Diversity and Biology of Terrestrial Orthopteroids (Insecta) in the Republic of Mordovia (Russia)
Abstract
1. Summary
2. Data Description
2.1. Description of the Data in the Dataset
2.2. Biodiversity of Terrestrial Orthopteroidea in the Republic of Mordovia
2.2.1. The Fauna of Terrestrial Orthopteroidea
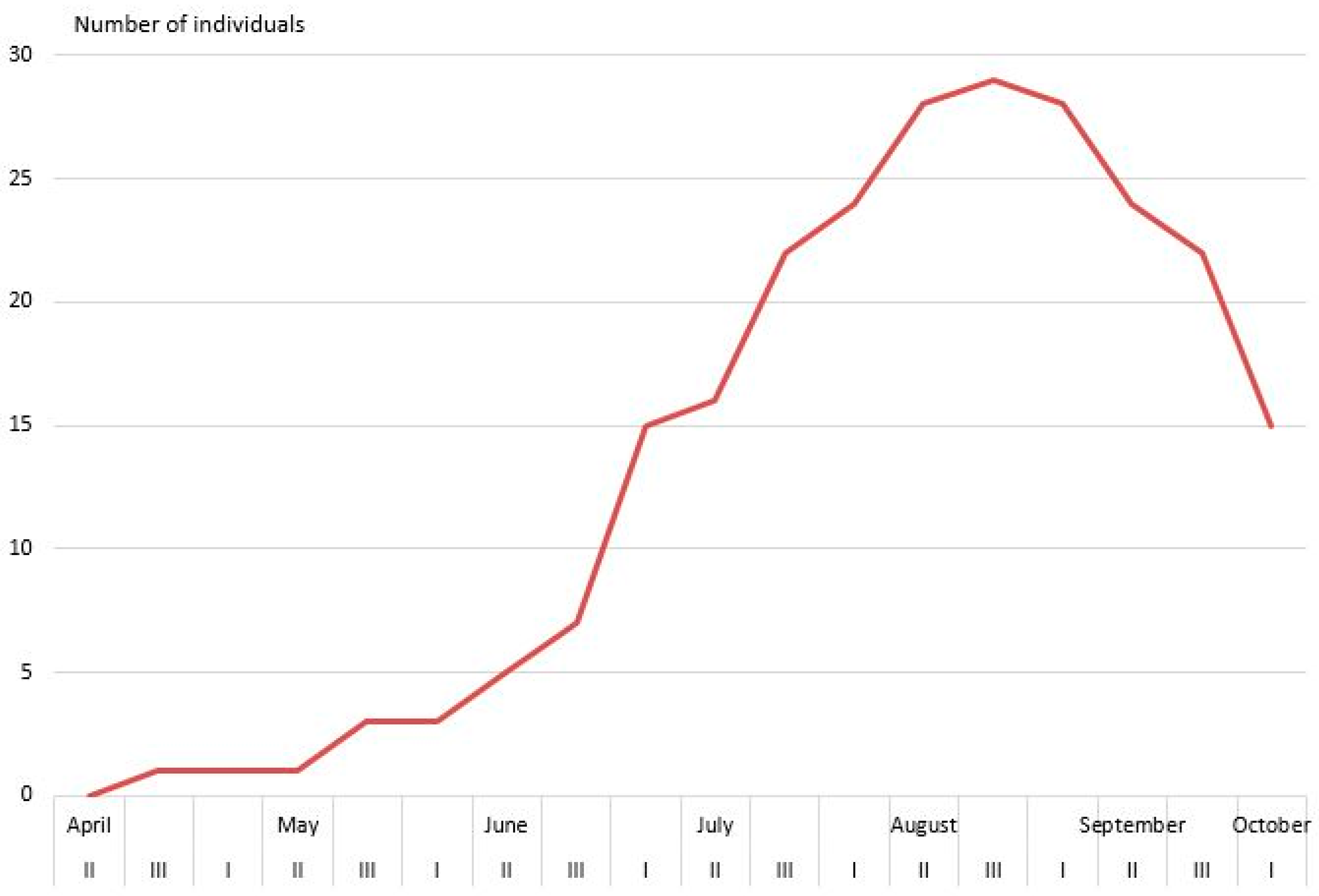
2.2.2. Dermaptera
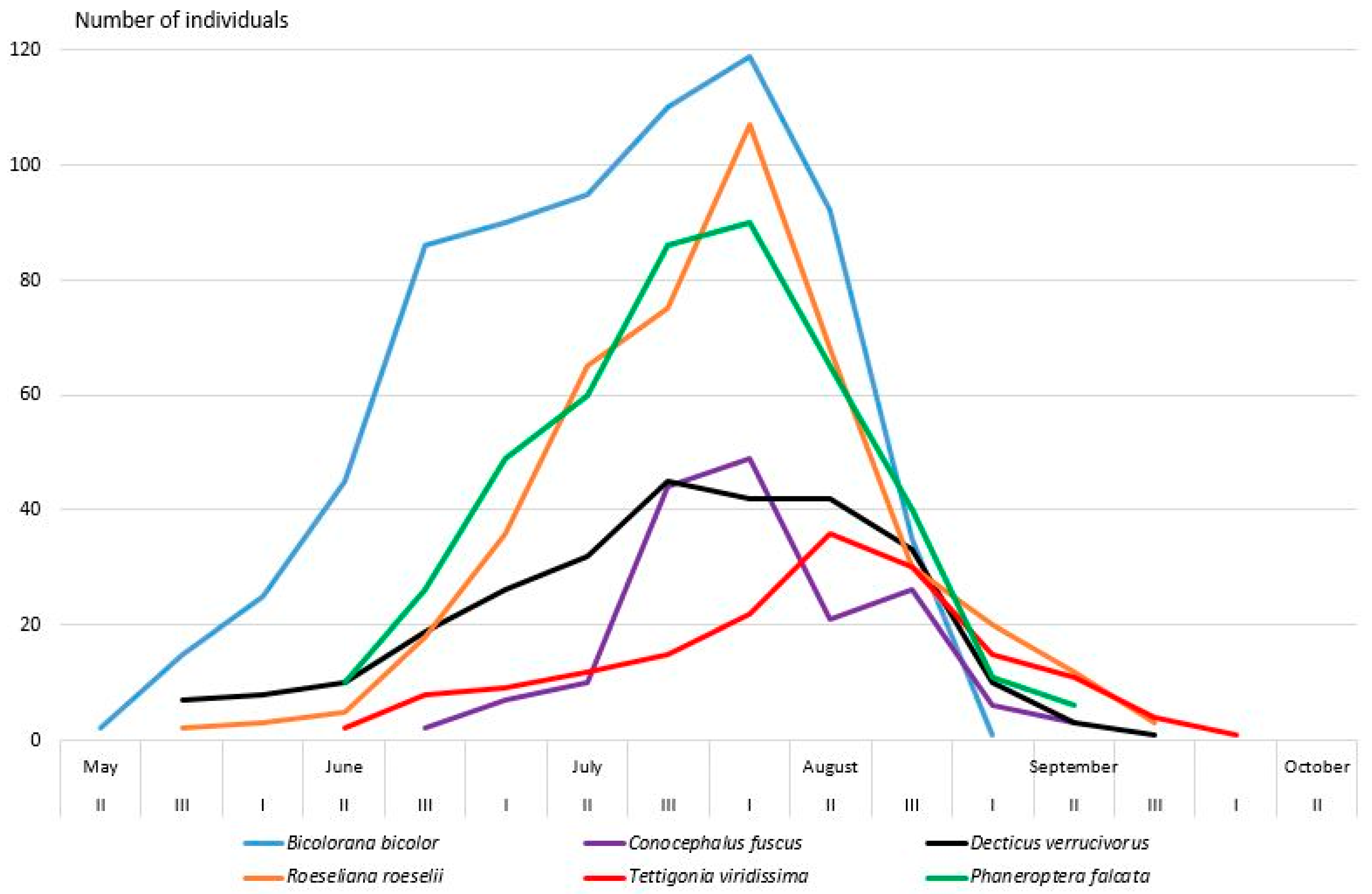
2.2.3. Orthoptera
2.2.4. Mantodea
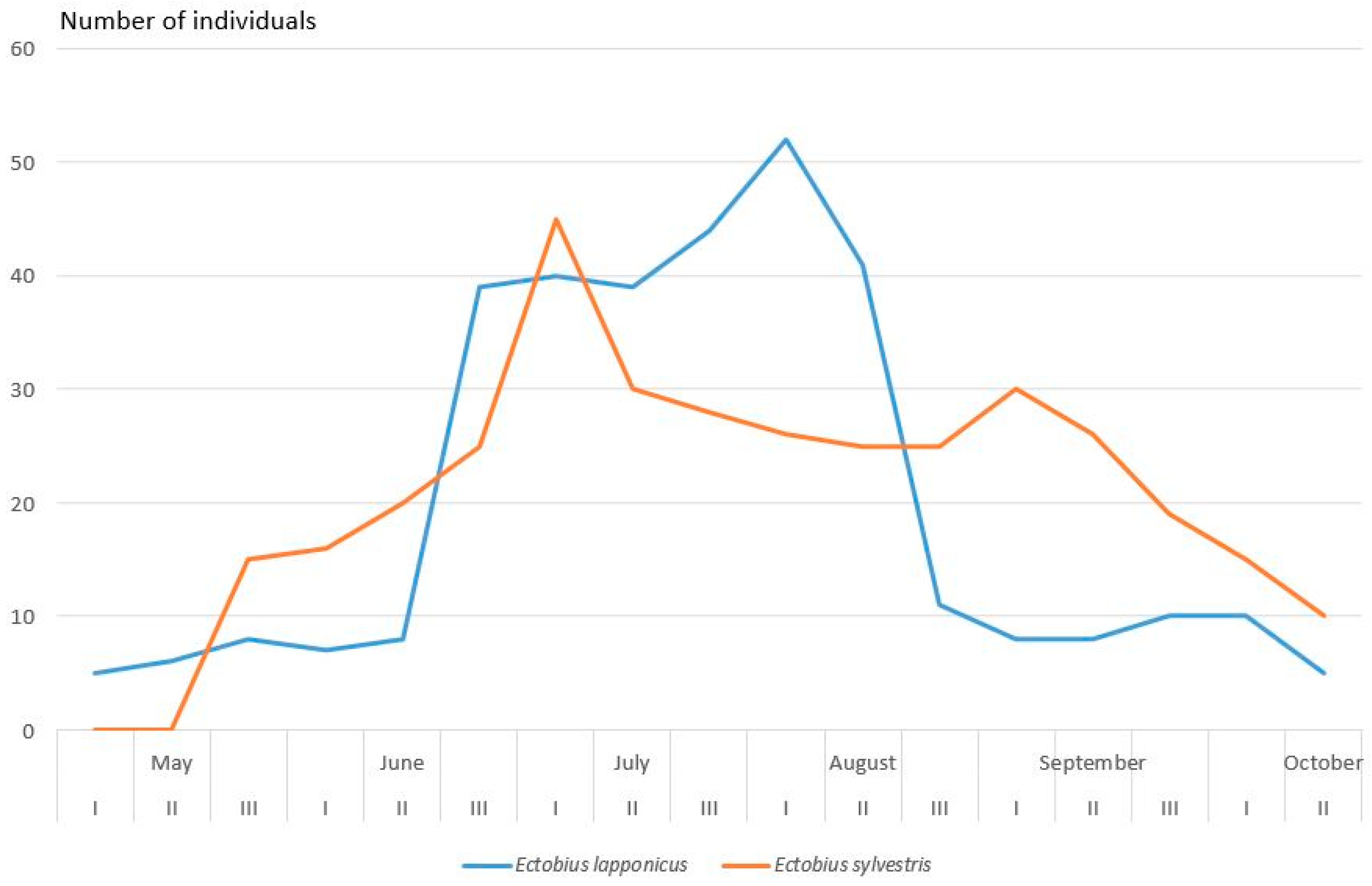
2.2.5. Blattodea
3. Methods
3.1. Study Area
3.2. Design of Research, Identification, and Taxonomic Position of Samples
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.-Q. Phylum Arthropoda. In: Zhang, Z.-Q. (Ed.) Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness (Addenda 2013). Zootaxa 2013, 3703, 17–26. [Google Scholar] [CrossRef]
- Nadeem, A.; Tahir, H.M.; Khan, A.A.; Hassan, Z.; Khan, A.M. Species composition and population dynamics of some arthropod pests in cotton fields of irrigated and semi-arid regions of Punjab, Pakistan. Saudi J. Biol. Sci. 2023, 30, 103521. [Google Scholar] [CrossRef] [PubMed]
- Lecoq, M.; Cease, A. What have we learned after millennia of locust invasions? Agronomy 2022, 12, 472. [Google Scholar] [CrossRef]
- Kietzka, G.J.; Lecoq, M.; Samways, J.M. Ecological and human diet value of locusts in a changing world. Agronomy 2021, 11, 1856. [Google Scholar] [CrossRef]
- Latchininsky, A.; Sword, G.; Sergeev, M.; Cigliano, M.M.; Lecoq, M. Locusts and grasshoppers: Behavior, ecology, and biogeography. Psyche J. Entomol. 2011, 2011, 4. [Google Scholar] [CrossRef]
- Kirstová, M.; Pyszko, P.; Kočárek, P. Factors influencing microhabitat selection and food preference of tree-dwelling earwigs (Dermaptera) in a temperate floodplain forest. Bull. Entomol. Res. 2019, 109, 54–61. [Google Scholar] [CrossRef]
- Storozhenko, S.Y. Studies on Podismopsis insularis (Orthoptera: Acrididae), endemic to the Shantar Islands National Park in the Sea of Okhotsk, Russia. Nat. Conserv. Res. 2021, 6, 98–102. [Google Scholar] [CrossRef]
- Tang, Q.; Bourguignon, T.; Willenmse, L.; De Coninck, E.; Evans, T. Global spread of the German cockroach, Blattella germanica. Biol. Invasions 2019, 21, 693–707. [Google Scholar] [CrossRef]
- Dosdall, L.M.; Cárcamo, H.; Olfert, O.; Meers, S.; Hartley, S.; Gavloski, J. Insect invasions of agroecosystems in the western Canadian prairies: Case histories, patterns, and implications for ecosystem function. Biol. Invasions 2011, 13, 1135–1149. [Google Scholar] [CrossRef]
- Karmazina, I.O.; Korb, S.K.; Mikhailenko, A.P.; Ruchin, A.B.; Shulaev, N.V.; Egorov, L.V.; Aleksanov, V.V. The last Pleistocene glaciations phylogeography episode of Phaneroptera falcata (Poda, 1761) (Orthoptera: Tettigoniidae) in the Volga River basin based on the mtDNA Cytochrome C Oxidase subunit 1 (COI) gene fragment. Acta Biol. Sib. 2020, 6, 279–291. [Google Scholar] [CrossRef]
- Uchida, K.; Ushimaru, A. Biodiversity declines due to abandonment and intensification of agricultural lands: Patterns and mechanisms. Ecol. Monogr. 2014, 84, 637–658. [Google Scholar] [CrossRef]
- Gankhuyag, E.; Dorjsuren, A.; Choi, E.H.; Hwang, U.W. An annotated checklist of grasshoppers (Orthoptera, Acridoidea) from Mongolia. Biodivers. Data J. 2023, 11, e96705. [Google Scholar] [CrossRef]
- Miskelly, J.; Paiero, S.M. Mantodea, Blattodea, Orthoptera, Dermaptera, and Phasmida of Canada. ZooKeys 2019, 819, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Ruchin, A.B. Contribution to the study of Orthoptera and Dermaptera (Insecta) of the Czech Republic. Proc. Mordovia State Nat. Reserve 2021, 26, 232–236. [Google Scholar]
- Suganya, M.; Manimegalai, K. Check List of Species Richness and Abundance of Orthoptera Fauna in Bharathi Park, Coimbatore, Tamil Nadu, India. Nat. Environ. Pollut. Technol. 2022, 21, 563–570. [Google Scholar] [CrossRef]
- Gray, C.; Hill, S.; Newbold, T.; Hudson, L.; Borger, L.; Contu, S.; Hoskins, A.J.; Ferrier, S.; Purvis, A.; Scharlemann, J. Local biodiversity is higher inside than outside terrestrial protected areas worldwide. Nat. Commun. 2016, 7, 12306. [Google Scholar] [CrossRef] [PubMed]
- Ejsmont-Karabin, J. Does the world need faunists? Based on rotifer (Rotifera) occurrence reflections on the role of faunistic research in ecology. Int. Rev. Hydrobiol. 2019, 104, 49–56. [Google Scholar] [CrossRef]
- Dedyukhin, S.V. Fauna and biotopic distribution of weevils (Coleoptera: Curculionoidea) of the Zhiguli State Nature Reserve, Russia. Nat. Conserv. Res. 2022, 7, 55–69. [Google Scholar] [CrossRef]
- Sushchuk, A.A.; Matveeva, E.M. Soil nematodes of coniferous forests in the Finnish-Russian Friendship Nature Reserve. Nat. Conserv. Res. 2021, 6 (Suppl. 1), 76–88. [Google Scholar] [CrossRef]
- Parhomenko, O.; Langraf, V.; Petrovičová, K.; Komlyk, V.; Brygadyrenko, V. Morphometric variability of ground beetles Bembidion minimum (Coleoptera, Carabidae): Who should change more, males or females? Nat. Conserv. Res. 2022, 7, 42–69. [Google Scholar] [CrossRef]
- Sundukov, Y.N.; Makarov, K.V. The ground beetles of the tribus Trechini (Carabidae) on the Southern Kuril Islands. Nat. Conserv. Res. 2021, 6, 15–51. [Google Scholar] [CrossRef]
- Zouaimia, A.; Adjami, Y.; Zebsa, R.; Youcefi, A.; Bensakhri, Z.; Bensouilah, S.; Amari, H.; Ouakid, M.-L.; Houhamdi, M.; Mahdjoub, H.; et al. Phenology of the regionally Critically Endangered dragonfly Urothemis edwardsii in the National Park of El Kala, Northeast of Algeria. Nat. Conserv. Res. 2022, 7, 1–9. [Google Scholar] [CrossRef]
- Anselmo, L.; Rizzioli, B. Side threats: Further possible effects of warming on the high alpine narrow endemic Carabus cychroides (Coleoptera: Carabidae). Nat. Conserv. Res. 2022, 7, 88–94. [Google Scholar] [CrossRef]
- Musolin, D.L. Insects in a warmer world: Ecological, physiological and life-history responses of true bugs (Heteroptera) to climate change. Glob. Chang. Biol. 2007, 13, 1565–1585. [Google Scholar] [CrossRef]
- Plavilshchikov, N.N. A list of insect species found on the territory of the Mordovia State Nature Reserve. Proc. Mordovia State Nat. Reserve 1964, 2, 105–134. [Google Scholar]
- Timraleev, Z.A.; Kamenev, A.G.; Bardin, O.D. Insects of Mordovia. Part 1; Mordovia University Press: Saransk, Russia, 2005; 104p. [Google Scholar]
- Ruchin, A.B. List of insect species of the National Park “Smolny”. Proc. Natl. Park Smolny 2008, 1, 151–180. [Google Scholar]
- Ruchin, A.B.; Mikhailenko, A.P. Fauna of mantids and orthopterans (Insecta: Mantodea, Orthoptera) of the Mordovia State Nature Reserve, Russia. Biodiversitas 2018, 19, 1194–1206. [Google Scholar] [CrossRef]
- Ruchin, A.; Aleksanov, V.; Karmazina, I.; Esin, M.; Lukiyanov, S.; Lobachev, E.; Artaev, O.; Ryzhov, M. Biodiversity of Orthopteroidea (Insecta) in the Republic of Mordovia (Russia). Joint Directorate of the Mordovia State Nature Reserve and National Park “Smolny”. Occurrence Dataset. 2023. Available online: www.GBIF.org (accessed on 29 May 2023). [CrossRef]
- Sergeev, M.G. Distribution Patterns of Orthoptera in North Asia of Northern Asia; Nauka: Novosibirsk, Russia, 1986; 238p. [Google Scholar]
- Hochkirch, A.; Nieto, A.; García Criado, M.; Cálix, M.; Braud, Y.; Buzzetti, F.M.; Tumbrinck, J. European Red List of Grasshoppers, Crickets and Bush-Crickets; Publications Office: Luxembourg, 2017. [Google Scholar] [CrossRef]
- Aleksanov, V.V. Life cycle and habitats of the European earwig Forficula auricularia L. (Dermaptera, Forficulidae) in Kaluga, Russia. Euroasian Entomol. J. 2015, 14, 285–292. [Google Scholar]
- Özgen, İ.; Ayaz, T.; Kitir, N. Dermaptera species in apricot orchards and its pest status in Malatya and Elazığ provinces of Eastern Anatolia, Turkey. Biharean Biol. 2016, 10, 58–59. [Google Scholar]
- Orpet, R.J.; Crowder, D.W.; Jones, V.P. Biology and management of European earwig in orchards and vineyards. J. Integr. Pest Manag. 2019, 10, 21. [Google Scholar] [CrossRef]
- Kirstová, M.; Pyszko, P.; Šipoš, J.; Drozd, P.; Kočárek, P. Vertical distribution of earwigs (Dermaptera: Forficulidae) in a temperate lowland forest, based on sampling with a mobile aerial lift platform. Entomol. Sci. 2017, 20, 57–64. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Egorov, L.V.; Khapugin, A.A.; Vikhrev, N.E.; Esin, M.N. The use of simple crown traps for the insects collection. Nat. Conserv. Res. 2020, 5, 87–108. [Google Scholar] [CrossRef]
- González-Miguéns, R.; Muñoz-Nozal, E.; Jiménez-Ruiz, Y.; Mas-Peinado, P.; Ghanavi, H.R.; García-París, M. Speciation patterns in the species complex: Cryptic and not so cryptic taxa across the western Palaearctic region. Zool. J. Linn. Soc. 2020, 190, 788–823. [Google Scholar] [CrossRef]
- Anlaş, S.; Haas, F.; Tezcan, S. Dermaptera (Insecta) fauna of Bozdaglar mountain, western Turkey. Linz. Biol. Beiträge 2010, 42, 389–399. [Google Scholar]
- Elisovetskaya, D.; Shleahtich, V.; Musleh, M.; Cristman, D. Influence of biorational pesticides on useful entomofauna in the Republic of Moldova. Olten. Stud. Comun. Stiintele Nat. 2014, 30, 89–97. [Google Scholar]
- Holuša, J.; Farkač, J. Occurrence of Labidura riparia (Dermaptera) in the Czech Republic. Acta Mus. Beskid. 2010, 2, 193. [Google Scholar]
- Dvorák, L. Confirmed occurrence of Labidura riparia (Pallas, 1773) on Cyprus (Dermaptera). Mun. Ent. Zool. 2017, 12, 361. [Google Scholar]
- Haas, F.; Henderickx, H. Dermaptera from Cyprus and Turkey. Beiträge Zur Entomol. 2002, 52, 235–239. [Google Scholar] [CrossRef]
- Grzędzicka, E.; Vahed, K. Habitat requirements of the endangered heath bush-cricket Gampsocleis glabra (Orthoptera, Tettigoniidae) in an isolated population. J. Insect Conserv. 2020, 24, 935–945. [Google Scholar] [CrossRef]
- Schirmel, J.; Blindow, I.; Fartman, T. The importance of habitat mosaics for Orthoptera (Caelifera and Ensifera) in dry heathlands. Eur. J. Entomol. 2010, 107, 129–132. [Google Scholar] [CrossRef]
- Karmazina, I.O.; Shulaev, N.V. Phenological Characteristics of Orthopterous Insects (Orthoptera) in the Volga-Kama State Nature Biosphere Reserve. Izv. Saratov Univ. Ser. Chem. Biol. Ecol. 2018, 18, 429–432. [Google Scholar] [CrossRef]
- Sergeev, M.G.; Vanjkova, I.A. Zonal-lanscape distribution of Podisma pedestris L. Orthoptera, Acrididae). Euroasian Entomol. J. 2003, 2, 157–165. [Google Scholar]
- Dreux, P. Recherches écologiques et biogéographiques sur les Orthoptères des Alpes françaises. Ann. Des Sci. Nat. 1962, 3, 323–766. [Google Scholar]
- Zinenko, N.V.; Korsunovskaya, O.S.; Striganova, B.R. Orthoptera and mantids of steppe biocenoses in Saratov region. Povolzhskiy Ekol. Zhurnal 2005, 1, 12–28. [Google Scholar]
- Karmazina, I.O.; Shulaev, N.V. Ecological and faunistic review of Orthoptera in the central part of the Volga-Kama region (Republic of Tatarstan). Entmol. Rev. 2015, 95, 832–851. [Google Scholar] [CrossRef]
- Hochkirch, A.; Gärtner, A.C.; Brandt, T. Effects of forest-dune ecotone management on the endangered heath grasshopper, Chorthippus vagans (Orthoptera: Acrididae). Bull. Entomol. Res. 2008, 98, 449–456. [Google Scholar] [CrossRef]
- Ikonnikov, N.F. To the knowledge of the straight-winged of the Russian Empire. Russian. Entomol. Rev. 1911, 11, 96–110. [Google Scholar]
- Hesoun, P. Occurrence of Stenobothrus stigmaticus (Orthoptera, Acrididae) in the Jindřichův Hradec region. Západočeské Entomol. Listy 2012, 3, 17–21. [Google Scholar]
- Fardisi, M.; Gondhalekar, A.D.; Ashbrook, A.R.; Scharf, M.E. Rapid evolutionary responses to insecticide resistance management interventions by the German cockroach (Blattella germanica L.). Sci. Rep. 2019, 9, 8292. [Google Scholar] [CrossRef] [PubMed]
- Abbar, S.; Cooper, R.; Ranabhat, S.; Pan, X.; Sked, S.; Wang, C. Prevalence of cockroaches, bed bugs, and house mice in low-income housing and evaluation of baits for monitoring house mouse infestations. J. Med. Entomol. 2022, 59, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Bei-Bienko, G. Blattodea (Nasekomye Tarakanovye); Academy Nauk: Moscow, Russia, 1950; 344p. [Google Scholar]
- Holuša, J.; Kočárek, P. Seasonal dynamics of the dusky cockroach Ectobius lapponicus (Blattodea, Blattellidae) in the eastern part of the Czech Republic. Biologia 2000, 55, 483–486. [Google Scholar]
- Clements, J.C.; Doucet, D.A.; McCorquodale, D.B. Establishment of a European cockroach, Ectobius lapponicus (L.) (Dictyoptera: Blattodea), in the Maritime Provinces of eastern Canada. J. Acadian Entomol. Soc. 2013, 9, 4–7. [Google Scholar]
- Yamashkin, A.A.; Ruzhenkov, V.V.; Yamashkin, A.A. Geography of the Republic of Mordovia; MGU Publ.: Saransk, Russia, 2004; 168p. [Google Scholar]
- Golub, V.B.; Tsurikov, M.N.; Prokin, A.A. Insect Collections: Collection, Processing and Storage of Material; KMK Scientific Press Ltd.: Moscow, Russia, 2012; 339p. [Google Scholar]
- Bei-Bienko, G.Y.; Mishenko, L.L. Locusts and Grasshoppers of the USSR and Adjacent Countries II. Keys to the Fauna of the USSR; 40; Academy Nauk: Moscow, Russia, 1964. [Google Scholar]
- Bei-Bienko, G.J.; Mistshenko, L.L. The Grasshopper of the Fauna of the USSR and Adjacent Countries, Part II.; Academy Nauk: Moscow, Russia, 1951; 668p. [Google Scholar]
- Bukhvalova, M.A. Acoustic signals and morphological features of some grasshoppers of the Chorthippus biguttulus group (Orthoptera, Acrididae) of Russia and adjacent territories. Entomol. Rev. 1995, 74, 56–67. [Google Scholar]
- Çiplak, B.; Heller, K.G.; Demirsoy, A. Review and key to species of Platycleis from Turkey (Orthoptera: Tettigoniidae) with descriptions of Yalvaciana subgen. n. and two new species. J. Nat. Hist. 2002, 36, 197–236. [Google Scholar] [CrossRef]
- Willemse, F.; von Helversen, O.; Odé, B. A review of Chorthippus species with angled pronotal lateral keels from Greece with special reference to transitional populations between some Peloponnesean taxa (Orthoptera, Acrididae). Zool. Meded. 2009, 83, 319–508. [Google Scholar]
- Cigliano, M.M.; Braun, H.; Eades, D.C.; Otte, D. Orthoptera Species File. Version 5.0/5.0. Available online: http://Orthoptera.SpeciesFile.org (accessed on 18 March 2023).





| Column Label | Column Description |
|---|---|
| eventID | An identifier for the set of information associated with an event (occurs in one place at one time). |
| occurrenceID | An identifier for the occurrence (as opposed to a particular digital record of the occurrence). |
| basisOfRecord | The specific nature of the data record: HumanObservation. |
| scientificName | The full scientific name including the genus name and the lowest level of taxonomic rank with the authority. |
| kingdom | The full scientific name of the kingdom in which the taxon is classified. |
| taxonRank | The taxonomic rank of the most specific name in the scientificName. |
| decimalLatitude | The geographic latitude of location in decimal degrees. |
| decimalLongitude | The geographic longitude of location in decimal degrees. |
| geodeticDatum | The ellipsoid, geodetic datum, or spatial reference system (SRS) upon which the geographic coordinates given in decimalLatitude and decimalLongitude are based. |
| country | The name of the country in which the location occurs. |
| countryCode | The standard code for the country in which the location occurs. |
| individualCount | The number of individuals represented present at the time of the occurrence. |
| eventDate | The date when material from the trap was collected or the range of dates during which the trap collected material. |
| year | The integer day of the month on which the event occurred. |
| month | The ordinal month in which the event occurred. |
| day | The integer day of the month on which the event occurred. |
| samplingProtocol | The names of, references to, or descriptions of the methods or protocols used during an event. |
| recordedBy | A person, group, or organization responsible for recording the original occurrence. |
| identifiedBy | A list of names of people who assigned the taxon to the subject. |
| Order, Family, Species | Meadows | Steppe Communities | Ruderal Communities | Forest Ecosystems | Agroecosystems | Human Habitations |
|---|---|---|---|---|---|---|
| DERMAPTERA | ||||||
| Forficulidae | ||||||
| Forficula auricularia Linnaeus, 1758 | + | + | + | + | + | + |
| Forficula tomis (Kolenati, 1846) | + | + | + | + | + | |
| Spongiphoridae | ||||||
| * Labia minor (Linnaeus, 1758) | + | + | ||||
| Labiduridae | ||||||
| Labidura riparia (Pallas, 1773) | + | |||||
| ORTHOPTERA | ||||||
| Tettigoniidae | ||||||
| Barbitistes constrictus (Brunner von Wattenwyl, 1878) | + | |||||
| Bicolorana bicolor (Philippi, 1830) | + | + | + | + | + | |
| Conocephalus dorsalis (Latreille, 1804) | + | + | + | |||
| Conocephalus fuscus (Fabricius, 1793) | + | + | + | + | + | |
| Decticus verrucivorus (Linnaeus, 1758) | + | + | + | + | + | |
| *Gampsocleis glabra (Herbst, 1786) | + | + | ||||
| Isophya modesta (Frivaldszky, 1868) | + | + | ||||
| * Leptophyes albovittata (Kollar, 1833) | + | |||||
| Metrioptera brachyptera (Linnaeus, 1761) | + | + | + | + | + | |
| * Montana montana (Kollar, 1833) | + | |||||
| Onconotus servillei Fischer von Waldheim, 1846 | + | |||||
| Phaneroptera falcata (Poda, 1761) | + | + | + | + | + | |
| Pholidoptera griseoaptera (De Geer, 1773) | + | + | + | + | ||
| * Platycleis albopunctata (Goeze, 1778) | + | + | ||||
| * Tessellana veyseli (Koçak, 1984) | + | + | ||||
| Poecilimon intermedius (Fieber, 1853) | + | + | + | |||
| Roeseliana roeselii (Hagenbach, 1822) | + | + | + | + | + | |
| Tettigonia caudata (Charpentier, 1845) | + | + | + | + | + | |
| Tettigonia cantans (Fuessly, 1775) | + | + | + | + | + | |
| Tettigonia viridissima (Linnaeus, 1758) | + | + | + | + | + | |
| Gryllidae | ||||||
| Acheta domesticus (Linnaeus, 1758) | + | |||||
| * Eumodicogryllus bordigalensis (Latreille, 1804) | + | |||||
| Gryllus campestris (Linnaeus, 1758) | + | + | + | |||
| Melanogryllus desertus (Pallas, 1771) | + | + | ||||
| Modicogryllus frontalis (Fieber, 1844) | + | + | + | + | + | + |
| Oecanthus pellucens (Scopoli, 1763) | + | + | + | + | + | |
| Tetrigidae | ||||||
| Tetrix bipunctata (Linnaeus, 1758) | + | + | + | + | + | |
| Tetrix subulata (Linnaeus, 1758) | + | + | + | + | + | |
| Tetrix tenuicornis (Sahlberg, 1893) | + | + | + | + | + | |
| Gryllotalpidae | ||||||
| Gryllotalpa gryllotalpa (Linnaeus, 1758) | + | + | + | |||
| Acrididae | ||||||
| ?Bryodemella tuberculatum (Fabricius, 1775) | + | |||||
| Calliptamus italicus (Linnaeus, 1758) | + | + | + | |||
| Chorthippus albomarginatus (De Geer, 1773) | + | + | + | + | + | |
| Chorthippus apricarius (Linnaeus, 1758) | + | + | + | + | + | |
| Chorthippus brunneus (Thunbberg, 1815) | + | + | + | + | + | |
| Chorthippus biguttulus (Linnaeus, 1758) | + | + | + | + | + | |
| * Chorthippus dichrous (Eversmann, 1859) | + | |||||
| Chorthippus dorsatus (Zetterstedt, 1821) | + | + | + | + | + | |
| * Chorthippus macrocerus (Fischer von Waldheim, 1846) | + | + | + | |||
| Chorthippus mollis (Charpentier, 1825) | + | + | + | + | + | |
| Chorthippus pullus (Philippi, 1830) | + | |||||
| * Chorthippus vagans (Eversmann, 1848) | + | |||||
| Chrysochraon dispar (Germar, 1834) | + | + | + | + | + | |
| Dociostaurus brevicollis (Eversmann, 1848) | + | + | + | |||
| Epacromius pulverulentus (Fischer von Waldheim, 1846) | + | + | ||||
| * Euchorthippus pulvinatus (Fischer von Waldheim, 1846) | + | + | ||||
| Euthystira brachyptera (Ocskay, 1826) | + | + | + | + | + | |
| Gomphocerippus rufus (Linnaeus, 1758) | + | + | + | + | ||
| * Gomphocerus sibiricus (Linnaeus, 1767) | + | |||||
| Locusta migratoria (Linnaeus, 1758) | + | + | + | |||
| Myrmeleotettix maculatus (Thunberg, 1815) | + | + | ||||
| Oedipoda caerulescens (Linnaeus, 1758) | + | + | + | + | ||
| Omocestus haemorrhoidalis (Charpentier, 1825) | + | + | + | + | + | |
| ?Omocestus rufipes (Zetterstedt, 1821) | + | |||||
| Omocestus viridulus (Linnaeus, 1758) | + | + | + | + | ||
| Podisma pedestris (Linnaeus, 1758) | + | |||||
| Pseudochorthippus parallelus (Zetterstedt, 1821) | + | + | + | + | + | |
| Psophus stridulus (Linnaeus, 1758) | + | + | + | |||
| Stenobothrus lineatus (Panzer, 1796) | + | + | + | + | + | |
| Stenobothrus nigromaculatus (Herrich-Schaffer, 1840) | + | |||||
| * Stenobothrus stigmaticus (Rambur, 1838) | + | + | ||||
| Sphingonotus caerulans (Linnaeus, 1767) | + | + | + | |||
| Stauroderus scalaris (Fischer von Waldheim, 1846) | + | + | ||||
| Stethophyma grossum (Linnaeus 1758) | + | + | ||||
| MANTODEA | ||||||
| Mantidae | ||||||
| Mantis religiosa (Linnaeus, 1758) | + | + | + | + | + | |
| BLATTODEA | ||||||
| Blattidae | ||||||
| Blatta orientalis Linnaeus, 1758 | + | |||||
| Blattella germanica (Linnaeus, 1767) | + | |||||
| Ectobiidae | ||||||
| Ectobius lapponicus (Linnaeus, 1758) | + | + | + | + | + | |
| Ectobius sylvestris (Poda, 1761) | + | + | + | + | + | |
| TOTAL | 43 | 53 | 43 | 61 | 33 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleksanov, V.V.; Karmazina, I.O.; Ruchin, A.B.; Esin, M.N.; Lukiyanov, S.V.; Lobachev, E.A.; Artaev, O.N.; Ryzhov, M.K. Diversity and Biology of Terrestrial Orthopteroids (Insecta) in the Republic of Mordovia (Russia). Diversity 2023, 15, 803. https://doi.org/10.3390/d15070803
Aleksanov VV, Karmazina IO, Ruchin AB, Esin MN, Lukiyanov SV, Lobachev EA, Artaev ON, Ryzhov MK. Diversity and Biology of Terrestrial Orthopteroids (Insecta) in the Republic of Mordovia (Russia). Diversity. 2023; 15(7):803. https://doi.org/10.3390/d15070803
Chicago/Turabian StyleAleksanov, Victor V., Inessa O. Karmazina, Alexander B. Ruchin, Mikhail N. Esin, Sergei V. Lukiyanov, Evgeniy A. Lobachev, Oleg N. Artaev, and Maxim K. Ryzhov. 2023. "Diversity and Biology of Terrestrial Orthopteroids (Insecta) in the Republic of Mordovia (Russia)" Diversity 15, no. 7: 803. https://doi.org/10.3390/d15070803
APA StyleAleksanov, V. V., Karmazina, I. O., Ruchin, A. B., Esin, M. N., Lukiyanov, S. V., Lobachev, E. A., Artaev, O. N., & Ryzhov, M. K. (2023). Diversity and Biology of Terrestrial Orthopteroids (Insecta) in the Republic of Mordovia (Russia). Diversity, 15(7), 803. https://doi.org/10.3390/d15070803







