Do Bats Avoid the Urban Core in the Breeding Season? A Case Study from Temperate Latitudes
Abstract
:1. Introduction
2. Materials and Methods
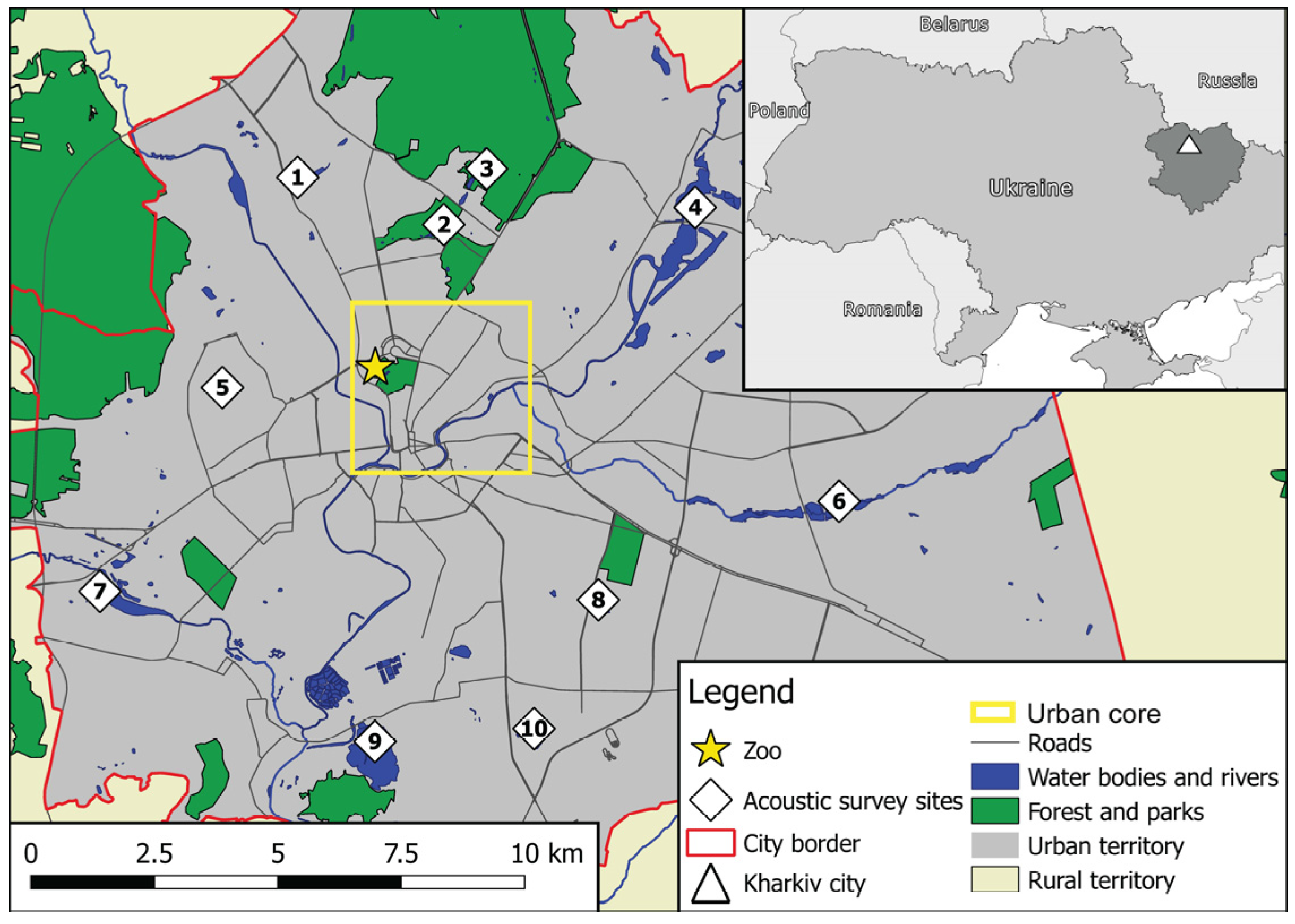
2.1. Study Area and Local Bat Fauna
2.2. Study Design and Periods of the Survey
2.3. Period of Survey and Bat Life Cycle Phenology
2.4. Data Collection
2.4.1. Acoustic Surveys
2.4.2. Mist-Netting
2.4.3. City Bat Records
2.4.4. Bat Measurements
2.5. Data Analysis
2.5.1. Analysis of Acoustic Data
2.5.2. Statistical Analysis
3. Results
3.1. General Findings
3.2. Acoustic Data
3.3. Mist-Netting
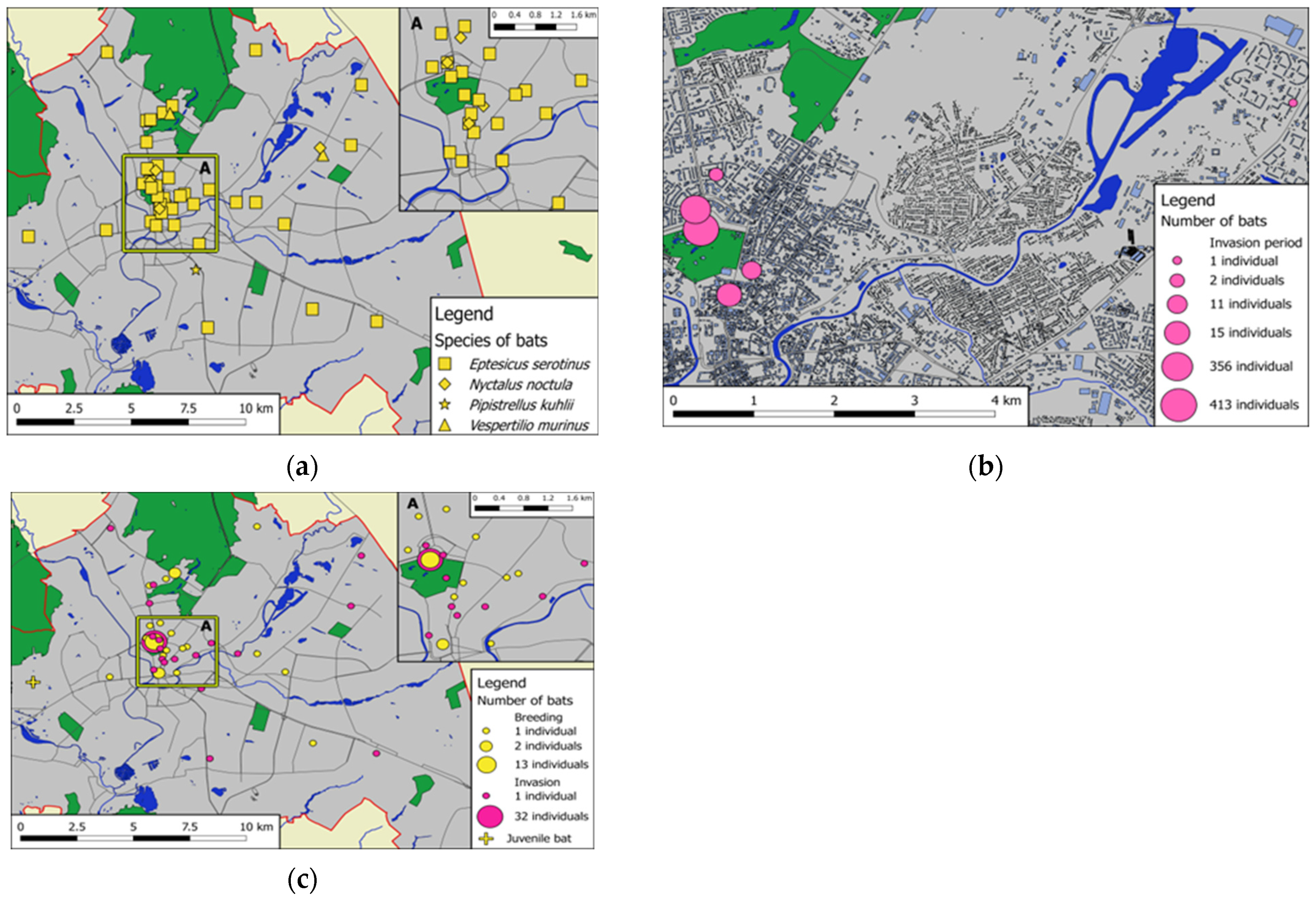
3.4. City Bat Records
3.5. Seasonal Changes in Bat Species Composition and Spatial Distribution of Records
3.6. Sex Ratio and Reproductive Status
4. Discussion
4.1. Methodology
4.2. Particularities of Species-Specific Urban Core Utilisation by Bat Species
4.3. Spatial and Seasonal Difference of Bat Sex/Age Groups of Urban Landscape Utilisation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seto, K.C.; Güneralp, B.; Hutyra, L.R. Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc. Natl. Acad. Sci. USA 2012, 109, 16083–16088. [Google Scholar] [CrossRef]
- United Nations, Department of Economic and Social Affairs, Population Division. World Urbanization Prospects: The 2014 Revision, Highlights (ST/ESA/SER.A/352); United Nations, Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2014. [Google Scholar]
- Grimm, N.B.; Faeth, S.H.; Golubiewski, N.E.; Redman, C.L.; Wu, J.; Bai, X.; Briggs, J.M. Global change and the ecology of cities. Science 2008, 319, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Pickett, S.T.A.; Cadenasso, M.L.; Grove, J.M.; Boone, C.G.; Groffman, P.M.; Irwin, E.; Kaushal, S.S.; Marshall, V.; McGrath, B.P.; Nilon, C.H.; et al. Urban ecological systems: Scientific foundations and a decade of progress. J. Environ. Manag. 2011, 92, 331–362. [Google Scholar] [CrossRef]
- Oke, T.R. The energetic basis of the urban heat island. Q. J. R. Meteorol. Soc. 1982, 108, 1–24. [Google Scholar] [CrossRef]
- Schneider, A.; Friedl, M.A.; Potere, D. A new map of global urban extent from MODIS satellite data. Environ. Res. Lett. 2010, 4, 044003. [Google Scholar] [CrossRef]
- Gaston, K.J.; Ávila-Jiménez, M.L.; Edmondson, J.L. Review: Managing urban ecosystems for goods and services. J. Appl. Ecol. 2013, 50, 830–840. [Google Scholar] [CrossRef]
- McKinney, M.L. Effects of urbanization on species richness: A review of plants and animals. Urban Ecosyst. 2008, 11, 161–176. [Google Scholar] [CrossRef]
- Sumasgutner, P.; Nemeth, E.; Tebb, G.; Krenn, H.W.; Gamauf, A. Hard times in the city—Attractive nest sites but insufficient food supply lead to low reproduction rates in a bird of prey. Front. Zool. 2014, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Russo, D.; Ancillotto, L. Sensitivity of bats to urbanization: A review. Mammal Biol. 2015, 80, 205–212. [Google Scholar] [CrossRef]
- Clergeau, P.; Savard, J.P.L.; Mennechez, G.; Falardeau, G. Bird abundance and diversity along an urban-rural gradient: A comparative study between two cities on different continents. Condor 1998, 100, 413–425. [Google Scholar]
- Evans, K.L.; Newson, S.E.; Gaston, K.J. Habitat influences on urban avian assemblages. Ibis 2009, 151, 19–39. [Google Scholar] [CrossRef]
- McDonnell, M.J.; Hahs, A.K. Adaptation and adaptedness of organisms to urban environments. Annu. Rev. Ecol. Evol. Syst. 2015, 46, 261–280. [Google Scholar] [CrossRef]
- Ancillotto, L.; Tomassini, A.; Russo, D. The fancy city life: Kuhl’s pipistrelle, Pipistrellus kuhlii, benefits from urbanisation. Wildl. Res. 2015, 42, 598–606. [Google Scholar] [CrossRef]
- Maxinova, E.; Kipson, M.; Naďo, L.; Hradická, P.; Uhrin, M. Foraging Strategy of Kuhl’s Pipistrelle at the Northern Edge of the Species Distribution. Act. Chiropt. 2016, 18, 215–222. [Google Scholar] [CrossRef]
- Jung, K.; Threlfall, C.G. Urbanisation and its effects on bats—A global meta-analysis. In Bats in the Anthropocene: Conservation of Bats in a Changing World; Voigt, C.C., Kingston, T., Eds.; Springer: Cham, Switzerland, 2016; pp. 13–33. [Google Scholar]
- Jones, G.; Jacobs, D.S.; Kunz, T.H.; Willig, M.R.; Racey, P.A. Carpe noctem: The importance of bats as bioindicators. Endanger. Species Res. 2009, 8, 93–115. [Google Scholar] [CrossRef]
- Russo, D.; Cistrone, L.; Jones, G. Sensory ecology of water detection by bats: A field experiment. PLoS ONE 2021, 6, e25555. [Google Scholar] [CrossRef]
- McIntyre, N.E. Ecology of urban arthropods: A review and a call to action. Ann. Entomol. Soc. Am. 2000, 93, 825–835. [Google Scholar]
- Piano, E.; De Wolf, K.; Bona, F.; Bonte, D.; Bowler, D.E.; Isaia, M.; Lens, L.; Merckx, T.; Mertens, D.; Van Kerckvoorde, M.; et al. Urbanization drives cross-taxon declines in abundance and diversity at multiple spatial scales. Glob. Chang. Biol. 2020, 26, 1196–1211. [Google Scholar] [CrossRef]
- Encarnação, J.A.; Kierdorf, U.; Holweg, D.; Jasnoch, U.; Wolters, V. Sex-related differences in roost-site selection by Daubenton’s bats Myotis daubentonii during the nursery period. Mammal Rev. 2005, 35, 285–294. [Google Scholar] [CrossRef]
- Moiseienko, M.; Vlaschenko, A. Quantitative evaluation of individual food intake by insectivorous vespertilionid bats (Chiroptera, Vespertilionidae). Biol. Open 2021, 10, bio058511. [Google Scholar] [CrossRef]
- Kunz, T.H.; Whitaker, J.O.; Wadanoli, M.D. Dietary energetics of the insectivorous Mexican free-tailed bat (Tadarida brasiliensis) during pregnancy and lactation. Oecologia 1995, 101, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Kravchenko, K.; Vlaschenko, A.; Prylutska, A.; Rodenko, O.; Hukov, V.; Shuvaev, V. Year-round monitoring of bat records in an urban area: Kharkiv (NE Ukraine), 2013, as a case study. Turk. J. Zool. 2017, 41, 530–548. [Google Scholar] [CrossRef]
- Vlaschenko, A.; Yatsiuk, Y.; Hukov, V.; Prylutska, A.; Straka, T.; Kravchenko, K. Urban forest preserves local bat species diversity, but not forest-dweller specialists—Renewed study 65 years later (Kharkiv city, Ukraine). Mammal Res. 2021, 66, 615–626. [Google Scholar] [CrossRef]
- Vlaschenko, A.; Shulenko, A.; But, A.; Yerofieiva, M.; Bohodist, V.; Petelka, M.; Vovk, A.; Zemliana, K.; Muzyka, D.; Kravchenko, K.; et al. The war-damaged urban environment becomes deadly trap for bats: Case from Kharkiv city (NE Ukraine) in 2022. J. Appl. Anim. Eth. Res. 2023, 5, 27–49. [Google Scholar] [CrossRef]
- Vlaschenko, A.S. Research history and list of records of bats (Chiroptera) in the Kharkov Region in the XIX and XX centuries. Plecotus 2011, 14, 26–54, (In Russian with English Summary). [Google Scholar]
- Prylutska, A.; Moiseienko, M.; Yerofieieva, M.; Hukov, V.; Vlaschenko, A. Northern record for Tadarida teniotis (NE Ukraine) far from known species range. J. Bat Res. Conserv. 2020, 13, 104–108. [Google Scholar] [CrossRef]
- Salvarina, I. Bats and aquatic habitats: A review of habitat use and anthropogenic impacts. Mammal Rev. 2016, 46, 131–143. [Google Scholar] [CrossRef]
- Vlaschenko, A.; Kovalov, V.; Hukov, V.; Kravchenko, K.; Rodenko, O. An example of ecological traps for bats in the urban environment. Eur. J. Wildl. Res. 2019, 65, 20. [Google Scholar] [CrossRef]
- Prylutska, A.; Yerofeieva, M.; Bohodist, V.; Shulenko, A.; But, A.; Kravchenko, K.; Prylutskyi, O.; Vlaschenko, A. The dataset of bat (Mammalia, Chiroptera) occurrences in Ukraine collected by the Ukrainian Bat Rehabilitation Center (2011–2022). Biodivers. Data J. 2023, 11, e99243. [Google Scholar] [CrossRef]
- Gukasova, A.; Vlaschenko, A. Effectiveness of mist-netting of bats (Chiroptera, Mammalia) during the non-hibernation period in oak forests of Eastern Ukraine. Acta Zool. Cracov. 2011, 54, 77–93. [Google Scholar] [CrossRef]
- Sikes, R.S. The Animal Care and Use Committee of the American Society of Mammalogists. Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. J. Mammal. 2016, 97, 663–688. [Google Scholar] [CrossRef]
- Prylutska, A.; Yerofeieva, M.; Bohodist, V.; Shulenko, A.; But, A.; Kravchenko, K.; Prylutskyi, O.; Vlaschenko, A. The Dataset of Bat (Chiroptera, Mammalia) Occurrences in Ukraine Collected by the Ukrainian Bat Rehabilitation Center (2011–2022), Version 1.4; Ukrainian Bat Rehabilitation Center: Kharkiv, Ukraine, 2022; Available online: https://GBIF.org (accessed on 17 May 2023). [CrossRef]
- Vlaschenko, A.; Prylutska, A.; Kravchenko, K.; Rodenko, O.; Hukov, V.; Timofieieva, O.; Holovchenko, O.; Moiseienko, M.; Kovalov, V. Regional recapture of bats (Chiroptera, Vespertilionidae) ringed in Eastern Ukraine. Zoodiversity 2020, 54, 53–66. [Google Scholar] [CrossRef]
- Zsebok, S.; Estók, P.; Görföl, T. Acoustic discrimination of Pipistrellus kuhlii and Pipistrellus nathusii and its application to assess changes in species distribution. Acta Zool. Acad. Sci. Hung. 2012, 58, 199–209. [Google Scholar]
- Vlaschenko, A.; Kravchenko, K.; Yatsiuk, Y.; Hukov, V.; Kramer-Schadt, S.; Radchuk, V. Bat Assemblages Are Shaped by Land Cover Types and Forest Age: A Case Study from Eastern Ukraine. Forests 2022, 13, 1732. [Google Scholar] [CrossRef]
- Naďo, L.; Lőbbová, D.; Hapl, E.; Ceľuch, M.; Uhrin, M.; Šara, M.; Kaňuch, P. Highly Selective Roosting of the Giant Noctule Bat and Its Astonishing Foraging Activity by GPS Tracking in a Mountain Environment. Mammal Res. 2019, 64, 587–594. [Google Scholar] [CrossRef]
- Bullen, R.D.; McKenzie, N.L.; Cruz-Neto, A.P. Characteristic flight speeds in bats. CEAS Aeronaut. J. 2016, 7, 621–643. [Google Scholar] [CrossRef]
- Roeleke, M.; Blohm, T.; Kramer-Schadt, S.; Yovel, Y.; Voigt, C.C. Habitat use of bats in relation to wind turbines revealed by GPS tracking. Sci. Rep. 2016, 6, 28961. [Google Scholar] [CrossRef]
- Roeleke, M.; Blohm, T.; Kramer-Schadt, S.; Yovel, Y.; Voigt, C.C.; Scholl, J.M.; Bauer, J.; Teige, T.; Gras, P. Movement responses of common noctule bats to the illuminated urban landscape. Landsc. Ecol. 2020, 35, 189–201. [Google Scholar] [CrossRef]
- Jung, K.; Threlfall, C.G. Trait-dependent tolerance of bats to urbanization: A global meta-analysis. Proc. R. Soc. B 2018, 285, 20181222. [Google Scholar] [CrossRef]
- Wolf, J.M.; Jeschke, J.M.; Voigt, C.C.; Itescu, Y. Urban affinity and its associated traits: A global analysis of bats. Glob. Chang. Biol. 2022, 28, 5667–5682. [Google Scholar] [CrossRef]
- Strelkov, P.P. Sex ratio in breeding season in adult individuals of long-distant migrant bats (Chiroptera, Vespertilionidae) of Eastern Europe and adjacent territories. Zool. Zhurnal 1999, 78, 1441–1454, (In Russian with English Summary). [Google Scholar]
- Ibáňez, C.; Guillén, A.; Agirre-Mandi, P.N.; Juste, J.; Schreur, G.; Cordero, A.I.; Popa-Lisseanu, A.G. Sexual segregation in Iberian Noctule Bats. J. Mammal. 2009, 90, 235–243. [Google Scholar] [CrossRef]
- Estók, P. Seasonal changes in the sex ratio of Nyctalus species in North-East Hungary. Acta Zool. Acad. Sci. Hung. 2007, 53, 89–95. [Google Scholar]
- Russo, D. Elevation affects the distribution of the two sexes in Daubenton’s bats Myotis daubentonii (Chiroptera, Vespertilionidae) from Italy. Mammalia 2002, 66, 543–551. [Google Scholar] [CrossRef]
- Nardone, V.; Cistrone, L.; Di Salvo, I.; Ariano, A.; Migliozzi, A.; Allegrini, C.; Ancillotto, L.; Fulco, A.; Russo, D. How to Be a Male at Different Elevations: Ecology of Intra-Sexual Segregation in the Trawling Bat Myotis daubentonii. PLoS ONE 2015, 10, e0134573. [Google Scholar] [CrossRef] [PubMed]
- McGuire, L.P.; Boyle, W.A. Altitudinal migration in bats: Evidence, patterns, and drivers. Biol. Rev. 2013, 88, 767–786. [Google Scholar] [CrossRef]
- Grindal, S.D.; Morissette, J.L.; Brigham, R.M. Concentration of bat activity in riparian habitats over an elevational gradient. Can. J. Zool. 1999, 77, 972–977. [Google Scholar] [CrossRef]
- Snit’ko, V.P. Seasonal Spatial Differentiation of Sex Groups in Populations of Resident Bat Species (Chiroptera, Vespertilionidae) in the Southern Urals. Russ. J. Ecol. 2007, 38, 334–340. [Google Scholar] [CrossRef]
- Senior, P.; Butlin, R.K.; Altringham, J.D. Sex and segregation in temperate bats. Proc. R. Soc. Lond. B Biol. Sci. 2005, 272, 2467–2473. [Google Scholar] [CrossRef]
- Neubaum, D.J.; O’Shea, T.J.; Wilson, K.R. Autumn Migration and Selection of Rock Crevices as Hibernacula by Big Brown Bats in Colorado. J. Mammal. 2006, 87, 470–479. [Google Scholar] [CrossRef]
- Patriquin, K.J.; Guy, C.; Hinds, J.; Ratcliffe, J.M. Male and female bats differ in their use of a large urban park. J. Urban Ecol. 2019, 5, juz015. [Google Scholar] [CrossRef]
- Coleman, J.L.; Barclay, R.M.R. Influence of urbanization on demography of Little Brown Bats (Myotis lucifugus) in the Prairies of North America. PLoS ONE 2011, 6, e20483. [Google Scholar] [CrossRef] [PubMed]
- Lintott, P.R.; Bunnefeld, N.; Fuentes-Montemayor, E.; Minderman, J.; Mayhew, R.J.; Olley, L.; Park, K.J. City life makes females fussy: Sex differences in habitat use of temperate bats in urban areas. R. Soc. Open Sci. 2014, 1, 140200. [Google Scholar] [CrossRef] [PubMed]
| Method | The Period of Bat Life Cycle | |
|---|---|---|
| Breeding | Autumn Migration | |
| Acoustic survey—zoo | 05.06.2015–23.07.2015 | 12.08.2015–10.09.2015 |
| Acoustic survey—water bodies | 04.06.2015–12.07.2015 | 16–25.08.2016 |
| Mist-netting—zoo | 19.05.2014–23.07.2014 07.05.2015–25.07.2015 | 10.08.2014–08.09.2014 13.08.2015–13.09.2015 |
| Bat findings | 01.05.2014–31.07.2014 01.05.2015–31.07.2015 | 01.08.2014–15.09.2014 01.08.2014–15.09.2015 |
| Sites | WB-1 | WB-2 | WB-3 | WB-4 | WB-5 | WB-6 | WB-7 | WB-8 | WB-9 | WB-10 | Zoo | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Breeding | E. serotinus | 16.8 | 62.1 | 31.7 | 50.3 | 50.0 | 73.1 | 27.7 | 0 | 27.6 | 55.3 | 39.5 |
| N. noctula | 77.6 | 32.4 | 59.0 | 46.9 | 0 | 26.9 | 72.3 | 0 | 56.3 | 31.6 | 0.5 | |
| P. pygmaeus | 0 | 1.4 | 0.4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| P. kuhlii/nathusii | 5.6 | 4.1 | 6.5 | 5.6 | 50.0 | 0 | 0 | 100 | 16.1 | 13.2 | 60.0 | |
| Number of sequences | 107 | 145 | 278 | 324 | 4 | 26 | 47 | 31 | 87 | 38 | 603 | |
| Species number | 3 | 4 | 4 | 3 | 2 | 2 | 2 | 1 | 3 | 3 | 3 | |
| Migration | E. serotinus | 7.4 | 18.4 | 32.7 | 46.9 | 19.2 | 33.8 | 41.6 | 17.2 | 58.5 | 16.1 | 12.1 |
| N. noctula | 72.3 | 49.1 | 41.8 | 38.3 | 76.0 | 58.4 | 46.8 | 77.4 | 41.5 | 70.1 | 45.2 | |
| P. pygmaeus | 0 | 0 | 2.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| P. kuhlii/nathusii | 20.2 | 32.5 | 23.5 | 14.8 | 4.8 | 7.8 | 11.6 | 5.4 | 0 | 13.8 | 42.7 | |
| Number of sequences | 94 | 114 | 98 | 162 | 146 | 77 | 173 | 93 | 41 | 87 | 681 | |
| Species number | 3 | 3 | 4 | 3 | 3 | 3 | 3 | 3 | 2 | 3 | 3 | |
| Total | Number of sequences | 201 | 259 | 376 | 486 | 150 | 103 | 220 | 124 | 128 | 125 | 1284 |
| Species number | 3 | 4 | 4 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |
| Chi-square | 12.3 | 65.3 | 25.3 | 12.8 | 18.2 | 12.7 | 11.9 | 101.4 | 14.9 | 21.2 | 379.6 | |
| Df | 2 | 3 | 3 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| p-value | <0.01 | <0.001 | <0.001 | <0.01 | <0.001 | <0.01 | <0.01 | <0.001 | <0.001 | <0.001 | <0.001 |
| Period | N. noctula | E. serotinus | P. kuhlii | P. nathusii | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Females | Males | N | Females | Males | N | Females | Males | N | Females | Males | ||||||||
| ad | sad | ad | sad | ad | sad | ad | sad | ad | sad | ad | sad | ad | sad | sad | |||||
| Breeding | 1 | - | - | 1 | - | 30 | 2 1 | - | 28 | - | 36 | 8 2 | - | 28 | - | 1 | 1 3 | - | |
| Migration | 255 | 34 | 155 | 7 | 59 | 7 | 1 | 2 | 3 | 1 | 29 | 1 | 6 | 10 | 12 | 2 | - | 1 | 1 |
| Total | 256 | 37 | 65 | 3 | |||||||||||||||
| Period | N. noctula | E. serotinus | V. murinus | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Females | Males | N | Females | Males | N | Males | |||||||||
| ad | sad | un | sad | un | ad | sad/juv | un | ad | sad/juv | un | sad | un | ||||
| Breeding | 0 | - | - | - | - | - | 35 | 2 ** | 1 | - | 28 | 3 | 1 | - | - | - |
| Migration | 784 | 8 | 282 | 44 | 317 | 39 | 49 | 2 | 8 | 2 | 5 | 26 | 2 | 2 | 1 | 1 |
| total | 784 | 84 | 2 | |||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlaschenko, A.; Rodenko, O.; Hukov, V.; Kovalov, V.; Prylutska, A.; Kravchenko, K. Do Bats Avoid the Urban Core in the Breeding Season? A Case Study from Temperate Latitudes. Diversity 2023, 15, 967. https://doi.org/10.3390/d15090967
Vlaschenko A, Rodenko O, Hukov V, Kovalov V, Prylutska A, Kravchenko K. Do Bats Avoid the Urban Core in the Breeding Season? A Case Study from Temperate Latitudes. Diversity. 2023; 15(9):967. https://doi.org/10.3390/d15090967
Chicago/Turabian StyleVlaschenko, Anton, Olena Rodenko, Vitalii Hukov, Viktor Kovalov, Alona Prylutska, and Kseniia Kravchenko. 2023. "Do Bats Avoid the Urban Core in the Breeding Season? A Case Study from Temperate Latitudes" Diversity 15, no. 9: 967. https://doi.org/10.3390/d15090967
APA StyleVlaschenko, A., Rodenko, O., Hukov, V., Kovalov, V., Prylutska, A., & Kravchenko, K. (2023). Do Bats Avoid the Urban Core in the Breeding Season? A Case Study from Temperate Latitudes. Diversity, 15(9), 967. https://doi.org/10.3390/d15090967








