Many midwater predators face vertically with their eyes oriented upward to hunt their prey from below [
1,
2,
3]. These predators look for the shadow of their prey from the faint downwelling light from the surface [
4,
5]. In response to this hunting behavior, midwater fish have adapted ventral photophores to hide their shadows, called counterillumination, as well as silver scales to obscure the shape of their bodies [
4,
5]. Orienting their body vertically in the water column not only diminishes their shadow from predators below, but it also allows them to look for shadows of their prey above [
1].
The order Myctophiformes contains 245 midwater species, with 99% possessing ventral photophores [
6]. The order Myctophiformes contains lanternfishes in the family Myctophidae and the blackchins in the family Neoscopelidae. Since the 1940s, there have been increasing interests in developing Myctophiformes fisheries for consumption, use as fishmeal, use as Omega-3 dietary supplements, and in bioprospecting for pharmaceuticals [
7,
8,
9]. Myctophiformes are also an important link in the food web between primary consumers, such as zooplankton and tertiary consumers, including tuna, sharks, whales, and dolphins [
7,
10]. Within the family Neoscopelidae, ventral photophores can be absent (in the derived genera
Scopelengys and
Solivomer) or present (in the stem blackchin genus
Neoscopelus) [
11]. Unlike lanternfish,
Neoscopelus contains a row of photophores on the tongue, and their purpose is unknown, in addition to ventral rows on the body [
12]. Blackchins are medium-sized fish that are black to silver in color, and their diet has not been studied in detail [
11]. The mouth contains multiple rows of small, filiform teeth, and the gill rakers are well developed, with the stomach of one fish caught off southern California containing copepods, suggesting they prey on pelagic crustaceans or small fishes similar to the lanternfishes [
11,
12,
13].
Visual surveys of underwater environments are useful for gaining insight into the behavior of midwater fishes [
14,
15], although no records have described blackchins in detail. In this report, we describe the in situ behavior of three species within the family Neoscopelidae, including
Neoscopelus macrolepidotus,
Neoscopelus microchir, and
Scopelengys tristis, based on observations using Remotely Operated Vehicles (ROVs) and Autonomous Underwater Vehicles (AUVs).
Blackchin observations were collected from four sources: the JAMSTEC E-library of Deep-Sea Images (J-EDI) [
16], the NOAA Ocean Exploration Video Portal [
17,
18,
19], cruise KM20-10C leg 2 to Ritto Seamount, and cruises DG5b and DG5e in the NORI-D area of the eastern Clarion-Clipperton Zone (CCZ). Blackchin behavior was divided into six categories: resting head upward, resting head downward, resting horizontally, swimming upward, swimming downward, and swimming horizontally. If the fish changed its behavior during the observation, only the initial behavior was recorded.
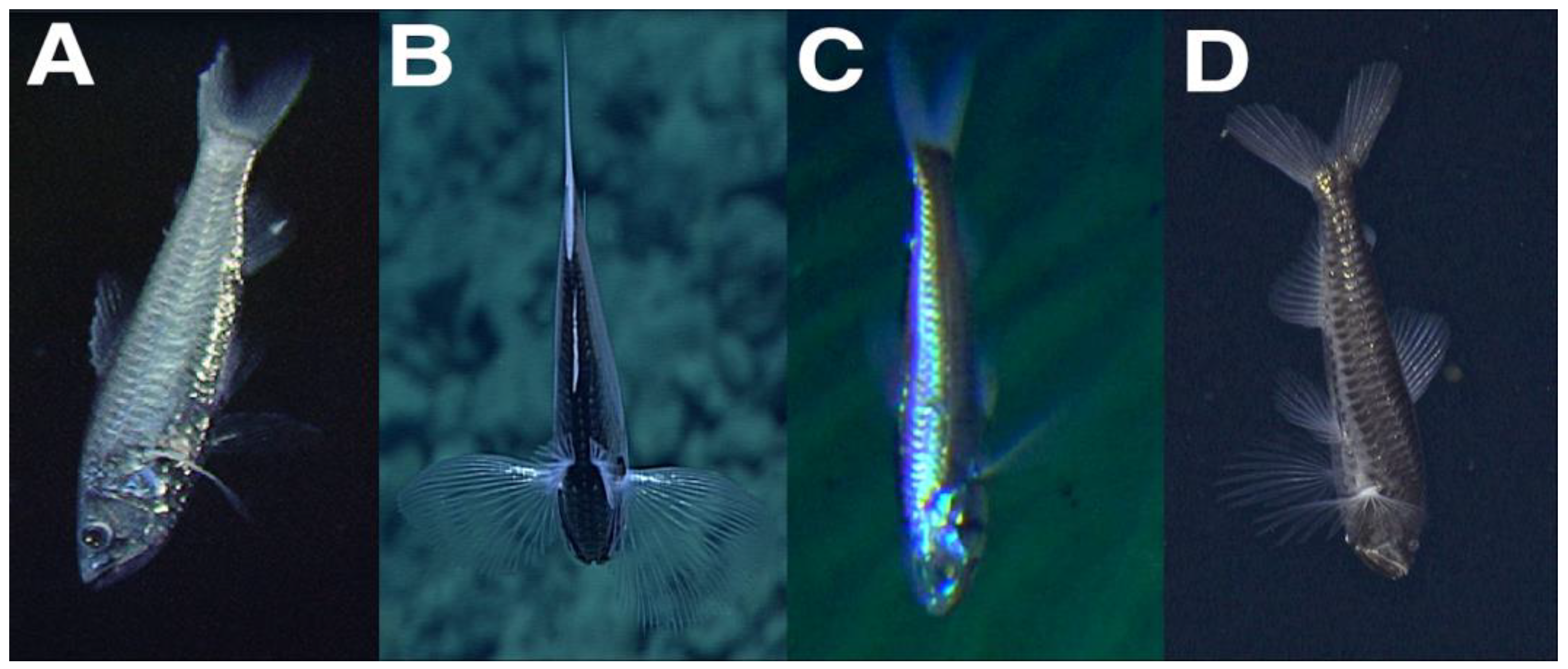
One video of
Neoscopelus macrolepidotus observed during the R/V ‘Natsushima’ cruise NT07-11 was selected from the J-EDI database. The ROV Hyper-Dolphin aboard the R/V ‘Natsushima’ cruise NT07-11 surveyed the hydrothermal vents at Minami-Ensei Knoll within the Okinawa Trough on 24 June 2007. The ROV Hyper-Dolphin was equipped with a high-definition Super HARP TV camera. During Dive HPD0703, one
N. macrolepidotus was observed resting head downward from 01:28:38 to 01:34:35 UTC (from 10:28:38 Japan Standard Time) between 698–700 m depth (Temperature: 7.5 °C; Salinity: 34.32; Dissolved Oxygen: 1.90 mL/L), five meters above the seafloor. The fish occasionally beat the extended fins to maintain its position and remained less than 20° removed from the vertical (in situ image in
Figure 1). The mouth was positioned slightly open during the observation, yet the gill operculum did not move. At the end of the observation, the fish swam downward and out of view with four consecutive tail beats.
Four observations of N. macrolepidotus from cruises EX1603, EX1605L3, and EX1606 were selected from the NOAA Ocean Exploration Video Portal. The Deep Discover ROV aboard the Okeanos Explorer was equipped with a high-definition Insite Titan Tilt and Rotate Color Zoom Camera (Insite Pacific Inc., San Diego, CA, USA). The observations of N. macrolepidotus during cruises EX1603 and EX1605L3 were resting head downward, while the two observations of N. macrolepidotus during cruise EX1603 DIVE06 were resting horizontally, directly above the seafloor.
During cruise EX1605L3 DIVE07 to Supply Reef on 23 June 2016, one N. macrolepidotus was observed resting head downward from 21:49:40–21:50:14 UTC (from 07:49:40 Chamorro Standard Time) between 941–957 m depth (Temperature: 4.3 °C; Salinity: 34.44; Dissolved Oxygen: 2.00 mL/L), approximately one meter above the seafloor. The fish remained completely motionless during the observation, with all fins fully extended, until it swam upward and out of view with six tail beats. Throughout the observation, the angle of the body changed from less than 20° removed from the vertical to 45° before abruptly changing its orientation and swimming upward. During cruise EX1606 DIVE08 to Wake Island on 10 August 2016, one N. macrolepidotus was observed from 3:03:23–3:07:27 UTC (from 15:03:23 Wake Time) between 775–779 m depth (Temperature: 4.8 °C; Salinity: 34.43; Dissolved Oxygen: 1.77 mL/L), one meter above the seafloor. The first changed its orientation during the observation, resting head downward less than 20° removed from the vertical to resting horizontally less than 10° removed from the horizontal. The fish occasionally beat its extended pectoral and caudal fin to maintain its position and navigate around the benthic terrain.
Twenty-eight observations of Neoscopelus microchir were recorded during the R/V ‘Kaimei’ cruise KM20-10C leg 2 with the KM-ROV and the AUV YOUZAN. The KM-ROV was equipped with a pan-tiltable high-definition Mini Zeus video camera, and the AUV YOUZAN was equipped with a 4K UMC-S3CA network camera with a FE 28 mm F2 lens (Sony, Tokyo, Japan). The KM-ROV was equipped with a Seabird SBE 49 Fast CAT CTD system, measuring depth, temperature, and salinity, while the AUV YOUZAN was equipped with a temperature and salinity sensor A7CT-USB (JFE Advantec, Takahatachou, Japan), a turbidity sensor ATUD-USB (JFE Advantec), and a pH sensor SPS14 (Kimoto Electronic, Osaka, Japan). During KM-ROV dive KM0129 to Ritto Seamount on 5 December 2020, two N. microchir were observed, resting head downward, and one was observed, swimming downward from 00:36:33–00:38:57 UTC (from 09:36:33 JST) between 696–698 m depth (Temperature: 5.7 °C; Salinity: 34.25), 1.5–1.8 m above the seafloor. During AUV YOUZAN Dive#2 to Ritto Seamount on 8 Dec 2020, twenty-five N. microchir were observed from 00:24:30–04:15:01 UTC (from 09:24:30 JST) between 699–808 m depth (Temperature 4.9–5.9 °C; Salinity: 34.57–34.73), 2.8–4.3 m above the seafloor. Twenty were observed resting head down, while five were resting horizontally directly above the seafloor.
Most observations were fleeting: two longer observations were selected for an in-depth behavioral analysis. During Dive KM0129, one
N. microchir was observed swimming downward between 00:37:21–00:37:26 UTC (from 09:37:21 JST) at 697 m depth (Temperature: 5.7 °C; Salinity: 34.25). The fish swam downward in three swimming bursts, with each burst using three caudal fin beats. Between swimming bursts was a short rest period where the fish rested head downward. During Dive#2, one
N. microchir was observed resting head downward between 2:04:01–2:04:06 UTC (from 11:04:01 JST) at 774 m depth (Temperature 5.0 °C; Salinity: 34.70), 3.0 m above the seafloor. The fish occasionally beat its extended fins to maintain its position and remained less than 10° removed from the vertical (
Supplementary Material Video S1). The mouth position of both fish could not be determined.
Fifty-one observations of Scopelengys tristis were recorded during cruises DG5b and DG5e. The ROV Odysseus aboard the Maersk Launcher was equipped with a pan-tilt-unit-mounted Mini Zeus 4K (Insite Pacific Inc.) with a 1/2.5 inch Exmor R CMOS sensor and a 20× Optical Zoom lens. Cruise DG5b was conducted between 28 February through 22 April 2021, while Cruise DG5e was conducted between 11 November though 19 December 2021. Scopelengys tristis were observed from 647–1122 m depth (Temperature 4.1–7.3 °C, Salinity 34.54–34.58, Dissolved Oxygen 0.05–1.04 mL/L), thousands of meters above the seafloor, which was at approximately 4200 m depth. All resting S. tristis were resting head downward. Scopelengys tristis preferred to be head downward when resting (χ2, p = 7.579 × 10−5) and swim downward when swimming (χ2, p = 8.423 × 10−6). Time of day had an effect on their overall activity level (one-way ANOVA, p = 0.0376), with more S. tristis swimming during the daytime. Time of day did not affect the direction of swimming. Depth, temperature, salinity, and dissolved oxygen had no effect on behavior.
Most observations of
S. tristis were fleeting; two longer observations were selected for an in-depth behavioral analysis to compare with
N. macrolepidotus and
N. microchir. During Dive OY034 on 5 December 2021, one
S. tristis was observed between 22:51:16–22:51:51 UTC (from 14:51:16 Pacific Standard time) at 724 m depth (Temperature: 5.8 °C; Salinity: 34.55; Dissolved Oxygen: 0.21 mL/L). The fish remained completely motionless throughout the observation, with all fins fully extended and the mouth closed, until it swam downward and out of view with one tail beat. During Dive OY039 on 15 December 2021, a second
S. tristis was observed between 19:11:31–19:12:51 UTC (from 11:11:31 PST) from 712–714 m depth (Temperature: 6.0 °C; Salinity: 34.55; Dissolved Oxygen: 0.09 mL/L). Similarly, the fish was oriented with its head down, mouth closed, and all fins extended, remaining completely motionless with its mouth closed (
Supplementary Material Video S2). The altitude of both remained less than 10° removed from the vertical.
During the OY034 and OY039 observations, bristlemouths (genus
Cyclothone) were observed near
S. tristis. A paralepid barracudina (
Magnisudis atlantica) was also observed near the OY039
S. tristis. The only record of predation on blackchins is from the Mediterranean Sea, where one
N. macrolepidotus was found in the stomach of the swallower
Chiasmodon niger [
20]. Considering that
M. atlantica eats krill, squid, and lanternfish [
21,
22], it is possible that it is a predator on
S. tristis, but this requires further study on the diet of
M. atlantica in the eastern CCZ.
Neoscopelus macrolepidotus and
S. tristis had similar swimming behavior, even though
Neoscopelus has ventral bioluminescent organs and a swim bladder, while
Scopelengys has neither. Both fish employed rapid escapes with a c-start [
23] before beating the caudal fin, the propulsive wave originating near the anal fin origin, and moving out of view of the ROV within a second. One caudal fin beat took only two frames to complete.
Neoscopelus microchir also had similar swimming behavior, but the short rest periods between swimming bursts were unique to this species. Both
N. macrolepidotus and
N. microchir used periodic pectoral fin beats to maintain position as they faced downward, whereas no
S. tristis moved their fins unless it was for escape.
S. tristis were observed in lower dissolved oxygen than
N. macrolepidotus, which may be why this species only moved to escape, although dissolved oxygen concentration did not affect their overall activity level. Contrastingly,
N. macrolepidotus is reported to have a well ossified skeleton and firm musculature, whereas
S. tristis has flabby muscles and a weakly ossified skeleton [
24], suggesting the two species have different swimming mechanics. This difference in anatomy may instead be explained in the two species having a different diet or foraging behavior, both of which have not been thoroughly studied.
Downward-facing behavior in deep-sea fishes is uncommon, yet it is reported in several species [
1,
14]. In the lanternfish
Stenobrachius leucopsarus and
Triphoturus mexicanus, head-up orientation is hypothesized to diminish their shadow from below [
1]. Despite lanternfish possessing ventral photophores to use in counterillumination, some predators with highly specialized vision can discern ambient light from bioluminescence, which turns their camouflage into a target [
4,
25]. By orienting their bodies vertically, rather than horizontally, their shadow becomes harder to spot for upward-facing predators. Considering that
S. tristis remained stationary during the observations, with only minimal fin movement in
N. macrolepidotus and
N. microchir, they are likely adopting a vertical position to avoid being seen by predators. The silvered scales on
N. macrolepidotus,
N. microchir, and
S. tristis further obscure the shape of the body when viewed from the side. Despite the added protection by orienting vertically, this still does not explain why blackchins rest head downward, rather than head upward.
The distance above the seafloor may affect the resting behavior of N. macrolepidotus and N. microchir. All blackchins resting horizontally were directly above the seafloor, although the exact distance is difficult to determine because only the ROV or AUV distance above the seafloor can be recorded. Moreover, some N. microchir observed in Dive#2 were also resting head downward directly above the seafloor, rather than at the same distance above the seafloor as the survey vehicle. The change from resting head down to resting horizontal in the EX1606 DIVE08 N. macrolepidotus observation may illustrate the effect distance from the seafloor has on behavior. However, this requires further examination of N. macrolepidotus and N. microchir in the midwater, far from the seafloor.
Time of day affecting the activity of midwater S. tristis likely has to do with changing prey abundance throughout the day. Scopelengys tristis do not undergo diel migration in the eastern CCZ, which is further underscored by the fact that time of day had no effect on swimming direction. If S. tristis has a diet similar to the lanternfishes, then their potential prey consists of vertically-migrating species which inhabit deeper areas during the day. By facing head downward, prey looking upward may not recognize the diminished shadow as a potential predator hunting from above. Moreover, their potential prey release bioluminescent signals, which are easier to see against a darker background when the fish is facing downward. Although all N. macrolepidotus and N. microchir within this study were observed near the seafloor and most had their mouth closed, the diminished shadow and the photophores on their tongue may be used in conjunction to lure bathypelagic prey looking upward.
Members of the family Neoscopelidae are a poorly studied group within the order Myctophiformes that are observed in many different areas around the Pacific Ocean. This study not only describes the in situ behavior of blackchins, but it also adds records of blackchins within the CCZ. The NORI-D area within the CCZ is a mining license area where deep-sea mining may commence as early as the end of 2024 [
26]. This study provides a baseline assessment of behavior and distribution of
S. tristis in NORI-D, which is important when assessing the potential effects of deep-sea mining on fishes within this region in the future.