Stable Isotopes and Herding Strategies in Middle Uruk Period in Tell Humeida (Syrian Euphrates Valley)
Abstract
1. Introduction
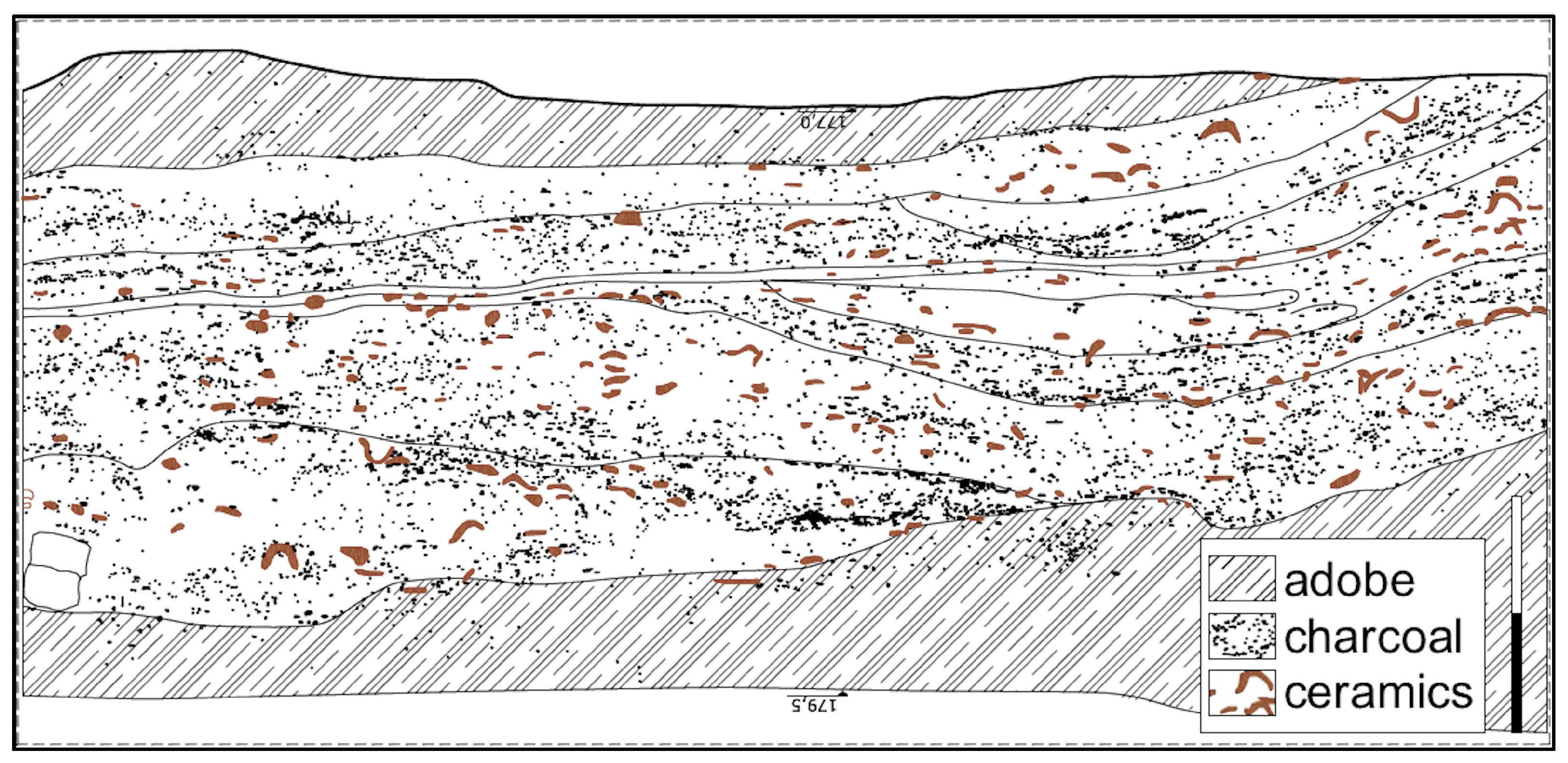
1.1. Tell Humeida and Its Archaeological Context

1.2. The Site and the Archaeological Surveys
1.3. Stable Isotopes Rationale
2. Materials and Methods
2.1. Samples
2.2. Separation and Identification of Plant Remains
2.3. Taxonomic Identification of Faunal Remains
2.4. Isotopic Analysis of Seeds and Bone Remains
3. Results
3.1. Chronological Framework
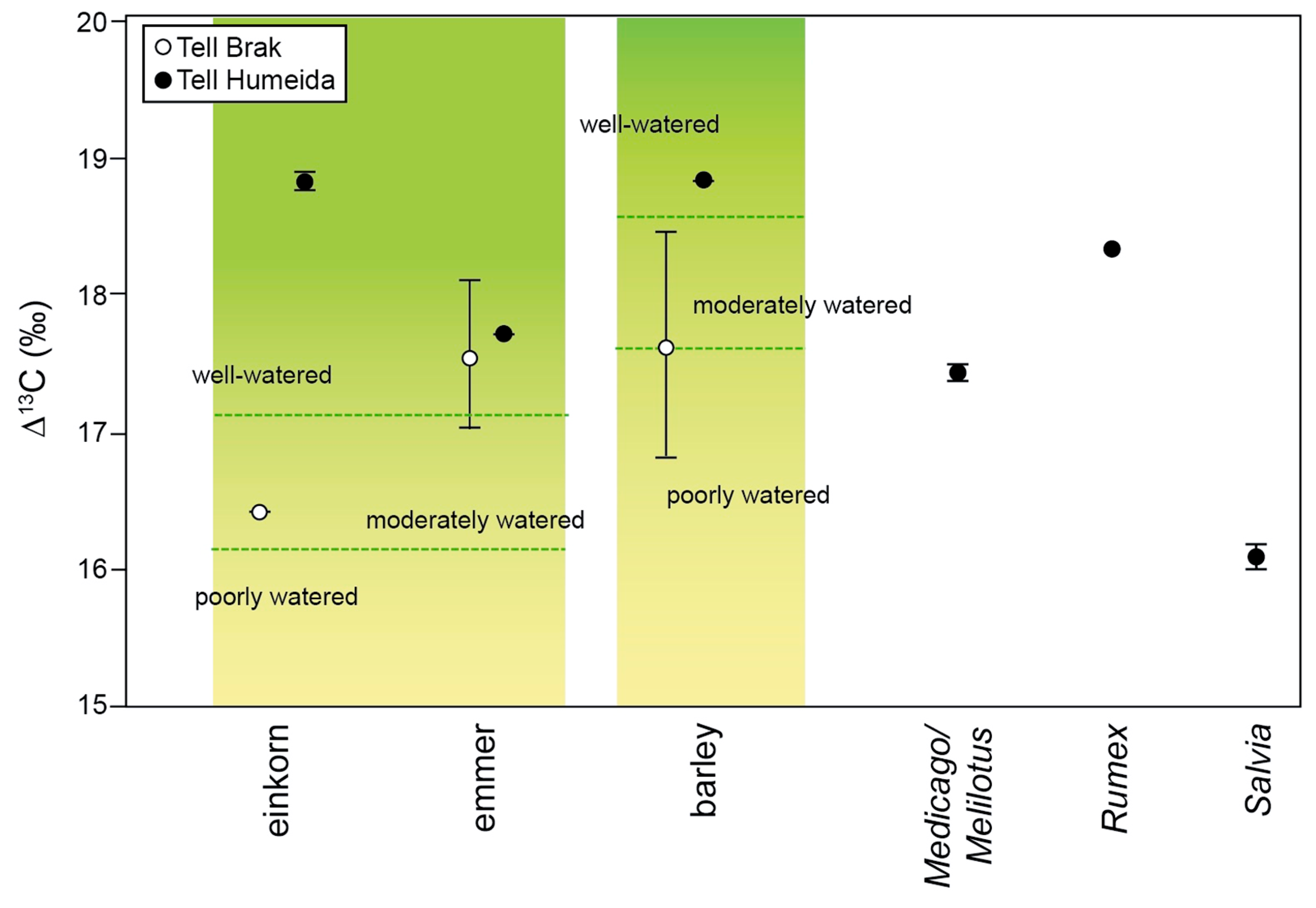
3.2. Study of Plant Remains
| Taxon (Number of Samples) | %C | %N | C:Nat | δ13CVPDV (‰) | δ13C (‰) −0.11 Offset | ∆13C | δ15NAIR (‰) | δ15N (‰) −0.31 Offset | δ13CVPDV (‰) Chaff | δ15NAIR (‰) Chaff |
|---|---|---|---|---|---|---|---|---|---|---|
| Triticum dicoccum (2) | 63.7 ± 0.5 | 3.4 ± 0.0 | 22.1 ± 0.0 | −23.5 ± 0.0 | −23.6 ± 0.0 | 17.7 ± 0.0 | 12.4 ± 0.0 | 12.1 ± 0.0 | −25.4 | 10.5 |
| Triticum monococcum (2) | 64.7 ± 2.0 | 3.6 ± 0.0 | 20.9 ± 0.6 | −24.6 ± 0.1 | −24.7 ± 0.1 | 18.8 ± 0.1 | 10.4 ± 0.3 | 10.1 ± 0.3 | −26.5 | 8.5 |
| Hordeum (2) | 66.9 ± 1.2 | 3.0 ± 0.9 | 26.0 ± 0.3 | −24.6±0.0 | −24.7 ± 0.0 | 18.7 ± 0.0 | 5.9 ± 0.1 | 5.6 ± 0.1 | −26.3 | 4.0 |
| Salvia (4) | 40.7 ± 5.7 | 2.8 ± 0.4 | 17.5 ± 0.2 | −22.4±0.2 | −22.5 ± 0.2 | 16.5 ± 0.2 | 6.6 ± 0.8 | 6.3 ± 0.8 | - | - |
| Rumex (1) | 42.5 | 2.0 | 24.5 | −24.7 | −24.8 | 18.9 | 11.6 | 11.3 | - | - |
| Medicago/Melilotus (2) | 41.1 ± 1.3 | 5.0 ± 0.3 | 9.6 ± 0.8 | −23.8 ± 0.1 | −23.9 ± 0.1 | 17.9 ± 0.1 | 3.8 ± 0.7 | 3.5 ± 0.7 | - | - |
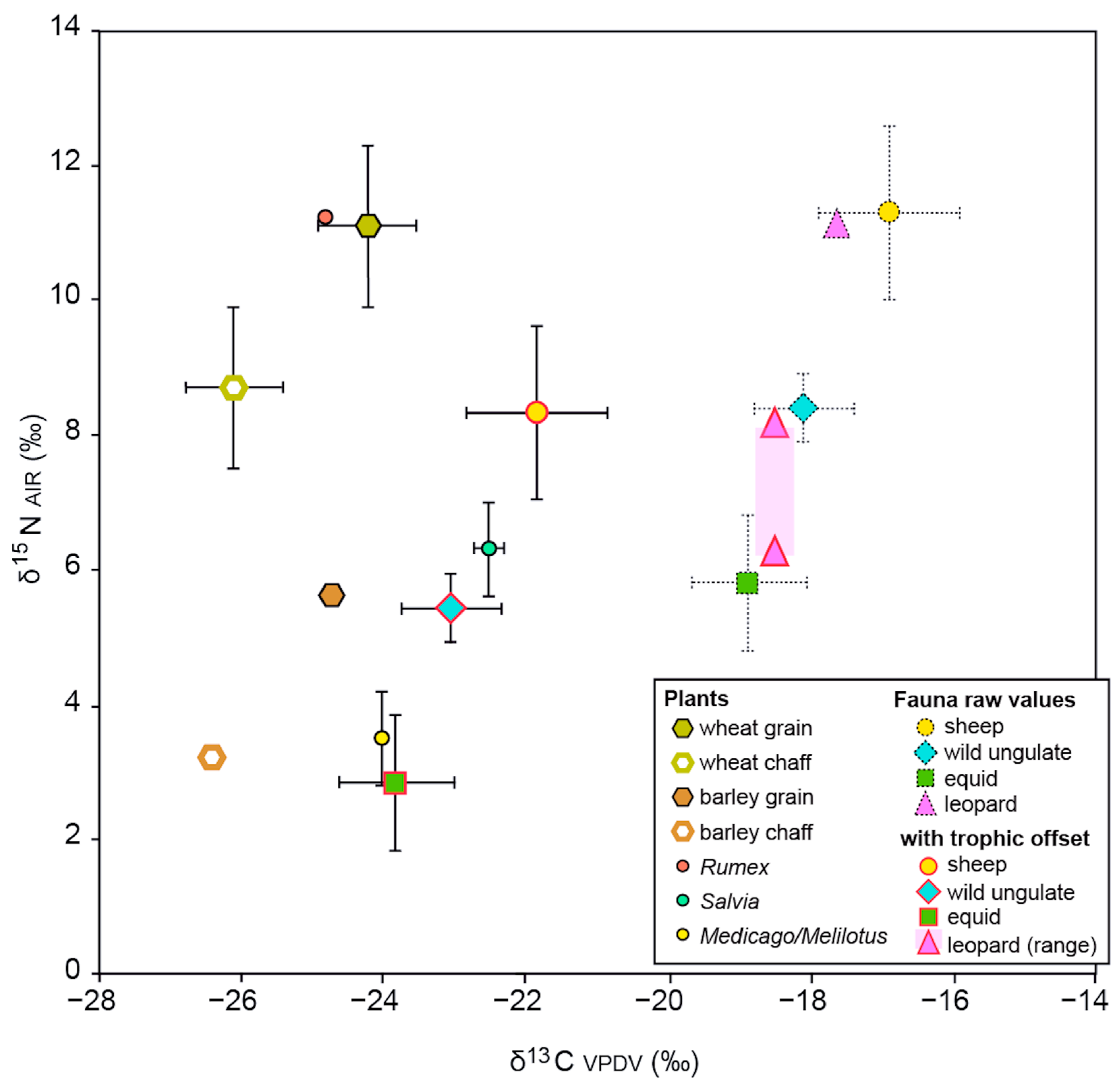
3.3. Study of Faunal Remains
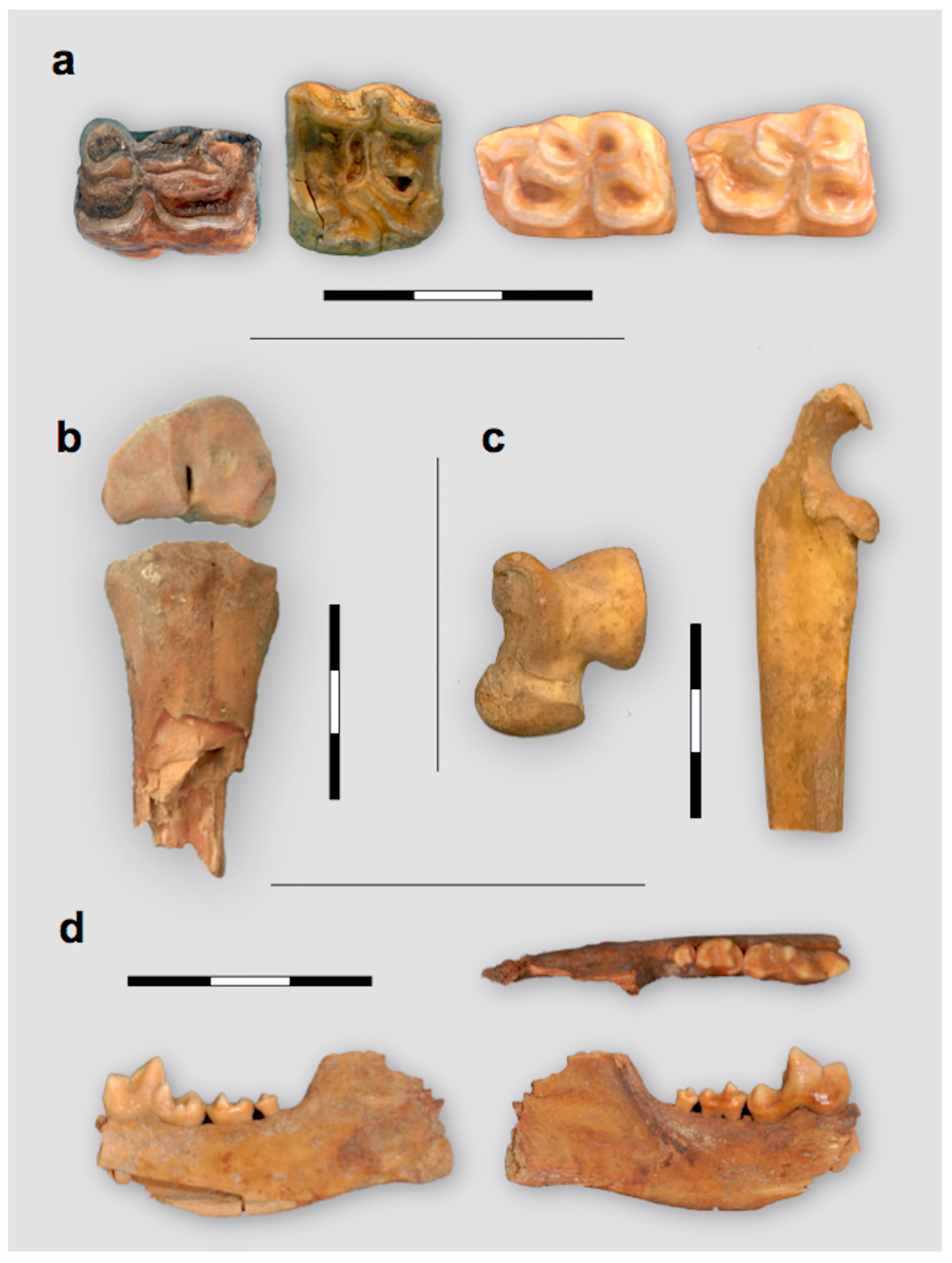
| Area | Code | Bone | Morphological id. | ZooMS id. | Yield (%) | % Ncol | % Ccol | C:Nat | δ15NAIR (‰) | δ13CVPDB (‰) |
|---|---|---|---|---|---|---|---|---|---|---|
| WP | TH09-H1 | Rib | N.I. | Ovis sp. | 11.2 | 13.3 | 36.9 | 3.2 | 10.5 | −17.3 |
| WP | TH09-H2 | Jaw | Fox | Vulpes sp. | 7.9 | 14.0 | 37.4 | 3.1 | 11.6 | −16.1 |
| WP | TH09-H3 | Tibia fgt. | Probably Equus | - | <0.5 | |||||
| WP | TH09-H4 | Rib | N.I. | Equus sp. | 9.4 | 12.9 | 35.3 | 3.2 | 6.5 | −18.6 |
| WP | TH09-H5 | Tibia fgt. | N.I. | Equus sp. | 2.3 | 13.0 | 35.5 | 3.2 | 5.5 | −19.1 |
| WP | TH09-H6 | Lower molar | Equus hemionus | - | 8.6 | 12.0 | 25.4 | 3.2 | 5.0 | −18.5 |
| WP | TH09-H7 | Long bone | N.I. | Cervid/Gazella | 9.3 | 12.0 | 32.8 | 3.2 | 8.6 | −17.2 |
| WP | TH09-H8 | Upper molar | Ovis/Capra | Ovis sp. | 11.2 | 12.4 | 26.5 | 3.1 | 12.5 | −15.8 |
| WP | TH09-H9 | Long bone | N.I. | Equus sp. | 4.6 | 14.6 | 39.9 | 3.2 | 6.2 | −19.3 |
| WP | TH09-H10 | Jaw | Ungulate, medium | Cervid/Gazella | 5.5 | 13.0 | 35.7 | 3.2 | 8.4 | −19.0 |
| WP | TH09-H11 | Rib | N.I. | Equus sp. | 10.4 | 13.6 | 37.3 | 3.2 | 5.2 | −19.0 |
| WP | TH09-H12 | Long bone | N.I. | Equus sp. | 10.3 | 13.1 | 35.9 | 3.2 | 4.4 | −18.6 |
| WP | TH09-H13 | Pelvis fgt. | N.I. | - | <0.5 | |||||
| WP | TH09-H14 | Tibia fgt. | Equus sp. | Equus sp. | 6.8 | 14.3 | 39.1 | 3.2 | 6.3 | −19.2 |
| WP | TH09-H15 | Pelvis fgt. | N.I. | Equus sp. | 3.9 | 14.0 | 37.8 | 3.2 | 3.9 | −19.8 |
| WP | TH09-H16 | Upper molar | Equus * | - | 9.8 | 11.2 | 29.2 | 3.0 | 6.7 | −18.3 |
| WP | TH09-H17 | Long bone | N.I. | Ovis sp. | 2.7 | 12.0 | 32.5 | 3.2 | 9.7 | −16.1 |
| WP | TH09-H18 | Humerus fgt. | N.I. | Equus sp. | 8.3 | 14.6 | 39.9 | 3.2 | 5.9 | −20.2 |
| WP | TH09-H19 | Long bone fgt. | Equus sp. | Equus sp. | 10.8 | 13.6 | 37.8 | 3.2 | 6.6 | −18.6 |
| WP | TH09-H20 | Metacarpal | N.I. | Ovis sp. | 5.6 | 11.9 | 33.1 | 3.2 | 5.8 | −19.0 |
| J-13 | TH11-1 | Long bone | N.I. | Ovis sp. | 2.6 | 12.0 | 34.0 | 3.3 | 11.4 | −15.9 |
| J-13 | TH11-2 | Long bone | N.I. | Ovis sp. | 1.5 | 11.4 | 32.8 | 3.3 | 12.9 | −17.1 |
| J-13 | TH11-3 | Long bone | N.I. | Ovis/Capra | 1.8 | 11.6 | 32.4 | 3.3 | 9.3 | −17.9 |
| J-13 | TH11-4 | Long bone | N.I. | Ovis/Capra | 2.1 | 13.3 | 37.2 | 3.3 | 9.5 | −17.3 |
| J-13 | TH11-5 | Long bone | N.I. | Unidentified | 5.2 | 13.8 | 39.1 | 3.3 | 14.4 | −17.2 |
| J-13 | TH11-6 | Long bone | N.I. | Capra sp. | 7.1 | 14.4 | 41.2 | 3.3 | 9.9 | −17.7 |
| J-13 | TH11-7 | Long bone | N.I. | Ovis sp. | 1,6 | 9.2 | 26.9 | 3.4 | 11.4 | −18.6 |
| J-13 | TH11-8 | Long bone | N.I. | Panthera sp. | 3.9 | 11.0 | 31.0 | 3.3 | 11.1 | −17.7 |
| J-13 | TH11-9 | Long bone | N.I. | no quality | 2.3 | 10.9 | 34.5 | 3.7 | ||
| J-13 | TH11-10 | Long bone | N.I. | - | <0.5 | |||||
| J-13 | TH11-11 | Long bone | N.I. | Cervid/Gazella | 7.8 | 12.6 | 34.7 | 3.2 | 9.1 | −18.1 |
| J-13 | TH11-12 | Long bone | N.I. | No quality | 1.6 | 3.8 | 13.4 | 4.1 | ||
| J-13 | TH11-13 | Long bone | N.I. | - | <0.5 | |||||
| J-13 | TH11-14 | Long bone | N.I. | Ovis sp. | 2.1 | 10.7 | 30.4 | 3.3 | 9.9 | −16.8 |
| J-13 | TH11-15 | Long bone | N.I. | No quality | 1.8 | 4.0 | 12.3 | 3.6 | ||
| J-13 | TH11-16 | Long bone | N.I. | Ovis sp. | 3.7 | 12.1 | 33.4 | 3.2 | 10.0 | −16.0 |
| J-13 | TH11-17 | Phalanx | Bos taurus | - | <0.5 | |||||
| J-13 | TH11-18 | Lower molar | Equus hydruntinus | Equus sp. | 1.5 | 13.1 | 35.8 | 3.2 | 7.4 | −17.0 |
| J-13 | TH11-19 | Long bone | N.I. | Cervid/Gazella | 6.2 | 12.8 | 36.1 | 3.3 | 8.3 | −18.5 |
| J-13 | TH11-20 | Long bone | N.I. | Ovis sp. | 7.4 | 10.7 | 29.6 | 3.2 | 13.5 | −16.3 |
| J-13 | TH11-21 | Long bone | N.I. | Cervid/Gazella | 8.3 | 11.7 | 32.5 | 3.2 | 7.8 | −17.9 |
| J-13 | TH11-22 | Long bone | N.I. | - | <0.5 | |||||
| J-13 | TH11-23 | Jaw | Gazella sp. | Ovis/Capra | 3.8 | 8.5 | 23.5 | 3.2 | 11.3 | −18.1 |
| J-13 | TH11-24 | Jaw | Ovis/Capra | Ovis sp. | 11.4 | 11.9 | 32.5 | 3.2 | 11.5 | −17.6 |
| J-13 | TH11-25 | Ulna | Caracal | Felis/Lynx | 10.7 | 12.1 | 33.5 | 3.2 | 9.7 | −15.9 |
| J-13 | TH11-26 | Jaw | Cervus | - | <0.5 |
| δ13CVPDB (‰) | δ15NAIR (‰) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | sd | Min | Max | Range | Mean | sd | Min | Max | Range | |
| Ovis | 11 | −17.0 | 1.1 | −19.0 | −15.8 | 4.2 | 10.8 | 2.1 | 5.8 | 13.5 | 7.7 |
| Ovis (without outlier) | 10 | −16.8 | 0.9 | −18.6 | −15.8 | 2.8 | 11.3 | 1.3 | 9.7 | 13.5 | 3.8 |
| Cervid/Gazella | 5 | −18.1 | 0.7 | −19.0 | −17.2 | 1.8 | 8.4 | 0.5 | 7.8 | 9.1 | 1.3 |
| Equus sp. | 12 | −18.9 | 0.8 | −20.2 | −17.0 | 3.2 | 5.8 | 1.0 | 3.9 | 7.4 | 3.5 |
| Carnivores | 3 | −16.6 | 1.0 | −17.7 | −15.9 | 1.8 | 10.8 | 1.0 | 9.7 | 11.6 | 1.9 |
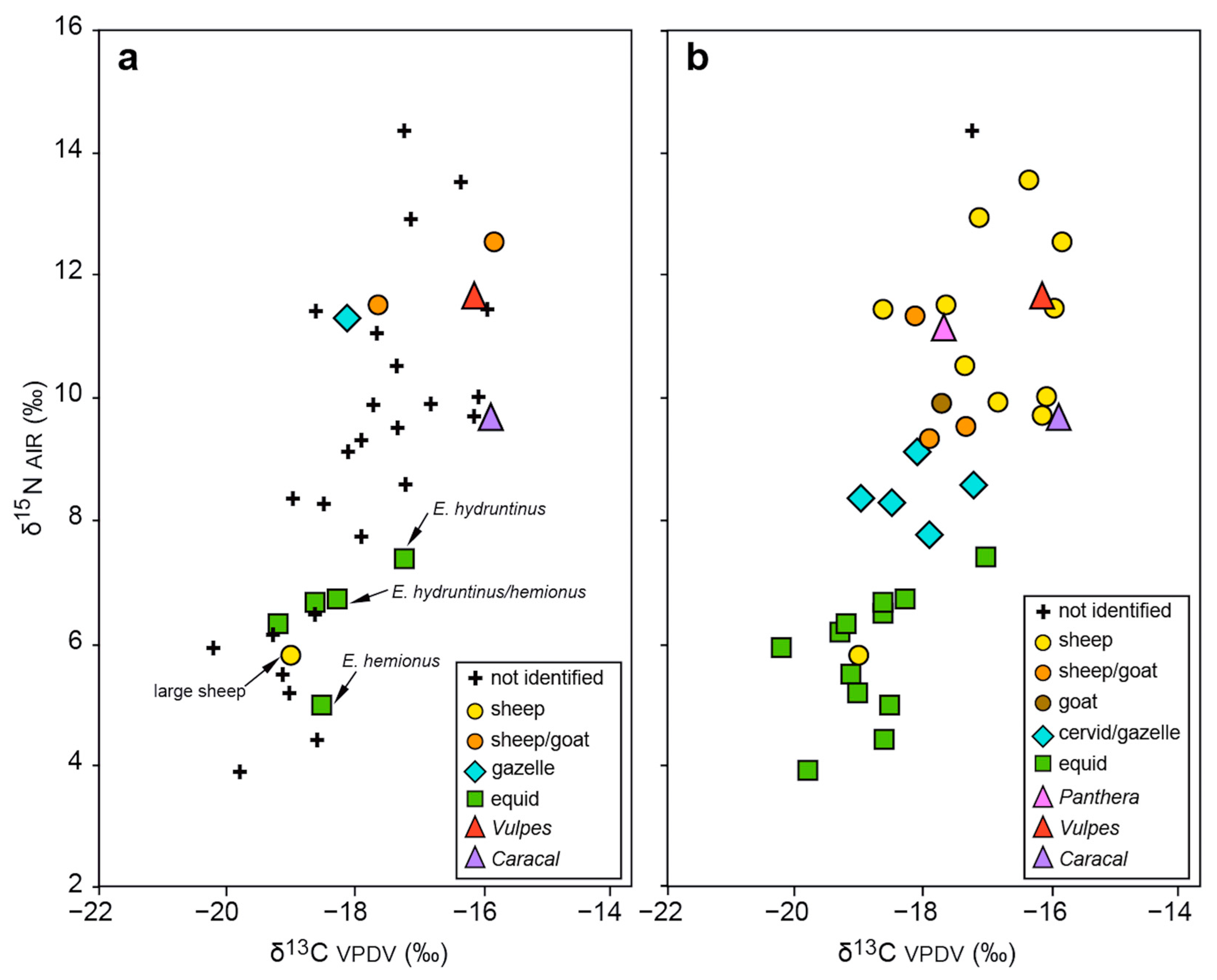
4. Discussion
4.1. Taxonomic Identification of the Fauna
4.2. Manuring and Watering of Crops?
4.3. Animal Diet and Husbandry
5. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montero Fenollós, J.L. Le défilé de Khanuqa. Géographie e histoire au Moyen Euphrate méridional. Isimu 2011, 13, 125–136. Available online: http://hdl.handle.net/10486/667759 (accessed on 9 December 2019).
- Fenollós, J.L.M.; Caramelo, F.; Masó, F. De Uruk a Bizancio. Investigaciones del Proyecto Arqueológico Medio Éufrates Sirio (2005–2011); Sociedade Luso-Galega de Estudos Mesopotámicos: Ferrol, Spain, 2012. [Google Scholar]
- Algaze, A. The Uruk World System: The Dynamics of Expansion of Early Mesopotamia; University of Chicago Press: Chicago, IL, USA, 2005. [Google Scholar]
- Butterlin, P. Architecture et Société au Proche-Orient Ancient; Picard: Paris, France, 2018; pp. 407–427. [Google Scholar]
- Montero Fenollós, J.L. De Uruk a Mari. Innovaciones tecnológicas de la Primera Revolución Urbana en el Medio Éufrates meridional. Anejos Nailos Estud. Interdiscip. Arqueol. 2014, 1, 139–155. Available online: https://nailos.org/index.php/nailos/article/view/81 (accessed on 9 December 2019).
- Sanjurjo Sánchez, J.; Montero Fenollós, J.L.; Prudêncio, M.I.; Barrientos, V.; Marques, R.; Dias, M.I. Geochemical study of beveled rim bowls from the Middle Syrian Euphrates sites. J. Archaeol. Sci. Rep. 2016, 7, 808–881. [Google Scholar] [CrossRef]
- Sanjurjo Sánchez, J.; Montero Fenollós, J.L.; Barrientos, V.; Polymeris, G.S. Assessing the firing temperature of Uruk pottery in the Middle Euphrates Valley (Syria): Bevelled rim bowls. Microchem. J. 2018, 142, 43–53. [Google Scholar] [CrossRef]
- Sanjurjo Sánchez, J.; Montero Fenollós, J.L.; Polymeris, G.S. Technological aspects of Mesopotamiam Uruk pottery: Esti-mating firing temperatures using mineralogical methods, thermal analysis and luminescence techniques. Archaeol. Anthropol. Sci. 2018, 10, 849–864. [Google Scholar] [CrossRef]
- Sanjurjo Sánchez, J.; Kaal, J.; Montero Fenollós, J.L. Organic matter from bevelled rim bowls of the Middle Euphrates: Results from molecular characterization using pyrolysis-GC–MS. Microchem. J. 2018, 141, 1–6. [Google Scholar] [CrossRef]
- Montero Fenollós, J.L.; Sanjurjo-Sánchez, J. El proceso de fabricación de los cuencos con borde biselado mesopotámicos. Nuevas aportaciones desde la arqueología experimental. Spal 2021, 30.2, 103–123. [Google Scholar] [CrossRef]
- Montero Fenollós, J.L.; Caramelo, F.; Márquez Rowe, I.; al-Abdalleh, Y. Excavaciones arqueológicas en Tall Humeida (Siria). In Informes y Trabajos 7. Excavaciones en el Exterior 2010; Ministerio de Educación, Cultura y Deporte de España: Madrid, Spain, 2012; pp. 309–315. Available online: https://www.libreria.culturaydeporte.gob.es/ebook/3746/free_download/ (accessed on 9 December 2019).
- Schwarcz, H.P.; Schoeninger, M.J. Stable isotope analyses in human nutritional ecology. Am. J. Phys. Anthr. 1991, 34, 283–321. [Google Scholar] [CrossRef]
- Hedges, R.E.M.; Reynard, L.M. Nitrogen isotopes and the trophic level of humans in archaeology. J. Archaeol. Sci. 2007, 34, 1240–1251. [Google Scholar] [CrossRef]
- Bogaard, A.; Heaton, T.H.E.; Poulton, P.; Merbach, I. The impact of manuring on nitrogen isotope ratios in cereals: Archaeological implications for reconstruction of diet and crop management practices. J. Archaeol. Sci. 2007, 34, 335–343. [Google Scholar] [CrossRef]
- Szpak, P. Complexities of nitrogen isotope biogeochemistry in plant-soil systems: Implications for the study of ancient agricultural and animal management practices. Front. Plant Sci. 2014, 5, 288. [Google Scholar] [CrossRef]
- Michener, R.; Lajtha, K. Stable Isotopes in Ecology and Environmental Science, 2nd ed.; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar] [CrossRef]
- Faure, G.; Mensing, T. Isotopes: Principles and Applications, 3rd ed.; Wiley: Delhi, India, 2004; p. 928. [Google Scholar]
- Koch, P.L.; Fogel, M.L.; Tuross, N. Tracing the diets of fossil animals using stable isotopes. In Stable Isotopes in Ecology and Environmental Science; Lajtha, K., Michener, R.H., Eds.; Blackwell Scientific Publications: Boston, MA, USA, 1994; pp. 63–92. [Google Scholar]
- Wallace, M.; Jones, G.; Charles, M.; Fraser, R.; Halstead, P.; Heaton, T.H.E.; Bogaard, A. Stable carbon isotope analysis as a direct means of inferring crop water status and water management practices. World Archaeol. 2013, 45, 388–409. [Google Scholar] [CrossRef]
- Van der Merwe, N.J. Carbon Isotopes, Photosynthesis, and Archaeology. Am. Sci. 1982, 70, 596–606. Available online: http://www.jstor.org/stable/27851731 (accessed on 17 December 2022).
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon Isotope Discrimination and Photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- O’Leary, M.H. Carbon isotope fractionation in plants. Phytochemistry 1981, 20, 553–567. [Google Scholar] [CrossRef]
- Araus, J.L.; Febrero, A.; Catala, M.; Molist, M.; Voltas, J.; Romagosa, I. Crop water availability in early agriculture: Evidence from carbon isotope discrimination of seeds from a tenth millennium BP site on the Euphrates. Glob. Change Biol. 1999, 5, 201–212. [Google Scholar] [CrossRef]
- Araus, J.L.; Ferrio, J.P.; Voltas, J.; Aguilera, M.; Buxó, R. Agronomic conditions and crop evolution in ancient Near East agriculture. Nat. Commun. 2014, 5, 3953. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, S.H.; Norr, L. Experimental Evidence for the Relationship of the Carbon Isotope Ratios of Whole Diet and Dietary Protein to Those of Bone Collagen and Carbonate. In Prehistoric Human Bone; Lambert, J.B., Grupe, G., Eds.; Springer: Heidelberg, Germany, 1993. [Google Scholar] [CrossRef]
- Bocherens, H.; Drucker, D. Trophic level isotopic enrichment of carbon and nitrogen in bone collagen: Case studies from recent and ancient terrestrial ecosystems. Int. J. Osteoarchaeol. 2003, 13, 46–53. [Google Scholar] [CrossRef]
- Amundson, R.; Austin, A.T.; Schuur, E.A.G.; Yoo, K.; Matzek, V.; Kendall, C.; Uebersax, A.; Brenner, D.; Baisden, W.T. Global patterns of the isotopic composition of soil and plant nitrogen. Glob. Biogeochem. Cycles 2003, 17, 1031. [Google Scholar] [CrossRef]
- Männel, T.T.; Auerswald, K.; Schnyder, H. Altitudinal gradients of grassland carbon and nitrogen isotope composition are recorded in the hair of grazers. Glob. Ecol. Biogeogr. 2007, 16, 583–592. [Google Scholar] [CrossRef]
- Dawson, T.E.; Mambelli, S.; Plamboeck, A.H.; Templer, P.H.; Tu, K.P. Stable Isotopes in Plant Ecology. Annu. Rev. Ecol. Syst. 2002, 33, 507–559. [Google Scholar] [CrossRef]
- Fraser, R.A.; Bogaard, A.; Heaton, T.; Charles, M.; Jones, G.; Christensen, B.T.; Halstead, P.; Merbach, I.; Poulton, P.R.; Sparkes, D.; et al. Manuring and stable nitrogen isotope ratios in cereals and pulses: Towards a new archaeobotanical approach to the inference of land use and dietary practices. J. Archaeol. Sci. 2011, 38, 2790–2804. [Google Scholar] [CrossRef]
- Heaton, T.H.E.; Vogel, J.C.; Von La Chevallerie, G.; Collett, G. Climatic influence on the isotopic composition of bone nitrogen. Nature 1986, 322, 822–823. [Google Scholar] [CrossRef]
- Cormie, A.; Schwarcz, H. Effects of climate on deer bone δ15N and δ13C: Lack of precipitation effects on δ15N for animals consuming low amounts of C4 plants. Geochim. Cosmochim. Acta 1996, 60, 4161–4166. [Google Scholar] [CrossRef]
- Hartman, G. Are elevated δ15N values in herbivores in hot and arid environments caused by diet or animal physiology? Funct. Ecol. 2010, 25, 122–131. [Google Scholar] [CrossRef]
- Boessneck, J. Osteological differences between sheep and goats. In Science in Archaeology, 2nd ed.; Brothwell, D., Higgs, E.S., Eds.; Thames and Hudsonpp: London, UK, 1969; pp. 331–358. [Google Scholar]
- Buckley, M.; Collins, M.; Thomas-Oates, J.; Wilson, J.C. Species identification by analysis of bone collagen using matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry. Rapid. Commun. Mass Spectrom. 2009, 23, 3843–3854. [Google Scholar] [CrossRef]
- Welker, F.; Hajdinjak, M.; Talamo, S.; Jaouen, K.; Dannemann, M.; David, F.; Julien, M.; Meyer, M.; Kelso, J.; Barnes, I.; et al. Palaeoproteomic evidence identifies archaic hominins associated with the Châtelperronian at the Grotte du Renne. Proc. Natl. Acad. Sci. USA 2016, 113, 11162–11167. [Google Scholar] [CrossRef]
- Bogaard, A.; Fraser, R.A.; Heaton, T.H.E.; Wallace, M.; Vaiglova, P.; Charles, M.; Jones, G.; Evershed, R.P.; Styring, A.K.; Andersen, N.H.; et al. Crop manuring and intensive land management by Europe’s first farmers. Proc. Natl. Acad. Sci. USA 2013, 110, 12589–12594. [Google Scholar] [CrossRef]
- Longin, R. New Method of Collagen Extraction for Radiocarbon Dating. Nature 1971, 230, 241–242. [Google Scholar] [CrossRef]
- Bocherens, H.; Billiou, D.; Patou-Mathis, M.; Bonjean, D.; Otte, M.; Mariotti, A. Paleobiological Implications of the Isotopic Signatures (13C,15N) of Fossil Mammal Collagen in Scladina Cave (Sclayn, Belgium). Quat. Res. 1997, 48, 370–380. [Google Scholar] [CrossRef]
- Ambrose, S.H. Preparation and characterization of bone and tooth collagen for isotopic analysis. J. Archaeol. Sci. 1990, 17, 431–451. [Google Scholar] [CrossRef]
- Van Klinken, G.J. Bone Collagen Quality Indicators for Palaeodietary and Radiocarbon Measurements. J. Archaeol. Sci. 1999, 26, 687–695. [Google Scholar] [CrossRef]
- DeNiro, M.J. Postmortem preservation and alteration of in vivo bone collagen isotope ratios in relation to palaeodietary reconstruction. Nature 1985, 317, 806–809. [Google Scholar] [CrossRef]
- Bocherens, H.; Drucker, D.; Billiou, D.; Moussa, I. Une nouvelle approche pour évaluer l’état de conservation de l’os et du collagène pour les mesures isotopiques (datation au radiocarbone, isotopes stables du carbone et de l’azote). L’Anthropologie 2005, 109, 557–567. [Google Scholar] [CrossRef]
- Schwarcz, H.P.; Nahal, H. Theoretical and observed C/N ratios in human bone collagen. J. Archaeol. Sci. 2021, 131, 105396. [Google Scholar] [CrossRef]
- Bronk Ramsey, C. Bayesian Analysis of Radiocarbon Dates. Radiocarbon 2009, 51, 337–360. [Google Scholar] [CrossRef]
- Reimer, P.J.; Austin, W.E.N.; Bard, E.; Bayliss, A.; Blackwell, P.G.; Ramsey, C.B.; Butzin, M.; Cheng, H.; Edwards, R.L.; Friedrich, M.; et al. The IntCal20 Northern Hemisphere Radiocarbon Age Calibration Curve (0–55 cal kBP). Radiocarbon 2020, 62, 725–757. [Google Scholar] [CrossRef]
- Hald, M.M.; Charles, M. Storage of crops during the fourth and third millennia b.c. at the settlement mound of Tell Brak, northeast Syria. Veg. Hist. Archaeobotany 2008, 17 (Suppl. S1), S35–S41. [Google Scholar] [CrossRef]
- Masi, A.; Sadori, L.; Balossi Restelli, F.; Baneschi, I.; Zanchetta, G. Stable carbon isotope analysis as a crop management indicator at Arslantepe (Malatya, Turkey) during the Late Chalcolithic and Early Bronze Age. Veg. Hist. Archaeobotany 2014, 23, 751–760. [Google Scholar] [CrossRef]
- Hunt, H.V.; Van der Linden, M.; Liu, X.; Motuzaite-Matuzeviciute, G.; Colledge, S.; Martin, K.; Jones, M.K. Millets across Eurasia: Chronology and context of early records of the genera Panicum and Setaria from archaeological sites in the Old World. Veg. Hist. Archaeobotany 2008, 17 (Suppl. S1), 5. [Google Scholar] [CrossRef]
- Nitsch, E.K.; Charles, M.; Bogaard, A. Calculating a statistically robust δ13C and δ15N offset for charred cereal and pulse seeds. STAR Sci. Technol. Archaeol. Res. 2015, 1, 1–8. [Google Scholar] [CrossRef]
- Farquhar, G.D.; O’Leary, M.H.; Berry, J.A. On the Relationship between Carbon Isotope Discrimination and the Intercellular Carbon Dioxide Concentration in Leaves. Funct. Plant Biol. 1982, 9, 121–137. [Google Scholar] [CrossRef]
- Fraser, R.A.; Bogaard, A.; Charles, M.; Styring, A.K.; Wallace, M.; Jones, G.; Ditchfield, P.; Heaton, T.H.E. Assessing natural variation and the effects of charring, burial and pre-treatment on the stable carbon and nitrogen isotope values of archaeobotanical cereals and pulses. J. Archaeol. Sci. 2013, 40, 4754–4766. [Google Scholar] [CrossRef]
- Parés Casanova, P.-M. Does Caballine Fold Define Caballines? J. Appl. Life Sci. Int. 2017, 12, 1–4. [Google Scholar] [CrossRef]
- Davis, S.J.M. Measurements of a Group of Adult Female Shetland Sheep Skeletons from a Single Flock: A Baseline for Zooarchaeologists. J. Archaeol. Sci. 1996, 23, 593–612. [Google Scholar] [CrossRef]
- Driesch, v.d.A. A Guide to the Measurement of Animal Bones from Archaeological Sites; Peabody Museum Bulletin 1; Harvard University Press: Cambridge, MA, USA, 1976. [Google Scholar]
- Levine, M. The use of crown height measurements and eruption wear sequences to age horse teeth. In Ageing and Sexing Animal Bones from Archaeological Sites; Wilson, B., Grigson, C., Payne, S., Eds.; BAR British Series: Oxford, UK, 1982; Volume 109, pp. 223–250. [Google Scholar]
- Clutton-Brock, J. Osteology of the equids from Sumer. In Equids in the Ancient World (Beihefte zum Tübinger Atlas des Vorderen Orients, Reihe A, Nr. 19/1); Meadow, R.H., Uerpmann, H.P., Eds.; Ludwig Reichert Verlag: Wiesbaden, Germany, 1986; pp. 207–229. [Google Scholar]
- Geigl, E.-M.; Grange, T. Eurasian wild asses in time and space: Morphological versus genetic diversity. Ann. Anat. Anat. Anz. 2012, 194, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Bennett, E.A.; Weber, J.; Bendhafer, W.; Champlot, S.; Peters, J.; Schwartz, G.M.; Thierry Grange, T.; Geigl, E.M. The genetic identity of the earliest human-made hybrid animals, the kungas of Syro-Mesopotamia. Sci. Adv. 2022, 8, eabm0218. [Google Scholar] [CrossRef] [PubMed]
- Twiss, K.C.; Wolfhagen, J.; Madgwick, R.; Foster, H.; Demirergi, G.A.; Russell, N.; Everhart, J.L.; Pearson, J.; Mulville, J. Horses, Hemiones, Hydruntines? Assessing the Reliability of Dental Criteria for Assigning Species to Southwest Asian Equid Remains. Int. J. Osteoarchaeol. 2016, 27, 298–304. [Google Scholar] [CrossRef]
- Orlando, L.; Mashkour, M.; Burke, A.; Douady, C.J.; Eisenmann, V.; Hänni, C. Geographic distribution of an extinct equid (Equus hydruntinus: Mammalia, Equidae) revealed by morphological and genetical analyses of fossils. Mol. Ecol. 2006, 15, 2083–2093. [Google Scholar] [CrossRef]
- Bennett, E.A.; Champlot, S.; Peters, J.; Arbuckle, B.S.; Guimaraes, S.; Pruvost, M.; Bar-David, S.; Davis, S.J.M.; Gautier, M.; Kaczensky, P.; et al. Taming the late Quaternary phylogeography of the Eurasiatic wild ass through ancient and modern DNA. PLoS ONE 2017, 12, e0174216. [Google Scholar] [CrossRef]
- Zarins, J. Equids associated with human burials in the third millennium BC Mesopotamia: Two complementary facets. In Equids in the Ancient World (Beihefte zum Tübinger Atlas des Vorderen Orients, Reihe A, Nr. 19/1); Meadow, R.H., Uerpmann, H.P., Eds.; Ludwig Reichert Verlag: Wiesbaden, Germany, 1986; pp. 165–191. [Google Scholar]
- Dolce, R. Equids as Luxury Gifts at the Centre of Interregional Economic Dynamics in the Archaic Urban Cultures of the Ancient Near East. Syria 2014, 91, 55–75. [Google Scholar] [CrossRef]
- Buckley, M.; Kansa, S.W.; Howard, S.; Campbell, S.; Thomas-Oates, J.; Collins, M. Distinguishing between archaeological sheep and goat bones using a single collagen peptide. J. Archaeol. Sci. 2010, 37, 13–20. [Google Scholar] [CrossRef]
- Arbuckle, B.S.; Hammer, E.L. The Rise of Pastoralism in the Ancient Near East. J. Archaeol. Res. 2019, 27, 391–449. [Google Scholar] [CrossRef]
- Saña Seguí, M. Arqueología de la Domesticación Animal. La Gestión de Los Recursos Animales en Tell Halula (Valle del Éufrates, Siria) Del 8800 al 7000 BP; Universitat Autònoma de Barcelona: Barcelona, Spain, 1999. [Google Scholar]
- Grigson, C. The fauna of Tell Nebi Mend (Syria) in the Bronze and Iron Age—A diachronic overview. Part 1: Stability and change—Animal husbandry. Levant 2015, 47, 5–29. [Google Scholar] [CrossRef]
- Van Neer, W.; Linseele, V.; Friedman, R.; De Cupere, B. More evidence for cat taming at the Predynastic elite cemetery of Hierakonpolis (Upper Egypt). J. Archaeol. Sci. 2014, 45, 103–111. [Google Scholar] [CrossRef]
- Masseti, M. Carnivores of Syria. In Animal Biodiversity in the Middle East, Proceedings of the First Middle Eastern Biodiversity Congress, Aqaba, Jordan, 20–23 October 2008; Neubert, E., Amr, Z., Taiti, S., Gümüs, B., Eds.; ZooKeys Special Issue 31; Pensoft: Sofia, Bulgaria, 2009; pp. 229–252. [Google Scholar] [CrossRef]
- Baryshnikov, G. Pleistocene Felidae (Mammalia, Carnivora) from the Kudaro Paleolithic cave sites in the Caucasus. Proc. Zool. Inst. RAS 2011, 315, 197–226. [Google Scholar] [CrossRef]
- Gourichon, L.; Helmer, D. Etude archéozoologique de Mureybet. In Tell Mureybet, un Site Néolithique Dans le Moyen Euphrate Syrien; Ibáñez, J.J., Ed.; BAR International Series 1843; Archeopress: Oxford, UK, 2008; pp. 115–227. [Google Scholar]
- Harrison, D.L.; Bates, P.J.J. The Mammals of Arabia; Harrison Zoological Museum: Kent, UK, 1991. [Google Scholar]
- Styring, A.K.; Charles, M.; Fantone, F.; Hald, M.M.; McMahon, A.; Meadow, R.H.; Nicholls, G.K.; Patel, A.K.; Pitre, M.C.; Smith, A.; et al. Isotope evidence for agricultural extensification reveals how the world’s first cities were fed. Nat. Plants 2017, 3, 17076. [Google Scholar] [CrossRef]
- Hartman, G.; Danin, A. Isotopic values of plants in relation to water availability in the Eastern Mediterranean region. Oecologia 2009, 162, 837–852. [Google Scholar] [CrossRef]
- Reinhardt, M.; Müller, B.; Gächter, R.; Wehrli, B. Nitrogen Removal in a Small Constructed Wetland: An Isotope Mass Balance Approach. Environ. Sci. Technol. 2006, 40, 3313–3319. [Google Scholar] [CrossRef]
- Gratton, C.; Donaldson, J.; Zanden, M.J.V. Ecosystem Linkages between Lakes and the Surrounding Terrestrial Landscape in Northeast Iceland. Ecosystems 2008, 11, 764–774. [Google Scholar] [CrossRef]
- Lindsay, I.M.; Macdonald, D.W. Behaviour and ecology of the Rüppell’s fox, Vulpes rueppelli, in Oman. Mammalia 1986, 50, 461–474. [Google Scholar] [CrossRef]
- Geffen, E.; Hefner, R.; Macdonald, D.W.; Ucko, M. Diet and Foraging Behavior of Blanford’s Foxes, Vulpes cana, in Israel. J. Mammal. 1992, 73, 395–402. [Google Scholar] [CrossRef]
- Farhadinia, M.S.; Akbari, H.; Beheshti, M.; Sadeghi, A. Ecology and status of the Caracal, Caracal caracal (Carnivora: Felidae), in the Abbasabad Naein Reserve, Iran. Zool. Middle East 2007, 41, 5–10. [Google Scholar] [CrossRef]
- Momeni, S.; Malekian, M.; Hemami, M.-R. Molecular versus morphological approaches to diet analysis of the caracal (Caracal caracal). Mammalia 2019, 83, 586–592. [Google Scholar] [CrossRef]
- Díez-Canseco, C.; Aguilera, M.; Tornero, C. Intra-tooth isotopic analysis (δ13C and δ15N) of dentine collagen in high-crowned teeth: A new experimental study with modern sheep specimens. Int. J. Osteoarchaeol. 2022, 32, 962–975. [Google Scholar] [CrossRef]
- Motuzaite-Matuzeviciute, G.; Staff, R.A.; Hunt, H.V.; Liu, X.; Jones, M.K. The early chronology of broomcorn millet (Panicum miliaceum) in Europe. Antiquity 2013, 87, 1073–1085. [Google Scholar] [CrossRef]
- Martin, L.; Messager, E.; Bedianashvili, G.; Rusishvili, N.; Lebedeva, E.; Longford, C.; Hovsepyan, R.; Bitadze, L.; Chkadua, M.; Vanishvili, N.; et al. The place of millet in food globalization during Late Prehistory as evidenced by new bioarchaeological data from the Caucasus. Sci. Rep. 2021, 11, 13124. [Google Scholar] [CrossRef]
- Liu, X.; Jones, P.J.; Matuzeviciute, G.M.; Hunt, H.V.; Lister, D.L.; An, T.; Przelomska, N.; Kneale, C.J.; Zhao, Z.; Jones, M.K. From ecological opportunism to multi-cropping: Mapping food globalisation in prehistory. Quat. Sci. Rev. 2019, 206, 21–28. [Google Scholar] [CrossRef]
- Sołtysiak, A.; Schutkowski, H. Continuity and change in subsistence at Tell Barri, NE Syria. J. Archaeol. Sci. Rep. 2015, 2, 176–185. [Google Scholar] [CrossRef]
- Nesbitt, M. Identification Guide for Near Eastern Grass Seeds; Routledge: Oxfordshire, UK, 2016. [Google Scholar] [CrossRef]
- Al-Jassem, W.; Arslan, A.; Al-Sied, F. Common weeds among fodder crops under saline conditions in Syria. In Sustainable Management of Saline Waters and Salt-Affected Soils for Agriculture, Proceedings of the Second Bridging Workshop, Aleppo, Syria, 15–18 November 2009; Qadir, M., Wichelns, D., Oster, J., Jacobsen, S.-E., Basra, S.M.A., Choukr-Allah, R., Eds.; International Center for Agricultural Research in the Dry Areas (ICARDA): Aleppo, Syria; International Water Management Institute (IWMI): Colombo, Sri Lanka, 2010; pp. 40–44. [Google Scholar]
- Rudov, A.; Mashkour, M.; Djamali, M.; Akhani, H. A Review of C4 Plants in Southwest Asia: An Ecological, Geo-graphical and Taxonomical Analysis of a Region with High Diversity of C4 Eudicots. Front. Plant Sci. 2020, 11, 546518. [Google Scholar] [CrossRef]
- Weber, J.A. Elite equids: Redefining equid burials of the mid- to late 3rd millennium BC from Umm el-Marra, Syria. In Archaeozoology of the Near East VIII. ACTES des Huitièmes Rencontres Internationales d’Archéozoologie de l’Asie du Sud-Ouest et Des Régions Adjacentes; Maison de l’Orient et de la Méditerranée Jean Pouilloux: Lyon, France, 2008; pp. 499–519. Available online: https://www.persee.fr/doc/mom_1955-4982_2008_act_49_1_2721 (accessed on 13 March 2021).
- Muller, M.-H.; Poncet, C.; Prosperi, J.M.; Santoni, S.; Ronfort, J. Domestication history in the Medicago sativa species complex: Inferences from nuclear sequence polymorphism. Mol. Ecol. 2006, 15, 1589–1602. [Google Scholar] [CrossRef] [PubMed]
- Michaud, R.; Lehman, W.; Rumbaugh, M.D. World Distribution and Historical Development. In Alfalfa and Alfalfa Improvement; Hanson, A.A., Barnes, D.K., Hill, R.R., Eds.; John Wiley and Sons, Inc.: Madison, NC, USA, 1988; pp. 25–91. [Google Scholar] [CrossRef]
- Slivinska, K.; Kopij, G. Diet of the Przewalski’s horse Equus przewalskii in the Chernobyl exclusion zone. Pol. J. Ecol. 2011, 59, 841–847. Available online: https://www.miiz.waw.pl/pliki/article/ar59_4_19.pdf (accessed on 24 August 2022).
- Abu Jayyab, K.; Glasser, A.; Albesso, M.; Gibbon, E.; Schwartz, I.; Taraqji, A.; Razzaz, S. Late Chalcolithic Occupation at Tell er-Ramadi (Syria): Results of the 2004–2006 Salvage Excavations. Paléorient 2020, 46, 133–160. [Google Scholar] [CrossRef]
- van der Plicht, J.; Akkermans, P.M.M.G.; Buitenhuis, H.; Kaneda, A.; Nieuwenhuyse, O.; Russell, A. Tell Sabi Abyad, Syria: An Interpretation of Stable Isotope Values of Faunal Bone Collagen. Radiocarbon 2012, 54, 281–289. [Google Scholar] [CrossRef]

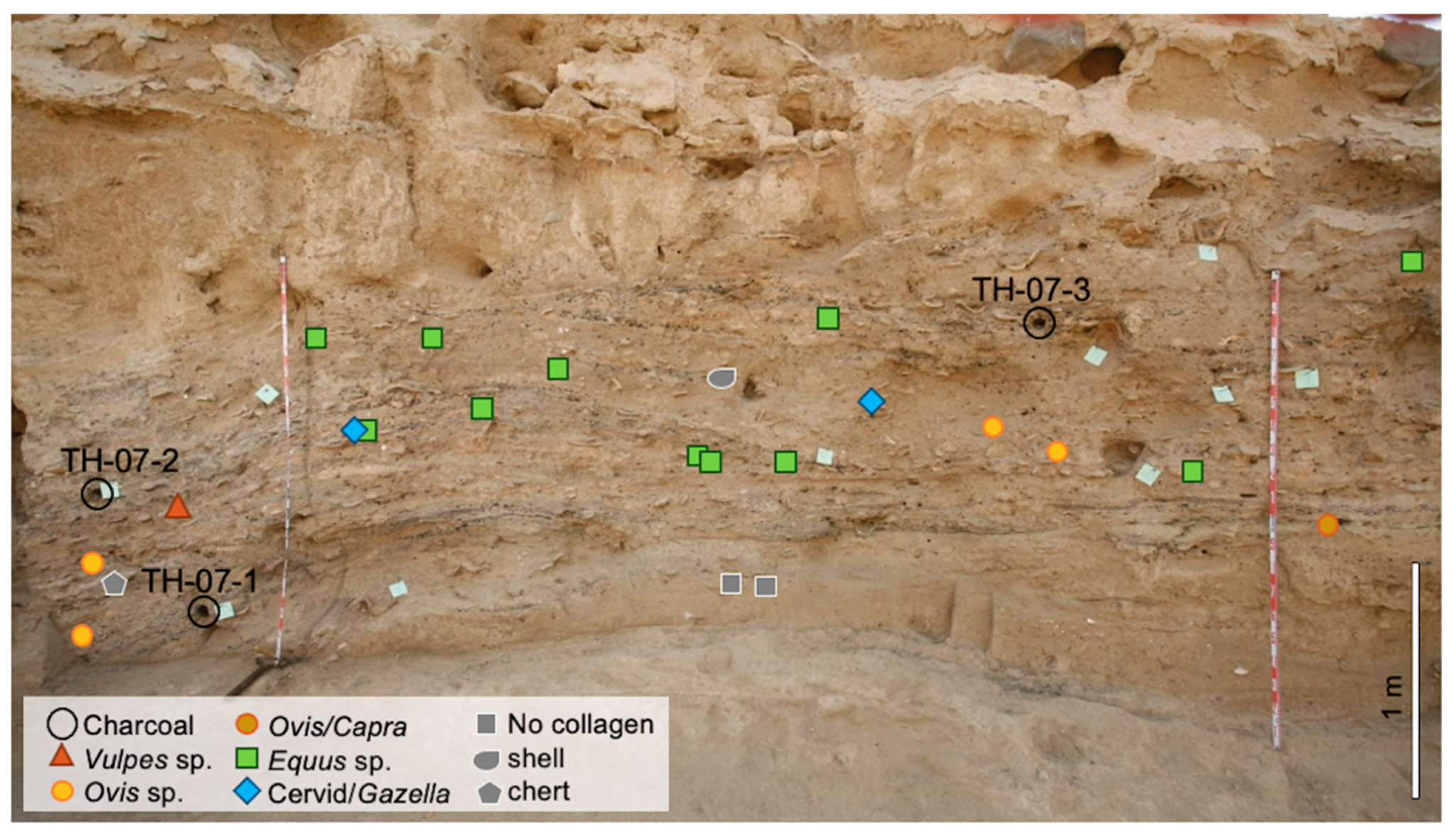


| Area | Depth/SU | Sample | Lab Code | 14C Age BP | Age Cal BC | |
|---|---|---|---|---|---|---|
| From | To | |||||
| WP | Bottom | TH.07.1 | Ua-35246 | 4 820 ± 50 | −3706 | −3383 |
| WP | Middle | TH.07.2 | Ua-35247 | 4 705 ± 45 | −3629 | −3371 |
| WP | Top | TH.07.3 | Ua-35248 | 4 810 ± 45 | −3697 | −3385 |
| J-13 | SU 1004 | TH.11.M4 | Ua-42141 | 4 772 ± 35 | −3640 | −3383 |
| J-13 | SU 1005 | TH.11.M5 | Ua-42143 | 4 706 ± 42 | −3629 | −3371 |
| J-13 | SU 1006 | TH.11.M1 | Ua-42140 | 4 811 ± 34 | −3645 | −3527 |
| J-13 | SU 1006 | TH.11.M2 | Ua-42142 | 4 835 ± 35 | −3698 | −3527 |
| J-13 | SU 1006 | TH.11.M3 | Ua-42144 | 4 917 ± 40 | −3780 | −3638 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grandal-d’Anglade, A.; García-Vázquez, A.; Moreno-García, M.; Peña-Chocarro, L.; Sanjurjo-Sánchez, J.; Montero-Fenollós, J.L. Stable Isotopes and Herding Strategies in Middle Uruk Period in Tell Humeida (Syrian Euphrates Valley). Diversity 2023, 15, 709. https://doi.org/10.3390/d15060709
Grandal-d’Anglade A, García-Vázquez A, Moreno-García M, Peña-Chocarro L, Sanjurjo-Sánchez J, Montero-Fenollós JL. Stable Isotopes and Herding Strategies in Middle Uruk Period in Tell Humeida (Syrian Euphrates Valley). Diversity. 2023; 15(6):709. https://doi.org/10.3390/d15060709
Chicago/Turabian StyleGrandal-d’Anglade, Aurora, Ana García-Vázquez, Marta Moreno-García, Leonor Peña-Chocarro, Jorge Sanjurjo-Sánchez, and Juan Luís Montero-Fenollós. 2023. "Stable Isotopes and Herding Strategies in Middle Uruk Period in Tell Humeida (Syrian Euphrates Valley)" Diversity 15, no. 6: 709. https://doi.org/10.3390/d15060709
APA StyleGrandal-d’Anglade, A., García-Vázquez, A., Moreno-García, M., Peña-Chocarro, L., Sanjurjo-Sánchez, J., & Montero-Fenollós, J. L. (2023). Stable Isotopes and Herding Strategies in Middle Uruk Period in Tell Humeida (Syrian Euphrates Valley). Diversity, 15(6), 709. https://doi.org/10.3390/d15060709






