Abstract
Invasive alien ant species pose serious threats to agricultural production, ecosystems, and human health in China. Solenopsis invicta Buren is the most destructive and aggressive invasive alien ant in China, causing serious agricultural and urban economic losses and public health concerns. Estimating its spatial distribution and ecological niche in China is crucial for S. invicta prevention and control. Based on 4195 occurrence records (4096 invasive occurrence records and 99 native occurrence records) and 10 environmental variables, we estimated the potential suitable area and ecological niche of S. invicta in China using the ensemble model and ‘ecospat’ package in R language. The mean AUC, KAPPA, and TSS values of the ensemble model were 0.989, 0.901, and 0.901, respectively, indicating that the ensemble model was better than the single-species distribution model for the simulation. Temperature, precipitation, and human factors are important variables that influence the distribution of S. invicta. Our results showed that the ecological niche similarity and equivalency test results showed that the ecological niches between native areas and China were not equivalent (D = 0.46, p = 0.001), but were more similar than would be expected by chance (p = 0.003). Under current climatic conditions, the total potential suitable area for S. invicta is 192.89 × 104 km2 in China, accounting for 20.09% of the land area in China; this land is mainly distributed in Hainan, Taiwan, Guangdong, Guangxi, Fujian, Zhejiang, Jiangsu, Anhui, Hubei, Hunan, Jiangxi, Guizhou, Yunnan, Chongqing, Sichuan, and Henan. Under future climatic conditions, the potential suitable areas of S. invicta will further increase, while the highly suitable areas will shift to higher latitudes. We suggest that early warning and monitoring of S. invicta in the central and northern areas of China should be strengthened to prevent its further spread.
1. Introduction
Biological invasion is a major global issue that has resulted in a significant reduction in biodiversity and caused huge economic losses [1,2]. Biological invasions may be directly or indirectly influenced by global warming [3,4]. Global warming changes the distribution patterns of invasive alien species (IAS), which aids in the establishment of new habitats or ecological niches [5,6]. At present, there are nearly 800 varieties of IAS in China, according to the survey statistics of the Ministry of Agriculture and Rural Affairs of the People’s Republic of China [7]. Invasive alien ants are an important part of the IAS because of their multiple lifestyles, complex social organization, nesting patterns, and dietary requirements [8]. Invasive alien ants can cause declines in biodiversity, reduced yields in agriculture and forestry, ecosystem deterioration, huge economic losses, and worse human health [9]. Among the invasive alien ants, Solenopsis invicta, Wasmannia auropunctata, Linepithema humile, Anoplolepis gracilipes, and Pheidole megacephala are listed in the “100 of the world’s worst invasive alien species” list by the International Union for Conservation of Nature and Natural Resources (IUCN) [10].
Solenopsis invicta Buren (Hymenoptera: Formicidae) is an omnivorous soil-dwelling ant native to South American countries, including Brazil, Argentina, and Paraguay [11]. It has spread across four continents (North America, Oceania, Africa, and Asia) and several islands worldwide owing to its powerful ability to reproduce and disperse [12]. Solenopsis invicta invaded the Taoyuan and Jiayi areas of Taiwan, China, in 2003 and Guangdong, Mainland China, in 2004 [13]. As of 20 June 2022, S. invicta has spread to 12 provinces (Zhejiang, Fujian, Jiangxi, Hubei, Hunan, Guangdong, Guangxi, Hainan, Chongqing, Sichuan, Guizhou, and Yunnan) and 579 counties (cities, districts). Solenopsis invicta can reduce biodiversity, decrease crop yields, cause significant economic losses, and harm human health. For instance, S. invicta has replaced native ants by competition in China, resulting in the reduction of native ant populations by 23% to 84% in regions affected by invasion [14]. Solenopsis invicta causes significant reductions in the germination rates of agricultural crop seeds by gnawing, causing direct damage to agricultural production in China [15]. The cost of S. invicta control was 22,710.93 million RMB in Guangdong Province from 2007 to 2020 [16]. Solenopsis invicta bit 93.8% of people in a village in Wuchuan, Guangdong, and, worse yet, there were two deaths caused by their bites in Guangdong [17,18]. Current research focuses on molecular identification [19], biological characteristics [20], and control measures for S. invicta [21]; however, few studies have examined their risk of invasion using ecological niche models in China. Early warning and risk assessment are the most useful strategies for preventing IAS invasion [22]. However, the ecological niches of species change with time and space, especially for IAS [23]. Based only on information about the native areas of IAS, their potential suitable areas in the invasion sites cannot be accurately predicted [24]. Therefore, discovering whether S. invicta gradually adapts to the climatic conditions of the invasion site and expands its ecological niche is extremely important for its management.
Species distribution models (SDMs) provide numerical tools for the correlation of species occurrence data with environmental variables and can be used to predict current and future potential suitable areas for the species [25,26]. There are several dozen SDMs nowadays, each with different principles, algorithms, and prediction performances [27]. Biomod2 can combine the results of several models and present them in one integrated result to improve the accuracy of predicting species distribution [28]. Biomod2 based on R software includes 10 single species distribution models: generalized additive (GAM), generalized boosted (GBM), generalized linear (GLM), classification tree analysis (CTA), multivariate adaptive regression splines (MARS), artificial neural networks (ANN), surface range envelope (SRE), flexible discriminant analysis (FDA), random forests (RF), and maximum entropy (MaxEnt) models [29]. The ensemble model has been widely used to study the potential suitable areas of invasive alien insects because of it has more reliable prediction results than a single model. For instance, ensemble models have been used to study current and future potential suitable areas for five exotic invasive pests in Europe and Pomacea canaliculata worldwide [30,31].
Currently, the distribution of S. invicta in counties (cities and districts) in China has increased by 29.24% and 242.60% compared with 2021 and 2013, respectively [32]. Therefore, this study is based on the global occurrence records of S. invicta and correlated environmental variables, using the ensemble model and ‘ecospat’ package to estimate the potential suitable areas and ecological niche dynamics of S. invicta in native areas and China. The main objectives of the study were to determine the following aspects: (1) important environmental variables influencing the potential suitable areas of S. invicta, (2) whether an ecological niche shift has occurred in S. invicta in China, (3) potential suitable areas for S. invicta in China under current and future climatic conditions, and (4) centroid shifts of S. invicta in highly suitable areas in China.
2. Materials and Methods
2.1. Global Occurrence Records of Solenopsis invicta
Global occurrence records of S. invicta were collected from the Global Biodiversity Information Facility (GBIF; https://www.gbif.org/, doi.org/10.15468/dl.q4zbxf/, accessed on 21 September 2022), Global Ant Biodiversity Informatics (GABI, https://antmaps.org/, accessed on 21 September 2022), Invasive Species Compendium of the Center for Agriculture and Bioscience International (CABI, https://www.cabi.org/, accessed on 21 September 2022), List of Agricultural Plant Quarantine Pest Distribution Administrative Areas in China (http://www.moa.gov.cn/, accessed on 23 September 2022), and documentary records. All occurrence records were selected using ENMtools to ensure that only one species occurrence record was retained in the 5 km × 5 km raster data [33]. Finally, 4195 occurrence records were retained for model construction, including 4096 invasion occurrence records and 99 native occurrence records (Figure 1).
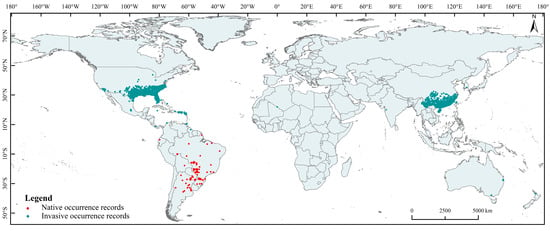
Figure 1.
Global occurrence records of Solenopsis invicta.
2.2. Selection of Environment Variables
A total of 21 environmental variables were selected for this study, including 19 bioclimatic variables, altitude, and human influence factors (Table 1). Current (1970–2000) and future (2030s and 2050s) bioclimatic variables and altitude used in this study were obtained from the World Climate Database (http://www.worldclim.org/, accessed on 12 October 2022) with a resolution of 2.5′. The future climate data (2030s and 2050s) were selected from the National Climate Center’s BCC-CSM2-MR global climate model using the three shared socioeconomic pathways (Table 2). SSP1-2.6, SSP2-4.5, and SSP5-8.5 are upgrades based on the RCP2.6, RCP4.5, and RCP8.5 scenarios of CMIP5, adding the relationship between climate change and socioeconomic development. The human influence index was obtained from the Global Human Influence Index (Geographic) (https://sedac.ciesin.columbia.edu/, accessed on 12 October 2022) with a resolution of 1 km × 1 km, which was created from nine global data layers, including human land use and infrastructure (nighttime lights, built-up areas, and land cover/use), human population pressure (population density), and human access (roads, railroads, coastlines, and navigable rivers). The human influence index was resampled to 5 km × 5 km in ArcGIS software to ensure consistent resolution of all environmental variables. Correlation analysis of 21 environmental variables was performed using ArcGIS software because of the presence of multicollinearity between environmental variables. If the correlation coefficient of two environmental variables exceeded 0.8 (|r| > 0.8), the more meaningful environmental variable was retained (Figure S1). Finally, 10 environmental variables were used to build the model: mean diurnal air temperature range (Bio2), mean temperature of warmest quarter (Bio10), mean temperature of coldest quarter (Bio11), precipitation of driest month (Bio14), precipitation seasonality (Bio15), precipitation of wettest quarter (Bio16), precipitation of warmest quarter (Bio18), precipitation of coldest quarter (Bio19), altitude, and human influence index (Hii).

Table 1.
Environmental variables correlated with distribution of Solenopsis invicta.

Table 2.
Three emission scenarios.
2.3. Construction of the Model
The potential suitable area of S. invicta was modeled using 10 single-species distribution models (GAM, GBM, GLM, CTA, MARS, ANN, SRE, FDA, RF, and MaxEnt) in biomod2 (R package) based on species occurrence records and environmental variables. In the single-species distribution model setup, 75% of the species occurrence records were used as training data and the remaining 25% as test data; 1000 pseudoabsence points were randomly selected, and the model was repeated 10 times. Finally, the results of 10 single-species distribution models were obtained, and 4 single models were selected by evaluation indicators (AUC, TSS, and KAPPA) to construct an ensemble model, which was used to predict the potential suitable area of S. invicta.
2.4. Ecological Niche Comparison Measures
The environmental principal components analysis (PCA-env) in the R language ‘ecospat’ package was used to study the ecological niche shifts of S. invicta in native and invasive countries [34]. Evaluation of ecological niche shifts of IAS requires the introduction of several ecological niche indicators, such as ecological niche overlap index D, equivalence, and similarity. Ecological niche overlap index D indicates the overlap rate of ecological niches of S. invicta in the native areas and China; the index has a value range from 0 to 1, with larger values indicating a higher overlap rate of ecological niches in the two areas [35]. Ecological niche equivalence analysis was performed by randomly dividing all occurrence records for the species into native and China data, whether native and China ecological niches are equivalents (i.e., whether ecological niches overlap when two occurrence records are randomly assigned in native and China). Randomly repeated 100 times (to ensure rejection of the null hypothesis with high confidence), the null hypothesis of ecological niche equivalence could not be rejected if the observed value of the overlap index D was within 95% of the simulated value. Ecological niche similarity tests whether ecological niches in the native and Chinese areas are more similar than randomly selected niches. Randomly repeated 100 times, the actual ecological niches in the native and Chinese were more similar than randomly assigned records if the observed ecological niche overlap index D was greater than 95% of the simulated value [36].
2.5. Model Evaluation and Potential Suitable Area Classification
The area under the receiver operating characteristic (ROC) curve (AUC), true skill statistic (TSS), and kappa coefficient (KAPPA) were used to evaluate model performance. AUC values were evaluated as follows: excellent (0.8 to 1), usable (0.5 to 0.8), and poor (<0.5) [37]. The values of KAPPA and TSS ranged from 0 to 1, with higher values indicating a higher model prediction accuracy.
The ASCII layer of the presence probability of S. invicta was obtained from the results of the ensemble model, with values ranging from 0 to 1000. The results were then clipped according to the Chinese vector boundary to obtain the potential suitable area of S. invicta in China. Finally, the potential suitable areas of S. invicta were classified into four types using ArcGIS: highly suitable area (600–1000), moderately suitable area (400–600), poorly suitable area (190–400), and unsuitable area (0–190).
As the geographical distribution of highly potential suitable areas of S. invicta is irregular and it is difficult to describe its migration tracks, we used the potential highly suitable area centroids to describe the migration tracks of S. invicta in China [38]. The centroids of highly suitable areas of S. invicta under current and future climatic conditions were obtained using the Feature to Point function of ArcGIS software.
3. Results
3.1. Evaluation of Model Accuracy
The results show that the GAM, GBM, GLM, and RF models generally performed most effectively (Figure 2). The mean AUC values for the GAM, GBM, GLM, and RF were 0.977, 0.979, 0.977, and 0.981, respectively. The mean KAPPA values for the GAM, GBM, GLM, and RF were 0.875, 0.857, 0.867, and 0.889, respectively. The mean TSS values for the GAM, GBM, GLM, and RF were 0.872, 0.868, 0.863, and 0.892, respectively. Therefore, we selected the GAM, GBM, GLM, and RF to construct the ensemble model. The mean AUC, KAPPA, and TSS values of the ensemble model were 0.989, 0.901, and 0.901, respectively. The results showed that the ensemble model was more accurate and stable in predicting the potential suitable area of S. invicta compared with the single-species distribution model.
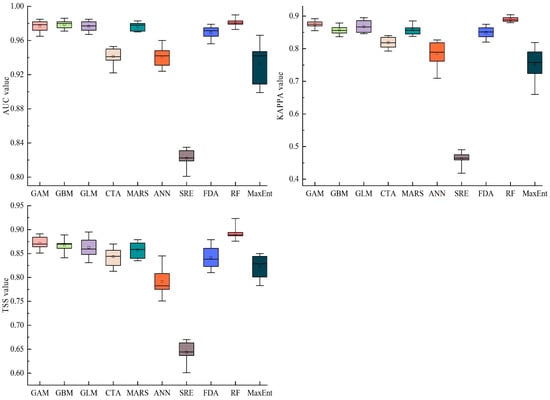
Figure 2.
Evaluation of AUC, KAPPA, and TSS for single-species distribution models.
3.2. Important Environmental Variables
In this study, the contribution values of each environmental variable were assessed using a single-species distribution model. Our results showed that the four environmental variables with the highest average contribution values were the precipitation of driest month (Bio14, 0.355), human influence index (Hii, 0.222), mean temperature of warmest quarter (Bio10, 0.212), and precipitation of wettest quarter (Bio16, 0.139) in GAM; precipitation of driest month (Bio14, 0.253), human influence index (Hii, 0.238), mean temperature of warmest quarter (Bio10, 0.117), and mean temperature of coldest quarter (Bio11, 0.091) in GBM; precipitation of driest month (Bio14, 0.360), mean temperature of warmest quarter (Bio10, 0.239), human influence index (Hii, 0.194), and mean temperature of coldest quarter (Bio11, 0.165) in GLM; and mean temperature of coldest quarter (Bio11, 0.146), human influence index (Hii, 0.143), mean temperature of warmest quarter (Bio10, 0.134), and precipitation of driest month (Bio14, 0.134) in RF (Figure 3). In summary, the important environmental variables influencing the potential suitable areas for S. invicta were the precipitation of driest month (Bio14), mean temperature of warmest quarter (Bio10), mean temperature of coldest quarter (Bio11), and human influence index (Hii).
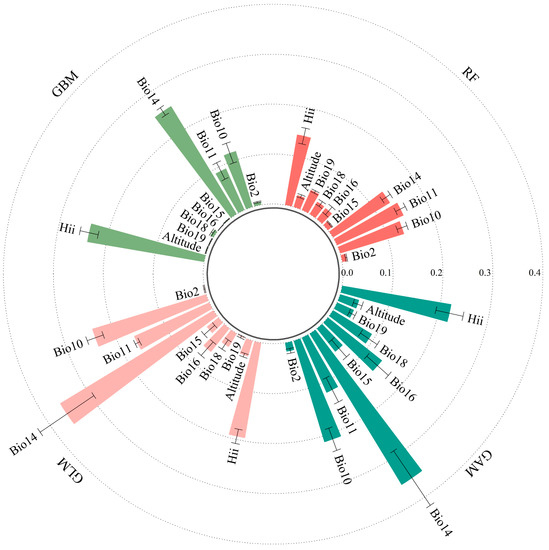
Figure 3.
Contribution values of environmental variables in GAM, GBM, GLM, and RF models.
3.3. Ecological Niche of Solenopsis invicta in China
The average values of the 10 environmental variables for S. invicta changed in native areas and China (Figure 4). The mean diurnal air temperature range (Bio2), mean temperature of warmest quarter (Bio10), altitude, and human influence index (Hii) changed less significantly in the native areas and China, whereas the other environmental variables changed significantly. The mean temperature of coldest quarter (Bio11) of S. invicta in China was 11.85 °C, which was lower than that of the native area, indicating that S. invicta is well-adapted to the mean temperature of coldest quarter. Meanwhile, S. invicta was more adapted to areas with a humid climate, with the precipitation seasonality (Bio15), precipitation of wettest quarter (Bio16), and precipitation of warmest quarter (Bio18) in China being higher than those of the native areas by 34.80–49.18%. Overall, S. invicta is better adapted to cold and humid climates in China.
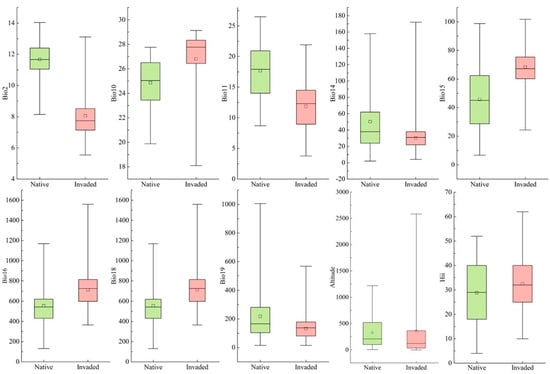
Figure 4.
Comparison of environmental variables of Solenopsis invicta in native areas and China.
The ecological niches of S. invicta in native areas and China shifted according to the comparative ecological space (Figure 5). The ecological niche overlap index D was 0.46, according to the simulation results based on the native area and Chinese occurrence records of S. invicta. The ecological niche of S. invicta in China compared with their native areas showed a certain degree of ecological niche expansion (NE = 0.29, only appears in the ecological niche space of China), while the ecological niche stability was higher in China (NS = 0.70, the ecological niche space appears both in China and native). The null hypothesis of equivalence (p = 0.001) was rejected, while the null hypothesis of similarity (p = 0.003) was not rejected for the ecological niches of S. invicta in native areas and China; thus, the ecological niches of the species in native areas and China are similar but not identical. In summary, the ecological niches of S. invicta in native areas and China are somewhat different, indicating that the ecological niches of the species have shifted; however, ecological niche expansion is smaller, and ecological niches generally maintain higher stability.
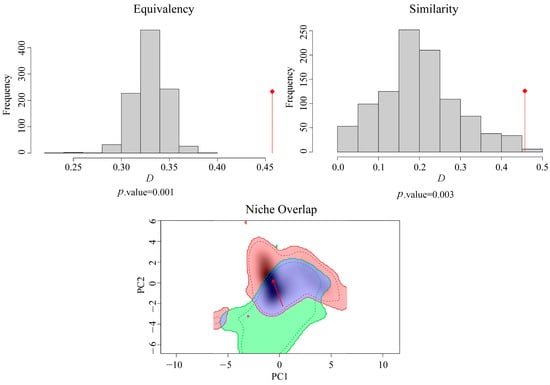
Figure 5.
Comparison of environmental variables of Solenopsis invicta between native areas and China. Red arrows indicate Schoener’s D. Red and green areas indicate expansion and unfilling, respectively, blue areas indicate overlapping ecological niches, and red arrows indicate changes in S. invicta’s ecological niche centers between native areas and China.
3.4. Potential Suitable Areas of Solenopsis invicta in China under Current Climatic Condition
The results showed that our model predictions were consistent with the actual distribution of S. invicta. (Figure 6). The total potential suitable area of S. invicta in China was 192.89 × 104 km2, accounting for 20.09% of the total area in China, and is mainly distributed in the southern area of China (Table S1). The highly suitable area was 93.35 × 104 km2, accounting for 9.72% of the total area in China, and mainly distributed in Yunnan, Guangdong, Guangxi, Hainan, Fujian, Taiwan, Zhejiang, Jiangxi, Hunan, Guizhou, Sichuan, and Chongqing. The moderately suitable area was 49.97 × 104 km2, accounting for 5.21% of the total area in China, and mainly distributed in Hainan, Taiwan, Jiangsu, Anhui, Hubei, and Guizhou. The poorly suitable area was 49.57 × 104 km2, accounting for 5.16% of the total area in China, and mainly distributed in Jiangsu, Anhui, Hubei, Henan, and Yunnan.
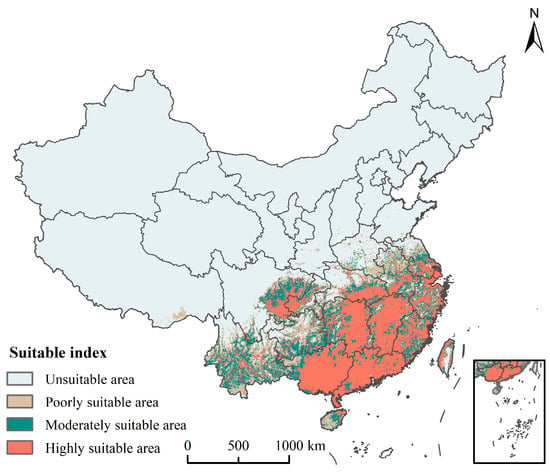
Figure 6.
Ensemble model-based simulation of potential suitable areas of Solenopsis invicta in China under current climatic conditions.
3.5. Potential Suitable Areas and Changes of Solenopsis invicta in China under Future Climatic Conditions
The potential suitable areas and changes to S. invicta under three future (2030s and the 2050s) climatic conditions (SSP1-2.6, SSP2-4.5, and SSP5-8.5) in China are shown in Figure S2 and Figure 7. The potential suitable areas for S. invicta in China were mainly distributed in Hainan, Taiwan, Guangdong, Guangxi, Fujian, Zhejiang, Jiangsu, Anhui, Henan, Hubei, Hunan, Jiangxi, Yunnan, Guizhou, Sichuan, and Chongqing (Figure S2). The land area of potential suitable areas of S. invicta increases under predicted future climatic conditions in China. The areas of increased distribution were mainly distributed in Henan, Jiangsu, Shandong, Hubei, and Guizhou; the areas of decreased distribution were mainly distributed in Yunnan, Hainan, and Taiwan.
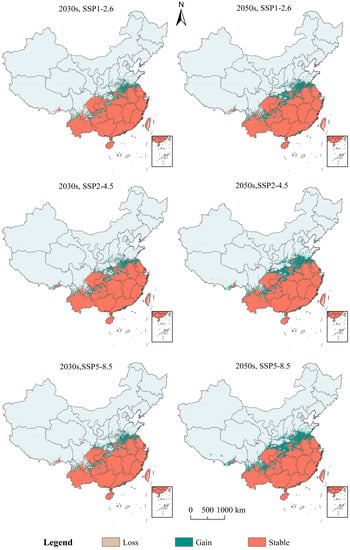
Figure 7.
Changes in potential suitable areas of Solenopsis invicta under future climatic conditions.
Under SSP1-2.6 in 2030s, the total, highly, moderately, and poorly suitable areas for S. invicta were 218.70 × 104 km2, 124.22 × 104 km2, 55.35 × 104 km2, and 39.13 × 104 km2, respectively, accounting for 22.78%, 12.94%, 5.76%, and 4.08% of the total area of China, respectively (Table S1). Under SSP1-2.6 in 2050s, the total, highly, moderately, and poorly suitable areas of S. invicta were 229.90 × 104 km2, 145.51 × 104 km2, 49.66 × 104 km2, and 34.73 × 104 km2, respectively, accounting for 23.95%, 15.16%, 5.17%, and 3.62% of the total area of China, respectively. The total and highly suitable areas gradually increased, the poorly suitable areas gradually decreased in the 2030s and the 2050s, and the moderately suitable areas increased in the 2030s and decreased in the 2050s.
Under the SSP1-2.6 in 2030s and 2050s, the gain areas were 25.26 × 104 km2 and 36.47 × 104 km2, respectively (Table S2), and mainly distributed in Henan, Jiangsu, Shandong, Hubei, Guizhou, and Yunnan. The loss areas were 0.49 × 104 km2 and 0.17 × 104 km2, respectively, and mainly distributed in Taiwan, Hainan, Yunnan, and Tibet (Figure 7).
Under SSP2-4.5 in 2030s, the total, highly, moderately, and poorly suitable areas for S. invicta were 224.08 × 104 km2, 128.51 × 104 km2, 56.99 × 104 km2, and 38.58 × 104 km2, respectively, accounting for 23.34%, 13.39%, 5.93%, and 4.02% of the total area of China, respectively. Under SSP2-4.5 in 2050s, the total, highly, moderately, and poorly suitable areas of S. invicta were 236.45 × 104 km2, 151.92 × 104 km2, 50.35 × 104 km2, and 34.18 × 104 km2, respectively, accounting for 24.63%, 15.83%, 5.24%, and 3.56% of the total area of China, respectively. The total, highly, and moderately suitable areas gradually increased, and poorly suitable areas gradually decreased in the 2030s and the 2050s.
Under the SSP2-4.5 in 2030s and 2050s, the gain areas were 28.43 × 104 km2 and 43.50 × 104 km2, respectively, and were mainly distributed in Henan, Jiangsu, Shandong, Hubei, Guizhou, and Shaanxi. The loss areas were 0.06 × 104 km2 and 0.43 × 104 km2, respectively, mainly distributed in Taiwan, Hainan, and Yunnan.
Under SSP5-8.5 in 2030s, the total, highly, moderately, and poorly suitable areas for S. invicta were 221.99 × 104 km2, 125.75 × 104 km2, 55.25 × 104 km2, and 40.99 × 104 km2, respectively, accounting for 23.12%, 13.10%, 5.75%, and 4.27% of the total area of China, respectively. Under SSP5-8.5 in 2050s, the total, highly, moderately, and poorly suitable areas for S. invicta were 241.47 × 104 km2, 167.64 × 104 km2, 44.17 × 104 km2, and 29.66 × 104 km2, respectively, accounting for 25.15%, 17.46%, 4.60%, and 3.09% of the total area of China, respectively. The total and highly suitable areas gradually increased, and the poorly suitable areas gradually decreased in the 2030s and the 2050s. Moderately suitable areas increased in the 2030s and decreased in the 2050s.
Under the SSP5-8.5 in 2030s and 2050s, the gain areas were 28.43 × 104 km2 and 48.57 × 104 km2, respectively, and were mainly distributed in Henan, Jiangsu, Shandong, Hubei, Guizhou Shaanxi, and Sichuan. The loss areas were 0.32 × 104 km2 and 0.29 × 104 km2, respectively, and were mainly distributed in Taiwan, Hainan, and Yunnan.
3.6. The Centroids Migration of Highly Suitable Areas of Solenopsis invicta
Under future climatic conditions, the centroid of the highly suitable areas of S. invicta shifted to higher latitudes and lower longitudes (Figure S3).
Under current climatic conditions, the centroid of highly suitable areas of S. invicta was located at point (112.78° E, 26.49° N). Under SSP1-2.6, the centroids of the highly suitable areas were located at points (112.53° E, 27.08° N) and (112.08° E, 27.31° N) in the 2030s and 2050s, respectively, and shifted between 0.25° E and 0.59° N from the current to the 2030s and between 0.45° E and 0.23° N from the 2030s to the 2050s. Under SSP2-4.5, the centroids of the highly suitable area were located at points (112.16° E, 27.23° N) and (112.12° E, 27.57° N) in the 2030s and 2050s, respectively, and shifted between 0.62° E and 0.26° N from the current to the 2030s and 0.04° E and 0.34° N from the 2030s to the 2050s. Under SSP5-8.5, the centroids of the highly suitable areas were located at points (112.41° E, 27.17° N) and (111.76° E, 27.58° N) in the 2030s and 2050s, respectively, and shifted between 0.37° E and 0.68° N from the current to the 2030s and between 0.65° E and 0.41° N from the 2030s to 2050s.
4. Discussion
Solenopsis invicta is one of the most dangerous invasive alien species in China, having rapidly spread to 12 provinces (cities, autonomous regions) and 579 counties (cities, districts) since its invasion of mainland China in 2004, which has caused high concern among government departments [13]. Predicting the potential suitable areas of S. invicta is an important approach to prevent further spread. Species distribution models can predict the potential geographic distribution of IAS in a region, determine the geographic range and specific locations of their colonization and distribution once invaded, and provide guidance for early warning, control, and prevention of IAS [39]. The fundamental assumption of SDMs is niche conservatism; therefore, whether the ecological niche of the IAS has shifted has an important impact on the predictive accuracy of SDMs [40]. Our results show that although the ecological niche of S. invicta has undergone a smaller expansion in China, the overall ecological stability has remained high. Finally, we predicted potential suitable areas of S. invicta under current and future climatic conditions in China based on 4195 global occurrence records and 10 environmental variables using ensemble model. Our results provide a scientific basis for the monitoring and controlling of S. invicta.
4.1. Important Environmental Variables Affecting the Distribution of Solenopsis invicta
Insects are typically poikilothermic animals, and global climate change has a significant impact on them [41,42]. Global warming affects insect distribution patterns, growth, and development, phenological synchronization with host plants, egg-laying rates, and genetic composition [41,43,44]. The results of the model showed that the mean temperature of warmest quarter (bio10), mean temperature of coldest quarter (bio11), precipitation of driest month (bio14), and human influence index (Hii) were the environmental variables with the highest contribution to the potential distribution of S. invicta in China. This finding shows that temperature and precipitation are the main factors affecting the distribution pattern of S. invicta, while the human influence index is also important [45,46].
Temperature is an important factor affecting S. invicta foraging: the soil temperature at a depth of 2 cm is an important indicator of foraging activity, while temperature regulates the foraging time of S. invicta and other urban ant species [47,48]. There was a significant correlation between the foraging activity and temperature of S. invicta [45]. The study used 17, 21, and 24 °C as starting temperatures to simulate the growth of S. invicta, and concluded that a temperature of 17 °C was most consistent with the development of larvae and pupae and the actual activity of the population [49]. Studies have shown that, as global temperatures rise, China’s temperatures will rise further in the future [50]. Overall, the southern area of China will provide suitable areas for the further spread of S. invicta.
Changes in precipitation can affect insect development time, pathogen transmission, and nest security, while precipitation itself can directly affect the lives of small insects [51,52]. Our study showed that precipitation factors limit the distribution of potential suitable areas for S. invicta in China. Continental areas with an average annual precipitation above 510 mm are suitable for S. invicta, whereas in areas with an average annual precipitation below 510 mm, only areas near rivers, lakes, and frequently irrigated areas can be colonized by S. invicta [53,54]. However, excessive precipitation has negative effects on this species, such as the possible destruction of nests, negative impacts on foraging, and even direct threats to their lives [7,55].
Human trade and travel break down the geographical limits of species, resulting in increased biological invasions. Studies have shown that recent human historical processes influenced the invasion of global ants [56]. Interestingly, the human influence index (Hii) also had a greater effect on the distribution of potential suitable areas for S. invicta. It has been shown that S. invicta can survive in human buildings and infrastructure [49]. According to the spread pattern of S. invicta, long-distance dispersal is only possible through human activity and major events [57]. Studies have shown that human activities are the main drivers of S. invicta invasion [58]. Therefore, studying the human influence index (Hii) that stimulates invasive ants may be key to understanding and controlling ant invasion.
4.2. Ecological Niche Shifts of Solenopsis invicta after Invasion in China
Ecological niches play an important role in predicting the distribution patterns of species; however, the native ecological niches of species do not include all suitable habitats for their survival, especially for IAS [59]. Invasive alien species undergo significant ecological niche shifts during invasion and after colonization, particularly among invertebrate, vertebrate, amphibian, and plant populations [60]. Invasive alien species adapt to new habitats and spatially expand their ecological niches after colonization through several methods, eventually resulting in differences between native and invasive ecological niches, such as thermal niche shift [61,62]. Solenopsis invicta spread rapidly after its invasion of mainland China in 2004; thus, accurately simulating and predicting the ecological niche of this species in response to climate change is of great significance.
Our study found that the ecological niche overlap index D is only 0.46 between the native and Chinese ecological niche of S. invicta, which indicates that the species has adapted to the Chinese climatic environment [63]. Due to the obvious differences between the ecological niche requirements and climatic environment of China after the invasion of S. invicta, the species has adapted to the climatic environment of the habitat. There are many reasons for ecological niche shifts in species, such as biotic interactions, dispersal constraints, and human factors [64]. These factors cause species to exceed the limits of their native ecological niches and occupy a variety of non-native ecological niches [65]. In conclusion, our results indicate that the ecological niche of S. invicta has shifted less after invasion in China; however, the overall ecological niche has maintained high stability.
4.3. Distribution of Potential Suitable Areas for Solenopsis invicta under Current and Future Climatic Conditions
Our study showed that under current climatic conditions, the potential suitable areas of S. invicta are mainly distributed in Hainan, Taiwan, Guangdong, Guangxi, Fujian, Zhejiang, Jiangsu, Anhui, Hubei, Hunan, Jiangxi, Guizhou, Yunnan, Chongqing, Sichuan, and Henan. These areas have suitable temperatures and adequate moisture, providing suitable habitats for the colonization and spread of S. invicta [45]. Our study results are consistent with the actual distribution, indicating the high accuracy of our model’s results [66]. Moreover, the potential suitable area for S. invicta will increase in China under future climatic conditions. One study predicted the potential suitable area of S. invicta in China based on the optimized MaxEnt model, with the area of potential suitable areas increasing under future climatic conditions [67]. There are also studies that used random forest (RF) models to predict the potential suitable area of S. invicta in Korea, which increased under the RCP8.5 scenario [68]. Although the SDMs used were different, the results of the above studies are consistent with those of our study.
Global warming will also cause a change in the distribution patterns of IAS, such as shifting to higher latitudes [69]. Our study showed that under future climatic conditions, the highly potential suitable area of S. invicta will shift to higher latitudes in China. The study shows that under future climatic conditions, the potential suitable areas of S. invicta, L. humile, and Helicoverpa zea will shift to higher latitudes in China [37,67,70]. The above study also confirms our results.
4.4. Measures for the Control and Management of Solenopsis invicta
The main methods for dispersal of S. invicta are natural and man-made [71]. Natural dispersal mainly includes mating flights, flow with floods, and ground migration, whereas man-made dispersal is mainly spread over long distances by gardening, waste soil, agricultural machinery, and transportation [72]. Solenopsis invicta has colonized China for nearly two decades, and control measures are mainly focused on the external prevention of invasion and internal prevention of spread. Customs departments should strengthen the quarantine of goods from countries with confirmed occurrence of S. invicta. Owing to the limited distance for natural dispersal of S. invicta, man-made dispersal is mainly prevented in the country [71]. The inspection of goods, vehicles, and agricultural machinery from S. invicta colonization areas should be strengthened to prevent and control man-made dispersal [14]. Areas colonized by S. invicta should also be removed, mainly through biological and chemical measures [66]. Biological measures include the use of parasitic flies and microsporidia to control S. invicta, with some positive results shown in [73,74]. Chemical measures include the use of chemicals for direct elimination of S. invicta, especially organic solvents, spinosyn, poison baits, and other chemicals; however, they are somewhat damaging to the ecosystem [75,76]. Finally, a complete monitoring, prevention, and control system should be established to prevent the continued spread of S. invicta in China.
5. Conclusions
In this study, the ensemble model was used to predict the potential suitable areas of S. invicta in China under current and future climatic conditions, and the overall prediction of the model was excellent. These predictions were based on the occurrence records from areas native and China, using the ‘ecospat’ package to research the ecological niche of the species. The most important environmental variables influencing the distribution of S. invicta were the mean temperature of warmest quarter (Bio10), mean temperature of coldest quarter (Bio11), precipitation of driest month (Bio14), and human influence index (Hii). We found that the ecological niche of S. invicta expanded to an extent; however, the overall ecological niche retains high stability. Under current climatic conditions, the total potential suitable area of S. invicta is 192.89 × 104 km2 in China, with these areas mainly located in southern China. Under future climatic conditions, the potential suitable areas for S. invicta will further increase, while highly suitable areas will shift to higher latitudes. Solenopsis invicta has colonized China for nearly 20 years, and customs and quarantine departments around the country should strengthen the inspection of foreign goods to prevent foreign importation and internal proliferation of S. invicta. The results of our study provide theoretical guidance for the prevention and control of S. invicta.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d15050607/s1. Figure S1. Correlation coefficient between environment variables. Figure S2. Ensemble model-based simulation of the potential suitable areas of Solenopsis invicta in China under future climatic conditions. Figure S3. The centroids migration of highly suitable areas of Solenopsis invicta under current and future climatic conditions. Table S1. Potential suitable areas and percentage (%) for Solenopsis invicta under different climate change conditions (104 km2). Table S2. Changes in potential suitable areas of Solenopsis invicta under future climatic conditions (104 km2).
Author Contributions
M.L., H.Z., R.W. and W.L.: conception and design of the research. M.L. and H.Z.: acquisition of data. M.L., H.Z. and X.X.: analysis and interpretation of data. M.L. and H.Z.: statistical analysis. M.L., H.Z. and X.X.: drafting manuscript. J.Z., B.C., T.J., R.W. and W.L.: manuscript revision. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by the National Key R&D Program of China (grant nos. 2021YFD1000500, 2021YFC2600400).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Diagne, C.; Leroy, B.; Vaissière, A.; Gozlan, R.E.; Roiz, D.; Jarić, I.; Salles, J.; Bradshaw, C.J.A.; Courchamp, F. High and rising economic costs of biological invasions worldwide. Nature 2021, 592, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Li, H.R.; Yan, J.; Du, C.; Yan, X.L. Current status and suggestions of research on invasive risk assessment of alien plants in China. Acta Ecol. Sin. 2022, 42, 6451–6463. [Google Scholar]
- Hulme, P.E. Climate change and biological invasions: Evidence, expectations, and response options. Biol. Rev. 2017, 92, 1297–1313. [Google Scholar] [CrossRef] [PubMed]
- Ryding, S.; Klaassen, M.; Tattersall, G.J.; Gardner, J.L.; Symonds, M.R.E. Shape-shifting: Changing animal morphologies as a response to climatic warming. Trends Ecol. Evol. 2021, 36, 1036–1048. [Google Scholar] [CrossRef]
- Gong, X.; Chen, Y.J.; Wang, T.; Jiang, X.F.; Hu, X.K.; Feng, J.M. Double-edged effects of climate change on plant invasions: Ecological niche modeling global distributions of two invasive alien plants. Sci. Total Environ. 2020, 740, 139933. [Google Scholar] [CrossRef]
- Bellard, C.; Thuiller, W.; Leroy, B.; Genovesi, P.; Bakkenes, M.; Courchamp, F. Will climate change promote future invasions? Glob. Chang. Biol. 2013, 19, 3740–3748. [Google Scholar] [CrossRef]
- Wang, R.; Huang, H.K.; Zhang, H.B.; Zhang, Y.P.; Xue, L.; Chen, B.X.; Yang, N.W.; Guo, J.Y.; Liu, W.X.; Wan, F.H. Analysis of gaps in regulations and management mechanisms for the prevention and control of invasive alien species in China. Plant. Quar. 2022, 48, 2–9. [Google Scholar]
- Bradshaw, C.J.A.; Leroy, B.; Bellard, C.; Roiz, D.; Albert, C.; Fournier, A.; Barbet-Massin, M.; Salles, J.; Simard, F.; Courchamp, F. Massive yet grossly underestimated global costs of invasive insects. Nat. Commun. 2016, 7, 12986. [Google Scholar] [CrossRef]
- Lv, X.Y.; Liu, X.; Zhang, Y. Inter-specific competition between invasive ant Anoplolepis gracilipes and native ant Oecophylla smaragdina (Hymenoptera: Formicidae) in Xishuangbanna, southwestern China. Acta Entomol. Sin. 2021, 64, 1196–1204. [Google Scholar]
- Luque, G.M.; Bellard, C.; Cleo, B.; Bonnaud, E.; Genovesi, P.; Simberloff, D.; Courchamp, F. The 100th of the world’s worst invasive alien species. Biol. Invasions 2014, 16, 981–985. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhao, C.Y.; Li, F.F.; Zhu, J.F.; Gao, K.X.; Hu, Y.B. Prediction of potential geographical distribution of Solenopsis invicta Buren in China based on MaxEnt. Plant. Quar. 2019, 33, 70–76. [Google Scholar]
- Allen, C.R.; Lutz, R.S.; Demarais, S. Red Imported Fire Ant Impacts on Northern Bobwhite Populations. Ecol. Appl. 1995, 5, 632–638. [Google Scholar] [CrossRef]
- Zeng, L.; Lu, Y.Y.; He, X.F.; Zhang, W.Q.; Liang, G.W. Identification of red imported fire ant Solenopsis invicta to invade mainland China and infestation in Wuchaun, Guangdong. Chin. J. Appl. Entomol. 2005, 42, 144–148. [Google Scholar]
- Lu, Y.Y.; Zeng, L. 10 years after red imported fire ant found to invade China: History, current situation and trend of its infestation. Plant Quar. 2015, 29, 1–6. [Google Scholar]
- Huang, J.; Xu, Y.J.; Liang, G.W.; Lu, Y.Y.; Zeng, L. Effects on the germination of two dry land crop seeds of Solenopsis invicta Buren. J. Biosaf. 2014, 23, 88–92. [Google Scholar]
- Fu, Q.Y.; Song, Z.D.; Zhao, Y.; Li, S.L.; Xu, Y.J. Analysis on the control cost of Solenopsis invicta in China’s mainland. J. Environ. Entomol. 2022, 44, 345–351. [Google Scholar]
- Wu, N.J.; Lu, W.C.; Luo, H.M.; He, Z.D.; He, J.F.; Liang, K.B.; Yang, C.; Ke, J.Y.; Xiao, K.S. A Survey on human bitten by Red Imported Fire Ants in Mainland for the First Time. Chin. J. Vector Biol. Control 2005, 16, 334–342. [Google Scholar]
- Zhang, Q.L.; Lin, L.F.; Chen, H.T.; Chen, P.H.; Lu, W.C.; Li, Y.J. An investigation on the first human death incident caused by the bite of red imported fire ants. Dis. Surveill. 2006, 21, 654–656. [Google Scholar]
- Chen, Y.; Huang, Y.; Zhu, S.F. A molecular identification of Solenopsis invicta based on partial Cyt b gene. Plant Quar. 2009, 23, 18–20. [Google Scholar]
- Zhang, R.Z.; Ren, L.; Liu, N. An introduction and strict precautions against red imported fire ant, Solenopsis invicta, for its potential invasion to the mainland of China. Chin. J. Appl. Entomol. 2005, 42, 6–10. [Google Scholar]
- Gao, X.W.; Gao, H.R. Chemical control of the red imported fire ant (RIFA), Solenopsis invicta Buren. Plant Quar. 2005, 31, 14–17. [Google Scholar]
- Kolar, C.S.; Lodge, D.M. Progress in invasion biology: Predicting invaders. Trends Ecol. Evol. 2001, 16, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Eastwood, M.M.; Donahue, M.J.; Fowler, A.E. Reconstructing past biological invasions: Niche shifts in response to invasive predators and competitors. Biol. Invasions 2007, 9, 397–407. [Google Scholar] [CrossRef]
- Fernández, M.; Hamilton, H. Ecological niche transferability using invasive species as a case study. PLoS ONE 2015, 10, e0119891. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Li, G.Q.; Du, S. Simulating the potential distribution of Elaeagnus angustifolia L. based on climatic constraints in China. Ecol. Eng. 2018, 113, 27–34. [Google Scholar] [CrossRef]
- Luo, M.; Wang, H.; Lv, Z. Evaluating the performance of species distribution model Biomod2 and MaxEnt using the giant panda distribution data. Chin. J. Appl. Ecol. 2017, 28, 4001–4006. [Google Scholar]
- Hao, T.X.; Elith, J.; Guillera-Arroita, G.; Lahoz-Monfort, J.J. A review of evidence about use and performance of species distribution modelling ensembles like BIOMOD. Divers. Distrib. 2019, 25, 839–852. [Google Scholar] [CrossRef]
- Lantschner, M.V.; Vega, G.; Corley, J.C. Predicting the distribution of harmful species and their natural enemies in agricultural, livestock and forestry systems: An overview. Int. J. Pest Manag. 2018, 65, 190–206. [Google Scholar] [CrossRef]
- Seidl, R.; Klonner, G.; Rammer, W.; Essl, F.; Moreno, A.; Neumann, M.; Dullinger, S. Invasive alien pests threaten the carbon stored in Europe’s forests. Nat. Commun. 2018, 9, 1626. [Google Scholar] [CrossRef]
- Lei, J.C.; Chen, L.A.; Li, H. Using ensemble forecasting to examine how climate change promotes worldwide invasion of the golden apple snail (Pomacea canaliculata). Environ. Monit. Assess. 2017, 189, 404. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.R.; Zhang, Q.Z.; Liu, R.F.; Sun, Y.; Xiao, J.H.; Gao, L.; Gao, X.; Wang, H.B. Impacts of changing climate on the distribution of Solenopsis invicta Buren in Mainland China: Exposed urban population distribution and suitable habitat change. Ecol. Indic. 2022, 139, 108944. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Broennimann, O.; Fitzpatrick, M.C.; Pearman, P.B.; Petitpierre, B.; Pellissier, L.; Yoccoz, N.G.; Thuiller, W.; Fortin, M.J.; Randin, C.; Zimmermann, N.E.; et al. Measuring ecological niche overlap from occurrence and spatial environmental data. Glob. Chang. Biol. 2012, 21, 481–497. [Google Scholar] [CrossRef]
- Schoener, T.W. Nonsynchronous Spatial Overlap of Lizards in Patchy Habitats. Ecology 1970, 51, 408–418. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. Environmental Niche Equivalency versus Conservatism: Quantitative Approaches to Niche Evolution. Evolution 2008, 62, 2868–2883. [Google Scholar] [CrossRef]
- Zhao, H.X.; Xian, X.Q.; Zhao, Z.H.; Zhang, G.F.; Liu, W.X.; Wan, F.H. Climate Change Increases the Expansion Risk of Helicoverpa zea in China According to Potential Geographical Distribution Estimation. Insects 2022, 13, 79. [Google Scholar] [CrossRef]
- Xian, X.Q.; Zhao, H.X.; Wang, R.; Zhang, H.B.; Cheng, B.X.; Huang, H.K.; Liu, W.X.; Wan, F.H. Predicting the potential geographical distribution of Ageratina adenophora in China using equilibrium occurrence data and ensemble model. Front. Ecol. Evol. 2022, 10, 973371. [Google Scholar] [CrossRef]
- Wei, B.; Liu, L.S.; Gu, C.J.; Yu, H.B.; Zhang, Y.L.; Zhang, B.H.; Cui, B.H.; Gong, D.Q.; Tu, Y.L. The climate niche is stable and the distribution area of Ageratina adenophora is predicted to expand in China. Biodivers. Sci. 2022, 30, 88–99. [Google Scholar] [CrossRef]
- Liu, C.L.; Wolter, C.; Courchamp, F.; Roura-Pascual, N.; Jeschke, J.M. Biological invasions reveal how niche change affects the transferability of species distribution models. Ecology 2022, 103, e3719. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, C.S. Effect of global warming on insect: A literature review. Acta Ecol. Sin. 2010, 30, 2159–2172. [Google Scholar]
- Ma, G.; Ma, C.S. The Impacts of Extreme High Temperature on Insect Populations under Climate Change: A review. Sci. Sin. 2016, 46, 556–564. [Google Scholar] [CrossRef]
- Hicking, R.; Roy, D.B.; Hill, J.K.; Thomas, C.D. A northward shift of range margins in British Odonata. Glob. Chang. Biol. 2005, 11, 502–506. [Google Scholar] [CrossRef]
- Menzel, F.; Feldmeyer, B. How does climate change affect social insects? Curr. Opin. Insect Sci. 2021, 46, 10–15. [Google Scholar] [CrossRef]
- Lei, Y.Y.; Jaleel, W.; Shahzad, M.F.; Ali, S.; Azad, R.; Ikram, R.M.; Ail, H.; Ghramh, H.A.; Khan, K.A.; Qiu, X.L.; et al. Effect of constant and fluctuating temperature on the circadian foraging rhythm of the red imported fire ant, Solenopsis invicta Buren (Hymenoptera: Formicidae). Saudi J. Biol. Sci. 2020, 28, 64–72. [Google Scholar] [CrossRef]
- Oi, D.H.; Atchison, R.A.; Chuzel, G.; Chen, J.; Henke, J.A.; Weeks, R.D. Effect of Irrigation on the Control of Red Imported Fire Ants (Hymenoptera: Formicidae) by Water-Resistant and Standard Fire Ant Baits. J. Econ. Entomol. 2021, 115, 266–272. [Google Scholar] [CrossRef]
- Porter, S.D.; Tschinkel, W.R. Foraging in Solenopsis invicta (Hymenoptera: Formicidae): Effects of Weather and Season. Environ. Entomol. 1987, 16, 802–808. [Google Scholar] [CrossRef]
- Roeder, K.A.; Roeder, D.V.; Kaspari, M. The role of temperature in competition and persistence of an invaded ant assemblage. Ecol. Entomol. 2018, 43, 774–781. [Google Scholar] [CrossRef]
- Sutherst, R.W.; Maywald, G. A Climate Model of the Red Imported Fire Ant, Solenopsis invicta Buren (Hymenoptera: Formicidae): Implications for Invasion of New Regions, Particularly Oceania. Environ. Entomol. 2005, 34, 317–335. [Google Scholar] [CrossRef]
- Duan, R.X.; Huang, G.H.; Li, Y.P.; Zheng, R.B.; Wang, G.Q.; Xin, B.Z.; Tian, C.Y.; Ren, J.Y. Ensemble Temperature and Precipitation Projection for Multi-Factorial Interactive Effects of GCMs and SSPs: Application to China. Front. Environ. Sci. 2021, 9, 382. [Google Scholar] [CrossRef]
- Scott, E.R.; Wei, J.P.; Li, X.; Han, W.Y.; Orians, C.M. Differing non-linear, lagged effects of temperature and precipitation on an insect herbivore and its host plant. Environ. Entomol. 2021, 46, 866–876. [Google Scholar] [CrossRef]
- Chen, C.; Harvey, J.A.; Biere, A.; Gols, R. Rain downpours affect survival and development of insect herbivores: The specter of climate change? Ecology 2019, 100, e02819. [Google Scholar] [CrossRef] [PubMed]
- Vinson, S.B. Invasion of the red imported fire ant (Hymenoptera: Formicidae): Spread, biology, and impact. Am. Entomol. 1997, 43, 23–39. [Google Scholar] [CrossRef]
- Morrison, L.W.; Porter, S.D.; Daniels, E.; Korzukhin, M.D. Potential Global Range Expansion of the Invasive Fire Ant, Solenopsis invicta. Biol. Invasions 2004, 6, 183–191. [Google Scholar] [CrossRef]
- Mertl, A.L.; Wilkie, K.T.R.; Traniello, J.F.A. Impact of Flooding on the Species Richness, Density and Composition of Amazonian Litter-Nesting Ants. Biotropica 2009, 41, 633–641. [Google Scholar] [CrossRef]
- Bertelsmeier, C.; Ollier, S.; Liebhold, A.; Keller, L. Recent human history governs global ant invasion dynamics. Nat. Ecol. Evol. 2017, 1, 0184. [Google Scholar] [CrossRef]
- Chen, S.; Ding, F.Y.; Hao, M.M.; Jiang, D. Mapping the Potential Global Distribution of Red Imported Fire Ant (Solenopsis invicta Buren) Based on a Machine Learning Method. Sustainability 2020, 12, 10182. [Google Scholar] [CrossRef]
- King, J.R.; Tschinkel, W.R.; Affiliations, A.I. Experimental evidence that human impacts drive fire ant invasions and ecological change. Proc. Natl. Acad. Sci. USA 2008, 105, 20339–20343. [Google Scholar] [CrossRef]
- Liu, C.L.; Wolter, C.; Xian, W.W.; Jeschke, J.M. Most invasive species largely conserve their climatic niche. Proc. Natl. Acad. Sci. USA 2020, 117, 23643–23651. [Google Scholar] [CrossRef]
- Pili, A.N.; Tingley, R.; Sy, E.Y.; Diesmos, M.L.L.; Diesmos, A.C. Niche shifts and environmental non-equilibrium undermine the usefulness of ecological niche models for invasion risk assessments. Sci. Rep. 2020, 10, 7972. [Google Scholar] [CrossRef]
- Zenni, R.D.; Bailey, J.K.; Simberloff, D. Rapid evolution and range expansion of an invasive plant are driven by provenance–environment interactions. Ecol. Lett. 2014, 17, 727–735. [Google Scholar] [CrossRef]
- Bujan, J.; Charavel, E.; Bates, O.K.; Gippet, J.M.W.; Darras, H.; Lebas, C.; Bertelsmeier, C. Increased acclimation ability accompanies a thermal niche shift of a recent invasion. J. Anim. Ecol. 2020, 90, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.D.; Tang, X.G.; Liu, M.Y.; Liu, X.F.; Tao, J. Species Distribution Models of the Spartina alterniflora Loisel in Its Origin and Invasive Country Reveal an Ecological Niche Shift. Front. Plant Sci. 2021, 12, 738769. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.Z.; Wang, C.J.; Tan, J.F.; Yu, F.H. Climatic niche divergence and habitat suitability of eight alien invasive weeds in China under climate change. Ecol. Evol. 2017, 7, 1541–1552. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.; Tricarico, E.; Panov, V.; Cardoso, A.; Katsanevakis, S. Pathways and gateways of freshwater invasions in Europe. Aquat. Invasions 2015, 10, 359–370. [Google Scholar] [CrossRef]
- CABI. Invasive Species Compendium; CAB International: Wallingford, UK, 2022; Available online: https://www.cabi.org/isc (accessed on 21 September 2022).
- Song, J.Y.; Zhang, H.; Li, M.; Han, W.H.; Yin, Y.X.; Lei, J.P. Prediction of Spatiotemporal Invasive Risk of the Red Import Fire Ant, Solenopsis invicta (Hymenoptera: Formicidae), in China. Insects 2021, 12, 874. [Google Scholar] [CrossRef]
- Sung, S.; Kwon, Y.S.; Lee, D.K.; Cho, Y. Predicting the Potential Distribution of an Invasive Species, Solenopsis invicta Buren (Hymenoptera: Formicidae), under Climate Change using Species Distribution Models. Entomol. Res. 2018, 48, 505–513. [Google Scholar] [CrossRef]
- Li, X.; Ge, X.Z.; Chen, L.H.; Zhang, L.J.; Wang, T.; Zong, S.C. Climate Change Impacts on the Potential Distribution of Eogystia hippophaecolus in China. Pest Manag. Sci. 2018, 75, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xian, X.Q.; Zhao, H.X.; Xue, L.; Chen, B.X.; Huang, H.K.; Wan, F.H.; Liu, W.X. Predicting the Potential Suitable Area of the Invasive Ant Linepithema humile in China under Future Climatic Scenarios Based on Optimized MaxEnt. Diversity 2022, 14, 921. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Liang, G.W.; Zeng, L. Study on Expansion Pattern of Red Imported Fire Ant, Solenopsis invicta Buren, in South China. Sci. Agric. Sin. 2008, 41, 1053–1063. [Google Scholar]
- Wang, L.; Chen, K.W.; Feng, X.D.; Wang, X.L.; Lu, Y.Y. Log-term predication of red imported fire ant (Solenopsis invicta Buren) expansion in Chinese mainland. J. Environ. Entomol. 2022, 44, 339–344. [Google Scholar]
- Graham, L.C.F.; Porter, S.D.; Pereira, R.M.; Dorough, H.D.; Kelley, A.T. Field releases of the decapitating fly Pseudacteon curvatus (Diptera: Phoridae) for control of imported fire ants (Hymenoptera: Formicidae) in Alabama, Florida, and Tennessee. Fla. Entomol. 2003, 86, 334–339. [Google Scholar] [CrossRef]
- Oi, D.H.; Briano, J.A.; Valles, S.M.; Williams, D.F. Transmission of Vairimorpha invictae (Microsporidia: Burenellidae) infections between red imported fire ant (Hymenoptera: Formicidae) colonies. J. Invertebr. Pathol. 2005, 88, 108–115. [Google Scholar] [CrossRef]
- Pang, X.Y.; Tao, Q.H.; Li, Q.T.; Zhang, F.C.; Lu, Y.Y.; Wang, L. Toxicity of five solvents against Solenopsis invicta. J. Environ. Entomol. 2021, 43, 1040–1046. [Google Scholar]
- Yu, X.; Wang, L.; Liang, G.W.; Zeng, L. Population dynamics of a Solenopsis invicta population and associated nontarget arthropod community in a lawn after pesticide. Chin. J. Appl. Entomol. 2015, 52, 1353–1360. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).