New Insights into the Taxonomy of Myotis Bats in China Based on Morphology and Multilocus Phylogeny
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Collection
2.2. Genetic and Morphological Data Acquisition
2.3. Phylogenetic Analysis
2.4. Genetic Divergence Evaluation
3. Results
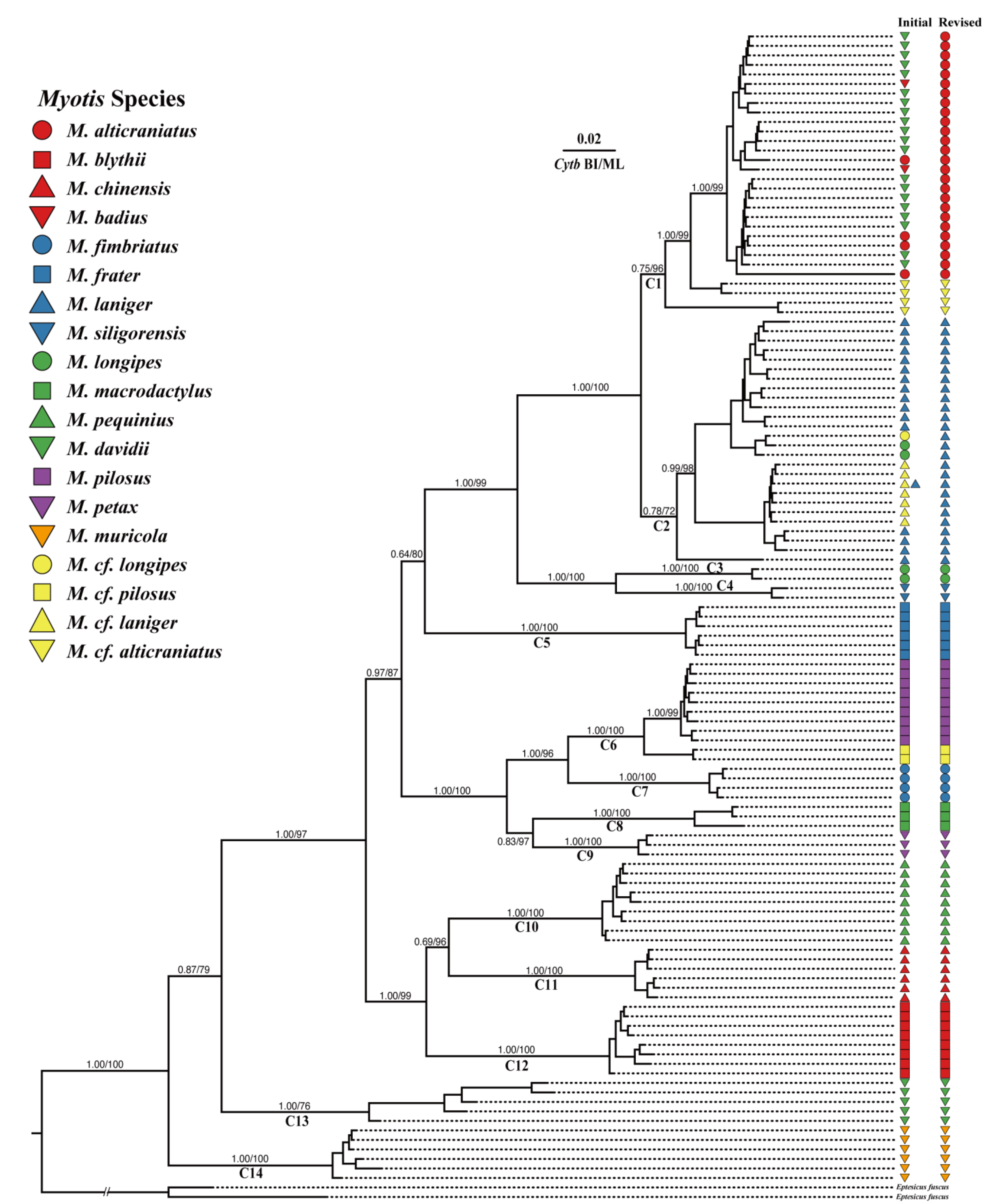
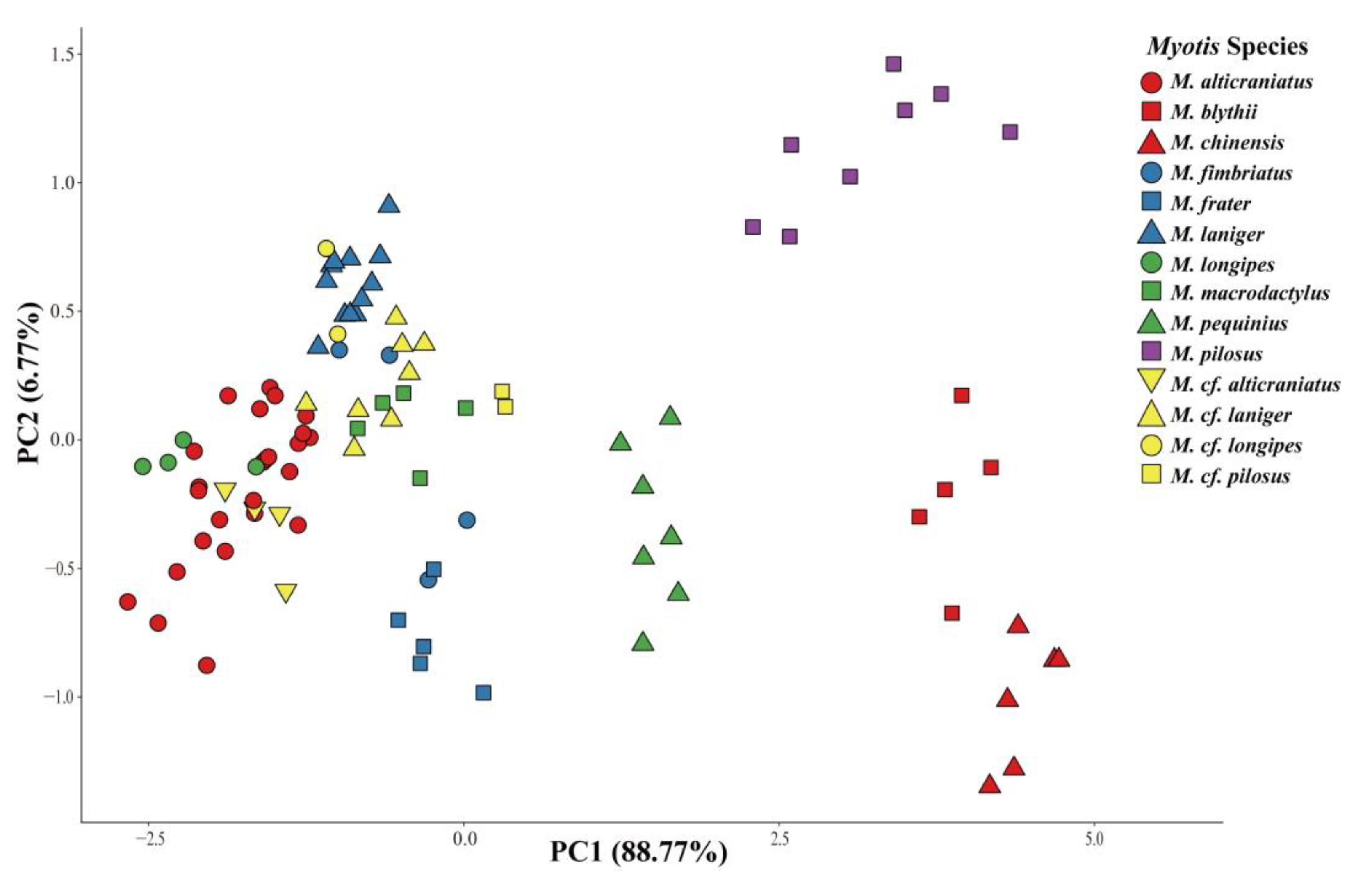
3.1. Taxonomic Revision
3.2. Phylogeny of Chinese Myotis
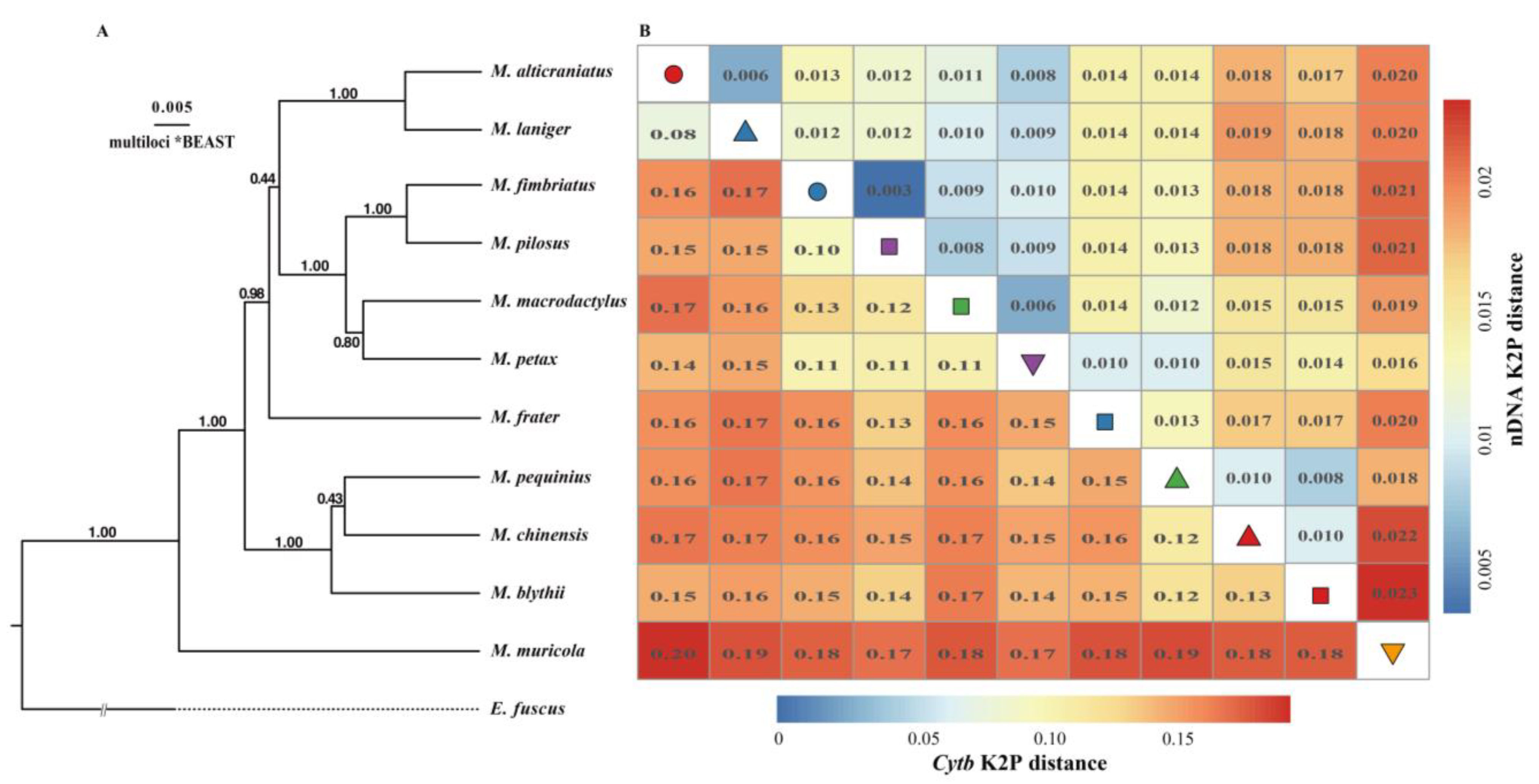
3.3. The Level of Genetic Divergence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Altringham, J.D. Bats: From Evolution to Conservation; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Frick, W.F.; Kingston, T.; Flanders, J. A review of the major threats and challenges to global bat conservation. Ann. N. Y. Acad. Sci. 2020, 1469, 5–25. [Google Scholar] [CrossRef]
- Dool, S.E. Conservation genetic studies in bats. In Conservation Genetics in Mammals; Ortega, J., Maldonado, J.E., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 29–62. [Google Scholar]
- Adams, R.A.; Pedersen, S.C. Bat Evolution, Ecology, and Conservation; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Zachos, F.E. Bats. In Handbook of the Mammals of the World; Wilson, D.E., Mittermeier, R.A., Eds.; Lynx Edicions: Barcelona, Spain, 2020; Volume 9. [Google Scholar]
- Burgin, C.J.; Colella, J.P.; Kahn, P.L.; Upham, N.S. How many species of mammals are there? J. Mammal. 2018, 99, 1–14. [Google Scholar] [CrossRef]
- Morales, A.E.; Ruedi, M.; Field, K.; Carstens, B.C. Diversification rates have no effect on the convergent evolution of foraging strategies in the most speciose genus of bats, Myotis. Evolution 2019, 73, 2263–2280. [Google Scholar] [CrossRef] [PubMed]
- Ruedi, M.; Stadelmann, B.; Gager, Y.; Douzery, E.J.P.; Francis, C.M.; Lin, L.-K.; Guillén-Servent, A.; Cibois, A. Molecular phylogenetic reconstructions identify East Asia as the cradle for the evolution of the cosmopolitan genus Myotis (Mammalia, Chiroptera). Mol. Phylogenetics Evol. 2013, 69, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Stadelmann, B.; Lin, L.-K.; Kunz, T.; Ruedi, M. Molecular phylogeny of New World Myotis (Chiroptera, Vespertilionidae) inferred from mitochondrial and nuclear DNA genes. Mol. Phylogenetics Evol. 2007, 43, 32–48. [Google Scholar] [CrossRef]
- Findley, J.S. Phenetic relationships among bats of the genus Myotis. Syst. Biol. 1972, 21, 31–52. [Google Scholar] [CrossRef]
- Rautenbach, I.; Bronner, G.; Schlitter, D. Karyotypic data and attendant systematic implications for the bats of southern Africa. Koedoe 1993, 36, 87–104. [Google Scholar] [CrossRef]
- Koopman, K.F. Chiroptera: Systematics. Handbook Zool. 1994, 8, 1–217. [Google Scholar]
- Tate, G.H.H.; Archbold, R. A Review of the Genus Myotis (Chiroptera) of Eurasia: With Special Reference to Species Occurring in the East Indies; American Museum of Natural History: New York, NY, USA, 1941. [Google Scholar]
- Bickham, J.W.; Patton, J.C.; Schlitter, D.A.; Rautenbach, I.L.; Honeycutt, R.L. Molecular phylogenetics, karyotypic diversity, and partition of the genus Myotis (Chiroptera: Vespertilionidae). Mol. Phylogenetics Evol. 2004, 33, 333–338. [Google Scholar] [CrossRef]
- Kawai, K.; Nikaido, M.; Harada, M.; Matsumura, S.; Lin, L.-K.; Wu, Y.; Hasegawa, M.; Okada, N. The status of the Japanese and East Asian bats of the genus Myotis (Vespertilionidae) based on mitochondrial sequences. Mol. Phylogenetics Evol. 2003, 28, 297–307. [Google Scholar] [CrossRef]
- Ruedi, M.; Mayer, F. Molecular systematics of bats of the Genus Myotis (Vespertilionidae) suggests deterministic ecomorphological convergences. Mol. Phylogenetics Evol. 2001, 21, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Fenton, M.B.; Bogdanowicz, W. Relationships between external morphology and foraging behaviour: Bats in the genus Myotis. Can. J. Zool. 2002, 80, 1004–1013. [Google Scholar] [CrossRef]
- Ghazali, M.; Moratelli, R.; Dzeverin, I. Ecomorph evolution in Myotis (Vespertilionidae, Chiroptera). J. Mamm. Evol. 2017, 24, 475–484. [Google Scholar] [CrossRef]
- Carstens, B.C.; Dewey, T.A. Species delimitation using a combined coalescent and information-theoretic approach: An example from North American Myotis bats. Syst. Biol. 2010, 59, 400–414. [Google Scholar] [CrossRef] [PubMed]
- Platt, R.N.; Faircloth, B.C.; Sullivan, K.A.; Kieran, T.J.; Glenn, T.C.; Vandewege, M.W.; Lee, T.E.; Baker, R.J.; Stevens, R.D.; Ray, D.A. Conflicting evolutionary histories of the mitochondrial and nuclear genomes in New World Myotis bats. Syst. Biol. 2018, 67, 236–249. [Google Scholar] [CrossRef]
- Ruedi, M.; Saikia, U.; Thabah, A.; Görföl, T.; Thapa, S.; Csorba, G. Molecular and morphological revision of small Myotinae from the Himalayas shed new light on the poorly known genus Submyotodon (Chiroptera: Vespertilionidae). Mamm. Biol. 2021, 101, 465–480. [Google Scholar] [CrossRef]
- Zhang, Z.; Tan, X.; Sun, K.; Liu, S.; Xu, L.; Feng, J. Molecular systematics of the Chinese Myotis (Chiroptera, Vespertilionidae) inferred from cytochrome-b sequences. Mammalia 2009, 73, 323–330. [Google Scholar] [CrossRef]
- Irwin, D.M.; Kocher, T.D.; Wilson, A.C. Evolution of the cytochrome b gene of mammals. J. Mol. Evol. 1991, 32, 128–144. [Google Scholar] [CrossRef]
- Lim, B.K.; Engstrom, M.D.; Bickham, J.W.; Patton, J.C. Molecular phylogeny of New World sheath-tailed bats (Emballonuridae: Diclidurini) based on loci from the four genetic transmission systems in mammals. Biol. J. Linn. Soc. 2007, 93, 189–209. [Google Scholar] [CrossRef]
- Swindell, S.R.; Plasterer, T.N. Seqman. In Sequence Data Analysis Guidebook; Springer: Berlin/Heidelberg, Germany, 1997; pp. 75–89. [Google Scholar]
- Hall, T.; Biosciences, I.; Carlsbad, C. BioEdit: An important software for molecular biology. GERF Bull. Biosci. 2011, 2, 60–61. [Google Scholar]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Xia, X. DAMBE5: A comprehensive software package for data analysis in molecular biology and evolution. Mol. Biol. Evol. 2013, 30, 1720–1728. [Google Scholar] [CrossRef]
- Team RC. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria. 2016. Available online: http://www.R-project.org/ (accessed on 25 October 2022).
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Lam-Tung, N.; Schmidt, H.A.; Arndt, V.H.; Quang, M.B. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar]
- Drummond, A.J.; Suchard, M.A.; Xie, D.; Rambaut, A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012, 29, 1969–1973. [Google Scholar] [CrossRef]
- Rambaut, A.; Suchard, M.; Xie, W.; Drummond, A. Tracer v. 1.6; Institute of Evolutionary Biology, University of Edinburgh: Edinburgh, UK, 2014. [Google Scholar]
- Subha, K.; Bui, Q.M.; Thomas, K.F.W.; Arndt, V.H.; Lars, S.J. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar]
- Rambaut, A. FigTree-v1.4.4. 2018. Available online: https://tree.bio.ed.ac.uk/software/Figtree/ (accessed on 19 August 2021).
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Sites, J.W., Jr.; Marshall, J.C. Delimiting species: A Renaissance issue in systematic biology. Trends Ecol. Evol. 2003, 18, 462–470. [Google Scholar] [CrossRef]
- Toews, D.P.; Brelsford, A. The biogeography of mitochondrial and nuclear discordance in animals. Mol. Ecol. 2012, 21, 3907–3930. [Google Scholar] [CrossRef]
- Ramsey, M.W.; Cairns, S.C.; Vaughton, G.V. Geographic variation in morphological and reproductive characters of coastal and tableland populations of Blandfordia grandiflora (Liliaceae). Plant Syst. Evol. 1994, 192, 215–230. [Google Scholar] [CrossRef]
- Singh, R.S.; Long, A.D. Geographic variation in Drosophila: From molecules to morphology and back. Trends Ecol. Evol. 1992, 7, 340–345. [Google Scholar] [CrossRef] [PubMed]
- How, R.; Schmitt, L.; Suyanto, A. Geographical variation in the morphology of four snake species from the Lesser Sunda Islands, eastern Indonesia. Biol. J. Linn. Soc. 1996, 59, 439–456. [Google Scholar] [CrossRef]
- Albrecht, G.H.; Miller, J. Geographic variation in primates. In Species, Species Concepts and Primate Evolution; Springer: Berlin/Heidelberg, Germany, 1993; pp. 123–161. [Google Scholar]
- Liu, Y.; Cotton, J.A.; Shen, B.; Han, X.; Rossiter, S.J.; Zhang, S. Convergent sequence evolution between echolocating bats and dolphins. Curr. Biol. 2010, 20, R53–R54. [Google Scholar] [CrossRef]
- Ellison, A.M.; Gotelli, N.J. Energetics and the evolution of carnivorous plants—Darwin’s ‘most wonderful plants in the world’. J. Exp. Bot. 2009, 60, 19–42. [Google Scholar] [CrossRef]
- Kelley, N.P.; Motani, R. Trophic convergence drives morphological convergence in marine tetrapods. Biol. Lett. 2015, 11, 20140709. [Google Scholar] [CrossRef]
- Moen, D.S.; Irschick, D.J.; Wiens, J.J. Evolutionary conservatism and convergence both lead to striking similarity in ecology, morphology and performance across continents in frogs. Proc. R. Soc. B Biol. Sci. 2013, 280, 20132156. [Google Scholar] [CrossRef]
- Chang, Y.; Song, S.; Li, A.; Zhang, Y.; Li, Z.; Xiao, Y.; Jiang, T.; Feng, J.; Lin, A. The roles of morphological traits, resource variation and resource partitioning associated with the dietary niche expansion in the fish-eating bat Myotis pilosus. Mol. Ecol. 2019, 28, 2944–2954. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Q.; Yang, C.; Liu, H.; Peng, Z.; Liang, J.; Peng, X.; He, X.; Ma, S.; Xiang, Z.; et al. Discovery of Kashmir Cave Myotis Myotis longipes in Guangdong Province (China) and its echolocation calls. Chin. J. Zool. 2017, 52, 521–529. [Google Scholar]
- Yu, Z.; Wu, Q.; Shi, S.; Ren, R.; Liu, Y.; Feng, L.; Deng, X. The Kashmir Cave Myotis (Myotis longipes) was found in Hengdong County Hunan Province, China. Chin. J. Zool. 2018, 53, 701–708. [Google Scholar]
- Bates, P. Bats of the Indian Subcontinent; Harrison Zoological Museum: Sevenoaks, UK, 1997; 258p. [Google Scholar]
- Topal, G. A new mouse-eared bat species, from Nepal, with statistical analyses of some other species of subgenus Leuconoe (Chiroptera, Vespertilionidae). Acta Zool. Acad. Sci. Hung. 1997, 43, 375–402. [Google Scholar]
- Smith, A.T.; Xie, Y.; Hoffmann, R.S.; Lunde, D.; MacKinnon, J.; Wilson, D.E.; Wozencraft, W.C.; Gemma, F. A Guide to the Mammals of China; Princeton University Press: Princeton, NJ, USA, 2008. [Google Scholar]
- Baker, R.; Bradley, R. Speciation in mammals and the genetic species concept. J. Mammal. 2006, 87, 643–662. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Sun, K.; Xu, L.; Wang, L.; Jiang, T.; Liu, S.; Lu, G.; Berquist, S.W.; Feng, J. Pleistocene glacial cycle effects on the phylogeography of the Chinese endemic bat species, Myotis davidii. BMC Evol. Biol. 2010, 10, 208. [Google Scholar] [CrossRef]
- You, Y.; Du, J.; Wang, L.; Jiang, T. Morphology variation among eleven local populations of the endemic Myotis davidii in China. Life Sci. J. 2021, 18, 54–63. [Google Scholar]
- Benda, P.; Gazaryan, S.; Vallo, P. On the distribution and taxonomy of bats of the Myotis mystacinus morphogroup from the Caucasus region (Chiroptera: Vespertilionidae). Turk. J. Zool. 2016, 40, 842–863. [Google Scholar] [CrossRef]
- Tinglei, J.; Huabin, Z.; Biao, H.; Libiao, Z.; Jinhong, L.; Ying, L.; Keping, S.; Wenhua, Y.; Yi, W.; Jiang, F. Research progress of bat biology and conservation strategies in China. Acta Theriol. Sin. 2020, 40, 539. [Google Scholar]
- Jones, G.; Parsons, S.; Zhang, S.; Stadelmann, B.; Benda, P.; Ruedi, M. Echolocation calls, wing shape, diet and phylogenetic diagnosis of the endemic Chinese bat Myotis pequinius. Acta Chiropterologica 2006, 8, 451–463. [Google Scholar] [CrossRef]
- Lu, G.; Lin, A.; Luo, J.; Blondel, D.V.; Meiklejohn, K.A.; Sun, K.; Feng, J. Phylogeography of the Rickett’s big-footed bat, Myotis pilosus (Chiroptera: Vespertilionidae): A novel pattern of genetic structure of bats in China. BMC Evol. Biol. 2013, 13, 241. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Jia, J.; Liu, L.; Wang, J.; Chen, W.; Miao, G.; Niu, Y.; Guo, W.; Zhang, K.; Sun, K.; et al. New Insights into the Taxonomy of Myotis Bats in China Based on Morphology and Multilocus Phylogeny. Diversity 2023, 15, 805. https://doi.org/10.3390/d15070805
Liu T, Jia J, Liu L, Wang J, Chen W, Miao G, Niu Y, Guo W, Zhang K, Sun K, et al. New Insights into the Taxonomy of Myotis Bats in China Based on Morphology and Multilocus Phylogeny. Diversity. 2023; 15(7):805. https://doi.org/10.3390/d15070805
Chicago/Turabian StyleLiu, Tong, Jiachen Jia, Lingyu Liu, Jie Wang, Wenjie Chen, Guiyin Miao, Yilin Niu, Wei Guo, Kangkang Zhang, Keping Sun, and et al. 2023. "New Insights into the Taxonomy of Myotis Bats in China Based on Morphology and Multilocus Phylogeny" Diversity 15, no. 7: 805. https://doi.org/10.3390/d15070805
APA StyleLiu, T., Jia, J., Liu, L., Wang, J., Chen, W., Miao, G., Niu, Y., Guo, W., Zhang, K., Sun, K., Yu, W., Zhou, J., & Feng, J. (2023). New Insights into the Taxonomy of Myotis Bats in China Based on Morphology and Multilocus Phylogeny. Diversity, 15(7), 805. https://doi.org/10.3390/d15070805





