Potential Effects of Habitat Change on Migratory Bird Movements and Avian Influenza Transmission in the East Asian-Australasian Flyway
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Waterfowl Capture and Marking
2.3. Telemetry Data Filtering
2.4. Movement Models
2.5. Habitat Change Layers
| Species | Capture Area | Marking Dates | Data Type | Transmitter Weights (g) | n | Fixes for dBBMM |
|---|---|---|---|---|---|---|
| Eurasian Wigeon | Mai Po (Hong Kong) | 8 December, 9 December | Argos, GPS | 12, 22 | 5 | 1087 |
| Northern Pintail | Mai Po (Hong Kong) | 8 December, 9 December | Argos, GPS | 12, 18, 22 | 9 | 3238 |
| Common Teal | Poyang Lake | 7 March | Argos | 12 | 3 | 187 |
| Falcated Teal | Poyang Lake | 7 March | Argos | 12, 18 | 4 | 249 |
| Garganey | Poyang Lake | 7 March | Argos | 12 | 1 | 69 |
| Swan Goose 1 | Mongol Daguur Complex | 8 July, 14 July | GPS, GSM | 30, 45 | 18 | 10,277 |
2.6. Forecasting Effects of Changing Rice Production
3. Results
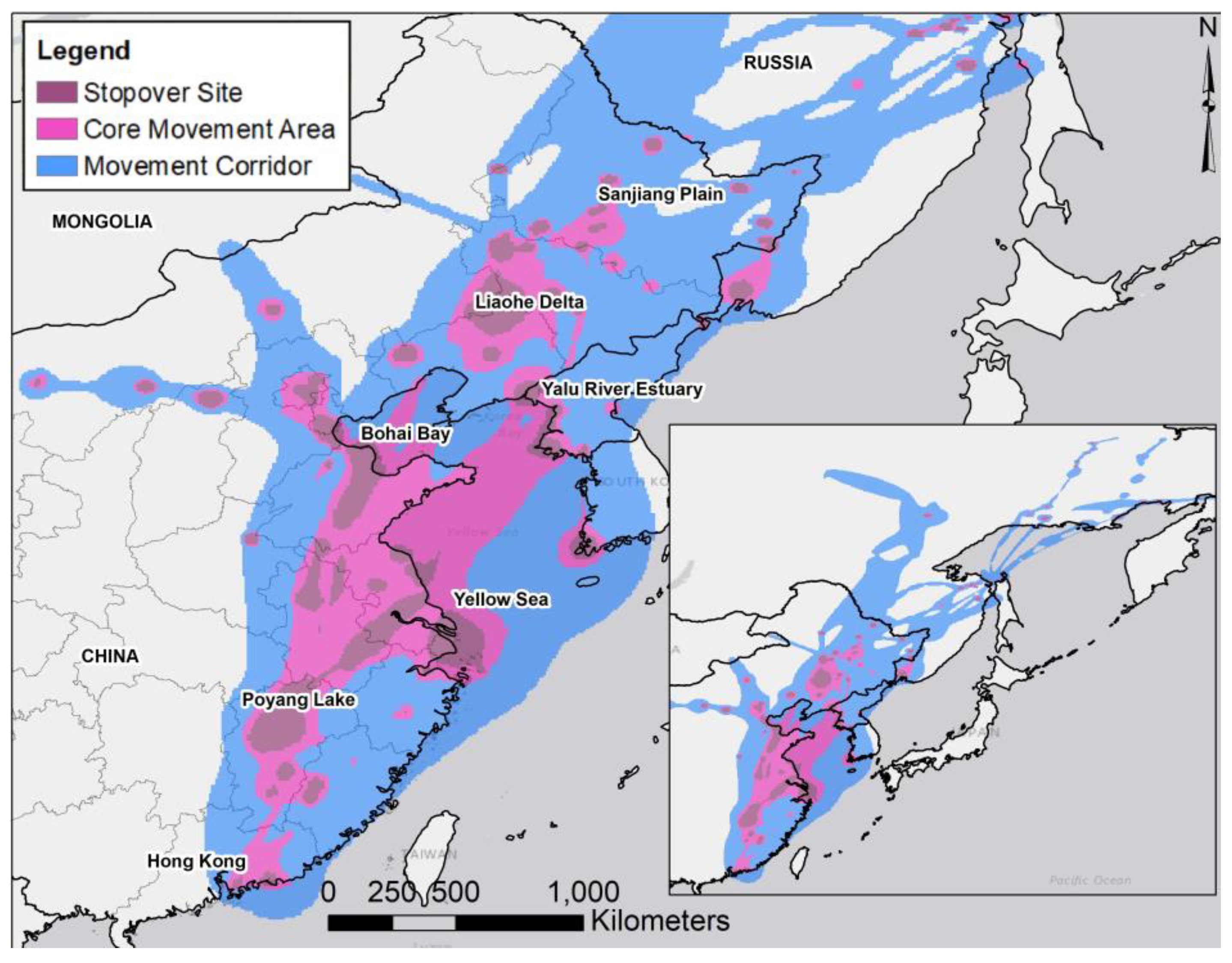
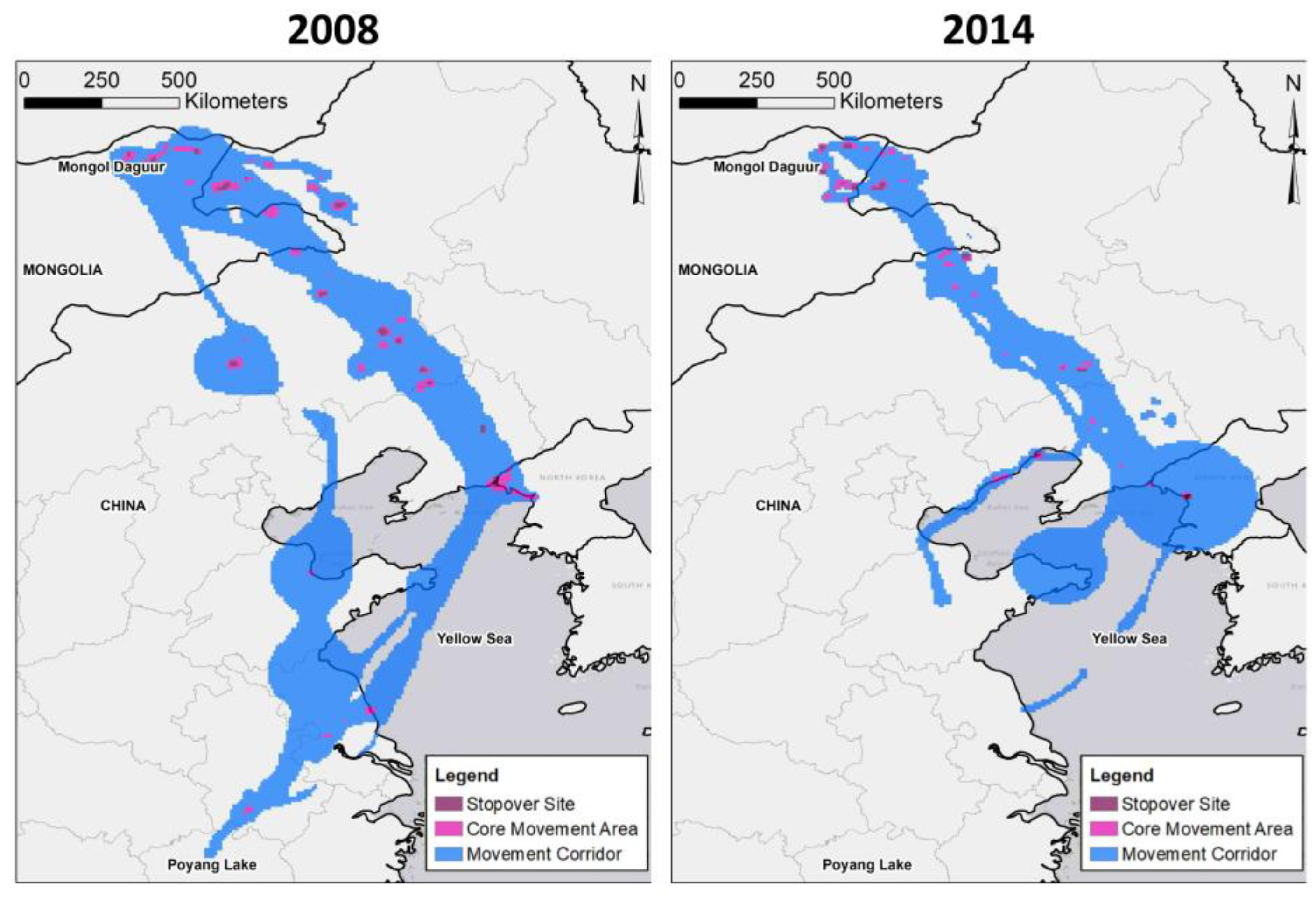
3.1. Transmitter Performance and Movement Paths
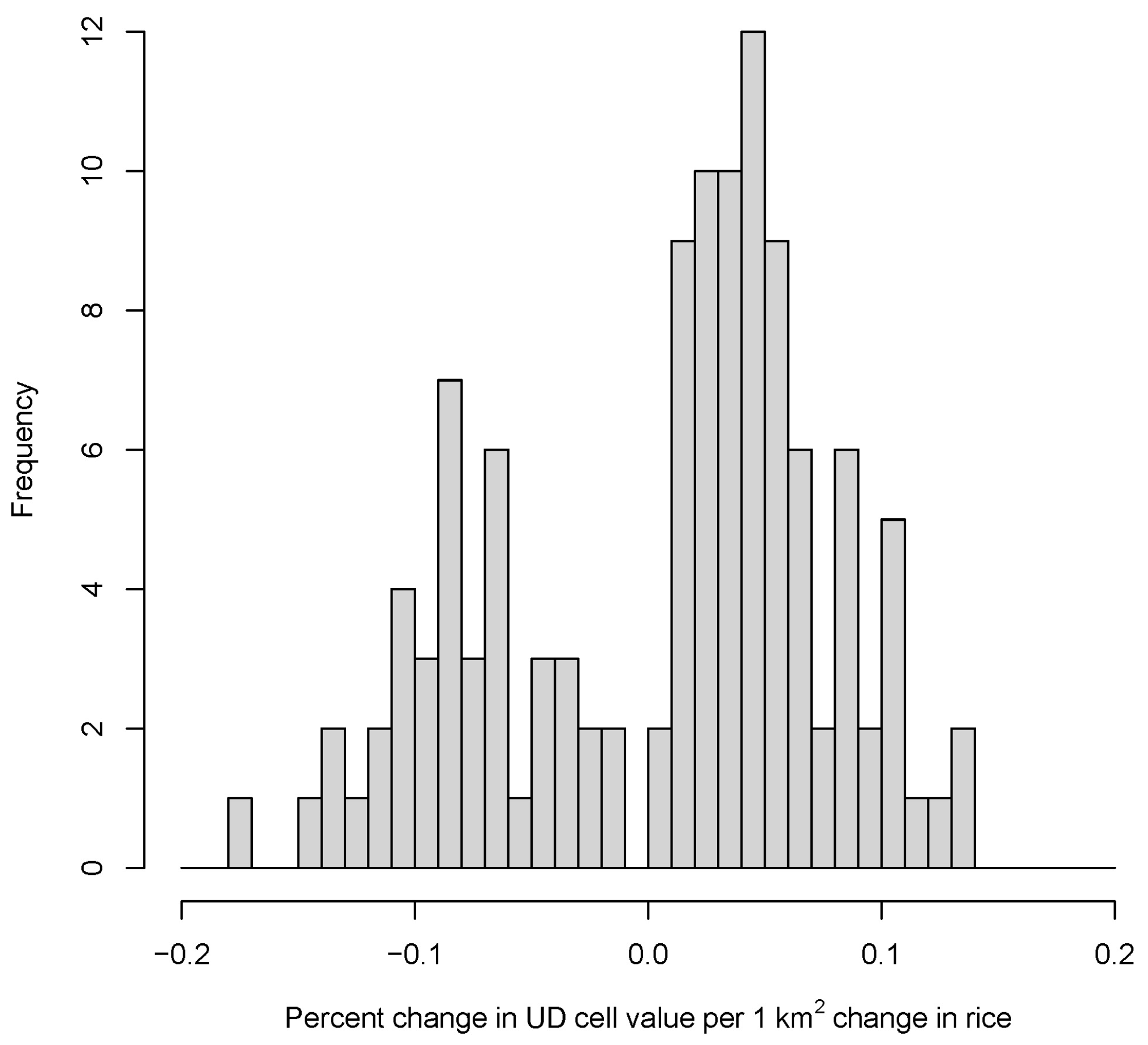
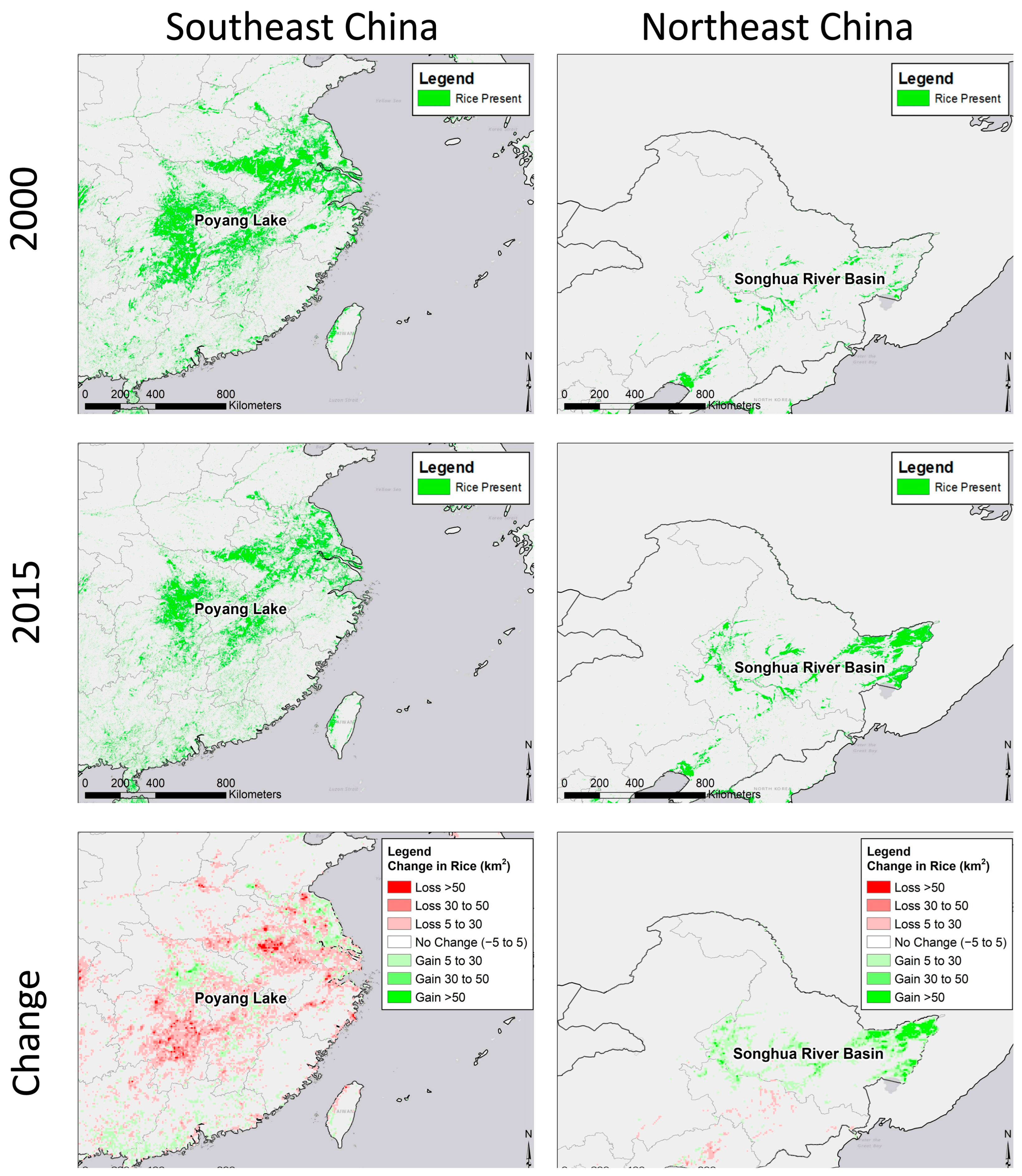
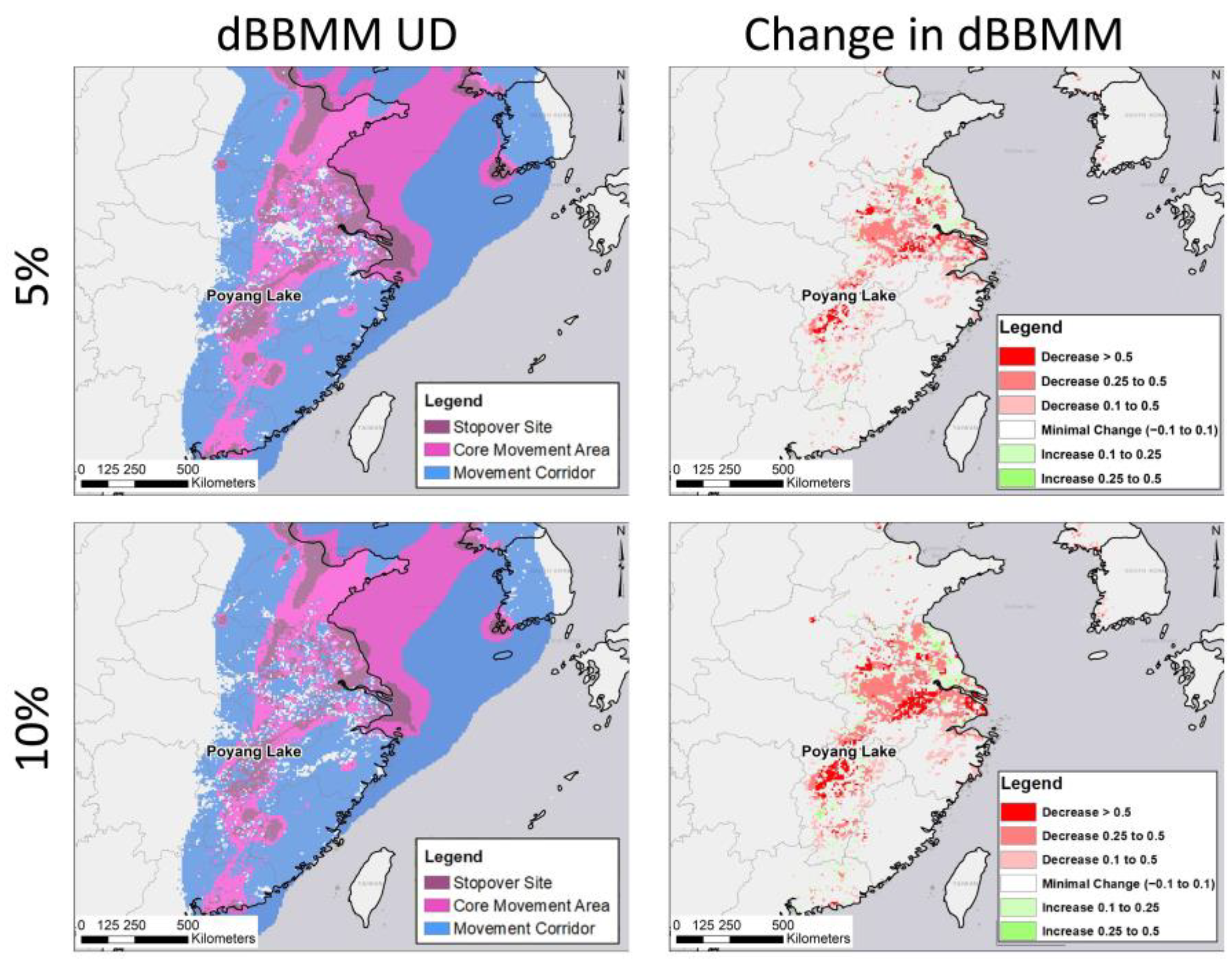
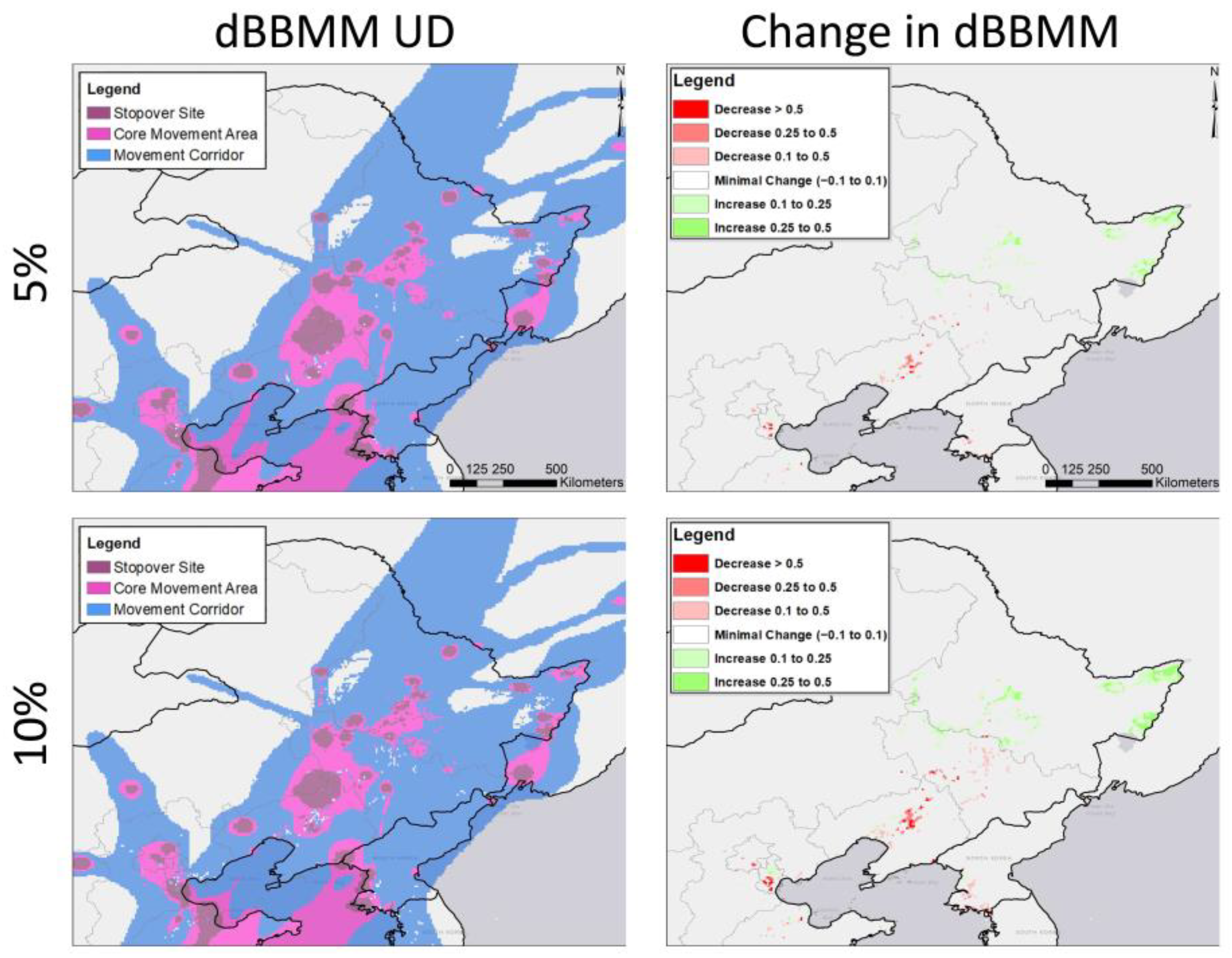
3.2. Potential Effects of Agricultural Shifts
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OIE. Update on Highly Pathogenic Avian Influenza in Animals (Type H5 and H7); OIE: Paris, France, 2017. [Google Scholar]
- Webster, R.G.; Peiris, M.; Chen, H.; Guan, Y. H5N1 Outbreaks and Enzootic Influenza. Emerg Infect Dis. 2006, 12, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.T.; Bryant, J.E.; Davis, C.T.; Nguyen, L.V.; Pham, L.T.; Loth, L.; Inui, K.; Nguyen, T.; Jang, Y.; To, T.L.; et al. Prevalence and Distribution of Avian Influenza A(H5N1) Virus Clade Variants in Live Bird Markets of Vietnam, 2011–2013. Avian Dis. 2014, 58, 599–608. [Google Scholar] [CrossRef]
- Chen, H.; Li, Y.; Li, Z.; Shi, J.; Shinya, K.; Deng, G.; Qi, Q.; Tian, G.; Fan, S.; Zhao, H.; et al. Properties and Dissemination of H5N1 Viruses Isolated during an Influenza Outbreak in Migratory Waterfowl in Western China. J. Virol. 2006, 80, 5976–5983. [Google Scholar] [CrossRef]
- Peiris, M.; Cowling, B.J.; Wu, J.T.; Feng, L.; Guan, Y.; Yu, H.; Leung, G.M. Interventions to Reduce Zoonotic and Pandemic Risks from Avian Influenza in Asia. Lancet Infect. Dis. 2016, 16, 252–258. [Google Scholar] [CrossRef]
- Gaidet, N.; Cattoli, G.; Hammoumi, S.; Newman, S.H.; Hagemeijer, W.; Takekawa, J.Y.; Cappelle, J.; Dodman, T.; Joannis, T.; Gil, P.; et al. Evidence of Infection by H5N2 Highly Pathogenic Avian Influenza Viruses in Healthy Wild Waterfowl. PLoS Pathog. 2008, 4, e1000127. [Google Scholar] [CrossRef]
- Gilbert, M.; Newman, S.H.; Takekawa, J.Y.; Loth, L.; Biradar, C.; Prosser, D.J.; Balachandran, S.; Subba Rao, M.V.; Mundkur, T.; Yan, B.; et al. Flying over an Infected Landscape: Distribution of Highly Pathogenic Avian Influenza H5N1 Risk in South Asia and Satellite Tracking of Wild Waterfowl. Ecohealth 2010, 7, 448–458. [Google Scholar] [CrossRef]
- Jeong, J.; Kang, H.-M.; Lee, E.-K.; Song, B.-M.; Kwon, Y.-K.; Kim, H.-R.; Choi, K.-S.; Kim, J.-Y.; Lee, H.-J.; Moon, O.-K.; et al. Highly Pathogenic Avian Influenza Virus (H5N8) in Domestic Poultry and Its Relationship with Migratory Birds in South Korea during 2014. Vet. Microbiol. 2014, 173, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhou, S.; Dong, L.; Van Boeckel, T.P.; Cui, Y.; Newman, S.H.; Takekawa, J.Y.; Prosser, D.J.; Xiao, X.; Wu, Y.; et al. Avian Influenza H5N1 Viral and Bird Migration Networks in Asia. PNAS2 2015, 112, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, N.M.; Brown, J.D.; Stallknecht, D.E.; Howerth, E.W.; Newman, S.H.; Swayne, D.E. Experimental Infection of Bar-Headed Geese (Anser Indicus) and Ruddy Shelducks (Tadorna Ferruginea) with a Clade 2.3.2 H5N1 Highly Pathogenic Avian Influenza Virus. Vet. Pathol. 2013, 50, 961–970. [Google Scholar] [CrossRef]
- Spackman, E.; Prosser, D.J.; Pantin-Jackwood, M.J.; Berlin, A.M.; Stephens, C.B. The Pathogenesis of Clade 2.3.4.4 H5 Highly Pathogenic Avian Influenza Viruses in Ruddy Duck (Oxyura Jamaicensis) and Lesser Scaup (Aythya Affinis). J. Wildl. Dis. 2017, 53, 832–842. [Google Scholar] [CrossRef]
- Van Den Brand, J.M.A.; Verhagen, J.H.; Veldhuis Kroeze, E.J.B.; Van De Bildt, M.W.G.; Bodewes, R.; Herfst, S.; Richard, M.; Lexmond, P.; Bestebroer, T.M.; Fouchier, R.A.M.; et al. Wild Ducks Excrete Highly Pathogenic Avian Influenza Virus H5N8 (2014–2015) without Clinical or Pathological Evidence of Disease Article. Emerg. Microbes Infect. 2018, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lycett, S.J.; Bodewes, R.; Pohlmann, A.; Banks, J.; Bányai, K.; Boni, M.F.; Bouwstra, R.; Breed, A.C.; Brown, I.H.; Chen, H.; et al. Role for Migratory Wild Birds in the Global Spread of Avian Influenza H5N8. Science 2016, 354, 213–217. [Google Scholar] [CrossRef]
- Lee, D.H.; Bertran, K.; Kwon, J.H.; Swayne, D.E. Evolution, Global Spread, and Pathogenicity of Highly Pathogenic Avian Influenza H5Nx Clade 2.3.4.4. J. Vet. Sci. 2017, 18, 269–280. [Google Scholar] [CrossRef]
- Saito, T.; Tanikawa, T.; Uchida, Y.; Takemae, N.; Kanehira, K.; Tsunekuni, R. Intracontinental and Intercontinental Dissemination of Asian H5 Highly Pathogenic Avian Influenza Virus (Clade 2.3.4.4) in the Winter of 2014–2015. Rev. Med. Viorolgy 2015, 25, 388–405. [Google Scholar] [CrossRef]
- Takekawa, J.Y.; Newman, S.H.; Xiao, X.; Prosser, D.J.; Spragens, K.A.; Palm, E.C.; Yan, B.; Li, T.; Lei, F.; Zhao, D.; et al. Migration of Waterfowl in the East Asian Flyway and Spatial Relationship to HPAI H5N1 Outbreaks. Avian Dis. 2010, 54, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Skidmore, A.K.; Wang, T.; De Boer, W.F.; Debba, P.; Toxopeus, A.G.; Li, L.; Prins, H.H.T. Spatio-Temporal Dynamics of Global H5N1 Outbreaks Match Bird Migration Patterns. Geospat. Health 2009, 4, 65–78. [Google Scholar] [CrossRef]
- Bahl, J.; Pham, T.T.; Hill, N.J.; Hussein, I.T.M.; Ma, E.J.; Easterday, B.C.; Halpin, R.A.; Stockwell, T.B.; Wentworth, D.E.; Kayali, G.; et al. Ecosystem Interactions Underlie the Spread of Avian Influenza A Viruses with Pandemic Potential. PLoS Pathog. 2016, 12, e1005620. [Google Scholar] [CrossRef]
- Alexander, D.J. An Overview of the Eipdemiology of Avian Influenza. Vaccine 2007, 25, 5637–5644. [Google Scholar] [CrossRef]
- Tian, H.; Xu, B. Persistence and Transmission of Avian Influenza A (H5N1): Virus Movement, Risk Factors and Pandemic Potential. Ann. GIS 2015, 21, 55–68. [Google Scholar] [CrossRef]
- Berthold, P.; Gwinner, E. Sonnenschein Avian Migration; Springer: Berlin, Germany, 2003. [Google Scholar]
- Warnock, N.; Takekawa, J.Y.; Bishop, M.A. Migration and Stopover Strategies of Individual Dunlin along the Pacific Coast of North America. Can. J. Zool. 2004, 82, 1687–1697. [Google Scholar] [CrossRef]
- Hill, N.J.; Takekawa, J.Y.; Ackerman, J.T.; Hobson, K.A.; Herring, G.; Cardona, C.J.; Runstadler, J.A.; Boyce, W.M. Migration Strategy Affects Avian Influenza Dynamics in Mallards (Anas Platyrhynchos). Mol. Ecol. 2012, 21, 5986–5999. [Google Scholar] [CrossRef] [PubMed]
- Boere, G.; Galbraith, C.; Stroud, D. Waterbirds around the World; The Stationary Office: Edingburgh, UK, 2006. [Google Scholar]
- Elphick, C.S. Why Study Birds in Rice Fields? Waterbirds 2010, 33, 1–7. [Google Scholar] [CrossRef]
- Hill, N.J.; Takekawa, J.Y.; Cardona, C.J.; Ackerman, J.T.; Schultz, A.K.; Spragens, K.A.; Boyce, W.M. Waterfowl Ecology and Avian Influenza in California: Do Host Traits Inform Us about Viral Occurrence? Avian Dis. 2010, 54, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.J.; Takekawa, J.Y.; Cardona, C.J.; Meixell, B.W.; Ackerman, J.T.; Runstadler, J.A.; Boyce, W.M. Cross-Seasonal Patterns of Avian Influenza Virus in Breeding and Wintering Migratory Birds: A Flyway Perspective. Vector-Borne Zoonotic Dis. 2012, 12, 243–253. [Google Scholar] [CrossRef]
- Gonzalez-Reiche, A.S.; Müller, M.L.; Ortiz, L.; Cordón-Rosales, C.; Perez, D.R. Prevalence and Diversity of Low Pathogenicity Avian Influenza Viruses in Wild Birds in Guatemala, 2010–2013. Avian. Dis. 2016, 60, 359–364. [Google Scholar] [CrossRef]
- Hill, N.J.; Ma, E.J.; Meixell, B.W.; Lindberg, M.S.; Boyce, W.M.; Runstadler, J.A. Transmission of Influenza Reflects Seasonality of Wild Birds across the Annual Cycle. Ecol. Lett. 2016, 19, 915–925. [Google Scholar] [CrossRef]
- Cao, L.E.I.; Meng, F.; Zhang, J.; Deng, X. Moving Forward: How Best to Use the Results of Waterbird Monitoring and Telemetry Studies to Safeguard the Future of Far East Asian Anatidae Species. Wildfowl 2020, 6, 293–319. [Google Scholar]
- Prosser, D.J.; Hungerford, L.L.; Erwin, R.M.; Ottinger, M.A.; Takekaw, J.Y.; Ellis, E.C. Mapping Avian Influenza Transmission Risk at the Interface of Domestic Poultry and Wild Birds. Front. Public Health 2013, 1, 28. [Google Scholar] [CrossRef]
- Artois, J.; Lai, S.; Feng, L.; Jiang, H.; Zhou, H.; Li, X.; Dhingra, M.S.; Linard, C.; Nicolas, G.; Xiao, X.; et al. H7N9 and H5N1 Avian Influenza Suitability Models for China: Accounting for New Poultry and Live-Poultry Markets Distribution Data. Stoch. Environ. Res. Risk Assess. 2017, 31, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.; Pfeiffer, D.U. Risk Factor Modelling of the Spatio-Temporal Patterns of Highly Pathogenic Avian Influenza (HPAIV) H5N1: A Review. Spat. Spatio-Temporal Epidemiol. 2012, 3, 173–183. [Google Scholar] [CrossRef]
- Prosser, D.J.; Wu, J.; Ellis, E.C.; Gale, F.; Van Boeckel, T.P.; Wint, W.; Robinson, T.; Xiao, X.; Gilbert, M. Modelling the Distribution of Chickens, Ducks, and Geese in China. Agric. Ecosyst Environ. 2011, 141, 381–389. [Google Scholar] [CrossRef]
- Choi, C.; Takekawa, J.Y.; Yue, X.; Ying, L.I.U.; Wikelski, M.; Heine, G.; Prosser, D.J.; Newman, S.H.; Edwards, J.; Guo, F.; et al. Tracking Domestic Ducks: A Novel Approach for Documenting Poultry Market Chains in the Context of Avian Influenza Transmission. J. Integr. Agric. 2016, 15, 1584–1594. [Google Scholar] [CrossRef]
- Gilbert, M.; Xiao, X.; Robinson, T.P. Intensifying Poultry Production Systems and the Emergence of Avian Influenza in China: A “One Health/Ecohealth” Epitome. Arch. Public Health 2017, 75, 1–7. [Google Scholar] [CrossRef]
- Bai, X.; Shi, P.; Liu, Y. Realizing China’s Urban Dream. Nature 2014, 509, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Nelson, G.C.; Bennett, E.; Berhe, A.A.; Cassman, K.; DeFries, R.; Dietz, T.; Dobermann, A.; Dobson, A.; Janetos, A.; Levy, M.; et al. Anthropogenic Drivers of Ecosystem Change: An Overview. Ecol. Soc. 2006, 11, 29. [Google Scholar] [CrossRef]
- Wang, J.; Lin, Y.; Glendinning, A.; Xu, Y. Land-Use Changes and Land Policies Evolution in China’s Urbanization Processes. Land Use Policy 2018, 75, 375–387. [Google Scholar] [CrossRef]
- Guan, X.; Wei, H.; Lu, S.; Dai, Q.; Su, H. Assessment on the Urbanization Strategy in China: Achievements, Challenges and Reflections. Habitat Int. 2018, 71, 97–109. [Google Scholar] [CrossRef]
- Kamaruddin, M.A.; Yusoff, M.S.; Rui, L.M.; Isa, A.M.; Zawawi, M.H.; Alrozi, R. An Overview of Municipal Solid Waste Management and Landfill Leachate Treatment: Malaysia and Asian Perspectives. Environ. Sci. Pollut. Res. 2017, 24, 26988–27020. [Google Scholar] [CrossRef]
- Shah, H.A.; Huxley, P.; Elmes, J.; Murray, K.A. Agricultural Land-Uses Consistently Exacerbate Infectious Disease Risks in Southeast Asia. Nat. Commun. 2019, 10, 4229. [Google Scholar] [CrossRef]
- Gibb, R.; Redding, D.W.; Chin, K.Q.; Donnelly, C.A.; Blackburn, T.M.; Newbold, T.; Jones, K.E. Zoonotic Host Diversity Increases in Human-Dominated Ecosystems. Nature 2020, 584, 398–402. [Google Scholar] [CrossRef]
- Jiang, L.; Deng, X.; Seto, K.C. The Impact of Urban Expansion on Agricultural Land Use Intensity in China. Land Use Policy 2013, 35, 33–39. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Z.; Tang, P.; Li, Z.; Wu, W.; Yang, P.; You, L.; Tang, H. Change Analysis of Rice Area and Production in China during the Past Three Decades. J. Geogr. Sci. 2013, 23, 1005–1018. [Google Scholar] [CrossRef]
- Zhang, G.; Xiao, X.; Biradar, C.M.; Dong, J.; Qin, Y.; Menarguez, M.A.; Zhou, Y.; Zhang, Y.; Jin, C.; Wang, J.; et al. Spatiotemporal Patterns of Paddy Rice Croplands in China and India from 2000 to 2015. Sci. Total Environ. 2017, 579, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hao, M.; Takekawa, J.Y.; Lei, F.; Yan, B.; Prosser, D.J.; Douglas, D.C.; Xing, Z.; Newman, S.H. Tracking the Autumn Migration of the Bar-Headed Goose (Anser Indicus) with Satellite Telemetry and Relationship to Environmental Conditions. Int. J. Zool. 2011, 2011, 323847. [Google Scholar] [CrossRef]
- Dong, J.; Xiao, X.; Kou, W.; Qin, Y.; Zhang, G.; Li, L.; Jin, C.; Zhou, Y.; Wang, J.; Biradar, C.; et al. Tracking the Dynamics of Paddy Rice Planting Area in 1986–2010 through Time Series Landsat Images and Phenology-Based Algorithms. Remote Sens. Env. 2015, 160, 99–113. [Google Scholar] [CrossRef]
- Xin, F.; Xiao, X.; Dong, J.; Zhang, G.; Zhang, Y.; Wu, X.; Li, X.; Zou, Z.; Ma, J.; Du, G.; et al. Large Increases of Paddy Rice Area, Gross Primary Production, and Grain Production in Northeast China during 2000–2017. Sci. Total Environ. 2020, 711, 135183. [Google Scholar] [CrossRef]
- Chen, X.; Wang, L.; Niu, Z.; Zhang, M.; Li, C.; Li, J. The Effects of Projected Climate Change and Extreme Climate on Maize and Rice in the Yangtze River Basin, China. Agric For. Meteorol. 2020, 282–283, 107867. [Google Scholar] [CrossRef]
- Xu, Y.; Si, Y.; Yin, S.; Zhang, W.; Grishchenko, M.; Prins, H.H.T.; Gong, P.; de Boer, W.F. Species-Dependent Effects of Habitat Degradation in Relation to Seasonal Distribution of Migratory Waterfowl in the East Asian–Australasian Flyway. Landsc. Ecol. 2019, 34, 243–257. [Google Scholar] [CrossRef]
- Muzaffar, S.B.; Takekawa, J.Y.; Prosser, D.J.; Newman, S.H.; Xiao, X. Rice Production Systems and Avian Influenza: Interactions between Mixed-Farming Systems, Poultry and Wild Birds. Waterbirds 2010, 33, 219–230. [Google Scholar] [CrossRef]
- Chen, H.; Smith, G.J.D.; Li, K.S.; Wang, J.; Fan, X.H.; Rayner, J.M.; Vijaykrishna, D.; Zhang, J.X.; Zhang, L.J.; Guo, C.T.; et al. Establishment of Multiple Sublineages of H5N1 Influenza Virus in Asia: Implications for Pandemic Control. Proc. Natl. Acad. Sci. USA 2006, 103, 2845–2850. [Google Scholar] [CrossRef]
- Cecchi, G.; Ilemobade, A.; Le Brun, Y.; Hogerwerf, L.; Slingenbergh, J. Agro-Ecological Features of the Introduction and Spread of the Highly Pathogenic Avian Influenza (HPAI) H5N1 in Northern Nigeria. Geospat. Health 2008, 3, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Caron, A.; Cumming, G.S.; Mundava, J.; Chiweshe, N.; Mundy, P.; de Garine-Wichatitsky, M. Report on a Five-Year Avian Influenza Survey in the Manyame Catchment. Honeyguide 2012, 58, 113–120. [Google Scholar]
- Caron, A.; Cappelle, J.; Cumming, G.S.; de Garine-Wichatitsky, M.; Gaidet, N. Bridge Hosts, a Missing Link for Disease Ecology in Multi-Host Systems. Vet. Res. 2015, 46, 11. [Google Scholar] [CrossRef] [PubMed]
- Partnership, E.A.-A.F. What Is a Flyway? Available online: https://www.eaaflyway.net/the-flyway/ (accessed on 1 January 2023).
- Yong, D.L.; Jain, A.; Liu, Y.; Iqbal, M.; Choi, C.Y.; Crockford, N.J.; Millington, S.; Provencher, J. Challenges and Opportunities for Transboundary Conservation of Migratory Birds in the East Asian-Australasian Flyway. Conserv. Biol. 2018, 32, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Billerman, S.M.; Keeney, B.K.; Rodewald, P.G.; Schulenberg, T.S. Birds of the World. Available online: https://birdsoftheworld.org/bow/home (accessed on 1 January 2023).
- Palm, E.C.; Newman, S.H.; Prosser, D.J.; Xiao, X.; Ze, L.; Batbayar, N.; Balachandran, S.; Takekawa, J.Y. Mapping Migratory Flyways in Asia Using Dynamic Brownian Bridge Movement Models. Mov. Ecol. 2015, 3, 3. [Google Scholar] [CrossRef]
- Peng, H.B.; Anderson, G.Q.A.; Chang, Q.; Choi, C.-Y.; Chowdhury, S.U.; Clark, N.A.; Gan, X.; Hearn, R.D.; Li, J.; Lappo, E.G.; et al. The Intertidal Wetlands of Southern Jiangsu Province, China—Globally Important for Spoon-Billed Sandpipers and Other Threatened Waterbirds, but Facing Multiple Serious Threats. Bird Conserv. Int. 2017, 27, 305–322. [Google Scholar] [CrossRef]
- Xia, S.; Yu, X.; Millington, S.; Liu, Y.; Jia, Y.; Wang, L.; Hou, X.; Jiang, L. Identifying Priority Sites and Gaps for the Conservation of Migratory Waterbirds in China’s Coastal Wetlands. Biol. Conserv. 2016, 210, 72–82. [Google Scholar] [CrossRef]
- Murray, N.J.; Clemens, R.S.; Phinn, S.R.; Possingham, H.P.; Fuller, R.A. Tracking the Rapid Loss of Tidal Wetlands in the Yellow Sea. Front. Ecol. Envion. 2014, 12, 267–272. [Google Scholar] [CrossRef]
- Melville, D.S.; Chen, Y.; Ma, Z. Shorebirds along the Yellow Sea Coast of China Face an Uncertain Future—A Review of Threats. Emu 2016, 116, 100–110. [Google Scholar] [CrossRef]
- Szabo, J.K.; Battley, P.F.; Buchanan, K.L.; Rogers, D.I. What Does the Future Hold for Shorebirds in the East Asian-Australasian Flyway? Emu 2016, 116, 95–99. [Google Scholar] [CrossRef]
- Wang, X.; Cao, L.; Fox, A.D.; Fuller, R.; Griffin, L.; Mitchell, C.; Zhao, Y.; Moon, O.K.; Cabot, D.; Xu, Z.; et al. Stochastic Simulations Reveal Few Green Wave Surfing Populations among Spring Migrating Herbivorous Waterfowl. Nat. Commun. 2019, 10, 3893. [Google Scholar] [CrossRef]
- Zhu, Q.; Hobson, K.A.; Zhao, Q.; Zhou, Y.; Damba, I.; Batbayar, N.; Natsagdorj, T.; Davaasuren, B.; Antonov, A.; Guan, J.; et al. Migratory Connectivity of Swan Geese Based on Species’ Distribution Models, Feather Stable Isotope Assignment and Satellite Tracking. Divers. Distrib. 2020, 26, 944–957. [Google Scholar] [CrossRef]
- Fang, L.; Zhang, J.; Zhao, Q.; Solovyeva, D.; Vangeluwe, D.; Rozenfeld, S.B.; Lameris, T.; Xu, Z.; Bysykatova-harmey, I. Two Distinct Flyways with Different Population Trends of Bewick’s Swan Cygnus Columbianus Bewickii in East Asia. Wildfowl 2020, Special Issue 6, 13–42. [Google Scholar]
- Batbayar, N.; Takekawa, J.Y.; Newman, S.H.; Prosser, D.J.; Natsagdorj, T.; Xiao, X. Migration Strategies of Swan Geese Anser Cygnoides from Northeast Mongolia. Wildfowl 2011, 61, 90–109. [Google Scholar]
- Kenward, R.E. Wildlife Radio Tagging: Equipment, Field Techniques and Data Analysis; Academic Press: London, UK, 1987. [Google Scholar]
- Douglas, D.C.; Weinzierl, R.; Davidson, S.C.; Kays, R.; Wikelski, M.; Bohrer, G. Moderating Argos Location Errors in Animal Tracking Data. Methods Ecol. Evol. 2012, 3, 999–1007. [Google Scholar] [CrossRef]
- Kranstauber, B.; Kays, R.; Lapoint, S.D.; Wikelski, M.; Safi, K. A Dynamic Brownian Bridge Movement Model to Estimate Utilization Distributions for Heterogeneous Animal Movement. J. Anim. Ecol. 2012, 81, 738–746. [Google Scholar] [CrossRef]
- Zhang, G.; Xiao, X.; Dong, J.; Xin, F.; Zhang, Y.; Qin, Y.; Doughty, R.B.; Moore, B. Fingerprint of Rice Paddies in Spatial–Temporal Dynamics of Atmospheric Methane Concentration in Monsoon Asia. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.D.; Takekawa, J.Y.; Spragens, K.A.; Newman, S.H.; Xiao, X.; Leader, P.J.; Smith, B.; Prosser, D.J. Waterfowl Spring Migratory Behavior and Avian Influenza Transmission Risk in the Changing Landscape of the East Asian-Australasian Flyway. Front. Ecol. Evol. 2018, 6, 1–14. [Google Scholar] [CrossRef]
- Choi, C.; Lee, K.-S.; Poyarkov, N.D.; Lee, H.; Takekawa, J.Y.; Smith, L.M.; Ely, C.R.; Wang, X.; Cao, L.; Fox, A.D.; et al. Low Survival Rates of Swan Geese (Anser Cygnoides) Estimated from Neck-Collar Resighting and Telemetry. Waterbirds 2016, 39, 277–286. [Google Scholar] [CrossRef]
- Yin, S.; Yanjie, X.; Batbayar, N.; Takakawa, J.Y.; Si, Y.; Prosser, D.; Newman, S.H.; Prins, H.H.T.; de Boer, W.F. Do Contrasting Patterns of Migration Movements and Disease Outbreaks between Congeneric Waterfowl Species Reflect Differing Immunity. Geospat. Health 2021, 16, 909. [Google Scholar] [CrossRef]
- Martin, V.; Pfeiffer, D.U.; Zhou, X.; Xiao, X.; Prosser, D.J.; Guo, F.; Gilbert, M. Spatial Distribution and Risk Factors of Highly Pathogenic Avian Influenza (HPAI) H5N1 in China. PLoS Pathog. 2011, 7, e1001308. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.; Prosser, D.J.; Zhang, G.; Artois, J.; Dhingra, M.S.; Tildesley, M.; Newman, S.H.; Guo, F.; Black, P.; Claes, F.; et al. Could Changes in the Agricultural Landscape of Northeastern China Have Influenced the Long-Distance Transmission of Highly Pathogenic Avian Influenza H5Nx Viruses? Front. Vet. Sci. 2017, 4, 225. [Google Scholar] [CrossRef] [PubMed]
- Gaidet, N.; Cappelle, J.; Takekawa, J.Y.; Prosser, D.J.; Iverson, S.A.; Douglas, D.C.; Perry, W.M.; Mundkur, T.; Newman, S.H. Potential Spread of Highly Pathogenic Avian Influenza H5N1 by Wildfowl: Dispersal Ranges and Rates Determined from Large-Scale Satellite Telemetry. J. Appl. Ecol. 2010, 47, 1147–1157. [Google Scholar] [CrossRef]
- Chen, X.; Li, C.; Sun, H.T.; Ma, J.; Qi, Y.; Qin, S.Y. Prevalence of Avian Influenza Viruses and Their Associated Antibodies in Wild Birds in China: A Systematic Review and Meta-Analysis. Microb. Pathog. 2019, 135, 103613. [Google Scholar] [CrossRef]
- Bevins, S.N.; Pedersen, K.; Lutman, M.W.; Baroch, J.A.; Schmit, B.S.; Kohler, D.; Gidlewski, T.; Nolte, D.L.; Swafford, S.R.; DeLiberto, T.J. Large-Scale Avian Influenza Surveillance in Wild Birds throughout the United States. PLoS ONE 2014, 9, e104360. [Google Scholar] [CrossRef]
- Van Dijk, J.G.B.; Hoye, B.J.; Verhagen, J.H.; Nolet, B.A.; Fouchier, R.A.M.; Klaassen, M. Juveniles and Migrants as Drivers for Seasonal Epizootics of Avian Influenza Virus. J. Anim. Ecol. 2014, 83, 266–275. [Google Scholar] [CrossRef]
- Anisfeld, S.C.; Hill, T.D. Fertilization Effects on Elevation Change and Belowground Carbon Balance in a Long Island Sound Tidal Marsh. Estuaries Coasts 2012, 35, 201–211. [Google Scholar] [CrossRef]
- Li, R.; Jiang, Z.; Xu, B. Global Spatiotemporal and Genetic Footprint of the H5N1 Avian Influenza Virus. Int. J. Health. Geogr. 2014, 13, 1–10. [Google Scholar] [CrossRef]
- Yin, S.; de Knegt, H.J.; de Jong, M.C.M.; Si, Y.; Prins, H.H.T.; Huang, Z.Y.X.; de Boer, W.F. Effects of Migration Network Configuration and Migration Synchrony on Infection Prevalence in Geese. J. Theor. Biol. 2020, 502, 110315. [Google Scholar] [CrossRef]
- Wu, T.; Perrings, C.; Shang, C.; Collins, J.P.; Daszak, P.; Kinzig, A.; Minteer, B.A. Protection of Wetlands as a Strategy for Reducing the Spread of Avian Influenza from Migratory Waterfowl. Ambio 2020, 49, 939–949. [Google Scholar] [CrossRef]
- An, S.; Zhang, X.; Song, S.; Zhao, H.; Jeelani, N. Wetlands: Ecosystem Services, Restoration and Wise Use; Springer: Berlin/Heidelberg, Germany, 2019; pp. 187–218. [Google Scholar]
- Si, Y.; Wei, J.; Wu, W.; Zhang, W.; Hou, L.; Yu, L.; Wielstra, B. Reducing Human Pressure on Farmland Could Rescue China’s Declining Wintering Geese. Mov. Ecol. 2020, 8, 35. [Google Scholar] [CrossRef]
- Yu, H.; Wang, X.; Cao, L.; Zhang, L.; Jia, Q.; Lee, H.; Xu, Z.; Liu, G.; Xu, W.; Hu, B.; et al. Are Declining Populations of Wild Geese in China ‘Prisoners’ of Their Natural Habitats? Curr. Biol. 2017, 27, R376–R377. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, J.T.; Takekawa, J.Y.; Orthmyer, D.L.; Fleskes, J.P.; Yee, J.L.; Kruse, K.L. Spatial Use by Wintering Greater White-Fronted Geese Relative to a Decade of Habitat Change in California’s Central Valley. J. Wildl. Manag. 2006, 70, 965–976. [Google Scholar] [CrossRef]
- Foden, W.B.; Butchart, S.H.M.; Stuart, S.N.; Vié, J.C.; Akçakaya, H.R.; Angulo, A.; DeVantier, L.M.; Gutsche, A.; Turak, E.; Cao, L.; et al. Identifying the World’s Most Climate Change Vulnerable Species: A Systematic Trait-Based Assessment of All Birds, Amphibians and Corals. PLoS ONE 2013, 8, e65427. [Google Scholar] [CrossRef]
- Morin, C.W.; Stoner-Duncan, B.; Winker, K.; Scotch, M.; Hess, J.J.; Meschke, J.S.; Ebi, K.L.; Rabinowitz, P.M. Avian Influenza Virus Ecology and Evolution through a Climatic Lens. Environ. Int. 2018, 119, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Vandegrift, K.J.; Sokolow, S.H.; Daszak, P.; Kilpatrick, A.M. Ecology of Avian Influenza Viruses in a Changing World. Ann. N. Y. Acad Sci. 2010, 1195, 113–128. [Google Scholar] [CrossRef]
- van Wijk, R.E.; Kölzsch, A.; Kruckenberg, H.; Ebbinge, B.S.; Müskens, G.J.D.M.; Nolet, B.A. Individually Tracked Geese Follow Peaks of Temperature Acceleration during Spring Migration. Oikos 2012, 121, 655–664. [Google Scholar] [CrossRef]
- Fei, Y.; ShiQi, Y.; YunQiang, Z.; JuanLe, W. Analysis on Livestock and Poultry Production and Nitrogen Pollution Load of Cultivated Land during Last 30 Years in China. Trans. Chin. Soc. Agric. Eng. 2013, 29, 1–11. [Google Scholar]
- Gilbert, M.; Conchedda, G.; Van Boeckel, T.P.; Cinardi, G.; Linard, C.; Nicolas, G.; Thanapongtharm, W.; D’Aietti, L.; Wint, W.; Newman, S.H.; et al. Income Disparities and the Global Distribution of Intensively Farmed Chicken and Pigs. PLoS ONE 2015, 10, 1–14. [Google Scholar] [CrossRef]
- Wood, C.; Qiao, Y.; Li, P.; Ding, P.; Lu, B.; Xi, Y. Implications of Rice Agriculture for Wild Birds in China. Waterbirds 2010, 33, 30–43. [Google Scholar] [CrossRef]
- Studds, C.E.; Kendall, B.E.; Murray, N.J.; Wilson, H.B.; Rogers, D.I.; Clemens, R.S.; Gosbell, K.; Hassell, C.J.; Jessop, R.; Melville, D.S.; et al. Rapid Population Decline in Migratory Shorebirds Relying on Yellow Sea Tidal Mudflats as Stopover Sites. Nat. Commun. 2017, 8, 14895. [Google Scholar] [CrossRef] [PubMed]
- Mackinnon, J.; Verkuil, Y.I.; Murray, N. IUCN Situation Analysis on East and Southeast Asian Intertidal Habitats, with Particular Reference to the Yellow Sea (Including the Bohai Sea); IUCN: Gland, Switzerland; Cambridge, UK, 2012. [Google Scholar]
- Melville, D.S.; Peng, H.-B.; Chan, Y.-C.; Bai, Q.; He, P.; Tan, K.; Chen, Y.; Zhang, S.; Ma, Z. Gaizhou, Liaodong Bay, Liaoning Province, China—A Site of International Importance for Great Knot Calidris Tenuirostris and Other Shorebirds. Stilt 2016, 69–70, 57–61. [Google Scholar]
- Li, Y.; Mao, D.; Wang, Z.; Wang, X.; Tan, X.; Jia, M.; Ren, C. Identifying Variable Changes in Wetlands and Their Anthropogenic Threats Bordering the Yellow Sea for Water Bird Conservation. Glob. Ecol. Conserv. 2021, 27, e01613. [Google Scholar] [CrossRef]
- Delany, S.; Scott, D. Waterbird Population Estimates; Wetlands International: Wageningen, The Netherlands, 2006. [Google Scholar]
- Murray, N.J.; Ma, Z.; Fuller, R.A. Tidal Flats of the Yellow Sea: A Review of Ecosystem Status and Anthropogenic Threats. Austral. Ecol. 2015, 40, 472–481. [Google Scholar] [CrossRef]
- Lameris, T.K.; Müskens, G.J.D.M.; Kölzsch, A.; Dokter, A.M.; van der Jeugd, H.P.; Nolet, B.A. Effects of Harness-Attached Tracking Devices on Survival, Migration, and Reproduction in Three Species of Migratory Waterfowl. Anim. Biotelemetry 2018, 6, 4–11. [Google Scholar] [CrossRef]
- Yin, S.; Xu, Y.; de Jong, M.C.M.; Huisman, M.R.S.; Contina, A.; Prins, H.H.T.; Huang, Z.Y.X.; de Boer, W. Habitat Loss Exacerbates Pathogen Spread: An Agent-Based Model of Avian Influenza Infection in Migratory Waterfowl. bioRxiv 2021, 18, e1009577. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, D.; Newman, S.; Mundkur, T.; Harris, P. Wild Birds and Avian Influenza: An Introduction to Applied Field Research and Disease Sampling Techniques; The Food and Agriculture Organization of the United Nations: Rome, Italy, 2017. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takekawa, J.Y.; Prosser, D.J.; Sullivan, J.D.; Yin, S.; Wang, X.; Zhang, G.; Xiao, X. Potential Effects of Habitat Change on Migratory Bird Movements and Avian Influenza Transmission in the East Asian-Australasian Flyway. Diversity 2023, 15, 601. https://doi.org/10.3390/d15050601
Takekawa JY, Prosser DJ, Sullivan JD, Yin S, Wang X, Zhang G, Xiao X. Potential Effects of Habitat Change on Migratory Bird Movements and Avian Influenza Transmission in the East Asian-Australasian Flyway. Diversity. 2023; 15(5):601. https://doi.org/10.3390/d15050601
Chicago/Turabian StyleTakekawa, John Y., Diann J. Prosser, Jeffery D. Sullivan, Shenglai Yin, Xinxin Wang, Geli Zhang, and Xiangming Xiao. 2023. "Potential Effects of Habitat Change on Migratory Bird Movements and Avian Influenza Transmission in the East Asian-Australasian Flyway" Diversity 15, no. 5: 601. https://doi.org/10.3390/d15050601
APA StyleTakekawa, J. Y., Prosser, D. J., Sullivan, J. D., Yin, S., Wang, X., Zhang, G., & Xiao, X. (2023). Potential Effects of Habitat Change on Migratory Bird Movements and Avian Influenza Transmission in the East Asian-Australasian Flyway. Diversity, 15(5), 601. https://doi.org/10.3390/d15050601








