Colonization in Artificial Seaweed Substrates: Two Locations, One Year
Abstract
1. Introduction
2. Materials and Methods
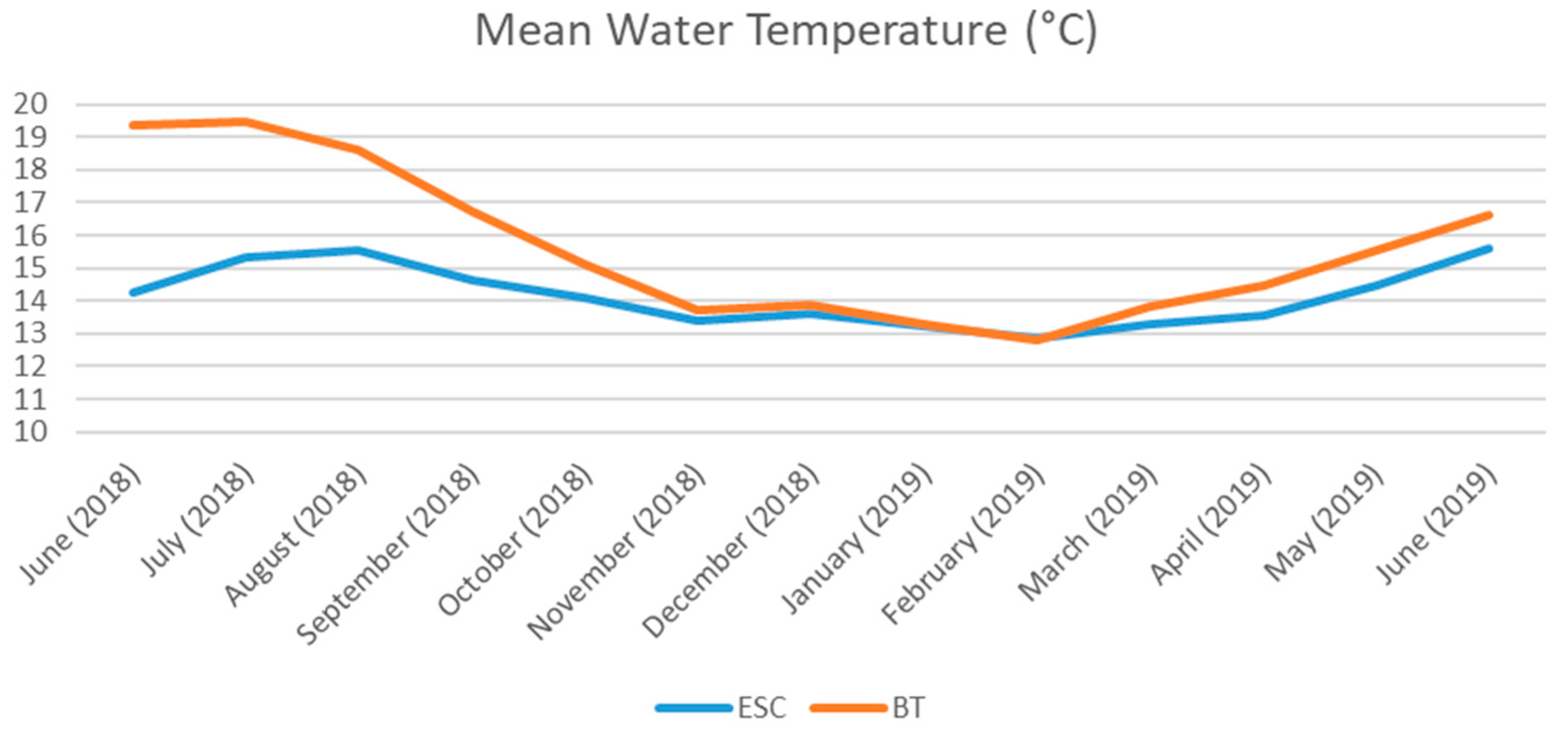
2.1. Study Area
2.2. Sampling Strategy
2.3. Data Analysis
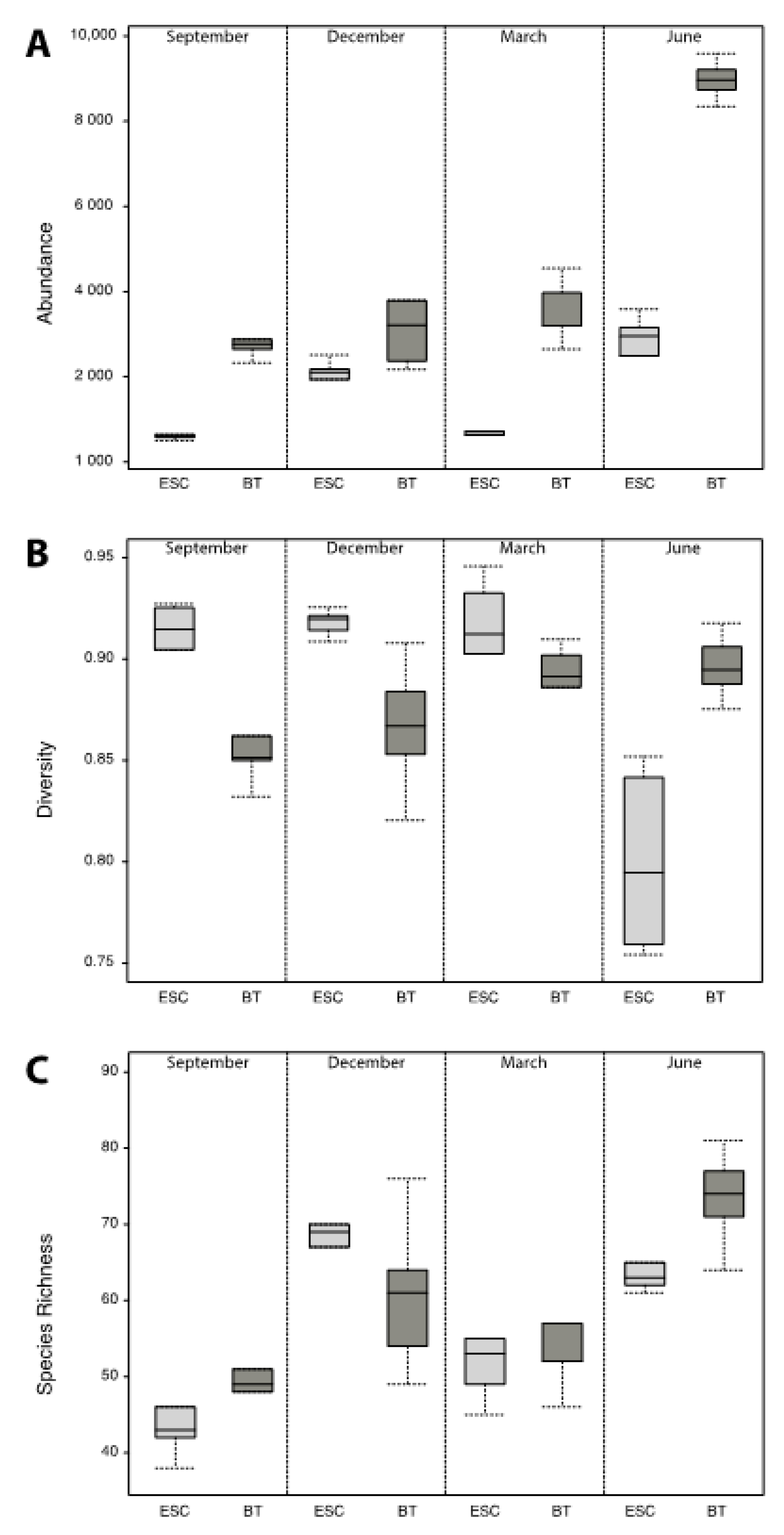
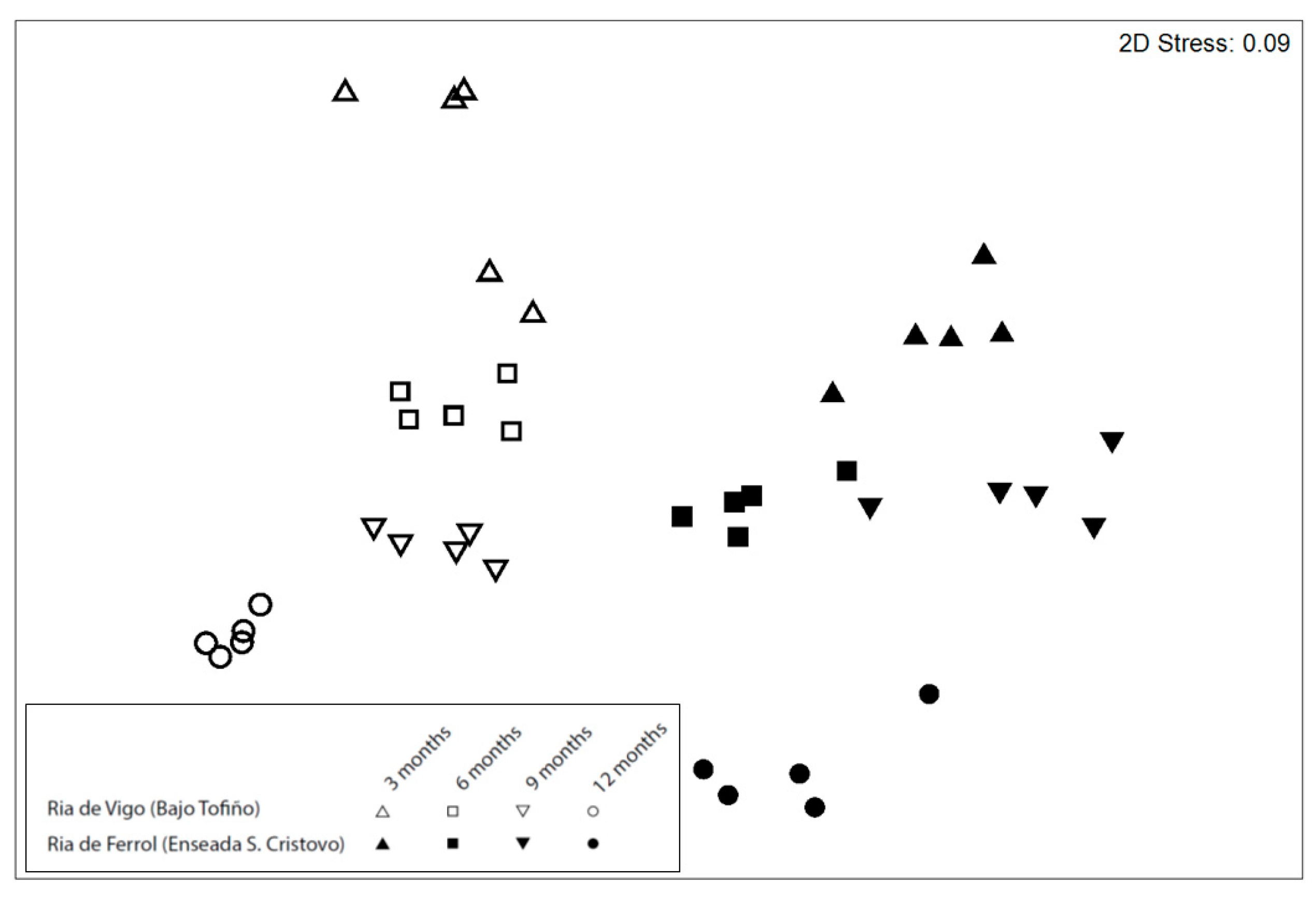
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antoniadou, C.; Voultsiadou, E.; Chintiroglou, C. Benthic colonization and succession on temperate sublittoral rocky cliffs. J. Exp. Mar. Biol. Ecol. 2010, 382, 145–153. [Google Scholar] [CrossRef]
- Antoniadou, C.; Voultsiadou, E.; Chintiroglou, C. Seasonal patterns of colonization and early succession on sublittoral rocky cliffs. J. Exp. Mar. Biol. Ecol. 2011, 403, 21–30. [Google Scholar] [CrossRef]
- Anderson, M.; Diebel, C.E.; Blom, W.M.; Landers, T.J. Consistency and variation in kelp holdfast assemblages: Spatial patterns of biodiversity for the major phyla at different taxonomic resolutions. J. Exp. Mar. Biol. Ecol. 2005, 320, 35–56. [Google Scholar] [CrossRef]
- Benedetti-Cecchi, L. Variability in abundance of algae and invertebrates at different spatial scales on rocky sea shores. Mar. Ecol. Prog. Ser. 2001, 215, 79–92. [Google Scholar] [CrossRef]
- Fraschetti, S.; Terlizzi, A.; Benedetti-Cecchi, L. From proof image to formal proof-a transformation. Mar. Ecol. Prog. Ser. 2005, 296, 13–29. [Google Scholar] [CrossRef]
- Underwood, A.J.; Chapman, M.G. International Association for Ecology Scales of Spatial Patterns of Distribution of Intertidal Invertebrates. Oecologia 1996, 107, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.; Arvanitidis, C.; Borja, A.; Frid, C.; Hiddink, J.G.; Krause, J.; Lorance, P.; Ragnarsson, S.Á.; Sköld, M.; Trabucco, B.; et al. Indicators for sea-floor integrity under the european marine strategy framework directive. Ecol. Indic. 2012, 12, 174–184. [Google Scholar] [CrossRef]
- Danovaro, R.; Carugati, L.; Berzano, M.; Cahill, A.E.; Carvalho, S.; Chenuil, A.; Corinaldesi, C.; Cristina, S.; David, R.; Dell’Anno, A.; et al. Implementing and Innovating Marine Monitoring Approaches for Assessing Marine Environmental Status. Front. Mar. Sci. 2016, 3, 213. [Google Scholar] [CrossRef]
- Gray, J.S. Marine biodiversity: Patterns, threats and conservation needs. Biodivers. Conserv. 1997, 175, 153–175. [Google Scholar] [CrossRef]
- Aneiros, F.; Moreira, J.; Troncoso, J.S. A functional approach to the seasonal variation of benthic mollusc assemblages in an estuarine-like system. J. Sea Res. 2014, 85, 73–84. [Google Scholar] [CrossRef]
- Beisiegel, K.; Darr, A.; Gogina, M.; Zettler, M.L. Benefits and shortcomings of non-destructive benthic imagery for monitoring hard-bottom habitats. Mar. Pollut. Bull. 2017, 121, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Veiga, P.; Redondo, W.; Sousa-Pinto, I.; Rubal, M. Relationship between structure of macrobenthic assemblages and environmental variables in shallow sublittoral soft bottoms. Mar. Environ. Res. 2017, 129, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Borja, A.; Franco, J.; Pérez, V. A marine Biotic Index to establish the ecological quality of soft-bottom benthos within European estuarine and coastal environments. Mar. Pollut. Bull. 2000, 40, 1100–1114. [Google Scholar] [CrossRef]
- Veiga, P.; Torres, A.C.; Aneiros, F.; Sousa-Pinto, I.; Troncoso, J.S.; Rubal, M. Consistent patterns of variation in macrobenthic assemblages and environmental variables over multiple spatial scales using taxonomic and functional approaches. Mar. Environ. Res. 2016, 120, 191–201. [Google Scholar] [CrossRef]
- Yakovis, E.L.; Artemieva, A.V.; Fokin, M.V.; Varfolomeeva, M.A.; Shunatova, N.N. Effect of habitat architecture on mobile benthic macrofauna associated with patches of barnacles and ascidians. Mar. Ecol. Prog. Ser. 2007, 348, 117–124. [Google Scholar] [CrossRef]
- Commito, J.A.; Como, S.; Grupe, B.M.; Dow, W.E. Species diversity in the soft-bottom intertidal zone: Biogenic structure, sediment, and macrofauna across mussel bed spatial scales. J. Exp. Mar. Biol. Ecol. 2008, 366, 70–81. [Google Scholar] [CrossRef]
- Terlizzi, A.; Anderson, M.J.; Fraschetti, S.; Benedetti-Cecchi, L. Scales of spatial variation in Mediterranean subtidal sessile assemblages at different depths. Mar. Ecol. Prog. Ser. 2007, 332, 25–39. [Google Scholar] [CrossRef]
- Underwood, A.J.; Chapman, M.G. Early development of subtidal macrofaunal assemblages: Relationships to period and timing of colonization. J. Exp. Mar. Biol. Ecol. 2006, 330, 221–233. [Google Scholar] [CrossRef]
- Kuklinski, P.; Balazy, P.; Porter, J.; Loxton, J.; Ronowicz, M.; Sokołowski, A. Experimental apparatus for investigating colonization, succession and related processes of rocky bottom epifauna. Cont. Shelf Res. 2022, 233, 104641. [Google Scholar] [CrossRef]
- Wollgast, S.; Lenz, M.; Wahl, M.; Molis, M. Effects of regular and irregular temporal patterns of disturbance on biomass accrual and species composition of a subtidal hard-bottom assemblage. Helgol. Mar. Res. 2008, 62, 309–319. [Google Scholar] [CrossRef]
- Baronio, M.D.A.; Bucher, D.J. Artificial crevice habitats to assess the biodiversity of vagile macro-cryptofauna of subtidal rocky reefs. Mar. Freshw. Res. 2008, 59, 661–670. [Google Scholar] [CrossRef]
- Dean, R.L.; Connell, J.H. Marine invertebrates in an algal succession. I. Variations in abundance and diversity with succession. J. Exp. Mar. Biol. Ecol. 1987, 109, 195–215. [Google Scholar] [CrossRef]
- Platt, W.J.; Connell, J.H. Natural disturbances and directional replacement of species. Ecol. Monogr. 2003, 73, 507–522. [Google Scholar] [CrossRef]
- Chapman, M.G. Colonization of novel habitat: Tests of generality of patterns in a diverse invertebrate assemblage. J. Exp. Mar. Biol. Ecol. 2007, 348, 97–110. [Google Scholar] [CrossRef]
- Olabarria, C. Role of colonization in spatio-temporal patchiness of microgastropods in coralline turf habitat. J. Exp. Mar. Biol. Ecol. 2002, 274, 121–140. [Google Scholar] [CrossRef]
- Aguado-Giménez, F.; Gairín, J.I.; Martinez-Garcia, E.; Fernandez-Gonzalez, V.; Ballester Moltó, M.; Cerezo-Valverde, J.; Sanchez-Jerez, P. Application of “taxocene surrogation” and “taxonomic sufficiency” concepts to fish farming environmental monitoring. Comparison of BOPA index versus polychaete assemblage structure. Mar. Environ. Res. 2015, 103, 27–35. [Google Scholar] [CrossRef]
- Van Der Linden, P.; Marchini, A.; Dolbeth, M.; Patrício, J.; Veríssimo, H.; Marques, J.C. The performance of trait-based indices in an estuarine environment. Ecol. Indic. 2015, 61, 378–389. [Google Scholar] [CrossRef]
- Salas, F.; Marcos, C.; Neto, J.M.; Patrı, J. User-friendly guide for using benthic ecological indicators in coastal and marine quality assessment. Ocean Coast. Manag. 2006, 49, 308–331. [Google Scholar] [CrossRef]
- Cacabelos, E.; Olabarria, C.; Incera, M.; Troncoso, J.S. Effects of habitat structure and tidal height on epifaunal assemblages associated with macroalgae. Estuar. Coast. Shelf Sci. 2010, 89, 43–52. [Google Scholar] [CrossRef]
- Christie, H.; Norderhaug, K.M.; Fredriksen, S. Macrophytes as habitat for fauna. Mar. Ecol. Prog. Ser. 2009, 396, 221–233. [Google Scholar] [CrossRef]
- Edgar, G.J. Distribution patterns of mobile epifauna associated with rope fibre habitats within the Bathurst Harbour estuary, south-western Tasmania. Estuar. Coast. Shelf Sci. 1991, 33, 589–604. [Google Scholar] [CrossRef]
- Edgar, G.J. Artificial algae as habitats for mobile epifauna: Factors affecting colonization in a Japanese Sargassum bed. Hydrobiologia 1991, 226, 111–118. [Google Scholar] [CrossRef]
- García-Sanz, S.; Navarro, P.G.; Tuya, F. Colonization of Prosobranch Gastropods onto Artificial Substrates: Seasonal Patterns between Habitat Patches. Am. Malacol. Bull. 2014, 32, 94–103. [Google Scholar] [CrossRef]
- Norderhaug, K.M.; Christie, H.; Rinde, E. Colonisation of kelp imitations by epiphyte and holdfast fauna: A study of mobility patterns. Mar. Biol. 2002, 141, 965–973. [Google Scholar] [CrossRef]
- Myers, A.A.; Southgate, T. Artificial substrates as a means of monitoring Rocky shore cryptofauna. J. Mar. Biol. Assoc. United Kingd. 1980, 60, 963–975. [Google Scholar] [CrossRef]
- Hylkema, A.; Debrot, A.O.; Pistor, M.; Postma, E.; Williams, S.M.; Kitson-Walters, K. High peak settlement of Diadema antillarum on different artificial collectors in the Eastern Caribbean. J. Exp. Mar. Biol. Ecol. 2022, 549, 151693. [Google Scholar] [CrossRef]
- García-Sanz, S.; Tuya, F.; Navarro, P.G.; Angulo-Preckler, C.; Haroun, R.J. Post larval, short-term, colonization patterns: The effect of substratum complexity across subtidal, adjacent, habitats. Estuar. Coast. Shelf Sci. 2012, 112, 183–191. [Google Scholar] [CrossRef]
- Carreira-Flores, D.; Neto, R.; Ferreira, H.; Cabecinha, E.; Díaz-Agras, G.; Gomes, P.T. Artificial substrates as sampling devices for marine epibenthic fauna: A quest for standardization. Reg. Stud. Mar. Sci. 2020, 37, 101331. [Google Scholar] [CrossRef]
- Zimmerman, T.L.; Martin, J.W. Artificial Reef Matrix Structures (Arms): An Inexpensive and Effective Method for Collecting Coral Reef-Associated Invertebrates. Gulf Caribb. Res. 2004, 16, 59–64. [Google Scholar] [CrossRef]
- Plaisance, L.; Caley, M.J.; Brainard, R.E.; Knowlton, N. The diversity of coral reefs: What are we missing? PLoS ONE 2011, 6, e25026. [Google Scholar] [CrossRef]
- Carreira-Flores, D.; Neto, R.; Ferreira, H.; Cabecinha, E.; Díaz-Agras, G.; Gomes, P.T. Two better than one: The complementary of different types of artificial substrates on benthic marine macrofauna studies. Mar. Environ. Res. 2021, 171, 105449. [Google Scholar] [CrossRef]
- Ransome, E.; Geller, J.B.; Timmers, M.; Leray, M.; Mahardini, A.; Sembiring, A.; Collins, A.G.; Meyer, C.P. The importance of standardization for biodiversity comparisons: A case study using autonomous reef monitoring structures (ARMS) and metabarcoding to measure cryptic diversity on Mo’orea coral reefs, French Polynesia. PLoS ONE 2017, 12, e0175066. [Google Scholar] [CrossRef] [PubMed]
- Pearman, J.K.; Leray, M.; Villalobos, R.; Machida, R.J.; Berumen, M.L.; Knowlton, N.; Carvalho, S. Cross-shelf investigation of coral reef cryptic benthic organisms reveals diversity patterns of the hidden majority. Sci. Rep. 2018, 8, 8090. [Google Scholar] [CrossRef] [PubMed]
- Obst, M.; Exter, K.; Allcock, A.L.; Arvanitidis, C.; Axberg, A.; Bustamante, M.; Cancio, I.; Carreira-Flores, D.; Chatzinikolaou, E.; Chatzigeorgiou, G.; et al. A Marine Biodiversity Observation Network for Genetic Monitoring of Hard-Bottom Communities (ARMS-MBON). Front. Mar. Sci. 2020, 7, 1031. [Google Scholar] [CrossRef]
- Prego, R.; Fraga, F. A simple model to calculate the residual flows in a Spanish Ría. Hydrographic consequences in the Ría of Vigo. Estuar. Coast. Shelf Sci. 1992, 34, 603–615. [Google Scholar] [CrossRef]
- Torres, A.C.; Veiga, P.; Rubal, M.; Sousa-Pinto, I. The role of annual macroalgal morphology in driving its epifaunal assemblages. J. Exp. Mar. Biol. Ecol. 2015, 464, 96–106. [Google Scholar] [CrossRef]
- DeCastro, M.; Gómez-Gesteira, M.; Prego, R.; Neves, R. Wind influence on water exchange between the ria of Ferrol (NW Spain) and the shelf. Estuar. Coast. Shelf Sci. 2003, 56, 1055–1064. [Google Scholar] [CrossRef]
- Cunha, X. Macrofauna Bentónica da Enseada de Santa Lucía (Ría de Ferrol, Galicia): Diversidade e Dinámica Temporal a Longo Prazo. Ph.D. Thesis, University of Santiago de Compostela, A Coruña, Spain, 2017. [Google Scholar]
- Díaz-Agras, G. Patrones de Distribución de Moluscos Gasterópodos en Sustratos Duros Intermareales Naturales y Artificiales en la Ría de Ferrol, Galicia. Ph.D. Thesis, University of Santiago de Compostela, A Coruña, Spain, 2015. [Google Scholar]
- Prego, R.; Filgueiras, A.; Santos-Echeandía, J. Temporal and spatial changes of total and labile metal concentration in the surface sediments of the Vigo Ría (NW Iberian Peninsula): Influence of anthropogenic sources. Mar. Pollut. Bull. 2008, 56, 1022–1031. [Google Scholar] [CrossRef]
- WoRMS Editorial Board. World Register of Marine Species. Available online: http://www.marinespecies.org (accessed on 29 September 2020).
- R Core Team R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Anderson, M.J. Permutational Multivariate Analysis of Variance (PERMANOVA). Wiley StatsRef Stat. Ref. Online 2017, 1–15. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v. 6: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2001. [Google Scholar]
- Anderson, M.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008; Volume 1. [Google Scholar]
- Tuya, F.; Wernberg, T.; Thomsen, M.S. Colonization of gastropods on subtidal reefs depends on density in adjacent habitats, not on disturbance regime. J. Molluscan Stud. 2008, 75, 27–33. [Google Scholar] [CrossRef]
- Russo, A.R. The role of seaweed complexity in structuring Hawaiian epiphytal amphipod communities. Hydrobiologia 1990, 194, 1–12. [Google Scholar] [CrossRef]
- Schreider, M.J.; Glasby, T.M.; Underwood, A.J. Effects of height on the shore and complexity of habitat on abundances of amphipods on rocky shores in New South Wales, Australia. J. Exp. Mar. Biol. Ecol. 2003, 293, 57–71. [Google Scholar] [CrossRef]
- Ashton, G.V.; Burrows, M.T.; Willis, K.J.; Cook, E.J. Seasonal population dynamics of the non-native Caprella mutica (Crustacea, Amphipoda) on the west coast of Scotland. Mar. Freshw. Res. 2010, 61, 549–559. [Google Scholar] [CrossRef]
- Hughes, L.E.; Ahyong, S.T. Collecting and processing amphipods. J. Crustac. Biol. 2016, 36, 584–588. [Google Scholar] [CrossRef]
- Connell, J.H.; Slatyer, R. 0 Mechanisms of Succession in Natural Communities and Their Role in Community Stability and Organization. Am. Nat. 1977, 111, 1119–1144. [Google Scholar] [CrossRef]
- Queiroga, H.; Cruz, T.; dos Santos, A.; Dubert, J.; González-Gordillo, J.I.; Paula, J.; Peliz, Á.; Santos, A.M.P. Oceanographic and behavioural processes affecting invertebrate larval dispersal and supply in the western Iberia upwelling ecosystem. Prog. Oceanogr. 2007, 74, 174–191. [Google Scholar] [CrossRef]
- Wooster, W.S.; Bakun, A.; McLain, D.R. The seasonal upwelling cycle along the eastern boundary of the North Atlantic. J. Mar. Res. 1976, 34, 131–141. [Google Scholar]
- Ponti, M.; Fava, F.; Perlini, R.A.; Giovanardi, O.; Abbiati, M. Benthic assemblages on artificial reefs in the northwestern Adriatic Sea: Does structure type and age matter? Mar. Environ. Res. 2015, 104, 10–19. [Google Scholar] [CrossRef]
- Satterthwaite, E.V.; Morgan, S.G.; Ryan, J.P.; Harvey, J.B.J.; Vrijenhoek, R.C. Seasonal and synoptic oceanographic changes influence the larval biodiversity of a retentive upwelling shadow. Prog. Oceanogr. 2020, 182, 102261. [Google Scholar] [CrossRef]
- Zupan, M.; Rumes, B.; Vaneverbeke, J.; Degraer, S.; Kerchof, F. Long-Term Succession on Offshore Wind Farms and the Role of Species Interactions. Diversity 2023, 15, 288. [Google Scholar] [CrossRef]
- Conradi, M.; López-González, P.J.; García-Gomez, C. The Amphipod Community as a Bioindicator in Algeciras Bay (Southern Iberian Peninsula) Based on a Spatio-Temporal Distribution. Mar. Ecol. 1997, 18, 97–111. [Google Scholar] [CrossRef]
- Brown, J. On the relationship between abundance and distribution of species. Am. Nat. 1984, 124, 255–279. [Google Scholar] [CrossRef]
- Tuya, F.; Cacabelos, E.; Duarte, P.; Jacinto, D.; Castro, J.J.; Silva, T.; Bertocci, I.; Franco, J.N.; Arenas, F.; Coca, J.; et al. Patterns of landscape and assemblage structure along a latitudinal gradient in ocean climate. Mar. Ecol. Prog. Ser. 2012, 466, 9–19. [Google Scholar] [CrossRef]
 Enseada de San Cristovo, Ría de Ferrol;
Enseada de San Cristovo, Ría de Ferrol;  Bajo Tofiño, Ría de Vigo.
Bajo Tofiño, Ría de Vigo.
 Enseada de San Cristovo, Ría de Ferrol;
Enseada de San Cristovo, Ría de Ferrol;  Bajo Tofiño, Ría de Vigo.
Bajo Tofiño, Ría de Vigo.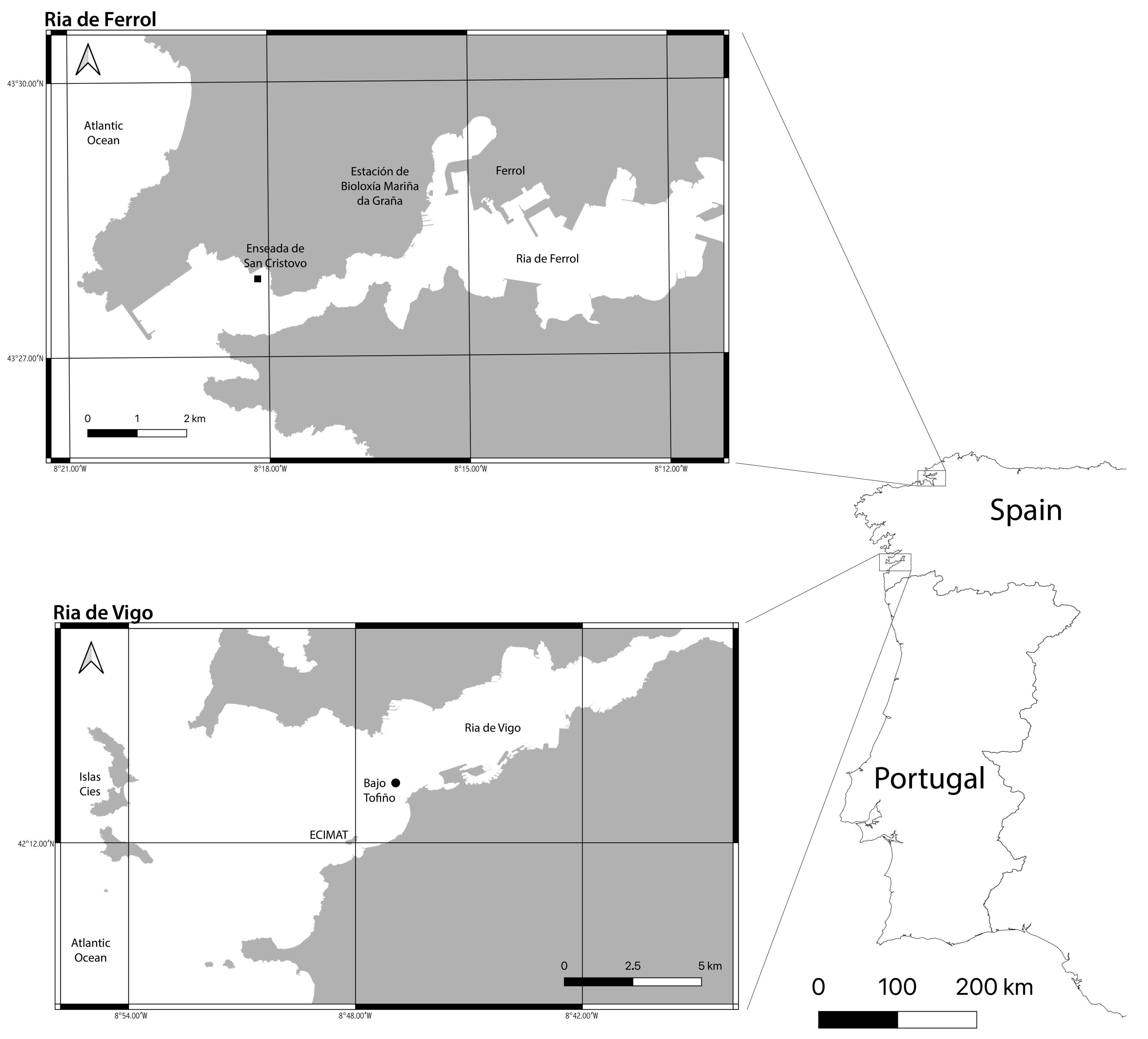
| Source | df | MS | Pseudo-F | P (perm) | Unique Perms |
|---|---|---|---|---|---|
| Location | 1 | 21603 | 6.4063 | 0.002 | 579 |
| Time | 3 | 5373.7 | 21.164 | 0.001 | 997 |
| Location × Time | 3 | 3372.1 | 13.281 | 0.001 | 999 |
| Residual | 32 | 253.91 | |||
| Total | 39 | ||||
| Permdisp | P (perm):0.371 |
| 3 Months | 6 Months | 9 Months | 12 Months | |
|---|---|---|---|---|
| t | t | t | t | |
| ESC vs. BT | 5.0261 * | 5.5261 * | 5.3744 * | 6.6794 ** |
| 3 Months | 6 Months | 9 Months | 12 Months | Total | |
|---|---|---|---|---|---|
| Common | 43 | 56 | 52 | 67 | 100 |
| Only ESC | 27 | 43 | 24 | 30 | 43 |
| Only BT | 26 | 27 | 35 | 26 | 19 |
| Total ESC | 70 | 99 | 76 | 95 | 143 |
| Total BT | 69 | 83 | 87 | 91 | 119 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carreira-Flores, D.; Neto, R.; Ferreira, H.R.S.; Cabecinha, E.; Díaz-Agras, G.; Rubal, M.; Gomes, P.T. Colonization in Artificial Seaweed Substrates: Two Locations, One Year. Diversity 2023, 15, 733. https://doi.org/10.3390/d15060733
Carreira-Flores D, Neto R, Ferreira HRS, Cabecinha E, Díaz-Agras G, Rubal M, Gomes PT. Colonization in Artificial Seaweed Substrates: Two Locations, One Year. Diversity. 2023; 15(6):733. https://doi.org/10.3390/d15060733
Chicago/Turabian StyleCarreira-Flores, Diego, Regina Neto, Hugo R. S. Ferreira, Edna Cabecinha, Guillermo Díaz-Agras, Marcos Rubal, and Pedro T. Gomes. 2023. "Colonization in Artificial Seaweed Substrates: Two Locations, One Year" Diversity 15, no. 6: 733. https://doi.org/10.3390/d15060733
APA StyleCarreira-Flores, D., Neto, R., Ferreira, H. R. S., Cabecinha, E., Díaz-Agras, G., Rubal, M., & Gomes, P. T. (2023). Colonization in Artificial Seaweed Substrates: Two Locations, One Year. Diversity, 15(6), 733. https://doi.org/10.3390/d15060733










