Abstract
This study aimed to assess the variations in the nutritional attributes of thirteen Moringa oleifera cultivars. Leaves from six-month-old plants were harvested and tested for various nutritional attributes. There were significant (p ≤ 0.05) differences in the carbohydrates, energy, some of the sugars, and fibre amongst the cultivars. The levels of moisture in the cultivars ranged from 7.10% to 8.20%. Additionally, there were significant (p ≤ 0.05) differences across the cultivars in microelements studied except for zinc (Zn). These data revealed that plants from different geographical provenances differed in their adapting to varied environments. In general, under the same cultivation, management, and environmental conditions, the main reasons for these differences occurring in cultivars could be associated with the genetic background of each M. oleifera germplasm. However, the study cautions on the differences in nutritional properties, as some of the cultivars have been reported not to be pharmacologically potent.
1. Introduction
Moringa oleifera Lam. is listed in the top 10% out of 500,000 plant species gaining popularity due to its broad spectrum of both nutritional and phytochemical profiles [1,2]. Moringa oleifera is the most utilised and cultivated species of the Moringaceae family (order Brassicales) because of its ability to grow under a wide range of conditions and its medico-nutritional properties [3]. Due to its widespread applications, many countries in Africa, South America, and Asia have intensified cultivation programs [4]. In Africa, for example, hope has been placed on the use of M. oleifera in alleviating malnutrition and unemployment, and bringing about changes to improve the lives of many individuals in the region [5].
It is arguably believed that M. oleifera was first brought to South Africa through the Kwazulu-Natal ports during the 1800s while the province was undergoing the development of sugar cane plantations in the subtropical coastal lowlands [6]. In the year 1860, Indian indentured laborers were imported to work in the plantations, and thereafter, traders and gardeners followed [6]. It is believed that at that time, the sugarcane settlements in which they were living in poor and lacked sanitary facilities, clean water, and medical care. This was exuberated by lower wages, which made access to nutritious food difficult [6]. However, because of the rich indigenous knowledge of Ayurveda, which is a Sanskrit word for science of life or practices of longevity, they were intuitive enough to rely on the various herbs and seeds that they brought with them from India, which they readily planted and harvested [6]. The use of these medicinal and nutritious plants, including M. oleifera, made the Indian settlers thrive despite their severe poverty. The use of such plants and their medical anecdotes have been passed down to generations including the rest of the South African tribes and communities.
In the present day, in South Africa, M. oleifeara is cultivated and processed into different commercial products, including powder, capsules, and tea bags used as either self-care products for lifestyle conditions or general nutritional supplements. The plant is currently cultivated by small-scale farmers in many parts of the country [7], and they mainly use domesticated cultivars. In the past few years, a lot of development has happened, and the known potential for considerable applications in the cosmeceutical and nutraceutical industries has led to large-scale cultivation of the species. In addition, the South African Government has formed a Moringa Flagship program to assist with the cultivation and development of the plant. Similarly, an association, the Moringa Development Association of South Africa (MDASA), was initiated in order to commercialise the plant products. There is, however, little knowledge on the diversity of the cultivars being cultivated, which is relevant for initiating research initiatives from which evidence-based large-scale commercialisation of the plant could emerge. Elucidation of the nutritional diversity of the cultivars may be relevant in this regard.
Several M. oleifera cultivars and germplasms suitable for several desirable traits such as leaf yield, seed production, medicinal properties, and nutritional levels have been produced throughout the world. Results from previous research indicated that these cultivars differ in many traits; for example, Zheng et al. [4] reported differences in the adaptability of eight cultivars in mainland China, while Ndhlala et al. [8] reported variations in antioxidant, antimicrobial properties, and phytochemical composition. In South Africa, some of the M. oleifera germplasm are housed at the Vegetable Industrial and Medicinal Plants facility of the Agricultural Research Council (ARC-VIMP) of South Africa and the University of Limpopo’s Green Biotechnologies Research Centre, where they are available for research and agricultural extension. The germplasm consists of several cultivars whose nutritional composition and relatedness are unknown. This study aimed to assess the variations in the nutritional attributes of thirteen M. oleifera cultivars obtained as seeds from different geographic locations worldwide and cultivated locally to select the most suitable variety for utilisation in programs designed to improve the nutritional benefits of local M. oleifera cultivation. It is important to note that understanding the ways that economically important domesticated plants, such as M. oleifera cultivars, differ from each other is essential for maximizing the benefits of these plants to humanity [9].
2. Materials and Methods
2.1. Experimental Site and Cultivars Used
Thirteen M. oleifera Lam. cultivar seeds originating from different geographical locations in the world were cultivated at the Agricultural Research Council (ARC) experimental farm, Roodeplaat, Pretoria (25°36′1.85″ S; 28°21′54.78″ E). The farm is located at an elevation of 1160 m a.s.l. The vegetation of the farm location is described as savanna [10], and Acocks [11] describes the area as Sourish Mixed Bushveld. The area receives annual precipitation ranging from 380 mm to 700 mm [12]. The average minimum and maximum temperatures in the summer range are 29 °C and 20 °C, respectively, while winter temperatures are 16 °C and 2 °C, respectively.
The details of the cultivars planted are presented in Table 1. The trial layout was a randomised block design fashion with all the cultivars receiving the same management practices of no fertilisers, watering, and constant weeding.

Table 1.
Details of the Moringa oleifera cultivars used in the study, obtained from different origins.
2.2. Sample Preparation and Methods of Analysis
Fresh leaf samples from each of the M. oleifera cultivars were harvested separately, and nutrients were fully preserved by carefully harvesting the leaflets, which were immediately placed in labelled envelopes and sealed in vessels containing liquid nitrogen for transportation to the freeze-drying facility. The harvested samples were freeze-dried for 48 h. and dried plant materials were ground into powder and used for analysis.
The protein, sugars, carbohydrates, fibre, ash, moisture, cholesterol, and fat contents were determined following the standards set by AOAC [13,14,15,16].
Determination of elemental micro-nutrients was carried out as reported by Mafokoane et al. [17]. In brief, a total of 10 g of dried leaf sample material was digested in 40 mL of 4% nitric acid (HNO3), followed by placing the container on a vortex to allow for the complete wetting of the mixture. The materials were magnetically stirred and thereafter incubated in a 95 °C water bath for 90 min, before being allowed to cool down at room temperature, filtered, and decanted into 50 mL tubes, which were covered with foil, and then selected nutrient elements were analysed using inductively coupled plasma optical emission spectrometry (ICPE-9000). The conditions of the analysis and the development of the mineral standard curve were as reported by Mafokoane et al. [17].
The analysis of vitamins was performed according to Patle et al. [18] on an HPLC (Shimadzu, Kyoto, Japan) coupled with a diode array detector (DAD). An aliquot of 20 µL extract sample/standard was injected into a Phenomenex Luna C18 column (100 × 2 mm, 2.5 μm particle size) for the quantification of vitamins. The separation and identification of vitamins were carried out by using a gradient solvent A (20 mM phosphate buffer; pH 3) and solvent B (acetonitrile) at room temperature. The flow rate was maintained at 0.5 mL min−1, and the gradient program started with 0.1–3 min, 100% A; 3–6.5 min, 98% A; 6.5–7 min, 100% A, with a total run time of 7 min. The vitamin analytes were measured at 275 nm.
2.3. Data Analysis
Data were subjected to statistical analysis using GenStat 18th version statistical package (VSN International, Hempstead, UK). The mean separation for significant treatments was achieved using a t-test at a significance level of 5%. We also determined the correlation among different measured attributes. Statistical analysis was carried out in the R statistical package version 3.6.3 [19].
3. Results and Discussion
The results of the carbohydrates, energy, sugars, and fibre are shown in Table 2. There were significant (p ≤ 0.05) differences in the carbohydrates, energy, some of the sugars, and fibre. The local domesticated cultivar from Limpopo Province, South Africa, exhibited higher (p ≤ 0.05) total sugars, energy, glycaemic carbohydrates, and glucose than the other cultivars. Cultivars TOT4977 and TOT5028 from Thailand showed lower (p ≤ 0.05) levels of total sugars, energy, glycaemic carbohydrates, and sucrose. There were, however, no significant differences (p ≥ 0.05) in the levels of glucose, lactose, and maltose detected in all the tested cultivars. In all the cultivars, very low amounts (<0.3 g/100 g) of lactose and maltose were detected.

Table 2.
Concentrations of various nutrients of Moringa oleifera found in different cultivars from several origins.
The Taiwanese cultivar TOT4100 (45.8 g/100 g) and Thai cultivars TOT5028 (45.7 g/100 g) and TOT5169 (45.8 g/100 g) had significantly (p ≤ 0.05) higher fibre composition compared to the rest of the tested cultivars, while the cultivars from Limpopo and TOT5330 from Thailand had the least (40.8 g/100 g for both). Higher fibre composition in the leaflet powders contributes significantly to the pharmacological attributes of the plant as it enhances normal bowel function and subsequently prevents diseases such as colon cancer [20].
Table 3 represents the results of moisture, ash, fat, cholesterol, and protein. There were significant (p ≤ 0.05) differences across all the cultivars in these parameters except for cholesterol. Higher amounts of moisture, ash, total saturated fat, total monounsaturated fat, and protein were exhibited by TOT4880, TOT5028, TOT4977, and Limpopo cultivars.

Table 3.
Moisture, ash, fat, cholesterol, and protein content of some cultivars of M. oleifera from different geographic origins.
The moisture levels in all the cultivars ranged from 7.10% (TOT4951) to 8.20% (TOT4880), which can be considered high. This may be due to the presence of sugars (fructose, glucose, maltose, lactose, and sucrose) as presented in Table 2, which shows the results in the MLP being hygroscopic, causing it to absorb moisture from the environment [21]. Higher moisture content in food ingredients reduces shelf-life; thus, care needs to be taken when storing raw powder or some products in South Africa, M. oleifera powder is consumed in many different forms, including capsules.
The locally domesticated cultivar from Limpopo Province, South Africa, displayed a noteworthy protein content of 28.9 g/100 g. This finding is highly encouraging and indicates that the cultivar can serve as an excellent protein supplement source for the local population. Moreover, considering that this cultivar is already domesticated in South Africa, its adoption and cultivation in other regions could provide a valuable solution to address protein deficiency. The results were comparable to those obtained by Fejer et al. [22] who investigated the nutritional properties of M. oleifera growing in the Caribbean. Protein is an essential nutrient that is often lacking in the diets of many South African individuals, as mentioned earlier. Instead, their diets tend to be dominated by high-sugar foods, alcoholic beverages, and sugary drinks. Therefore, the identification of a cultivar with elevated protein levels, such as the local Limpopo cultivar, presents an opportunity to bridge this nutritional gap. Conversely, the Thai cultivar TOT5169 exhibited the lowest protein content of 20.8 g/100 g among the studied cultivars. This disparity highlights the variation in protein composition across different M. oleifera cultivars.
Table 4 shows the levels of microelements in various cultivars of M. oleifera. Significant differences (p ≤ 0.05) were observed among the cultivars for all the studied microelements, except for zinc (Zn). Notably, cultivar TOT5028 from Thailand exhibited the highest calcium content (51,893 mg/100 g), while the domesticated Limpopo cultivar displayed the lowest calcium levels (31,363 mg/100 g). Regarding iron (Fe) and potassium (K), cultivar TOT5077 demonstrated the highest Fe content, and cultivar SH showed the highest K content. Conversely, cultivars TOT7266 and TOT5028 had the lowest levels of Fe and K, respectively.

Table 4.
Microelements content of some cultivars of M. oleifera from different geographic origins.
Microelements such as calcium (Ca), iron (Fe), potassium (K), and zinc (Zn) play vital physiological roles in maintaining overall body health [23]. For instance, calcium is essential for bone growth and development in infants as well as the normal formation of fetal bones. Daily intake of microelements is crucial to support these physiological processes. It is worth mentioning that green leafy vegetables, including M. oleifera as presented in Table 4, are natural sources of calcium.
Table 5 presents the variation of vitamins observed in the thirteen cultivars of M. oleifera. Notable differences were observed among the cultivars in terms of vitamin content. TOT5169 exhibited the highest (p ≤ 0.05) levels of folic acid (vitamin B) and vitamin A. Conversely, TOT5077 and the Limpopo cultivar showed the highest (p ≤ 0.05) amounts of vitamin C. It is worth noting that none of the cultivars contained detectable levels of vitamin B12.

Table 5.
Vitamin composition of some cultivars of M. oleifera from different geographic origins.
Vitamin A, comprising retinol, retinal, and retinoic acids, plays a crucial role in various physiological processes such as enhancing vision, promoting skin development, boosting the immune system, and supporting embryonic growth. Remarkably, M. oleifera was found to contain higher levels of vitamin A compared to carrots, as reported by Fahey [24]. This highlights the potential of M. oleifera as a valuable source of vitamin A in the diet [24]. Because folates, a B-vitamin category, are required for DNA synthesis and cell division, inadequate folate consumption during pregnancy may result in neural defects in newborns’ brains and spinal cords [19]. To support optimal foetal growth, it is strongly advised to add folate-rich foods or supplements into the diet throughout pregnancy [25]. TOT5169′s high folic acid content highlights its potential as a valuable cultivar source of natural folic acid. In addition, Vitamin C, also known as ascorbic acid, was found in substantial amounts in M. oleifera, above 200 mg/100 g, surpassing the levels found in oranges, as reported by Ramachandran et al. [26]. Vitamin C is known for its role in reducing blood cholesterol levels and aiding in iron absorption. Furthermore, vitamin C acts as an antioxidant, combating harmful reactive oxygen species (ROS) known as free radicals.
These findings highlight M. oleifera’s nutritional importance, notably in terms of vitamin composition. The variations discovered among the cultivars allow for the selection of certain cultivars high in required vitamins for a variety of nutritional demands. M. oleifera can help meet vitamin requirements while also potentially providing extra health advantages due to its antioxidant qualities.
Figure 1 indicates the correlogram of various nutrients, minerals, and fats found in M. oleifera. There was mostly no correlation between the different measured attributes, except for a few including total sugar and sucrose; glucose and fructose; moisture and glycemic carbohydrates; phosphorus and calcium; phosphorus and magnesium; calcium and ash; total fat and saturated fat; magnesium and ash; magnesium and calcium, while there were significant negative relationships between the following pairs: calcium and total sugar; calcium and sucrose; ash and total sugar; ash and sucrose; magnesium and total sugar; total polyunsaturated fats and total monosaturated fats. These significant relationships indicate the potential of using models to predict some of these nutrients, fats, and minerals. This would significantly reduce the expense used in wet chemical analysis for measuring these attributes.
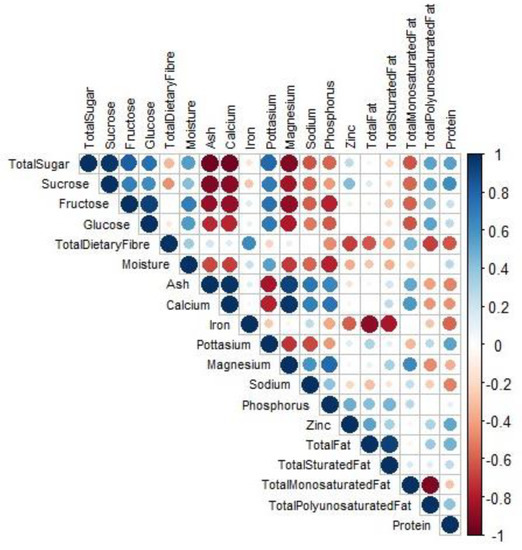
Figure 1.
Correlogram indicating the relationship between various nutrients found in different Moringa oleifera variants. X indicates a non-significant correlation at p < 0.05.
The identification of these correlations provides important insights into the interrelationships of numerous components in M. oleifera. These findings are consistent with prior research by Pekel et al. [27], which found comparable associations in different plant components. Furthermore, these linkages imply that certain traits can be used as proxies for estimating others, allowing for the development of rapid and cost-effective prediction models.
Using predictive models based on these relationships has tremendous potential for expediting the assessment of M. oleifera nutritional content, mineral composition, and fat profiles. By entering easily measurable attributes into the model, such as total sugar or ash content, it is feasible to estimate related components, such as sucrose or calcium levels, without requiring a lengthy laboratory examination. This not only decreases the time and cost of traditional wet chemical analysis but also allows for high-throughput screening of huge sample sets.
South Africa does not only suffer from the prevalence of infectious diseases associated with underdevelopment, poverty, and undernutrition, but there is also an emerging epidemic of chronic diseases linked to over-nutrition and Western types of diet and lifestyle [28]. An example is that in South Africa, nearly 30% of dietary energy is supplied by sugar, fat, and alcohol, which have a high energy density but are very low in other nutrient densities. Rapid urbanisation, rising incomes, and poor dietary choices have acted as drivers for this emerging epidemic of chronic lifestyle diseases. The net result is an increased burden on the national healthcare system because of the effects of the two forms of malnutrition, which are undernutrition and over-nutrition.
The high nutritional content of M. oleifera leaflets makes the plant an extraordinarily attractive tool for addressing malnutrition throughout the developing world, including South Africa. The selection of cultivars with the advantages of high nutritional content or any other factor is critical in malnutrition alleviation programs. For example, in South Africa, the National Government, through the Department of Science and Innovation, Directorate of Indigenous Knowledge-based Technology Innovation, initiated a M. oleifera Flagship made up of several communities that are encouraged to grow the tree for food and nutritional security. These communities are linked to researchers from universities and science councils to assist them with cultivation and quality control and product formulations. Therefore, it is critical to understand variants within M. oleifera cultivars to select those that simultaneously have high nutritional content for better nutraceutical effects and high medicinal properties.
4. Conclusions
The differences observed among the studied cultivars can be attributed to the unique genetic makeup of each M. oleifera germplasm, resulting in distinct characteristics even when cultivated under identical environmental conditions. In support of this, Makita et al. [29] reported that not all M. oleifera varieties contain the crucial flavonoid rutinoside, which is responsible for the plant’s nutritional and therapeutic properties. Therefore, considering the presence of these secondary metabolites will significantly enhance the identification of cultivars with high nutritional and pharmacological potential. By understanding the genetic variations and secondary metabolite profiles of different M. oleifera germplasms, researchers and breeders can make informed decisions to develop improved varieties with desired traits for various applications in agriculture, nutrition, and medicine.
Author Contributions
A.R.N. carried out the study concept, nutrition work, analysed the data and drafting of the manuscript. T.T. study design, assisted in data analysis and interpretation as well as drafting of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Department of Science and Innovations (DSI)—Indigenous Knowledge System-based Tech Innovation, Pretoria, grant number DSI/CON C2235/2021.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors acknowledge The Agricultural Research Council (Vegetable and Ornamental Plants) for the plant material used.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Karthy, E.S.; Ranjitha, P.; Mohankumar, A. Antimicrobial potential of plant seed extracts against Multidrug Resistant Methicillin Resistant Staphylococcus aureus (MDR—MRSA). Int. J. Biol. 2009, 1, 34–40. [Google Scholar] [CrossRef]
- Tshabalala, T.; Ncube, B.; Madala, N.E.; Nyakudya, T.T.; Moyo, H.P.; Sibanda, M.; Ndhlala, A.R. Scribbling the cat: A Case of the “miracle” plant, Moringa oleifera. Plants 2019, 8, 510. [Google Scholar] [CrossRef]
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Cultivation, genetic, ethnopharmacology, phytochemistry and pharmacology of Moringa oleifera leaves: An overview. Int. J. Mol. Sci. 2015, 16, 12791–12835. [Google Scholar] [CrossRef]
- Zheng, Y.; Sun, H.; Zhang, Y.; Wu, J. Evaluation of the adaptability, productivity, and leaf powder quality of eight Moringa oleifera cultivars introduced to a dry-hot climate of Southwest China. Ind. Crops Prod. 2019, 128, 199–205. [Google Scholar] [CrossRef]
- Habtemariam, S. The African Moringa is to change the lives of millions in Ethiopia and far beyond. Asian Pac. J. Trop. Biomed. 2016, 6, 355–356. [Google Scholar] [CrossRef]
- Naidoo, K.K.; Coopoosamy, R.M. Review on herbal remedies used by the 1860 South African Indian settlers. Afr. J. Biotechnol. 2011, 10, 8533–8538. [Google Scholar]
- Mashamaite, C.V.; Pieterse, P.J.; Mothapo, P.N.; Phiri, E.E. Moringa oleifera in South Africa: A review on its production, growing conditions and consumption as a food source. S. Afr. J. Sci. 2021, 117, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Ndhlala, A.; Mulaudzi, R.; Ncube, B.; Abdelgadir, H.; du Plooy, C.; Van Staden, J. Antioxidant, antimicrobial and phytochemical variations in thirteen Moringa oleifera Lam. cultivars. Molecules 2014, 19, 10480. [Google Scholar] [CrossRef] [PubMed]
- Chodur, G.M.; Olson, M.E.; Wade, K.L.; Stephenson, K.K.; Nouman, W.; Garima, J.; Fahey, J.W. Wild and domesticated Moringa oleifera differ in taste, glucosinolate composition, and antioxidant potential, but not myrosinase activity or protein content. Sci. Rep. 2018, 8, 7995. [Google Scholar] [CrossRef]
- Mucina, L.; Rutherford, M.C. (Eds.) The Vegetation of South Africa, Lesotho and Swaziland; South African National Biodiversity Institute: Pretoria, South Africa, 2006. [Google Scholar]
- Acocks, J.P.H. Veld Types of South Africa; Department of Agriculture Technical Services: Pretoria, South Africa, 1988. [Google Scholar]
- Panagos, M.D.; Westfall, R.H.; van Staden, J.M.; Zacharias, P.J.K. The plant communities of the Roodeplaat Experimental Farm, Gauteng, South Africa and the importance of classification verification. S. Afr. J. Bot. 1998, 64, 44–61. [Google Scholar] [CrossRef]
- AOAC. Association of Analytical Chemists. Official Methods of Analysis; AOAC International: Gaithersburg, ML, USA, 2000. [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Analytical Chemists International, 17th ed.; AOAC International: Gaithersburg, ML, USA, 2003. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars related substance. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Langemeier, J.M.; Rogers, D.E. Rapid method for sugar analysis of doughs and baked products. Cereal Chem. 1995, 72, 349–351. [Google Scholar]
- Mafokoane, A.M.; Mphosi, M.S.; Shadung, K.G. Effect of time-based oven-drying on the essential mineral elements of cowpea (Vigna unguiculata) leaves. Res. Crops 2019, 20, 50–54. [Google Scholar]
- Patle, T.K.; Shrivas, K.; Patle, A.; Patel, S.; Harmukh, N.; Kumar, A. Simultaneous determination of B1, B3, B6 and C vitamins in green leafy vegetables using reverse phase-high performance liquid chromatography. Microchem. J. 2022, 176, 107249. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.R-project.org (accessed on 13 September 2021).
- Omale, J.; Adeyemi, A.R.; Omajali, J.B. Phytoconstituents, proximate and nutrient investigations of Saba florida (Benth.) from Ibaji forest. Int. J. Nutr. Metab. 2010, 2, 88–92. [Google Scholar]
- Mathlouthi, M. Water content, watter activity, water structure and stability of foodstuffs. Food Control 2001, 12, 409–417. [Google Scholar] [CrossRef]
- Fejér, J.; Kron, I.; Pellizzeri, V.; Pl’uchtová, M.; Eliašová, A.; Campone, L.; Gervasi, T.; Bartolomeo, G.; Cicero, N.; Babejová, A. First Report on Evaluation of Basic Nutritional and Antioxidant Properties of Moringa oleifera Lam. from Caribbean Island of Saint Lucia. Plants 2019, 8, 537. [Google Scholar] [CrossRef] [PubMed]
- Pravina, P.; Sayaji, D.; Avinash, M. Calcium and its Role in Human Body. Int. J. Res. Pharm. Biomed. Sci. 2013, 4, 659–668. [Google Scholar]
- Fahey, J.W. Moringa oleifera: A review of the medical evidence for its nutritional, therapeutic and prophylactic properties. Trees Life J. 2005, 1, 5. [Google Scholar]
- Imbard, A.; Benoist, J.F.; Blom, H.J. Neural tube defects, folic acid and methylation. Int. J. Environ. Res. Public Health 2013, 10, 4352–4389. [Google Scholar] [CrossRef]
- Ramachandran, C.; Peter, K.V.; Gopalakrishnan, P.K. Drumstick (Moringa oleifera): A multipurpose Indian vegetable. Econ. Bot. 1980, 34, 276–283. [Google Scholar] [CrossRef]
- Pekel, A.Y.; Alık, A.C.; Alatas, M.S.; Kuter, E.; Cengiz, O.; Omurtag, G.Z.; Inan, G. Evaluation of Correlations Between Nutrients, Fatty Acids, Heavy Metals, and Deoxynivalenol in Corn (Zea mays L.). J. Appl. Poult. Res. 2019, 28, 94–107. [Google Scholar] [CrossRef]
- Nyakudya, T.T.; Tshabalala, T.; Dangarembizi, R.; Erlwanger, K.H.; Ndhlala, A.R. The Potential Therapeutic Value of Medicinal Plants in the Management of Metabolic Disorders. Molecules 2020, 25, 2669. [Google Scholar] [CrossRef] [PubMed]
- Makita, C.; Madala, N.E.; Cukrowska, E.; Abdelgadir, H.; Chimuka, L.; Steenkamp, P.; Ndhlala, A.R. Variation in pharmacologically potent rutinoside-bearing flavonoids amongst twelve Moringa oleifera Lam. Cultivars. S. Afr. J. Bot. 2017, 112, 270–274. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).