Evidence for Conductivity- and Macroinvertebrate-Driven Segregation of Ostracod Assemblages in Endorheic Depression Wetlands in North West Province of South Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area, Location, and Selection of the Sampling Sites
2.2. Sampling, Identification of Biota, and Environmental Characterization
2.3. Linking Ostracod Assemblage Analysis to Environmental Variables
3. Results
3.1. Environmental Characteristics of the Studied Sites
3.2. Regional Ostracod Diversity
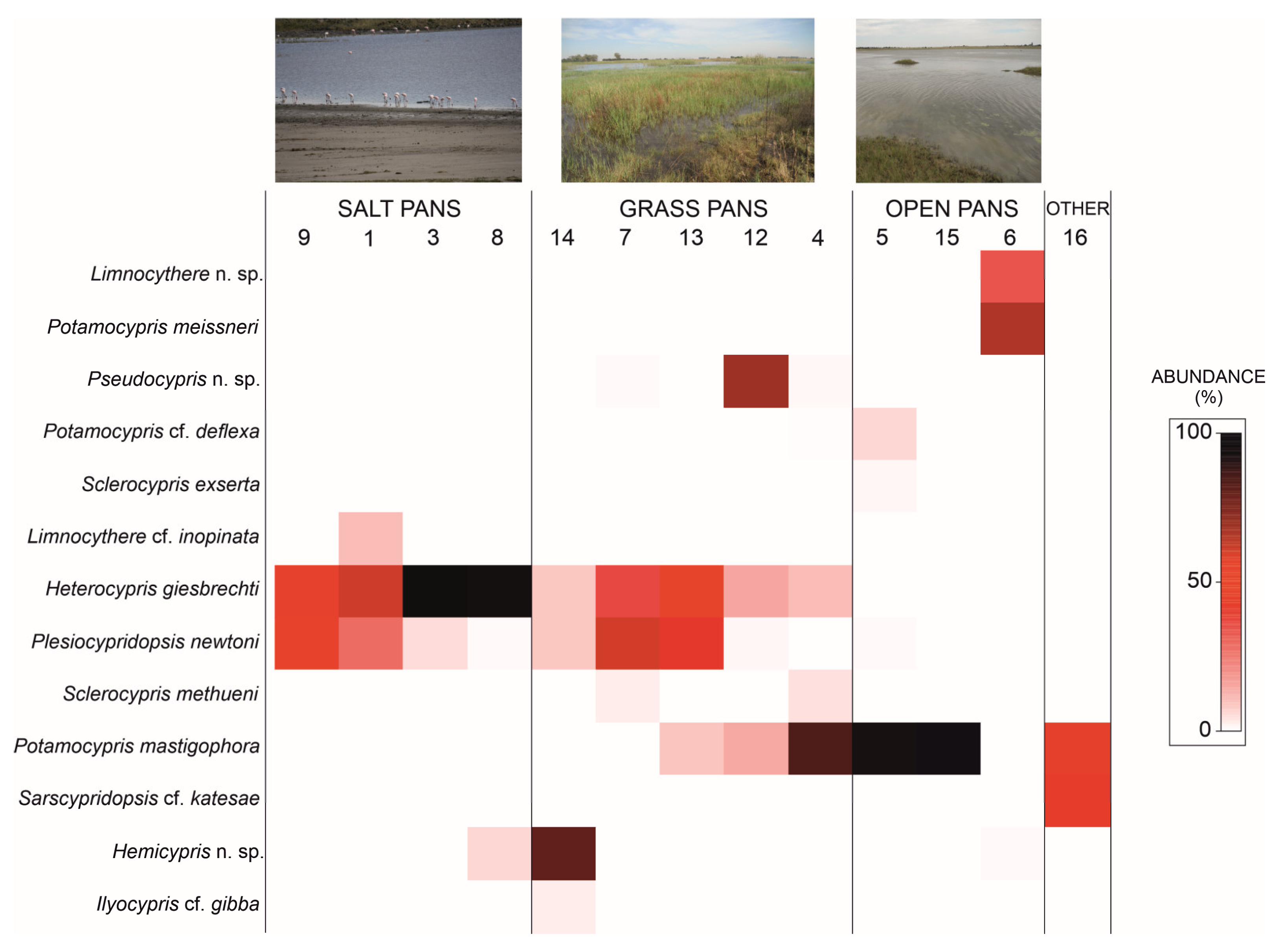
3.3. Ostracod Species and Assemblage Distribution across Pan Types
3.4. Macroinvertebrate Communities and Functional Feeding Groups
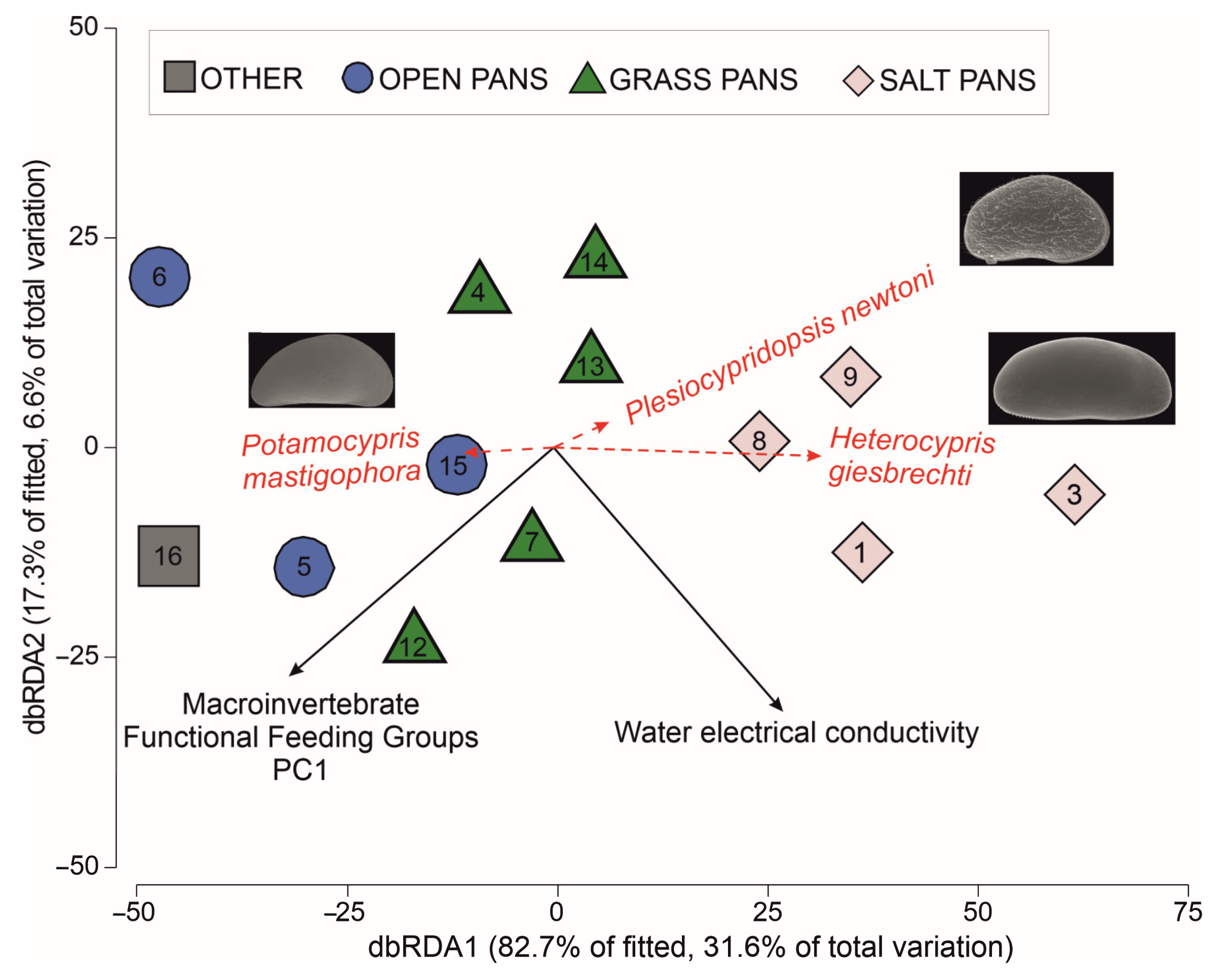
3.5. Effect of Environmental Variables on Ostracod Diversity and Assemblage Composition
4. Discussion
4.1. Ostracods of the North West Province
4.2. Ostracods-Environment Relationships
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doods, W.; Whiles, M. Freshwater Ecology: Concepts and Environmental Applications of Limnology, 2nd ed.; Elsevier: Burlington, MA, USA; San Diego, CA, USA; London, UK, 2020; p. 981. [Google Scholar]
- Williams, D.D. Environmental constraints in temporary fresh waters and their consequences for the insect fauna. J. N. Am. Benthol. Soc. 1996, 15, 634–650. [Google Scholar] [CrossRef]
- Meintjes, S. Seasonal changes in the invertebrate community of small shallow ephemeral pans at Bain’s Vlei, South Africa. Hydrobiologia 1996, 317, 51–64. [Google Scholar] [CrossRef]
- Blaustein, L.; Schwartz, S.S. Why study ecology in temporary pools? Isr. J. Zool. 2001, 47, 303–312. [Google Scholar] [CrossRef]
- De Meester, L.; Declerck, S.; Stoks, R.; Louette, G.; Van De Meutter, F.; De Bie, T.; Michels, E.; Brendonck, L. Ponds and pools as model systems in conservation biology, ecology and evolutionary biology. Aquat. Conserv. Mar. Freshw. Ecosyst. 2005, 15, 715–725. [Google Scholar] [CrossRef]
- Allan, D.G.; Seaman, M.T.; Kalejta, B. The endorheic pans of South Africa. In Wetlands of South Africa; Cowan, G.I., Ed.; Department of Environmental Affairs and Tourism: Pretoria, South Africa, 1995; pp. 75–101. [Google Scholar]
- Williams, D.D. The role of dormancy in the evolution and structure of temporary water invertebrate communities. Arch. Hydrobiol. Spec. Issues Advanc. Limnol. 1998, 52, 109–124. Available online: https://hdl.handle.net/1807/781 (accessed on 25 June 2022).
- Williams, D.D. Biotic adaptations in temporary lentic waters, with special reference to those in semi-arid and arid regions. In Perspectives in Southern Hemisphere Limnology, Proceedings of a Symposium, Wilderness, South Africa, 3–13 July 1984; Davies, B.R., Walmsley, R.D., Eds.; Springer: Berlin/Heidelberg, Germany, 1985; pp. 85–110. [Google Scholar]
- Brendonck, L.; de Meester, L. Egg banks in freshwater zooplankton: Evolutionary and ecological archives in the sediment. Hydrobiologia 2003, 491, 65–84. [Google Scholar] [CrossRef]
- Hairston, N.G., Jr.; Cáceres, C.E. Distribution of crustacean diapause: Micro- and macroevolutionary pattern and process. Hydrobiologia 1996, 320, 27–44. [Google Scholar] [CrossRef]
- Williams, D.D. The Biology of Temporary Waters; University Press: Oxford, UK, 2006; p. 348. [Google Scholar]
- Rosa, J.; Martens, K.; Higuti, J. Dried aquatic macrophytes are floating egg banks and potential dispersal vectors of ostracods (Crustacea) from pleuston communities. Hydrobiologia 2023, 850, 1319–1329. [Google Scholar] [CrossRef]
- Nhiwatiwa, T.; Dalu, T. Seasonal variation in pans in relation to limno-chemistry, size, hydroperiod, and river connectivity in a semi-arid subtropical region. Phys. Chem. Earth 2017, 97, 37–45. [Google Scholar] [CrossRef]
- Dini, J.; Cowan, G.; Goodman, P. Proposed Wetland Classification System for South Africa. Appendix W1: Ecoregional Typing for Wetland Ecosystems; DWAF (Department of Water Affairs and Forestry): Pretoria, South Africa, 1998; pp. 3–19. [Google Scholar]
- Castillo-Escriva, A.; Valls, L.; Rochera, C.; Camacho, A.; Mesquita-Joanes, F. Spatial and environmental analysis of an ostracod metacommunity from endorheic lakes. Aquat. Sci. 2016, 78, 707–716. [Google Scholar] [CrossRef]
- Marchegiano, M.; Gliozzi, E.; Ceschin, S.; Mazzini, I.; Adatte, T.; Mazza, R.; Gliozzi, S.; Ariztegui, D. Ecology and distribution of living ostracod assemblages in a shallow endorheic lake: The example of Lake Trasimeno (Umbria, central Italy). J. Limnol. 2017, 76, 469–487. [Google Scholar] [CrossRef]
- Rosa, J.; de Campos, R.; Martens, K.; Higuti, J. Spatial variation of ostracod (Ostracoda, Crustacea) egg banks in temporary lakes of a tropical floodplain. Mar. Freshw. Res. 2020, 72, 26–34. [Google Scholar] [CrossRef]
- Meisch, C.; Smith, R.J.; Martens, K. A subjective global checklist of the extant non-marine Ostracoda (Crustacea). Eur. J. Taxon. 2019, 492, 1–135. [Google Scholar] [CrossRef]
- Martens, K. Ostracoda. In Guides to the Freshwater Invertebrates of Southern Africa; Water Research Commission Report TT 148/01; Day, J.A., de Moor, I.J., Stewart, B.A., Louw, A.E., Eds.; Water Research Commission: Pretoria, South Africa, 2001; Volume 3, pp. 9–77. [Google Scholar]
- Cours, M.; Vanaverbeke, J.; Parmentier, K.; Knockaert, M.; Higuti, J.; Martens, K.; Schön, I. Water chemistry and not urbanization influences community structure of non-marine Ostracoda (Crustacea) in northern Belgium. Belg. J. Zool. 2021, 151, 149–167. [Google Scholar] [CrossRef]
- Namiotko, T.; de Moor, F.C.; Barber-James, H.M.; Schön, I.; Martens, K. Environmental correlates of non-marine ostracod (Crustacea) assemblages of the Eastern Cape (South Africa). Hydrobiologia, 2023; in press. [Google Scholar]
- Barnes, K. Important bird areas of the North West Province. In The Important Bird Areas of Southern Africa; Barnes, K.N., Ed.; BirdLife: Johannesburg, South Africa, 1998; pp. 93–122. [Google Scholar]
- Henri, A.J.; Ferreira, M.; Malherbe, W.; de Necker, L.; Wepener, V.; van Vuren, J.H.J. The Hatching Success of Egg Banks of Selected Endorheic Wetland (Pan) Fauna and a Suggested Water Quality Classification of Pans; Water Research Commission Report 2190/1/14; Water Research Commission: Pretoria, South Africa, 2014; 191p. [Google Scholar]
- Foster, L.; Malherbe, W.; Ferreira, M.; van Vuren, J.H.J. Macroinvertebrate variation in endorheic depression wetlands in North West and Mpumalanga provinces, South Africa. Afr. J. Aquat. Sci. 2015, 40, 287–297. [Google Scholar] [CrossRef]
- Kabanda, T.H. An approach to land capability evaluation for agriculture using remote sensing and GIS in Barberspan, North West Province of South Africa. Afr. J. Sci. Technol. Innov. Dev. 2015, 7, 453–461. [Google Scholar] [CrossRef]
- Malherbe, W.; Ferreira, M.; van Vuren, J.H.J.; Wepener, V.; Smit, N.J. The Aquatic Biodiversity and Tourism Value of Selected South African Ramsar Wetlands; Water Research Commission Report TT 732/17; Water Research Commission: Pretoria, South Africa, 2017; 164p. [Google Scholar]
- Mathews, J.A.; Kruger, L.; Wentink, G.J. Climate-smart agriculture for sustainable agricultural sectors: The case of Mooifontein. Jàmbá. J. Disaster. Risk. Stud. 2018, 10, a492. [Google Scholar] [CrossRef]
- De Klerk, A.D.; De Klerk, L.P.; Oberholster, P.J.; Ashton, P.J.; Dini, J.A.; Holness, S.D. A Review of Depressional Wetlands (Pans) in South Africa, including a Water Quality Classification System; Water Research Commission Report No. 2230/1/16; Water Research Commission: Pretoria, South Africa, 2016; 21p. [Google Scholar]
- Sars, G.O. The fresh-water Entomostraca of Cape Province (Union of South Africa). Part 2. Ostracoda. Ann. S. Afr. Mus. 1924, 20, 105–193. [Google Scholar]
- Sars, G.O. The contributions to a knowledge of the fauna of South-West Africa (Union of South Africa). Part 3. Ann. S. Afr. Mus. 1924, 20, 194–211. [Google Scholar]
- Martens, K. Revision of African Limnocythere s.s. Brady, 1867 (Crustacea, Ostracoda), with special reference to the Rift Valley Lakes: Morphology, taxonomy, evolution and (palaeo) ecology. Arch. Für Hydrobiol. 1990, 4, 453–524. [Google Scholar]
- Savatenalinton, S.; Martens, K. Generic revision of Cypricercinae McKenzie, 1971 (Crustacea, Ostracoda), with the description of three new genera and one new species and a phylogenetic analysis of the subfamily. Hydrobiologia 2009, 632, 1–48. [Google Scholar] [CrossRef]
- Rumes, B. Regional Diversity, Ecology and Palaeoecology of Aquatic Invertebrate Communities in East African Lakes. Ph.D. Thesis, Ghent University, Ghent, Belgium, 2010. [Google Scholar]
- Seaman, M.T.; Kok, D.J.; Watson, M. Cladocera. In Guides to the Freshwater Invertebrates of Southern Africa; Water Research Commission Report TT 121/00; Day, J.A., Stewart, B.A., de Moor, I.J., Louw, A.E., Eds.; Water Research Commission: Pretoria, South Africa, 1999; Volume 2, pp. 81–110. [Google Scholar]
- Rayner, N. Copepods. In Guides to the Freshwater Invertebrates of Southern Africa; Water Research Commission Report TT 148/01; Day, J.A., de Moor, I.J., Stewart, B.A., Louw, A.E., Eds.; Water Research Commission: Pretoria, South Africa, 2001; Volume 3, pp. 78–123. [Google Scholar]
- Appleton, C.C. Mollusca. In Guides to the Freshwater Invertebrates of Southern Africa; Water Research Commission Report TT 182/02; de Moor, I.J., Day, J.A., Eds.; Water Research Commission: Pretoria, South Africa, 2002; Volume 6, pp. 42–125. [Google Scholar]
- Van Hoven, W.; Day, J.A. Oligochaeta. In Guides to the Freshwater Invertebrates of Southern Africa; Water Research Commission Report TT 167/02; Day, J.A., de Moor, I.J., Eds.; Water Research Commission: Pretoria, South Africa, 2002; Volume 5, pp. 203–236. [Google Scholar]
- Day, J.A.; Harrison, A.D.; de Moor, I.J. Diptera. In Guides to the Freshwater Invertebrates of Southern Africa; Water Research Commission Report TT 201/02; Water Research Commission: Pretoria, South Africa, 2003; Volume 9, 200p. [Google Scholar]
- De Moor, F.C.; Day, J.A. Introduction. In Guides to the Freshwater Invertebrates of Southern Africa; Water Research Commission Report TT 207/03; de Moor, I.J., Day, J.A., de Moor, F.C., Eds.; Water Research Commission: Pretoria, South Africa, 2003; Volume 7, pp. 1–15. [Google Scholar]
- Henning, S.F. Lepidoptera. In Guides to the Freshwater Invertebrates of Southern Africa; Water Research Commission Report TT 214/03; de Moor, I.J., Day, J.A., de Moor, F.C., Eds.; Water Research Commission: Pretoria, South Africa, 2003; Volume 8, pp. 182–188. [Google Scholar]
- Reavel, P.A. Hemiptera. In Guides to the Freshwater Invertebrates of Southern Africa; Water Research Commission Report TT 214/03; de Moor, I.J., Day, J.A., de Moor, F.C., Eds.; Water Research Commission: Pretoria, South Africa, 2003; Volume 8, pp. 16–71. [Google Scholar]
- Samways, M.J.; Wilmot, B.C. Odonata. In Guides to the Freshwater Invertebrates of Southern Africa; Water Research Commission Report TT 207/03; de Moor, I.J., Day, J.A., de Moor, F.C., Eds.; Water Research Commission: Pretoria, South Africa, 2003; Volume 7, pp. 160–212. [Google Scholar]
- Stals, R. Introduction to aquatic Coleoptera. In Guides to the Freshwater Invertebrates of Southern Africa; Water Research Commission Report TT 320/07; Stals, R., de Moor, I.J., Eds.; Water Research Commission: Pretoria, South Africa, 2007; Volume 10, pp. 1–38. [Google Scholar]
- Biström, O. Dytiscidae. In Guides to the Freshwater Invertebrates of Southern Africa; Water Research Commission Report TT 320/07; Stals, R., de Moor, I.J., Eds.; Water Research Commission: Pretoria, South Africa, 2007; Volume 10, pp. 69–84. [Google Scholar]
- Stals, R.; Endrödy-Younga, S. Hydrophilidae: Hydrophilinae. In Guides to the Freshwater Invertebrates of Southern Africa; Water Research Commission Report TT 320/07; Stals, R., de Moor, I.J., Eds.; Water Research Commission: Pretoria, South Africa, 2007; Volume 10, pp. 101–112. [Google Scholar]
- Courtney, G.W.; Cranston, P.S. Order Diptera. In Ecology and General Biology. Thorp and Covich’s Freshwater Invertebrates, 4th ed.; Thorp, J.H., Rogers, C., Eds.; Elsevier: London, UK; San Diego, CA, USA; Waltham, MA, USA; Oxford, UK, 2015; Volume 1, pp. 1043–1058. [Google Scholar]
- Cover, M.R.; Bogan, M.T. Minor Insect Orders. In Ecology and General Biology. Thorp and Covich’s Freshwater Invertebrates, 4th ed.; Thorp, J.H., Rogers, C., Eds.; Elsevier: London, UK; San Diego, CA, USA; Waltham, MA, USA; Oxford, UK, 2015; Volume 1, pp. 1059–1072. [Google Scholar]
- Pyron, M.; Brown, K.M. Introduction to Mollusca and the Class Gastropoda. In Ecology and General Biology. Thorp and Covich’s Freshwater Invertebrates, 4th ed.; Thorp, J.H., Rogers, C., Eds.; Elsevier: London, UK; San Diego, CA, USA; Waltham, MA, USA; Oxford, UK, 2015; Volume 1, pp. 383–421. [Google Scholar]
- Sartori, M.; Brittain, J.E. Order Ephemeroptera. In Ecology and General Biology. Thorp and Covich’s Freshwater Invertebrates, 4th ed.; Thorp, J.H., Rogers, C., Eds.; Elsevier: London, UK; San Diego, CA, USA; Waltham, MA, USA; Oxford, UK, 2015; Volume 1, pp. 873–891. [Google Scholar]
- Merritt, T.; Cummins, K.W.; Berg, M.B. Trophic Relationships of Macroinvertebrates. In Methods in Stream Ecology, 3rd ed.; Hauer, F.R., Lamberti, G.A., Eds.; Elsevier: London, UK; San Diego, CA, USA; Waltham, MA, USA; Oxford, UK, 2017; Volume 1, pp. 413–433. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v7: User Manual/Tutorial; PRIMER-E Ltd.: Plymouth, UK, 2015; 296p. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for Primer: Guide to Software and Statistical Methods, 1st ed.; PRIMER-E Ltd.: Plymouth, UK, 2008; 214p. [Google Scholar]
- Clarke, K.R.; Gorley, R.N.; Somerfield, P.J.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 3rd ed.; PRIMER-E Ltd.: Plymouth, UK, 2014; 259p. [Google Scholar]
- Hutchinson, G.E.; Pickford, G.E.; Schuurman, F.M. A contribution to the hydrobiology of pans and other inland waters of South Africa. Arch. Hydrobiol. 1932, 24, 1–154. [Google Scholar]
- Klie, W. Drei neue Süβwasser-Ostracoden aus Südafrica. Zool. Anz. 1933, 102, 65–74. [Google Scholar]
- Roode, M.C.; van Eeden, J.A. ’N kwantitatiewe en kwalitatiewe ondersoek van die bentos in Barberspan, Transvaal. Wetenskaplike Bydraes van die Potchefstroomse Universiteit vir. C.H.O. Reeks B Natuurwetenskappe 1970, 18, 1–16. [Google Scholar]
- Martens, K.; Davies, B.R.; Baxter, A.J.; Meadows, M.E. A contribution to the taxonomy and ecology of the ostracoda (Crustacea) from Verlorenvlei (Western Cape, South Africa). S. Afr. J. Zool. 1996, 31, 23–36. [Google Scholar] [CrossRef]
- Martens, K. On two new crenobiont ostracod genera (Crustacea, Ostracoda, Herpetocypridinae) from Africa and Asia Minor, with the description of a new species from dolomitic springs in South Africa. S. Afr. J. Sci. 1997, 93, 542–554. [Google Scholar]
- Martens, K.; Rossetti, G. On two new species of Darwinula Brady and Robertson, 1885 (Crustacea, Ostracoda) from South African dolomitic springs. Bull. Het K. Belg. Inst. Voor Nat. Biol. 1997, 67, 57–66. [Google Scholar]
- Rossetti, G.; Martens, K. Taxonomic revision of the recent and holocene representatives of the Family Darwinulidae (Crustacea, Ostracoda), with a description of three new genera. Bull. Het K. Belg. Inst. Voor Nat. Biol. 1998, 68, 55–110. [Google Scholar]
- Martens, K. Taxonomy of the Herpetocypridinae (Ostracoda, Cyprididae). Crustaceana 2001, 74, 295–308. [Google Scholar] [CrossRef]
- Park, L.E.; Martens, K.; Cohen, A.S. Phylogenetic relationships of Gomphocythere (Ostracoda) in Lake Tanganyika, East Africa. J. Crustac. Biol. 2002, 22, 15–27. [Google Scholar] [CrossRef]
- Van Doninck, L.; Schön, I.; De Bruyn, L.; Martens, K. A general purpose genotype in an ancient asexual. Oecologia 2002, 132, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Van Doninck, L.; Schön, I.; Maes, F.; De Bruyn, L.; Martens, K. Ecological strategies in the ancient asexual animal group Darwinulidae (Crustacea, Ostracoda). Freshw. Biol. 2003, 4, 1285–1294. [Google Scholar] [CrossRef]
- Schön, I.; Kamiya, T.; Van den Berghe, T.; Van den Broecke, L.; Martens, K. Novel Cardinium strains in non-marine ostracod (Crustacea) hosts from natural populations. Mol. Phylogenet. Evol. 2019, 130, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Szwarc, A.; Martens, K.; Namiotko, T. Two new Cypridopsinae Kaufmann, 1900 (Crustacea, Ostracoda) from southern Africa. ZooKeys 2021, 1076, 83–107. [Google Scholar] [CrossRef]
- Martens, K.; Hamer, M.; Coke, M. A preliminary account of the diversity of non-marine ostracoda (Crustacea) in KwaZulu-Natal, South Africa. Lammergeyer 1998, 45, 17–31. [Google Scholar]
- Szwarc, A.; Namiotko, T. Biodiversity of Non-Marine Ostracoda (Crustacea) of Botswana: An Annotated Checklist with Notes on Distribution. Water 2022, 14, 1441. [Google Scholar] [CrossRef]
- Martens, K. Annotated checklist of non-marine ostracods (Ostracoda, Crustacea) from African inland waters. K. Mus. Midden-Afr., Zool. Doc. 1984, 20, 1–51. [Google Scholar]
- Martens, K. On the freshwater ostracods (Crustacea, Ostracoda) of the Sudan, with special reference to the Red Sea Hills, including a description of a new species. Hydrobiologia 1984, 110, 137–161. [Google Scholar] [CrossRef]
- Holmes, J.A.; Fothergill, P.A.; Street-Perrott, F.A.; Perrott, R.A. A high-resolution Holocene ostracod record from the Sahel zone of Northeastern Nigeria. J. Paleolimnol. 1998, 20, 369–380. [Google Scholar] [CrossRef]
- Meisch, C. Revision of the Recent West European Species of the Genus Potamocypris. Part II: Species with long swimming setae on the second antennae. Trav. Sci. Mus Hist. Nat. Luxemb. 1985, 6, 1–95. [Google Scholar]
- Meisch, C. Freshwater Ostracoda of Western and Central Europe; Spektrum Akademischer Verlag: Heidelberg, Germany, 2000; 522p. [Google Scholar]
- Bird, M.S.; Mlambo, M.C.; Wasserman, R.J.; Dalu, T.; Holland, A.J.; Day, J.A.; Villet, M.H.; Bilton, D.T.; Barber-James, H.M.; Brendonck, L. Deeper knowledge of shallow waters: Reviewing the invertebrate fauna of southern African temporary wetlands. Hydrobiologia 2019, 827, 89–121. [Google Scholar] [CrossRef]
- Jocqué, M.; Martens, K.; Riddoch, B.; Brendonck, L. Faunistics of ephemeral rock pools in southeastern Botswana. Arch. Hydrobiol. 2006, 165, 415–431. [Google Scholar] [CrossRef]
- Ferreira, M.; Wepener, V.; van Vuren, J.H.J. Aquatic invertebrate communities of perennial pans in Mpumalanga, South Africa: A diversity and functional approach. Afr. Invertebr. 2012, 53, 751–768. [Google Scholar] [CrossRef]
- Barnard, K.H. Scientific results of the Vernay-Lang Kalahari Expedition, March to September, 1930. Crustacea. Ann. Transvaal Mus. 1935, 16, 481–492. Available online: https://decapoda.nhm.org/pdfs/3673/3673.pdf (accessed on 20 June 2022).
- McKenzie, K.G. Entomostraca of Aldabra, with special reference to the genus Heterocypris (Crustacea, Ostracoda). Philos. Trans. R. Soc. Lond. B Biol. Sci. 1971, 260, 257–297. [Google Scholar]
- Tudorancea, C.; Baxter, R.M.; Fernando, C.H. A comparative limnological study of zoobenthic associations in lakes of the Ethiopian Rift Valley. Arch. Hydrobiol. Supp. 1989, 83, 121–174. [Google Scholar]
- Verschuren, D.; Tibby, J.; Sabbe, K.; Roberts, N. Effects of depth, salinity, and substrate on the 1170 invertebrate community of a fluctuating tropical lake. Ecology 2000, 81, 164–182. [Google Scholar] [CrossRef]
- Martens, K. Ostracoda in the Zwai-Shala-Awasa Basins. In Ethiopian Rift Valley Lakes; Tudorancea, C., Taylor, W.D., Eds.; Backhuys Publishers: Leiden, The Netherlands, 2002; pp. 163–166. [Google Scholar]
- Rumes, B.; Van der Meeren, T.; Martens, K.; Verschuren, D. Distribution and community structure of Ostracoda (Crustacea) in shallow waterbodies of southern Kenya. Afr. J. Aquat. Sci. 2016, 41, 377–387. [Google Scholar] [CrossRef]
- Van der Meeren, T.; Ito, E.; Laird, K.R.; Cumming, B.F.; Verschuren, D. Ecohydrological evolution of Lake Naivasha (central Rift Valley, Kenya) during the past 1650 years, as recorded by ostracod assemblages and stable-isotope geochemistry. Quat. Sci. Rev. 2019, 223, 105–906. [Google Scholar] [CrossRef]
- Mesquita-Joanes, F.; Smith, A.J.; Viehberg, F.A. The ecology of Ostracoda across levels of biological organisation from individual to ecosystem: A review of recent developments and future potential. In Ostracoda as Proxies for Quaternary Climate Change; Horne, D.J., Holmes, J.A., Rodriguez-Lazaro, J., Viehberg, F.A., Eds.; Developments in Quaternary Science; Elsevier: Amsterdam, The Netherlands; Oxford, UK; Burlington, MA, USA; San Diego, CA, USA, 2012; Volume 17, pp. 15–35. [Google Scholar] [CrossRef]
- Vandekerkhove, J.; Namiotko, T.; Hallmann, E.; Martens, K. Predation by macroinvertebrates on Heterocypris incongruens (Ostracoda) in temporary ponds: Impacts and responses. Fundam. Appl. Limnol. 2012, 181, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Ohba, S.-Y. Mouth morphology of the diving beetle Hyphydrus japonicus (Dytiscidae: Hydroporinae) is specialized for predation on seed shrimps. Biol. J. Linn. Soc. 2018, 125, 315–320. [Google Scholar] [CrossRef]
- Havel, J.; Link, J.; Niedzwiecki, J. Selective predation by Lestes (Odonata, Lestidae) on littoral microcrustacean. Freshw. Biol. 1993, 29, 47–58. [Google Scholar] [CrossRef]
- Müller, G.W. Ergebnisse einer zoologischen Forschungsreise in Madagaskar und Ost-Africa 1889–1895 von Dr. A. Voeltzkow: Die Ostracoden. Abh. Senckenberg. Nat. Ges. 1898, 21, 255–296. [Google Scholar]
- Methuen, P.A. On a collection of freshwater Crustacea from the Transvaal. Proc. Zool. Soc. Lond. 1910, 80, 148–166. [Google Scholar] [CrossRef]
- Uiblein, F.; Roca, J.R.; Danielopol, D.L. Experimental observations on the behaviour of the ostracode Cypridopsis vidua. Verh. Internat. Ver. Limnol. 1994, 25, 2418–2420. [Google Scholar] [CrossRef]
- Roca, J.R.; Baltanás, A.; Uiblein, F. Adaptive responses in Cypridopsis vidua (Crustacea: Ostracoda) to food and shelter offered by a macrophyte (Chara fragilis). Hydrobiologia 1993, 262, 127–131. [Google Scholar] [CrossRef]
- Riessen, H.P. Predator-induced life history shifts in Daphnia: A synthesis of studies using meta-analysis. Can. J. Fish. Aquat. Sci. 1999, 56, 2487–2494. [Google Scholar] [CrossRef]
- Modig, H.; van de Bund, W.J.; Ólafsson, E. Uptake of phytodetritus by three ostracod species from the Baltic Sea: Effects of amphipod disturbance and ostracod density. Mar. Ecol. Prog. Ser. 2000, 202, 125–134. [Google Scholar] [CrossRef]
- Blaustein, L.; Schwartz, S.S. Species richness and the proportion of predatory animal species in temporary freshwater pools: Relationships with habitat size and permanence. Ecol. Lett. 1999, 2, 157–166. [Google Scholar]
- Zokan, M.; Drake, J.M. The effect of hydroperiod and predation on the diversity of temporary pond zooplankton communities. Ecol. Evol. 2015, 5, 3066–3074. [Google Scholar] [CrossRef] [PubMed]
- Schmit, O.; Bode, S.N.S.; Camacho, A.; Horne, D.J.; Lamatsch, D.K.; Martens, K.; Martins, M.J.F.; Namiotko, T.; Rossetti, G.; Rueda-Sevilla, J.; et al. Linking present environment and the segregation of reproductive modes (geographical parthenogenesis) in Eucypris virens (Crustacea: Ostracoda). J. Biogeogr. 2013, 40, 2396–2408. [Google Scholar] [CrossRef]
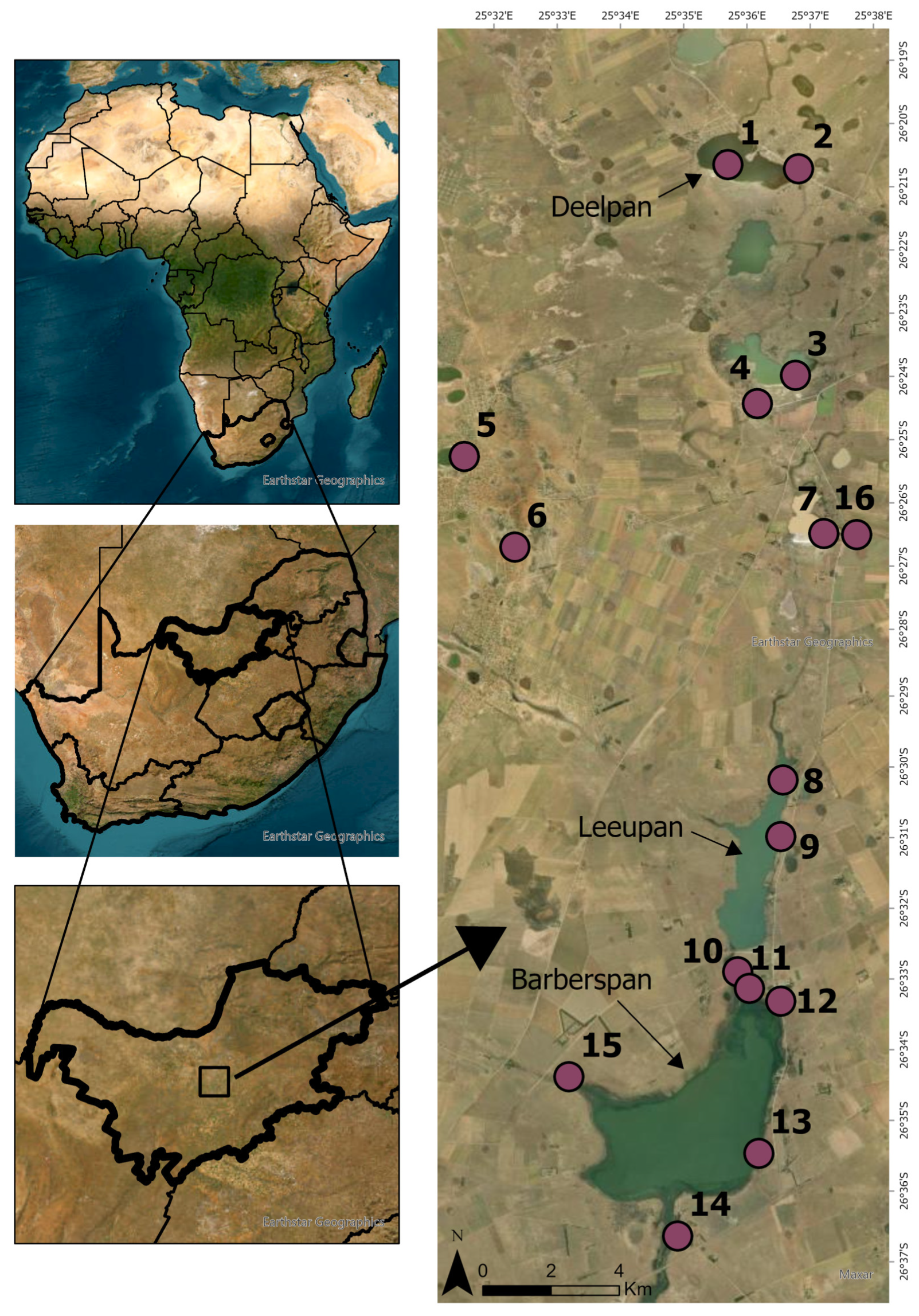
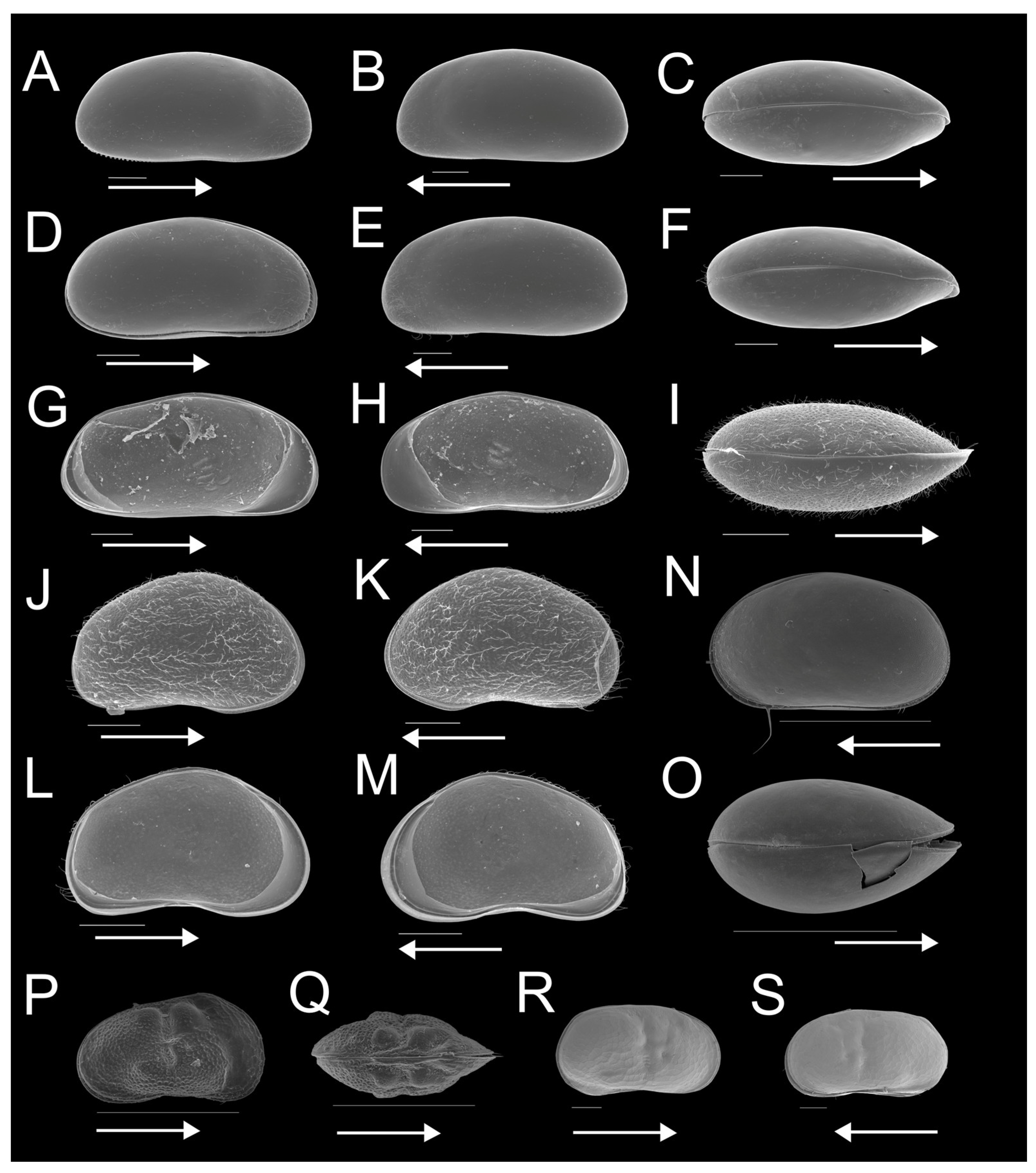
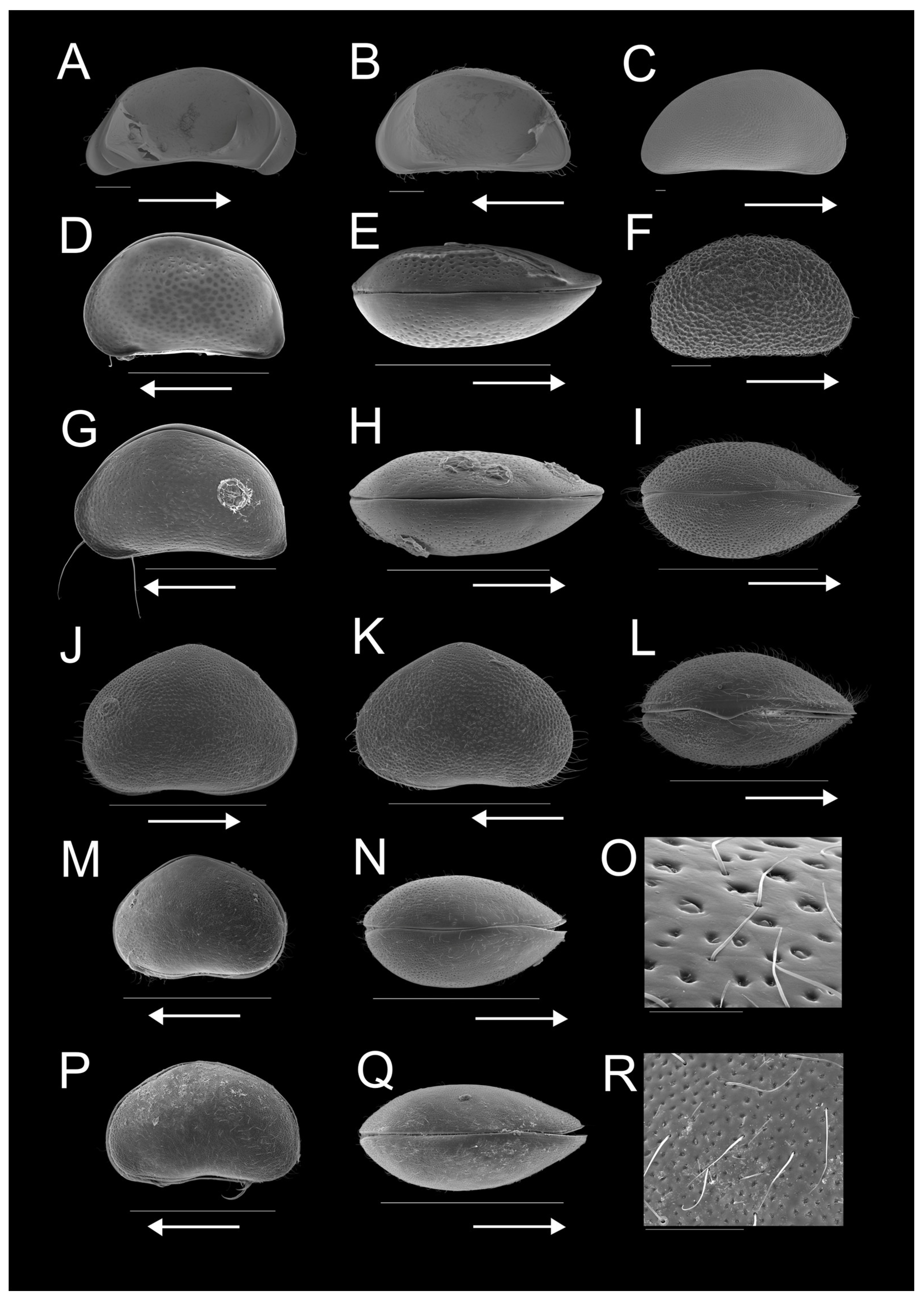
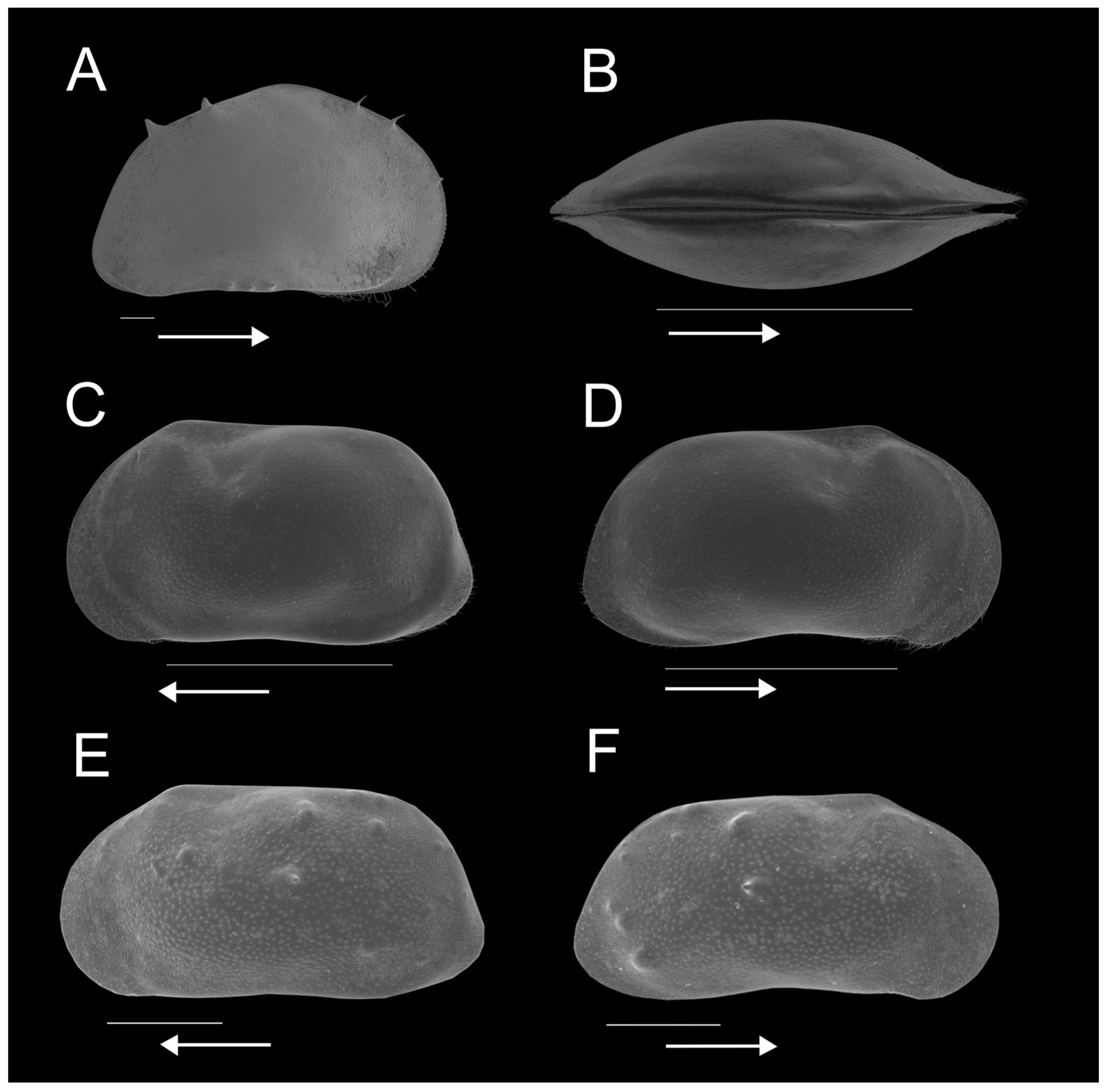
| Site | Longitude E | Latitude S | Altitude (m A.S.L.) | Water Temperature (°C) | pH | Electrical Conductivity (µS/cm) | Pan Type | Land Use | Fish Presence (Yes/No) | Macrophyte Coverage (%) | Substrate Type | Surface Area (km2) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 26°20′39.00″ | 25°35′42.00″ | 1347 | 27.5 | 10.0 | 10,670 | salt | urban/degraded | N | 10 | muddy | 0.97 |
| 2 | 26°20′43.00″ | 25°36′49.00″ | 1354 | 26.7 | 8.5 | 2670 | grass | urban/degraded | N | 40 | sandy-muddy | <0.01 |
| 3 | 26°23′59.00″ | 25°36′46.00″ | 1353 | 22.4 | 9.5 | 16,760 | salt | natural | N | 0 | muddy | 0.73 |
| 4 | 26°24′26.47″ | 25°36′10.02″ | 1357 | 29.3 | 8.5 | 358 | grass | natural | N | 90 | sandy | 0.08 |
| 5 | 26°25′16.00″ | 25°31′32.00″ | 1371 | 25.1 | 10.0 | 1123 | open | urban/degraded | N | 25 | muddy | 0.26 |
| 6 | 26°26′42.00″ | 25°32′20.00″ | 1372 | 25.8 | 7.0 | 36 | open | urban/degraded | N | 0 | sandy-muddy | 0.05 |
| 7 | 26°26′29.00″ | 25°37′13.00″ | 1345 | 21.4 | 8.5 | 2700 | grass | natural | N | 40 | sandy | 0.56 |
| 8 | 26°30′11.25″ | 25°36′34.61″ | 1354 | 32.7 | 8.5 | 3900 | salt | natural | Y | 70 | sandy | 0.11 |
| 9 | 26°30′59.10″ | 25°36′32.92″ | 1354 | 32.7 | 8.5 | 3900 | salt | natural | Y | 70 | sandy | 4.05 |
| 10 | 26°32′54.00″ | 25°35′51.00″ | 1344 | 22.8 | 8.0 | 1109 | grass | natural | Y | 95 | sandy-muddy | 0.04 |
| 11 | 26°33′08.00″ | 25°36′02.00″ | 1358 | 25.9 | 7.5 | 1112 | grass | natural | Y | 95 | sandy-muddy | 0.04 |
| 12 | 26°33′19.00″ | 25°36′32.00″ | 1357 | 27.7 | 8.5 | 3010 | grass | natural | N | 80 | sandy | 0.01 |
| 13 | 26°35′28.70″ | 25°36′11.08″ | 1347 | 22.3 | 8.5 | 1101 | grass | natural | Y | 45 | sandy | 17.3 |
| 14 | 26°36′38.00″ | 25°34′54.00″ | 1347 | 22.5 | 7.0 | 519 | grass | natural | Y | 90 | sandy-muddy | 1.47 |
| 15 | 26°34′23.00″ | 25°33′11.00″ | 1371 | 19.1 | 7.0 | 1165 | open | natural | N | 75 | sandy | 0.04 |
| 16 | 26°26′30.00″ | 25°37′44.00″ | 1360 | 25.4 | 9.5 | 512 | other | natural | N | 0 | concrete/artificial | <0.001 |
| Species | References |
|---|---|
| Family Cyprididae Baird, 1845 | |
| Subfmily Cypridinae Baird, 1845 | |
| Pseudocypris expansa Sars, 1924 | 1, 3 |
| Pseudocypris sp. | 16 |
| Subfmily Cypridopsinae Kaufmann, 1900 | |
| Plesiocypridopsis inaequivalva (Klie, 1933) | 1 and 2 (as Cypridopsis inaequivalva Klie) |
| Plesiocypridopsis newtoni (Brady & Robertson, 1870) | 15, 16 |
| Potamocypris cf. deflexa (Sars, 1924) | 16 |
| Potamocypris cf. gibbula (Sars, 1924) | 15 |
| Potamocypris mastigophora (Methuen, 1910) | 15, 16 |
| Potamocypris meissneri Szwarc et al. 2021 | 14, 16 |
| Sarscypridopsis aculeata (Costa, 1847) | 15 |
| Sarscypridopsis elizabethae (Sars, 1924) | 15 |
| Sarscypridopsis cf. katesae (Hartmann, 1957) | 16 |
| Subfamily Cyprinotinae Bronstein, 1947 | |
| Hemicypris congenera (Vávra, 1897) | 1 (as Cyprinotus congener Vávra, doubtful identification, may refer to Heterocypris congenera (Vávra, 1897)), |
| Hemicypris sp. | 14 (as Hemicypris cf. inversa (Daday, 1913)), 16 |
| Heterocypris giesbrechti (G.W. Müller, 1898) | 15, 16 |
| Subfamily Herpetocypridinae Kaufmann, 1900 | |
| Chrissia levetzovi Hartmann, 1957 | 9 |
| Humphcypris greenwoodi Martens, 1997 | 5, 8 |
| Subfamily Megalocypridinae Rome, 1965 | |
| Sclerocypris exserta Sars, 1924 | 16 |
| Sclerocypris methueni (Kempf, 2015) | 16 |
| Sclerocypris tuberculata (Sars, 1924) | 1 (as Megalocypris tuberculata Sars) |
| Family Ilyocyprididae Kaufmann, 1900 | |
| Ilyocypris cf. gibba (Ramdohr, 1808) | 16 |
| Family Limnocytheridae Sars, 1925 | |
| Limnocythere sp. ex gr. stationis | 14 (as Limnocythere cf. stationis Vávra, 1897), 16 |
| Limnocythere cf. inopinata (Baird, 1843) | 16 |
| Gomphocythere capensis G.W. Müller, 1914 | 4, 5, 10 |
| Family Darwinulidae Brady & Robertson, 1885 | |
| Alicenula inversa (Martens & Rossetti, 1997) | 6 (as Darwinula inversa), 7, 8 |
| Darwinula stevensoni (Brady & Robertson, 1870) | 8 |
| Vestalenula molopoensis (Martens & Rossetti, 1997) | 6 (as Darwinula molopoensis), 7, 8, 11, 12, 13 |
| Salt Pans | Grass Pans | Open Pans | Salt vs. Grass Pans | Salt vs. Open Pans | Grass vs. Open Pans | ||||
|---|---|---|---|---|---|---|---|---|---|
| AvSim = 66.36 | AvSim = 23.34 | AvSim = 30.54 | AvDiss = 66.16 | AvDiss = 99.66 | AvDiss = 85.43 | ||||
| Species | AvA | CSim | AvA | CSim | AvA | CSim | CDiss | CDiss | CDiss |
| Heterocypris giesbrechti | 74.09 | 89.65 | 24.04 | 56.77 | 0.00 | 0.00 | 37.99 | 37.17 | 14.07 |
| Plesiocypridopsis newtoni | 21.73 | 10.33 | 22.40 | 27.31 | 0.24 | 0.00 | 18.85 | 10.78 | 13.03 |
| Potamocypris mastigophora | 0.04 | 0.00 | 21.42 | 13.83 | 63.88 | 100.00 | 16.18 | 32.04 | 33.20 |
| Marginal Tests Variable | Sum of Squares (Trace) | Pseudo-F | P | R2 |
|---|---|---|---|---|
| Pan type | 19,113 | 2.703 | 0.013 | 0.4740 |
| Conductivity | 9762 | 3.514 | 0.014 | 0.2421 |
| FFGs PC1 | 8747 | 3.047 | 0.030 | 0.2169 |
| Macroinvertebrate PC1 | 6716 | 2.198 | 0.051 | 0.1666 |
| Surface area | 8696 | 1.375 | 0.209 | 0.2157 |
| Substrate | 11,679 | 1.223 | 0.257 | 0.2896 |
| Fish presence | 7312 | 1.108 | 0.345 | 0.1813 |
| pH | 3195 | 0.946 | 0.407 | 0.0792 |
| Human impact | 2266 | 0.655 | 0.622 | 0.0562 |
| Vegetation cover | 3694 | 0.504 | 0.881 | 0.0916 |
| Best Models Variable Selection | No variables | AICc | RSS | R2 |
| Conductivity | 1 | 106.11 | 30,562 | 0.2421 |
| FFGs PC1 | 1 | 106.54 | 31,578 | 0.2169 |
| Conductivity and FFGs PC1 | 2 | 106.93 | 24,926 | 0.3819 |
| Macroinvertebrate PC1 | 1 | 107.35 | 33,608 | 0.1666 |
| pH and FFGs PC1 | 2 | 108.21 | 27,502 | 0.3180 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szwarc, A.; Martens, K.; Meissner, W.; Namiotko, T. Evidence for Conductivity- and Macroinvertebrate-Driven Segregation of Ostracod Assemblages in Endorheic Depression Wetlands in North West Province of South Africa. Diversity 2023, 15, 614. https://doi.org/10.3390/d15050614
Szwarc A, Martens K, Meissner W, Namiotko T. Evidence for Conductivity- and Macroinvertebrate-Driven Segregation of Ostracod Assemblages in Endorheic Depression Wetlands in North West Province of South Africa. Diversity. 2023; 15(5):614. https://doi.org/10.3390/d15050614
Chicago/Turabian StyleSzwarc, Agata, Koen Martens, Włodzimierz Meissner, and Tadeusz Namiotko. 2023. "Evidence for Conductivity- and Macroinvertebrate-Driven Segregation of Ostracod Assemblages in Endorheic Depression Wetlands in North West Province of South Africa" Diversity 15, no. 5: 614. https://doi.org/10.3390/d15050614
APA StyleSzwarc, A., Martens, K., Meissner, W., & Namiotko, T. (2023). Evidence for Conductivity- and Macroinvertebrate-Driven Segregation of Ostracod Assemblages in Endorheic Depression Wetlands in North West Province of South Africa. Diversity, 15(5), 614. https://doi.org/10.3390/d15050614







