + | + | - |
| Petrocephalus cf. frieli Lavoué, 2012 | - | N | - | - | - | - | - | - | - | - | - | N | - | - | - | NE | - | + | - | - |
| Petrocephalus sauvagii (Boulenger, 1887) | - | N | - | - | - | - | - | - | - | - | - | N | - | - | - | NE | + | - | + | - |
| Petrocephalus simus Sauvage, 1879 | - | - | - | - | N | + | - | - | - | - | - | - | + | - | - | LC | + | + | + | + |
| Pollimyrus cuandoensis Kramer et al., 2013 | - | - | - | N | - | - | - | - | - | - | - | - | N | - | - | NE | - | - | - | - |
| Pollimyrus fasciaticeps (Boulenger, 1920) | - | N | - | - | N | - | - | - | - | - | N | N | - | - | LC | - | - | - | - | |
| Pollimyrus osbornii (Nichols and Griscom, 1917) | - | N | - | N | N | N | - | - | - | N | - | N | N | - | N | NE | + | + | + | - |
| Pollimyrus tumifrons (Boulenger, 1902) | - | + | - | - | - | - | - | - | - | - | - | + | - | - | - | LC | + | - | + | - |
| Clupeiformes | ||||||||||||||||||||
| Clupeidae (2/4) | ||||||||||||||||||||
| Microthrissa congica (Regan, 1917) | - | - | - | + | - | - | - | N | - | - | - | - | + | N | - | LC | + | - | + | - |
| Microthrissa royauxi Boulenger, 1902 | - | - | - | + | - | - | - | - | - | - | - | - | + | - | - | LC | - | - | + | - |
| Microthrissa whiteheadi Gourène and Teugels, 1988 | - | - | - | + | - | - | - | - | - | - | - | - | + | - | - | LC | + | - | - | - |
| Odaxothrissa losera Boulenger, 1899 | - | - | - | + | - | - | - | - | - | - | - | - | + | - | - | LC | + | - | - | - |
| Gonorynchiformes | ||||||||||||||||||||
| Kneriidae (2/11) | ||||||||||||||||||||
| Kneria katangae Poll, 1976 | - | + * | - | - | - | - | - | - | - | - | - | + * | - | - | - | LC | - | - | - | - |
| Kneria sp. ‘kalule’ | - | - | N E * | - | - | - | - | N E * | - | - | - | N E * | - | N E * | - | NE | - | - | - | - |
| Kneria sp. ‘kalumengongo’ | N E * | - | - | - | - | - | - | - | - | - | - | N E * | - | - | - | NE | - | - | - | - |
| Kneria sp. ‘lusinga’ | - | - | - | - | - | - | - | - | - | - | E * | - | - | - | E * | NE | - | - | - | - |
| Kneria sp. ‘luena’ | - | - | - | - | - | - | N E * | - | - | - | - | - | - | N E * | - | NE | - | - | - | - |
| Parakneria lufirae Poll, 1965 | - | E * | - | - | - | - | - | - | - | - | - | E * | - | - | - | DD | - | - | - | - |
| Parakneria sp. ‘kilwezi’ | - | E * | - | - | - | - | - | - | - | - | - | E * | - | - | - | NE | - | - | - | - |
| Parakneria sp. ‘fungwe’ | - | - | - | - | - | N E * | - | - | - | - | - | - | N E * | - | - | NE | - | - | - | - |
| Parakneria sp. ‘kalule’ | - | - | - | - | - | - | - | N E * | - | - | - | - | - | N E * | - | NE | - | - | - | - |
| Parakneria sp. ‘luvilombo’ | - | - | - | - | - | - | - | - | - | N E * | - | - | - | - | N E * | NE | - | - | - | - |
| Parakneria thysi Poll, 1965 | - | N E * | - | - | - | - | - | - | N | N E * | - | N E * | - | - | N | DD | - | - | - | - |
| Cypriniformes | ||||||||||||||||||||
| Cyprinidae (4/51) | ||||||||||||||||||||
| Clypeobarbus congicus (Boulenger, 1899) | - | - | - | N | - | - | - | - | - | - | - | - | N | - | - | LC | + | - | - | - |
| Clypeobarbus pleuropholis (Boulenger, 1899) | - | - | - | + | N | N | N | - | - | - | - | - | + | N | - | LC | + | - | + | - |
| Clypeobarbus sp. ‘mwanza’ | - | - | - | - | N E * | - | - | - | - | - | - | - | N E * | - | - | NE | - | - | - | - |
| Enteromius afrovernayi (Nichols and Boulton, 1927) | - | N | - | - | - | - | - | - | - | N | - | N | - | - | N | LC | + | + | ||
| Enteromius eutaenia (Boulenger, 1904) | - | + | - | - | - | + | - | - | - | + | + | - | + | - | + | DD | - | + | + | + |
| Enteromius janssensi (Poll, 1976) | - | - | - | - | - | + * | - | - | - | - | - | - | + * | - | - | LC | - | - | - | - |
| Enteromius kamolondoensis (Poll, 1938) | - | + | - | - | - | N E | - | N | - | - | - | + | N E | N | - | LC | + | - | - | - |
| Enteromius kerstenii (Peters, 1868) | - | + | - | N | N | + | - | N | N | N | - | + | + | N | N | LC | + | + | + | + |
| Enteromius kessleri (Steindachner, 1866) | - | - | - | - | - | - | - | - | - | - | N | - | - | - | N | LC | + | + | + | + |
| Enteromius lineomaculatus (Boulenger, 1903) | - | - | - | - | - | - | - | - | N | N | - | - | - | - | N | LC | + | + | + | + |
| Enteromius lukusiensis (David and Poll, 1937) | - | N | - | N | - | + | - | - | - | - | - | N | + | - | - | LC | + | + | - | - |
| Enteromius luluae (Fowler, 1930) | - | - | - | + | - | - | - | - | - | - | - | - | + | - | - | LC | + | + | + | - |
| Enteromius miolepis (Boulenger, 1902) | - | + | - | - | - | - | - | - | - | - | - | + | - | - | - | LC | + | + | + | + |
| Enteromius cf. mocoensis (Trewavas, 1936) | - | - | - | - | - | - | - | N | - | - | - | - | - | N | - | DD | - | - | + | - |
| Enteromius multilineatus (Worthington, 1933) | - | N | - | - | - | - | - | - | - | N | - | N | - | - | N | LC | - | + | - | + |
| Enteromius neefi (Greenwood, 1962) | - | + | + | + | - | N | - | + | - | - | - | + | + | + | - | LC | + | + | + | + |
| Enteromius paludinosus (Peters, 1852) | - | N | - | - | - | N | - | - | + | + | + | N | N | - | + | LC | - | + | + | + |
| Enteromius radiatus (Peters, 1853) | - | + | - | - | - | - | - | - | N | N | - | + | - | - | N | LC | + | + | + | + |
| Enteromius taeniopleura (Boulenger, 1917) | - | + | - | - | - | + | - | - | - | - | - | + | + | - | - | LC | + | - | - | - |
| Enteromius trimaculatus (Peters, 1852) | - | + | - | - | - | - | - | - | + | + | - | + | - | - | + | LC | - | + | - | + |
| Enteromius sp. ‘kalumengongo’ | N E * | - | - | - | - | - | - | - | - | - | - | N E * | - | - | - | NE | - | - | - | - |
| Enteromius sp. ‘ngulungu’ | - | - | + * | - | - | - | - | + * | - | - | - | + * | - | + * | - | NE | - | - | - | - |
| Enteromius sp. ‘luena’ | - | + * | - | - | - | - | - | - | - | - | - | + * | - | - | - | NE | - | - | - | - |
| Enteromius thespesios Katemo Manda et al., 2020 | - | - | - | - | - | - | - | N * | - | N | - | - | - | N * | N | NE | - | + | - | - |
| Enteromius unitaeniatus (Günther, 1867) | - | + | - | - | - | - | - | - | + | - | - | + | - | - | + | LC | + | + | + | + |
| Enteromius urostigma (Boulenger, 1917) | - | - | - | - | - | - | - | - | - | - | + | - | - | - | + | LC | - | - | - | - |
| Labeo annectens Boulenger, 1903 | - | + | - | - | - | - | - | - | - | - | - | + | - | - | - | LC | + | + | + | + |
| Labeo cylindricus Peters, 1852 | - | N | - | - | N | - | - | N | - | - | - | N | N | N | - | LC | + | + | - | + |
| Labeo dhonti Boulenger, 1920 | - | - | - | N | - | - | - | - | - | - | - | - | N | - | - | LC | + | - | + | + |
| Labeo greeni Boulenger, 1920 | - | N | - | N | - | - | - | - | - | - | - | N | N | - | - | LC | + | - | + | - |
| Labeo kibimbi Poll, 1949 | - | + | - | + | - | - | - | - | - | - | - | + | + | - | - | LC | + | - | - | - |
| Labeo lineatus Boulenger, 1898 | - | + | - | + | - | - | - | - | - | - | - | + | + | - | - | LC | + | + | + | - |
| Labeo longipinnis Boulenger, 1898 | - | - | - | N | - | - | - | - | + | - | - | - | N | - | + | LC | + | - | + | - |
| Labeo lualabaensis Tshibwabwa, 1997 | - | - | - | N | - | - | - | - | - | - | - | - | N | - | - | LC | + | - | - | - |
| Labeo parvus Boulenger, 1902 | - | N | - | - | - | N | - | N | N | N | - | N | N | N | N | LC | + | - | + | - |
| Labeo simpsoni Ricardo-Bertram, 1943 | - | N | - | - | - | - | - | N | - | - | N | - | N | LC | + | + | + | - | ||
| Labeo rosae Steindachner, 1894 | - | - | - | - | - | - | - | - | N | - | - | - | - | - | N | LC | - | - | - | - |
| Labeo weeksii Boulenger, 1909 | - | + | - | + | - | - | - | N | - | - | - | + | + | N | - | LC | + | + | + | - |
| Labeobarbus altipinnis (Banister and Poll, 1973) | - | E * | - | - | - | - | - | - | - | - | - | E * | - | - | - | LC | - | - | - | - |
| Labeobarbus gestetneri (Banister and Bailey, 1979) | E * | - | - | - | - | - | - | - | - | - | - | E * | - | - | - | DD | - | - | - | - |
| Labeobarbus iphthimostoma (Banister and Poll, 1973) | - | E * | - | - | - | - | - | - | - | - | - | E * | - | - | - | DD | - | - | - | - |
| Labeobarbus sp. ‘lower_lufira’ | - | N E * | - | - | - | - | - | - | - | - | - | N E * | - | - | - | NE | - | - | - | - |
| Labeobarbus sp. ‘middle_lufira’ | - | N E * | - | - | - | - | - | - | N E * | N E * | N E * | N E * | - | - | N E * | NE | - | - | - | - |
| Labeobarbus sp. ‘kalumengongo’ | N E * | - | - | - | - | - | - | - | - | - | - | N E * | - | - | - | NE | - | - | - | - |
| Labeobarbus sp. ‘kapepe’ | - | - | - | - | - | - | - | N E * | - | - | - | N E * | - | - | - | NE | - | - | - | - |
| Labeobarbus sp. ‘kayo’ | - | - | N E * | - | - | - | - | N E * | - | - | - | N E * | - | N E * | - | NE | - | - | - | - |
| Labeobarbus sp. ‘ngulungu’ | - | - | N E * | - | - | - | - | N E * | - | - | - | N E * | - | N E * | - | NE | - | - | - | - |
| Labeobarbus sp. ‘thick lip’ | - | + | - | - | - | - | - | N | - | - | - | + | - | N | - | NE | + | + | + | - |
| Labeobarbus sp. ‘nshila’ | - | - | - | - | - | - | - | N E * | - | - | - | - | - | N E * | - | NE | - | - | - | - |
| Labeobarbus upembensis (Banister and Bailey, 1979) | E * | - | - | - | - | - | - | - | - | - | - | E * | - | - | - | DD | - | - | - | - |
| Labeobarbus wittei (Banister and Poll, 1973) | - | E * | - | - | - | - | - | - | - | - | - | E * | - | - | - | DD | - | - | - | - |
| Danionidae (4/9) | ||||||||||||||||||||
| Chelaethiops congicus (Nichols and Griscom, 1917) | - | N | - | + | - | - | - | N | - | N | - | N | + | N | N | LC | + | + | + | - |
| Chelaethiops elongatus Boulenger, 1899 | - | - | - | + | - | - | - | - | N | - | - | - | + | - | N | LC | + | - | + | - |
| Leptocypris lujae (Boulenger, 1909) | - | - | - | + | - | - | - | - | - | - | - | - | + | - | - | LC | + | - | - | - |
| Leptocypris modestus Boulenger, 1900 | - | - | - | + | - | - | - | - | - | - | - | - | + | - | - | LC | + | - | + | - |
| Leptocypris weynsii (Boulenger, 1899) | - | - | - | + | - | - | - | - | - | - | - | - | + | - | - | LC | + | - | + | - |
| Opsaridium leleupi (Matthes, 1965) | - | - | - | N | - | - | - | - | - | - | - | - | N | - | - | DD | - | - | - | - |
| Opsaridium ubangiense (Pellegrin, 1901) | - | + | - | - | - | - | - | N | - | - | - | + | - | N | - | LC | + | + | + | - |
| Opsaridium zambezense (Peters, 1852) | - | N | - | - | - | - | - | - | N | N | - | N | - | - | N | LC | - | + | + | + |
| Raiamas marqueti Katemo Manda et al., 2018 | - | - | - | - | - | - | - | N * | - | - | - | - | - | N * | - | NE | + | - | - | - |
| Characiformes | ||||||||||||||||||||
| Citharinidae (1/3) | ||||||||||||||||||||
| Citharinus congicus Boulenger, 1897 | - | - | - | + | - | - | - | - | - | - | - | - | + | - | - | LC | + | - | + | - |
| Citharinus gibbosus Boulenger, 1899 | - | + | - | + | - | - | - | - | - | - | - | + | + | - | - | LC | + | - | + | - |
| Citharinus macrolepis Boulenger, 1899 | - | - | - | + | - | - | - | - | - | - | - | - | + | - | - | LC | + | - | - | - |
| Distichodontidae (7/18) | ||||||||||||||||||||
| Distichodus antonii Schilthuis, 1891 | - | N | - | + | - | - | - | - | - | - | - | N | + | - | - | LC | + | + | + | - |
| Distichodus fasciolatus Boulenger, 1898 | - | + | - | + | - | - | - | - | - | - | - | + | + | - | - | LC | + | - | + | - |
| Distichodus lusosso Schilthuis, 1891 | - | + | - | + | - | - | - | - | - | - | - | + | + | - | - | LC | + | - | + | - |
| Distichodus maculatus Boulenger, 1898 | - | + | - | + | - | - | - | N | - | N | - | + | + | N | N | LC | + | + | + | - |
| Distichodus polli Abwe et al., 2019 | - | N * | - | N * | - | - | - | - | - | - | - | - | N * | - | - | NE | - | - | + | - |
| Distichodus sexfasciatus Boulenger, 1897 | - | + | - | - | - | - | - | - | - | - | - | + | - | - | - | LC | + | - | + | - |
| Eugnathichthys eetveldii Boulenger, 1898 | - | + | - | - | - | N | - | - | - | N | - | + | N | - | N | LC | + | - | + | - |
| Eugnathichthys macroterolepis Boulenger, 1899 | - | N | - | - | - | - | - | - | - | - | - | N | - | - | - | LC | + | - | + | - |
| Ichthyborus congolensis (Giltay, 1930) | - | - | - | N | - | N | - | - | - | - | - | - | N | - | - | NE | + | - | - | - |
| Mesoborus crocodilus Pellegrin, 1900 | - | - | - | + | - | - | - | - | - | - | - | - | + | - | - | LC | + | - | + | - |
| Microstomatichthyoborus katangae David and Poll, 1937 | - | - | - | + * | - | - | - | - | - | - | - | - | + * | - | - | LC | + | - | - | - |
| Nannocharax brevis Boulenger, 1902 | - | + | - | - | - | - | - | - | - | - | - | + | - | - | - | LC | + | - | + | - |
| Nannocharax chochamandai Katemo Manda et al., 2023 | - | + | - | - | N * | + | - | - | - | - | - | + | + | - | - | NE | + | - | - | - |
| Nannocharax elongatus Boulenger, 1900 | - | - | - | N | - | - | - | - | - | - | - | - | N | - | - | LC | + | - | + | - |
| Nannocharax hadros Katemo Manda et al., 2021 | - | - | - | - | - | - | - | N E * | - | - | - | - | - | N E * | - | NE | - | - | - | - |
| Nannocharax sp. ‘lovoi’ | - | - | - | N | - | - | - | - | - | - | - | - | N | - | - | NE | - | - | - | - |
| Phago boulengeri Schilthuis, 1891 | - | + | - | - | - | - | - | - | - | - | - | + | - | - | - | LC | + | - | + | - |
| Phago intermedius Boulenger, 1899 | - | + | - | - | - | - | - | - | - | - | - | + | - | - | - | LC | + | - | - | - |
| Alestidae (8/18) | ||||||||||||||||||||
| Alestes liebrechtsii Boulenger, 1898 | - | N | - | + | - | - | - | - | - | - | - | N | + | - | - | LC | + | + | + | + |
| Alestes macrophthalmus Günther, 1867 | - | + | - | + | - | - | - | N | - | N | - | + | + | N | N | LC | + | + | + | + |
| Brachypetersius cadwaladeri (Fowler, 1930) | - | - | - | + | - | - | - | - | - | - | - | - | + | - | - | LC | + | - | + | - |
| Brycinus bimaculatus (Boulenger, 1899) | - | - | - | + | - | - | - | - | - | - | - | - | + | - | - | LC | + | - | - | - |
| Brycinus grandisquamis (Boulenger, 1899) | - | N | - | - | - | - | - | N | - | N | - | N | - | N | N | LC | + | + | + | - |
| Brycinus imberi (Peters, 1852) | - | + | - | + | - | + | - | N | - | - | - | + | + | N | - | LC | + | + | + | + |
| Brycinus lateralis (Boulenger, 1900) | - | N | - | - | - | - | - | - | + | - | - | N | - | - | + | LC | - | + | + | + |
| Brycinus macrolepidotus Valenciennes, 1850 | - | + | - | - | - | - | - | - | - | - | - | + | - | - | - | LC | + | + | + | - |
| Bryconaethiops boulengeri Pellegrin, 1900 | - | N | - | - | - | - | - | - | - | N | - | N | - | - | N | LC | + | + | + | - |
| Bryconaethiops macrops Günther, 1873 | - | N | - | - | - | - | - | - | - | - | - | - | - | - | - | LC | + | - | ||
| Bryconaethiops microstoma Günther, 1873 | - | N | - | - | - | - | - | - | - | N | - | N | - | - | N | LC | + | - | + | - |
| Hydrocynus vittatus Castelnau, 1861 | - | + | - | + | - | - | - | N | - | - | - | + | + | N | - | LC | + | + | + | + |
| Micralestes acutidens (Peters, 1852) | - | + | - | N | - | - | - | N | - | N | - | + | N | N | N | LC | + | + | + | + |
| Micralestes humilis Boulenger, 1899 | - | N | - | + | N | + | - | - | - | - | - | N | + | - | - | LC | + | + | + | + |
| Micralestes lualabae Poll, 1967 | - | - | - | + | - | - | - | - | - | - | - | - | + | - | - | LC | + | - | - | - |
| Micralestes stormsi Boulenger, 1902 | - | + | - | - | - | - | - | N | N | N | - | + | - | N | N | LC | + | - | + | - |
| Phenacogrammus aurantiacus (Pellegrin, 1930) | - | - | - | N | - | - | - | - | - | - | - | - | N | - | - | LC | + | - | + | - |
| Rhabdalestes rhodesiensis (Ricardo-Bertram, 1943) | - | - | - | N | - | - | - | - | - | - | - | - | N | - | - | LC | - | + | - | - |
| Siluriformes | ||||||||||||||||||||
| Bagridae (1/1) | ||||||||||||||||||||
| Bagrus ubangensis Boulenger, 1902 | - | + | - | - | - | - | - | N | - | - | - | + | - | N | - | LC | + | - | + | - |
| Clariidae (2/8) | ||||||||||||||||||||
| Clarias buthupogon Sauvage, 1879 | - | + | - | N | - | + | N | N | + | + | - | + | N | N | + | LC | + | + | + | - |
| Clarias cf. dumerilii Steindachner, 1866 | - | - | N | - | - | - | - | N | - | - | - | N | - | N | - | LC | + | + | + | + |
| Clarias cf. liocephalus Boulenger, 1898 | N | + | - | - | - | - | - | - | - | - | - | N | - | - | - | LC | + | + | + | - |
| Clarias gariepinus (Burchell, 1822) | - | N | - | + | + | + | - | - | + | + | - | N | + | - | + | LC | + | + | + | + |
| Clarias ngamensis Castelnau, 1861 | - | - | - | - | - | - | - | - | - | N | - | - | - | - | N | LC | - | + | + | + |
| Claraias stappersii Boulenger, 1915 | - | - | - | - | - | - | - | - | N | - | - | - | - | - | N | LC | - | + | + | - |
| Clarias theodorae Weber, 1897 | - | - | - | + | + | - | - | - | + | - | + | - | - | - | + | LC | - | + | + | + |
| Heterobranchus longifilis Valenciennes, 1840 | - | + | - | + | - | - | - | N | - | - | - | + | + | N | - | LC | + | + | + | + |
| Amphiliidae (5/17) | ||||||||||||||||||||
| Amphilius cryptobullatus Skelton, 1986 | - | N | - | - | - | - | - | - | - | - | N | N | - | - | N | NE | - | + | - | - |
| Amphilius frieli Thomson and Page, 2015 | - | N | - | - | - | - | - | - | - | - | - | N | - | - | - | NE | - | + | - | - |
| Amphilius sp. ‘lufira’ | - | N | - | - | - | - | - | - | + | + | - | N | - | - | + | NE | - | - | - | - |
| Amphilius sp. ‘kayo_above’ | - | - | - | - | - | - | - | N E * | - | - | - | - | - | N E * | - | NE | - | - | - | - |
| Amphilius sp. ‘kayo_below’ | - | - | N E * | - | - | - | - | N E * | - | - | - | N E * | - | N E * | - | NE | - | - | - | - |
| Amphilius sp. ‘kalumengongo’ | N E * | - | - | - | - | - | - | - | - | - | - | N E * | - | - | - | NE | - | - | - | - |
| Amphilius sp. ‘mwanza’ | - | - | - | - | N E * | - | - | - | - | - | - | - | N E * | - | - | NE | - | - | - | - |
| Belonoglanis tenuis Boulenger, 1902 | - | + | - | - | N | + | - | - | - | - | - | + | + | - | - | LC | + | - | + | - |
| Congoglanis cf. alula (Nichols and Griscom, 1917) | - | - | - | - | - | - | - | - | N | - | - | - | - | - | N | LC | - | - | - | - |
| Congoglanis sagitta Ferraris, Vari and Skelton, 2011 | N | - | - | - | - | - | - | - | + | - | - | N | - | - | + | NE | - | + | + | - |
| Tetracamphilius notatus (Nichols and Griscom, 1917) | - | + | - | - | - | - | - | - | - | - | - | + | - | - | - | LC | - | - | + | - |
| Zaireichthys brevis (Boulenger, 1915) | - | - | - | - | + | + | - | + | - | - | - | - | + | + | - | LC | + | + | - | - |
| Zaireichthys rotundiceps (Hilgendorf, 1905) | - | + | - | + | - | - | - | - | + | + | - | + | + | - | + | LC | - | + | - | + |
| Zaireichthys sp. ‘upemba’ | - | - | N E * | - | - | - | - | N E | - | - | - | N E * | - | N E | - | NE | - | - | - | - |
| Zaireichthtys sp. ‘ngulungu’ | - | - | N E * | - | - | - | - | - | - | - | - | N E * | - | - | - | NE | - | - | - | - |
| Zaireichthtys sp. ‘mwanza’ | - | - | - | - | N E * | - | - | - | - | - | - | - | N E * | - | - | NE | - | - | - | - |
| Zaireichthtys sp. ‘dikulwe’ | - | - | - | - | - | - | - | - | N E * | - | - | - | - | - | N E * | NE | - | - | - | - |
| Malapteruridae (1/3) | ||||||||||||||||||||
| Malapterurus melanochir Norris, 2002 | - | + | - | - | - | - | - | - | - | - | - | + | - | - | - | LC | + | - | + | - |
| Malapterurus microstoma Poll and Gosse, 1969 | - | + | - | + | - | - | - | - | - | - | - | + | + | - | - | LC | + | - | + | - |
| Malapterurus monsembeensis Roberts, 2000 | - | + | - | - | - | N | - | N | - | - | - | + | N | N | - | NE | + | - | + | - |
| Mochokidae (4/26) | ||||||||||||||||||||
| Atopochilus christyi Boulenger, 1920 | - | - | - | N | - | - | - | - | - | - | - | - | N | - | - | LC | - | - | + | - |
| Chiloglanis lufirae Poll, 1976 | - | E * | - | - | - | - | - | - | - | - | - | E * | - | - | - | DD | - | - | - | - |
| Chiloglanis microps Matthes, 1965 | - | E * | - | - | - | - | - | - | - | - | - | E * | - | - | - | LC | - | - | - | - |
| Chiloglanis msirii Kashindye et al., 2021 | - | - | - | - | N E * | N E | - | - | - | - | - | - | N E * | - | - | NE | + | - | - | - |
| Chiloglanis pojeri Poll, 1944 | - | N | - | - | - | - | - | - | - | - | - | N | - | - | - | LC | + | - | - | - |
| Chiloglanis sp. ‘dikulwe’ | - | - | - | - | - | - | - | - | N E * | - | - | - | - | - | N E * | NE | - | - | - | - |
| Chiloglanis sp. ‘mwanza’ | - | - | - | - | N E * | N E | - | - | - | - | - | - | N E * | - | - | NE | - | - | - | - |
| Chiloglanis sp. ‘kalule’ | - | - | - | - | - | - | - | N E * | - | - | - | - | - | N E * | - | NE | - | - | - | - |
| Chiloglanis sp. ‘kalumengongo_1’ | N E * | - | - | - | - | - | - | - | - | - | - | N E * | - | - | - | NE | - | - | - | - |
| Chiloglanis sp. ‘kalumengongo_2’ | N E * | - | - | - | - | - | - | - | - | - | - | N E * | - | - | - | NE | - | - | - | - |
| Euchilichthys royauxi Boulenger, 1902 | - | + | - | - | - | - | - | N | - | N | - | + | - | N | N | LC | + | + | + | - |
| Synodontis acanthomias Boulenger, 1899 | - | + | - | + | - | - | - | - | - | - | - | + | + | - | - | LC | + | - | + | - |
| Synodontis alberti Schilthuis, 1891 | - | N | - | + | - | - | - | - | - | - | - | N | + | - | - | LC | + | - | + | - |
| Synodontis angelicus Schilthuis, 1891 | - | - | - | N | - | - | - | - | - | - | - | - | N | - | - | LC | + | - | + | - |
| Synodontis congicus Poll, 1971 | - | N | - | N | - | + | - | - | - | - | - | N | N | - | - | LC | + | - | + | - |
| Synodontis decorus Boulenger, 1899 | - | + | - | N | - | - | - | N | - | N | - | + | N | N | N | LC | + | - | + | - |
| Synodontis denticulatus Kasongo Ilunga et al., 2019 | - | - | - | - | - | - | - | - | N * | - | - | - | - | - | N * | NE | - | - | - | - |
| Synodontis dorsomaculatus Poll, 1971 | - | N | - | + | N | + | - | N | - | N | - | N | + | N | N | EN | + | - | - | - |
| Synodontis greshoffi Schilthuis, 1891 | - | N | - | + | - | + | - | N | - | - | - | N | + | N | - | LC | + | - | + | - |
| Synodontis lufirae Poll, 1971 | - | - | - | - | - | - | - | - | + | - | - | - | - | - | + | VU | - | - | - | - |
| Synodontis notatus Vaillant, 1893 | - | - | - | + | - | N | - | - | - | - | - | - | + | - | - | LC | + | - | + | - |
| Synodontis nummifer Boulenger, 1899 | - | - | - | + | - | - | - | - | - | - | - | - | + | - | - | LC | + | - | + | - |
| Synodontis pleurops Boulenger, 1897 | - | + | - | - | - | - | - | - | - | - | - | + | - | - | - | LC | + | - | + | - |
| Synodontis polystigma Boulenger, 1915 | - | - | - | + | - | - | - | - | - | - | - | - | + | - | - | LC | - | + | - | - |
| Synodontis smiti Boulenger, 1902 | - | - | - | + | - | - | - | - | - | - | - | - | + | - | - | LC | + | - | + | - |
| Synodontis sp. ‘kifita’ | - | - | - | N * | - | N * | - | - | - | - | - | - | N * | - | - | LC | - | + | - | - |
| Claroteidae (3/10) | ||||||||||||||||||||
| Auchenoglanis occidentalis (Valenciennes, 1840) | - | + | - | + | - | - | - | N | - | - | - | + | + | N | - | LC | + | + | + | - |
| Chrysichthys congicus (Boulenger, 1899) | - | - | - | + | - | - | - | - | - | - | - | - | + | - | - | NE | - | - | - | - |
| Chrysichthys cranchii (Leach, 1818) | - | N | - | + | - | - | - | N | - | - | - | N | + | N | - | LC | + | - | + | - |
| Chrysichthys delhezi Boulenger, 1899 | - | + | - | - | - | - | - | - | - | - | - | + | - | - | - | LC | + | - | - | - |
| Chrysichthys longipinnis (Boulenger, 1899) | - | - | - | N | - | - | - | - | - | - | - | - | N | - | - | LC | + | - | + | - |
| Chrysichthys macropterus Boulenger, 1920 | - | + | - | - | - | - | - | - | - | - | - | + | - | - | - | DD | - | - | + | - |
| Chrysichthys sharpii Boulenger, 1901 | - | N | - | - | - | - | - | - | - | N | - | N | - | - | N | LC | + | + | - | - |
| Chrysichthys thonneri Steindachner, 1912 | - | - | - | + | - | - | - | - | - | - | - | - | + | - | - | LC | + | - | - | - |
| Parauchenoglanis punctatus (Boulenger, 1902) | - | + | - | - | - | - | - | - | + | - | - | + | - | N | + | LC | - | - | + | - |
| Parauchenoglanis sp. ‘kalule’ | - | - | - | - | - | - | - | N E | - | - | - | - | - | N | - | NE | - | - | - | - |
| Schilbeidae (3/5) | ||||||||||||||||||||
| Parailia congica Boulenger, 1899 | - | - | - | + | - | - | - | - | - | - | - | - | + | - | - | LC | + | - | + | - |
| Pareutropius debauwi (Boulenger, 1900) | - | - | - | + | - | - | - | - | - | - | - | - | + | - | - | LC | + | - | + | - |
| Pareutropius mandevillei Poll, 1959 | - | + | - | - | - | - | - | - | - | - | - | + | - | - | - | LC | + | + | - | - |
| Schilbe grenfelli (Boulenger, 1900) | - | N | - | - | - | - | - | - | - | N | - | N | - | - | N | LC | + | - | + | - |
| Schilbe intermedius Rüppell, 1832 | - | N | - | + | - | N | - | N | + | N | - | N | + | N | + | LC | + | + | + | + |
| Gobiiformes | ||||||||||||||||||||
| Eleotridae (1/1) | ||||||||||||||||||||
| Kribia nana (Boulenger, 1901) | - | - | - | - | N | + | - | - | - | - | - | - | + | - | - | LC | + | - | - | - |
| Synbranchiformes | ||||||||||||||||||||
| Mastacembelidae (1/3) | ||||||||||||||||||||
| Mastacembelus congicus Boulenger, 1896 | - | + | - | N | N | N | - | N | - | - | - | + | N | N | - | LC | + | + | + | - |
| Mastacembelus frenatus Boulenger, 1901 | - | + | - | - | - | - | - | - | - | - | - | + | - | - | - | LC | + | + | + | + |
| Mastacembelus sp. ‘lufiraensis’ | - | N E * | - | - | - | - | - | - | + | N | - | N E * | - | - | + | NE | - | - | - | - |
| Anabantiformes | ||||||||||||||||||||
| Anabantidae (2/4) | ||||||||||||||||||||
| Ctenopoma multispine Peters, 1844 | - | - | - | N | - | N | - | - | - | - | - | - | N | - | - | LC | + | + | + | + |
| Ctenopoma muriei (Boulenger, 1906) | - | - | - | + | - | - | - | - | - | - | - | - | + | - | - | LC | - | + | - | - |
| Microctenopoma intermedium (Pellegrin, 1920) | - | - | - | + | - | - | - | - | - | - | - | - | + | - | - | LC | - | + | + | + |
| Microctenopoma ocellifer (Nichols, 1928) | - | - | - | + | - | - | - | - | - | - | - | - | + | - | - | LC | + | - | - | - |
| Channidae (1/1) | ||||||||||||||||||||
| Parachanna obscura (Günther, 1861) | - | - | - | + | - | - | - | - | - | - | - | - | + | - | - | LC | - | - | + | - |
| Carangiformes | ||||||||||||||||||||
| Latidae (1/1) | ||||||||||||||||||||
| Lates niloticus (Linnaeus, 1758) | - | N | - | + | - | - | - | - | - | N | - | N | + | - | N | LC | + | - | + | - |
| Cichliformes | ||||||||||||||||||||
| Cichlidae (9/16) | ||||||||||||||||||||
| Coptodon rendalli (Boulenger, 1897) | - | + | - | + | - | N | - | N | + | + | - | + | + | N | + | LC | + | + | + | + |
| Coptodon zillii (Gervais, 1848) | - | - | - | N | - | - | - | - | - | - | - | N | - | - | LC | + | - | - | - | |
| Lamprologus mocquardi Pellegrin, 1903 | - | + | - | - | - | - | - | - | - | - | - | + | - | - | - | LC | + | - | + | - |
| Lamprologus symoensi Poll, 1976 | - | * E | - | * E | - | - | - | - | - | - | - | + * | - | - | - | DD | - | - | - | - |
| Oreochromis macrochir (Boulenger, 1912) | - | + | - | N | - | - | - | - | + | - | - | + | N | - | + | VU | + | + | + | + |
| Oreochromis upembae (Thys van den Audenaerde, 1964) | - | N | - | + | - | N | - | - | - | - | - | N | + | - | - | LC | + | - | - | - |
| Orthochromis kimpala Schedel et al., 2018 | - | - | - | - | - | - | - | N E * | - | - | - | - | N E * | - | NE | - | - | - | - | |
| Orthochromis sp. ‘lufira’ | - | N | - | - | - | - | - | - | N | - | - | N | - | - | N | NE | + | - | - | - |
| Orthochromis torrenticola (Thys van den Audenaerde, 1963) | - | + * | - | - | - | - | - | - | - | + | - | + * | - | - | + | LC | + | - | - | - |
| Pseudocrenilabrus nicholsi (Pellegrin, 1928) | - | - | - | N | - | N | N | - | - | - | - | - | N | - | - | LC | + | - | - | - |
| Pseudocrenilabrus philander (Weber, 1897) | - | + | - | + | - | + | - | - | + | + | - | + | + | - | + | LC | + | + | + | + |
| Sargochromis sp. ‘lufira’ | - | - | - | - | - | - | - | - | N | - | - | - | - | - | N | NE | - | - | - | - |
| Serranochromis macrocephalus (Boulenger, 1899) | - | - | - | - | - | - | - | - | N | - | - | - | - | - | N | LC | + | + | + | + |
| Tilapia sparrmanii Smith, 1840 | - | + | - | + | N | N | - | N | + | + | - | + | + | N | + | LC | + | + | + | + |
| Tylochromis regani Stiassny, 1989 | - | N | - | N | - | + | - | - | - | N | - | N | + | - | N | NT | + | + | - | - |
| Tylochromis variabilis Stiassny, 1989 | - | + | - | N | - | - | - | - | - | - | - | + | N | - | - | LC | + | + | - | - |
| Cyprinodontiformes | ||||||||||||||||||||
| Nothobranchiidae (1/1) | ||||||||||||||||||||
| Nothobranchius brieni Poll, 1938 | - | - | - | * E | - | - | - | - | - | - | - | - | * E | - | - | LC | - | - | - | - |
| Procatopodidae (2/2) | ||||||||||||||||||||
| Lacustricola lualabaensis (Poll, 1938) | - | - | - | - | - | N * | - | - | - | - | - | - | N * | - | - | LC | - | - | - | - |
| Micropanchax petnehazyi Nagy and Vreven, 2018 | - | - | - | + | - | - | - | - | - | + | - | - | + | - | + | LC | - | + | - | - |
| Tetraodontiformes | ||||||||||||||||||||
| Tetraodontidae (1/2) | ||||||||||||||||||||
| Tetraodon mbu Boulenger, 1899 | - | + | - | - | - | - | - | - | - | - | - | + | - | - | - | LC | + | - | + | - |
| Tetraodon miurus Boulenger, 1902 | - | + | - | - | - | - | - | - | - | - | - | + | - | - | - | LC | - | - | + | - |
| Lepidosireniformes | ||||||||||||||||||||
| Protopteridae (1/2) | ||||||||||||||||||||
| Protopterus aethiopicus Heckel, 1851 | - | - | - | + | - | - | - | - | - | - | - | - | + | - | - | LC | + | - | - | - |
| Protopterus annectens (Owen, 1839) | - | - | - | N | N | - | - | - | - | - | - | - | N | - | - | LC | - | + | - | + |
| Total number of species | 10 | 130 | 9 | 113 | 24 | 48 | 5 | 56 | 40 | 49 | 9 | 144 | 135 | 58 | 74 | 149 | 82 | 125 | 43 | |
| Number of endemic species | 8 | 15 | 6 | 3 | 6 | 5 | 1 | 13 | 4 | 3 | 2 | 29 | 11 | 12 | 5 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katemo Manda, B.; Snoeks, J.; Chocha Manda, A.; Abwe, E.; Mukweze Mulelenu, C.; Ilunga Kayaba, M.K.; Kiwele Mutambala, P.; Ngoy Kalumba, L.; Vreven, E.J.W.M.N. The Upemba National Park (Upper Congo Basin, DR Congo): An Updated Checklist Confirming Its Status as an African Fish Biodiversity Hotspot. Diversity 2023, 15, 966. https://doi.org/10.3390/d15090966
Katemo Manda B, Snoeks J, Chocha Manda A, Abwe E, Mukweze Mulelenu C, Ilunga Kayaba MK, Kiwele Mutambala P, Ngoy Kalumba L, Vreven EJWMN. The Upemba National Park (Upper Congo Basin, DR Congo): An Updated Checklist Confirming Its Status as an African Fish Biodiversity Hotspot. Diversity. 2023; 15(9):966. https://doi.org/10.3390/d15090966
Chicago/Turabian StyleKatemo Manda, Bauchet, Jos Snoeks, Auguste Chocha Manda, Emmanuel Abwe, Christian Mukweze Mulelenu, Micheline Kasongo Ilunga Kayaba, Pacifique Kiwele Mutambala, Lewis Ngoy Kalumba, and Emmanuel J. W. M. N. Vreven. 2023. "The Upemba National Park (Upper Congo Basin, DR Congo): An Updated Checklist Confirming Its Status as an African Fish Biodiversity Hotspot" Diversity 15, no. 9: 966. https://doi.org/10.3390/d15090966
APA StyleKatemo Manda, B., Snoeks, J., Chocha Manda, A., Abwe, E., Mukweze Mulelenu, C., Ilunga Kayaba, M. K., Kiwele Mutambala, P., Ngoy Kalumba, L., & Vreven, E. J. W. M. N. (2023). The Upemba National Park (Upper Congo Basin, DR Congo): An Updated Checklist Confirming Its Status as an African Fish Biodiversity Hotspot. Diversity, 15(9), 966. https://doi.org/10.3390/d15090966







