Abstract
Bahrain is a cluster of islands in the mid-section of the Arabian Gulf that serves as an important wintering and stop-over ground for many migratory shorebirds in the Central Asian Flyway (CAF). However, natural and anthropogenic factors have had a significant impact on these ecosystems over the last few decades. Long-term, systematic studies based on standardized survey observations are needed to understand the population dynamics and diversity changes of shorebirds in these critical sites. We systematically surveyed the shorebird population and community in Bahrain between 2010 January to 2021 December. This is the first comprehensive study from the entire Kingdom of Bahrain, and covered 13 sites over 12 years to establish the results. A total of 39 species were encountered during the study period from all 13 sites in Bahrain, of which 27 species were common and regular migrants to all the study sites; these were selected to analyze the population trend. Five species represented 77% or more of the total wintering shorebird population. All the shorebird species assessed exhibited significant declining trends over the years, and majority of them had over 1% relative abundance. Shorebirds in Bahrain were severely threatened at these sites, indicating that their population trend in the area could be crucially affected. Further conservation efforts are needed, aided by an understanding of the mechanisms driving the decline and diversity changes of shorebirds in the most stressed coastal regions of Bahrain. Further studies organized throughout the country’s coasts may aid in establishing improved conservation measures to protect the shorebirds of the CAF in Bahrain.
1. Introduction
Shorebirds are a group species of major concern for conservation that would benefit from an increased understanding of their demographic patterns and the underlying factors [1]. Shorebirds are among the most rapidly declining groups of birds around the globe, especially the long-distance migrant species, which rely on habitats and resources along their entire migratory route [2]. This emphasizes the need to identify where and why certain challenges exist in order to adopt effective conservation strategies [3,4]. Poor habitat quality, affected by anthropogenic activities and global climate change, is the broad cause of their decline [2,5]. Considering the limitations involved in following and documenting individual variation in demographic rates over time, population-level studies have been carried out at different locations along the migratory pathways [6]. Long-term population studies reveal the shifts in species distribution and shed light on how accelerating environmental changes reshape vital ecosystems [7].
In order to successfully finish their extraordinary long-distance migration flights, constrained within a narrow window of time, shorebirds must accumulate large fuel reserves in the form of fat and protein [8]. Hence, the critical wintering and stop-over sites of shorebirds are characterized by increased primary productivity and high abundance of macrobenthic prey items, which are favorably influenced by suitable environmental variables, organic matter, and nutrients from terrestrial and marine sources [9,10]. Thus, for successful migration, they require high-quality wintering and stop-over sites that allow efficient and opportunistic foraging strategies for refueling which, thus, ensure the survival and timely departure of the migratory shorebirds from wintering sites [11]. Food preferences and foraging strategies adopted by different species vary considerably [12], which has important implications on the effect of habitat degradation.
Among the world’s major migratory corridors, research on the CAF is comparably underrepresented [13,14,15,16]. The CAF covers a large continental area of Eurasia bound by the Arctic and the Indian Oceans [17] which covers 30 countries, including the Arabian Gulf countries. Though CAF is the shortest flyway covering 30 countries, significant comprehensive knowledge on important shorebird habitats, demographic patterns, and the threats faced by the shorebirds in the CAF has not yet been achieved. Catastrophic declines in shorebirds over the years have been reported in the Central Asian Flyway [9,14], primarily due to prey depletion driven by changes in multiple environmental factors, such as relative humidity, rainfall, water temperature, air temperature, and salinity [15]. This food shortage was observed to be affecting the feeding rate, which in turn affects their survival and breeding success [18].
The Arabian Gulf countries are characterized by extreme environmental conditions, with a high-latitude geographical position, relative shallowness, and high evaporation rates. Hence, the marine organisms in the Arabian Gulf are dwelling close to the limits of their environmental tolerance due to the extreme sea temperatures and salinity levels, which fluctuate seasonally [19]. The Kingdom of Bahrain is a cluster of islands in the mid-section of the Arabian Gulf and falls under CAF, where their most productive coastal mudflat habitats typically harbor large numbers of migratory shorebirds [20]. These ecosystems have been exposed to anthropogenic impacts during the last few decades [21]. The major sources of pollution in the Arabian Gulf include oil spills, brine water intrusion from desalination plants, and industrial sewage. Bahrain is heavily susceptible to oil spills and has already witnessed a number of major and minor oil spill incidents [22]. Reductions in the density, biomass, and diversity of benthic fauna have been linked to petroleum hydrocarbons. Aarif et al. [15] suggested that salinity and invertebrate prey abundance influenced shorebird abundance and departure dates. Long-term studies on the abundance of shorebirds, the top-level predators in the coastal habitat, helps us to better understand the effects and potential impacts of environmental stress and anthropogenic pressure on the ecological integrity of this fragile ecosystem [19,23].
Despite its significance as a documented wintering site for globally declining avifauna, no long-term study has been conducted on shorebird assemblages in Bahrain. A significant amount of the data on birds and shorebird counts, in particular in the country, is fragmented, and much is outdated [24]. Specific focus on shorebirds using intertidal coastal areas is sparse, and there is no continuous monitoring [25,26]. Hence, to understand the alterations in the wintering shorebird populations in Bahrain, we collected and analyzed shorebird abundance data from all major intertidal and other wetland sites in the main island of Bahrain and assessed changes in the shorebird diversity and abundance in a recent 12-year period. Further, driving factors that may have affected these shorebird populations are also discussed. This is the first comprehensive study from the entire Kingdom of Bahrain which covered 13 sites over 12 years; hence, this data provides first-hand information on the abundance, diversity shifts, and population trends of common long-distance migrant shorebirds of Bahrain. This information can be utilized for formulating various conservation measures to be employed in this important wintering site of CAF.
2. Materials and Method
2.1. Study Area
The long-term survey was carried out at a total of 13 sites in the main island of Bahrain (Figure 1), which covered almost all areas of wintering grounds for shorebirds in the Kingdom of Bahrain except for remote islands such as Hawar Islands, which host one of the world’s largest colonies of the vulnerable Socotra cormorant (Phalacrocorax nigrogularis) as well as other regionally and internationally important waterbird species.
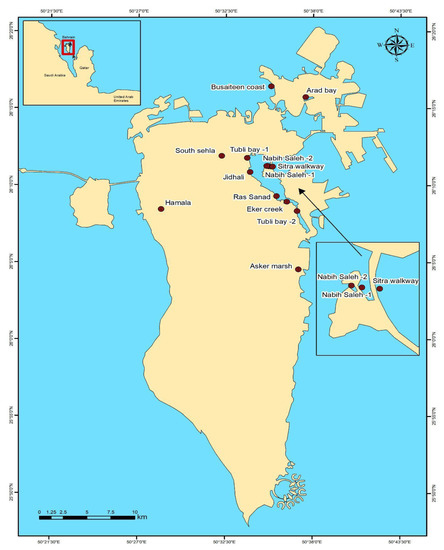
Figure 1.
A map showing major sites for wintering, staging, and over-summering shorebirds in the main island of Bahrain.
Tubli Bay 1 (N 26.196255 E 50.56432) is one of the two designated Ramsar site in the country that hosts large numbers of wintering and migratory shorebirds [20], and the intertidal habitat provides feeding and roosting grounds for shorebird populations. This study site occupies the outfall of Tubli Water Pollution Control Centre, the main sewage treatment plant in Bahrain. We conducted the survey within an area of approximately 1 km2.
Tubli Bay 2 (N 26.138836 E 50.616594) is a bay in the east of Bahrain, between Bahrain Island and Sitra Island, and is directly south of the capital, Manama. We covered an area of about 2.5 km2 for the survey.
Jidhali lies (N 26.180335 E 50.567305) adjacent to Tubli Water Pollution Control Centre (TWCC). We carried out the survey within a 1 km2 area.
Eker Creek (N 26.149035 E 50.605947) is a shallow, sheltered bay in an urban/industrial area with saltmarsh vegetation, extensive mudflats, and a large area of mangroves. The survey was carried out within a 3 km2 area.
Nabih Saleh (1 and 2) (N 26.187249 E 50.587021 and N 26.187624 E 50.584665), lies in Tubli Bay, east of Bahrain Island. We covered an area of about 1.5 km2 for our shorebird survey.
Sitra Walkway (N 26.186958 E 50.590864) connects the island of Nabih Saleh and Sitra to the capital city of Manama, across Tubli Bay. The main habitats in these shallow waters consist of sand, rock, and coral reefs, which form a barrier to tidal water movements and wind-driven wave action [27]. We conducted our survey in this leisure zone featuring a 2 km walkway and green spaces.
Ras Sanad (N 26.15495 E 50.595024) is a sheltered lagoon hosting the last remaining natural mangrove ecosystems in Bahrain. In addition, it is considered as a foraging and roosting ground for many migratory birds. The largest concentration of mangrove forests is located in the Ras Sanad Lagoon, one of the most protected areas of the coast of Bahrain [28]. We carried out the survey within a 2 km2 area.
Arad Bay (N 26.262325 E 50.625393) is a natural intertidal mudflat and a sheltered bay located (approximately 1 km in length) in the northeast of Bahrain. The bay is a very shallow, low-lying tidal flat adjacent to the Bahrain International Airport. This is a classic example of a mudflat providing a feeding ground for shorebird populations [20,29]. This mudflat bay was designated as a natural marine protected area in 2003. The estimated area of this bay is around 0.5 km2, and it was considered for our survey.
The Busaiteen coastal area (N 26.273966 E 50.589215) is the first typical coastline in Muharraq with an area of more than 4.5 km2. Shorebirds congregate in large numbers to forage on the exposed mudflats during low tide. We covered an area of about 1 km2 for the survey.
Asker Marsh (N 26.075857 E 50.618081) is situated west of Jazīrat ash Shaykh and northwest of Ra’s Ḩayyān. Mudflats in this area provide feeding and resting areas for wader populations.
Hamala agricultural and farming land (N 26.1406464 E 50.4742752) is situated on the western coast of Bahrain Island, which offers wintering grounds for shorebirds.
South Sehla (N 26.1984497 E 50.5376197), the eastern part of Sehla, is an agricultural land. It has a network of canals that make the area fertile, and many birds thrive in this canal.
2.2. Shorebird Survey
Direct observation [30] and point count methods were employed to obtain the total count of shorebirds from each study area from January 2010 to December 2021. The survey was carried out once a week during low tide, between 06:00 to 12:00 h, using 10 × 50 Swarovski binoculars and three sets of video cameras (CX 130 E Sony, p1000 Nikon Bridge Camera; Canon EOS-1DX Mark II with lens Canon EF 400mm f/2.8 IS II USM + Canon Extender EF 2X III). The wintering period between September and April and the over-summering period between the first week of June and the second week of July were considered (a total of six weeks in a year). Maximum care was taken to avoid disturbance during the surveys. The observations recorded while moving from one scanning point to another were entered as incidental records.
2.3. Statistical Analysis
Species that were common and regular migrants to all the study sites were chosen from among the shorebirds encountered during the study period to investigate population trends.
The Shannon–Weiner diversity index and the Gini-Simpson index were used to assess the changes in diversity, and the Margalef index was employed to check the species richness. A one-way ANOVA was run to determine the differences in the Margalef richness index, and Tukey’s post hoc multiple comparisons test was used to test the diversity changes. Confidence limits were calculated and pairwise comparisons between years for each diversity index were carried out using bootstrap methods with replacement, using PAST version 4.03 [31]. Linear regressions were carried out to test the population trends of each of the 27 analyzed shorebird species. ANOVA and regression analyses were performed using R version 4.0.5 [32]. Significance for all statistical tests was set at 0.05.
3. Results
3.1. Species Account
A total of thirty-nine species were encountered during our survey between 2010 and 2021, of which thirty-four species were winter visitors and three species were breeding residents, these being the Black-winged Stilt (Himantopus himantopus), Kentish Plover (Charadrius alexandrinus), and Red-wattled Lapwing (Vanellus indicus) (Table 1). Kentish Plovers were breeding in almost all the areas in Bahrain, viz., Amwaj, Asker, Busaiteen, Sanad, and Tubli Bay. The Black-winged Stilt bred mostly at the Asker coast, whereas the Red-wattled Lapwing had been regularly breeding at two sites (Hamala and South Sehla, near Adhari park) for past several years. Crab Plover (Dromas ardeola) and Beach Stone-curlew (Esacus magnisrostris) were recorded as resident species, but breeding had not yet been observed in Bahrain.

Table 1.
IUCN, regional, and migratory status of shorebirds in Bahrain’s intertidal habitats.
Among the observed species, the Great Knot (Calidris tenuirostris) was categorized as endangered (EN), and eight other species, i.e., Beach Stone-curlew, Curlew Sandpiper (Calidris ferruginea), Bar-tailed Godwit (Limosa lapponica), Black-tailed Godwit (Limosa limosa), Eurasian Curlew (Numenius arquata), Eurasian Oystercatcher (Haematopus ostralegus), and Great Snipe (Gallinago media) were listed as near-threatened (NT) in the IUCN Red List. Of the thirty-nine species of shorebirds, twenty-five species were common winter visitors and nine species were uncommon winter visitors. Three (i.e., Little Stint (Calidris minuta), Lesser Sand Plover (Charadrius mongolus), and Dunlin (Calidris alpina) are often dominant species during winter, and peak counts were significantly more highly documented for them at one time. When it comes to seasonal abundance, shorebird numbers begin to rise in November and reach a peak in January.
Nine species of regularly over-summering shorebirds were documented from different sites of Bahrain. They were the Greater Sand Plover (Charadrius leschenaultii), Whimbrel (Numenius phaeopus), Eurasian Curlew, Bar-tailed Godwit, Curlew sandpiper, Common Redshank (Tringa totanus), Common Greenshank (Tringa nebularia), Ruddy Turnstone (Arenaria interpres), and Eurasian Oystercatcher. The Bar-tailed Godwit was the most abundant and dominant species, followed by the Common Greenshank, while the Eurasian Oystercatcher was the least abundant. Three of these species fall under IUCN near-threatened category: they are Eurasian Curlew, Curlew sandpiper, and Eurasian Oystercatcher.
We documented forty-six oiled individuals belonging to six species, namely, sixteen oil-spilled Dunlin, twelve Wood Sandpiper, six Eurasian Oystercatcher, four Curlew Sandpiper (Figure 2), four Sanderling, and two Whimbrel during the study period in different sites of Bahrain. They were all exhausted and unfit to fly.

Figure 2.
Oiled IUCN red-listed Curlew sandpiper (Calidris ferruginea) from Nabih Saleh.
3.2. Diversity, Relative Abundance, and Species Trends over the Years
We performed a one-way ANOVA to determine the difference in the Shannon diversity index (H′) between 2010 and 2021, which showed significant variation in diversity indices (p < 0.05). Tukey’s post hoc multiple comparisons tests showed that diversity significantly changed in 2019 to 2021 compared to 2010 to 2016, and in 2021, a significant decrease was evident between 2010 and 2020 (Table 2).

Table 2.
Comparison of the changes in Shannon’s index, the Gini–Simpson index, and richness in relation to year (2010–2021) in Bahrain intertidal habitats.
A one-way ANOVA was performed to determine the difference in the Margalef richness index (d) between 2010 and 2021, which showed a significant increase (p < 0.05). Tukey post hoc multiple comparisons tests showed that the Margalef richness index significantly changed between 2012 and 2021.
Shannon–Weiner index values ranged between 1.81 and 1.91, with significant variation between the years and a generally declining trend, although a slight fluctuation was reported from 2014 to 2017 (Table 2, bootstrap, p<0.05 in all pairwise comparisons between years). Margalef richness index varied between 2.13 and 2.31, indicating a gradual increase in the species richness over the years (Table 2). The Gini–Simpson index values ranged between 0.66 and 0.70, with significant fluctuations occurring between years and a declining trend. Five species represented 77% or more of the total wintering shorebird population, namely, Little Stint, Dunlin, Curlew Sandpiper, Common Redshank, and Marsh Sandpiper (Tringa stagnatilis) (Table 3). Little Stint was the most dominant species (54.26% of total shorebirds) throughout the duration of the study (Table 3). Although all the shorebirds we assessed exhibited declining trends over the years (Figure 3 and Figure 4, Table 3, p < 0.05 in all cases), eight species among them, namely, Wood Sandpiper (Tringa glareola), Terek Sandpiper (Xenus cinereus), Sanderling (Calidris alba), Red-necked Phalarope (Phalaropus lobatus), Pacific Golden Plover (Pluvialis fulva), Green Sandpiper (Tringa ochropus), Eurasian Oystercatcher, Common Snipe (Gallinago gallinago), Common Sandpiper (Actitis hypoleucos), Common ringed Plover (Charadrius hiaticula), Broad-billed Sandpiper (Limicola falcinellus), and Black-tailed Godwit, exhibited less than 1% relative abundance (Figure 3b).

Table 3.
Comparison of population trends in shorebirds in Bahrain intertidal flats.
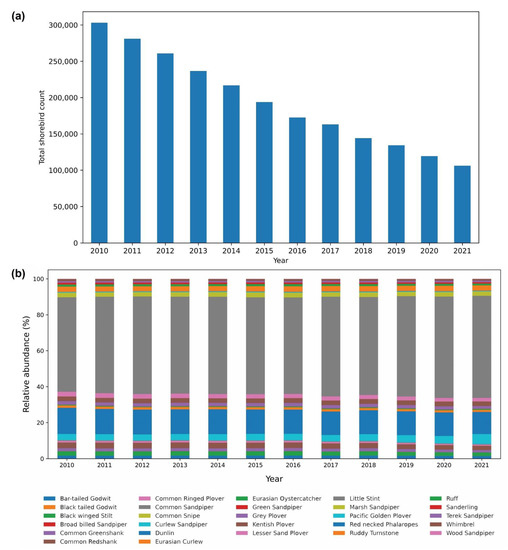
Figure 3.
(a) The total count of the shorebird population, showing a steady decline over the years in the study area; (b) the relative abundance of shorebirds (%) in entire individual sites in relation to the year.
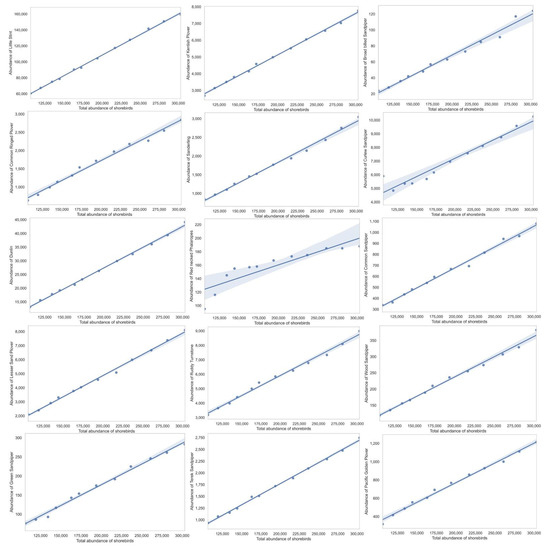
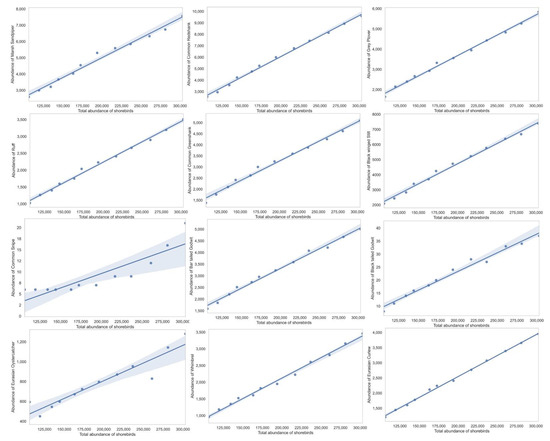
Figure 4.
Population trend analysis of 27 most common migrant shorebirds in Bahrain intertidal habitats using regression analysis.
4. Discussion
By systematically surveying 13 important sites during 2010–2021, our findings provide the first detailed account of and insights into the composition of shorebirds and their population trends in Bahrain. All the observed migratory species showed significant declining trends during the study period, which highlights the importance of conservation of the studied crucial habitats for migratory shorebirds in the CAF(Figure 5).
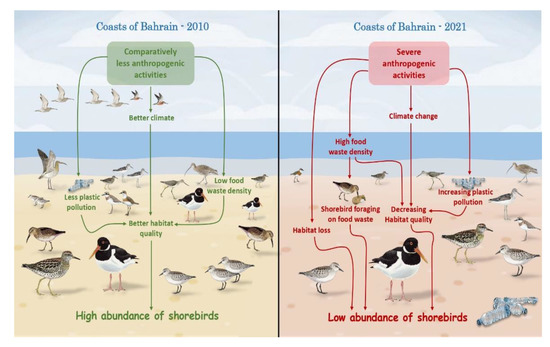
Figure 5.
Overview of population trends and diversity shifts among shorebirds in the Kingdom of Bahrain and their driving factors.
Among the thirty-nine species of shorebirds recorded during the present study, thirty-four species were winter visitors, including eight species that are IUCN red listed. Out of the five resident species, just three (the Black-winged Stilt, Kentish Plover, and Red-wattled Lapwing) were found to be breeding in the Kingdom. There are breeding reports of the other two resident species, namely, Beach Stone-curlew and Crab Plover, from other countries of the Arabian Gulf [33,34,35], but none from Bahrain so far. The study also documented nine species of regularly over-summering shorebirds from different sites of Bahrain, including the Eurasian Oystercatcher, Curlew Sandpiper, Bar-tailed Godwit, and the near-threatened Eurasian Curlew. Therefore, the coasts of Bahrain are critical habitats that act as “nurseries” for long-distance migratory shorebirds. Bar-tailed Godwits were found to be the most dominant over-summering species, followed by the Common Greenshank. This may be due to the tendency of a higher over-summering index for large-bodied, long-distance migrant shorebirds due to their delayed maturity [36], even though different factors, such as adverse weather conditions [37], could be responsible. Detailed studies on sex and age-related patterns of over-summering shorebird abundance can greatly aid in implementing the year-round conservation strategies which need to be adopted in the habitat.
The present study delineated that in the Kingdom of Bahrain, more than 77% of the total wintering population of shorebirds was composed of five species, namely the Little Stint, Dunlin, Curlew Sandpiper, Common Redshank, and Marsh Sandpiper. Among these, the first three species are surface-pecking small shorebirds. Along with their traditionally recognized macrobenthic prey items, these small-bodied shorebirds were observed as consuming large quantities of intertidal biofilms [38], containing essential fatty acids [39], providing fuel, and improving flight performance during migration [40]. The other two species, being medium-sized shorebirds, seem to have a significant degree of niche overlap with the small shorebirds in terms of prey and foraging behavior [41]. As the costs of availing macrobenthic invertebrates are high in certain habitats and are dominated by planktonic prey items, these shorebirds have been observed to employ surface tension prey transport, which aids in small prey profitability and is a strategy unavailable to other birds [42]. The dominance of shorebirds depending directly on the lowest trophic level identifies the coast of Bahrain as a region with various factors favoring high primary productivity. These regions might have evolved as important wintering grounds for shorebirds in the CAF due to these favorable environmental variables and high primary productivity, which contribute to the abundance of macrobenthic organisms as well.
We observed a steadily declining trend across the years in the total count of all the shorebirds in the Kingdom of Bahrain. The species diversity and richness varied over the years, with a general declining trend similar to that observed in the coastal zones of southwest India [14,15] in the CAF, which was experiencing long-term fluctuations in water and sediment quality [43] and declining primary productivity [44]. The diversity and abundance of shorebirds were largely influenced by the quality, quantity, and availability of their crucial nutritional resources, such as planktonic prey items, intertidal biofilms, and macrobenthos in the stop-over sites [9,45,46,47]. Coastal habitat conditions in Bahrain displayed serious conservation problems and direct habitat loss due to climate change, coastal reclamation, desalination, and oil and gas activities [29,48], which led to a general trend of reductions in both diversity and abundance of macrobenthos [49]. Changes in habitat quality driven by anthropogenic disturbances, including habitat degradation by developmental activities and environmental pollution by oil spills [50], have caused changes in individual bird behavior [51], in a way affecting their fitness [52].
Among the 27 species of shorebirds showing a significant downward trend in abundance, ten of them were observed feeding on discarded food waste during the study period, especially in the Busaiteen region (Figure 6). These were Little Stint, Dunlin, Curlew Sandpiper, Broad-billed Sandpiper, Ruddy Turnstone, Grey Plover, Common Redshank, Common Greenshank, Eurasian Curlew, and Bar-tailed Godwit. Although direct evidence is sparse due to a lack of studies, this alteration from their specialized diet will induce negative effects on shorebirds’ physiology in the long term due to the gradual accumulation of harmful chemicals. Effluents containing high levels of ammonia, nitrate, phosphate, and other heavy metals are discharged from sewage treatment plants in the Tubli bay and cause reductions in the biodiversity, richness, and evenness of macrobenthos [53]. A similar impact can be observed in areas adjacent to seawater desalination plants, where high-temperature discharged brine water with containing heavy metals and other chemical pollutants limits the survival of most macrobenthos, except for a few opportunistic species [53]. This change in the community structure of key prey items, such as polychaetes and crabs, might have accounted for the diversity changes in the shorebird population along the coasts of Bahrain.

Figure 6.
Dunlin (Calidris alpina) foraging on food waste on the Busaiteen coast (a,b).
Since the Arabian Gulf is the largest reserve of oil in the world, the coasts of Bahrain and its biodiversity are under a permanent threat of oil pollution [49]. This can have a direct or indirect, lethal or sublethal impact on the shorebirds. We documented forty-six oiled individuals belonging to six species during the study period from different sites of Bahrain. Oil on feathers causes direct, immediate mortality of shorebirds due to hypothermia [54]. Oil ingestion due to preening of oiled feathers or by foraging in contaminated habitats and consumption of oiled prey might cause acute toxic effects on internal organs and lead to mortality [55]. Since migratory shorebirds have strong site-fidelity to wintering and stop-over sites [56,57], they are more vulnerable to repeated exposure to oil, and this can ultimately lead to population-level impacts through diminished health and reproductive success [58]. Similar to the impacts of sewage and desalination plant effluents, the oil pollution also causes mortality and community changes in macrobenthic prey items [49]. This affects the refueling rates of shorebirds, causing subsequent weight loss and delaying their departure [15] from wintering grounds. Studies should prioritize the understanding of this pollution-induced decline in refueling rates, which contributes to the over-summering abundance of large-sized shorebirds in Bahrain.
Finally, one of the most important challenges confronting coastal bird assemblages in general is the persistent threat of habitat degradation and loss linked to coastal land reclamation. Sea land reclamation activities are widespread in both the Arabian Gulf and in many other areas in Asia [59,60], and in Bahrain, reclamation activities have led to the loss of many important intertidal mudflat areas. As a small archipelago country, coastal reclamation continues to be an option for securing land to meet the needs of the expanding population and sprawling urban areas in Bahrain [29]. Given the trend for continued extensive coastal development in Bahrain, intertidal coastal and marine habitats may continue to be significantly degraded by reclamation works. This threat is one of the most important problems for a wide range of coastal wildlife, including specialized shorebird species that feed, rest, and roost directly in intertidal areas and coastal wetlands [29,61]. However, Bahrain has designated several bird grounds as nationally and internationally protected areas. These areas include Tubli Bay and Hawar Islands, which are declared as Ramsar sites. Finally, with careful planning, ecological restoration, and habitat assessment with specific concern for migratory and over-summering shorebirds, there may be opportunities to reverse the losses and mitigate degradation [62,63]. This will require increased knowledge and understanding of intertidal coastal biodiversity, conservation evaluation studies, and political will for the conservation and rehabilitation of key coastal wetland sites. More effort should be made to secure and protect specific important areas for coastal birds in Bahrain [48].
Long-term, systematic studies based on standardized survey observations are necessary to understand the factors responsible for both the decline and diversity changes of shorebirds in the most stressed coasts of Bahrain. Further studies organized throughout the country’s coasts may aid in establishing improved conservation measures to protect the shorebirds of Bahrain in the CAF.
Author Contributions
H.S.: data curation; K.A.R.: writing and editing; H.A.N.: writing and editing; T.R.A.: writing and editing; A.K.S.: writing and editing; A.H.A.: writing and editing; S.Z.: writing and editing; O.F.A.-S.: writing and editing; Y.X.: writing and editing; A.N.: writing and editing; D.R.G.: writing and editing; S.B.M.: review and editing; K.M.A.: writing, editing, software, conceptualization, and overall supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not Applicable.
Data Availability Statement
Upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hua, N.; Tan, K.; Chen, Y.; Ma, Z. Key research issues concerning the conservation of migratory shorebirds in the Yellow Sea region. Bird Conserv. Int. 2015, 25, 38–52. [Google Scholar] [CrossRef]
- Koleček, J.; Reif, J.; Šálek, M.; Hanzelka, J.; Sottas, C.; Kubelka, V. Global population trends in shorebirds: Migratory behaviour makes species at risk. Sci. Nat. 2021, 108, 9. [Google Scholar] [CrossRef]
- Ellis, K.S.; Anteau, M.J.; Cuthbert, F.J.; Gratto-Trevor, C.L.; Jorgensen, J.G.; Newstead, D.J.; Powell, L.A.; Ring, M.M.; Sherfy, M.H.; Swift, R.J.; et al. Impacts of extreme environmental disturbances on piping plover survival are partially moderated by migratory connectivity. Biol. Conserv. 2021, 264, 109371. [Google Scholar] [CrossRef]
- Rashiba, A.P.; Jishnu, K.; Byju, H.; Shifa, C.T.; Anand, J.; Vichithra, K.; Xu, Y.; Nefla, A.; Bin Muzaffar, S.; Aarif, K.M.; et al. The Paradox of Shorebird Diversity and Abundance in the West Coast and East Coast of India: A Comparative Analysis. Diversity 2022, 14, 885. [Google Scholar] [CrossRef]
- Joulami, L.; El Hamoumi, R.; Daief, Z.; Bazairi, H.; Lopes, R. Impact of shorebird predation on intertidal macroinvertebrates in a key North African Atlantic wintering site: An experimental approach. Afr. J. Mar. Sci. 2019, 41, 1–9. [Google Scholar] [CrossRef]
- Norris, D.R. Carry-over effects and habitat quality in migratory populations. Oikos 2005, 109, 178–186. [Google Scholar] [CrossRef]
- Anderson, C.; Lenore, F.; Rausch, J.; Martin, J.-L.; Daufresne, T.; Smith, P.A. Climate-related range shifts in Arc-tic-breeding shorebirds. Ecol. Evol. 2023, 13, e9797. [Google Scholar] [CrossRef]
- Placyk, J.S.; Harrington, B.A. Prey abundance and habitat use by migratory shorebirds at coastal stopover sites in Connecticut. J. Field Ornithol. 2004, 75, 223–231. [Google Scholar] [CrossRef]
- Butler, R.W.; Davidson, N.C.; Morrison, R.I.G. Global-Scale Shorebird Distribution in Relation to Productivity of Near-Shore Ocean Waters. Waterbirds 2001, 24, 224–232. [Google Scholar] [CrossRef]
- Norazlimi, N.; Ramli, R. Temporal variation of shorebirds population in two different mudflats areas. Int. J. Biol. Agric. Food Biotechnol. Eng. 2014, 8, 1314–1320. [Google Scholar] [CrossRef]
- Smith, R.V.; Stafford, J.D.; Yetter, A.P.; Horath, M.M.; Hine, C.S.; Hoover, J.P. Foraging Ecology of Fall-Migrating Shorebirds in the Illinois River Valley. PLoS ONE 2012, 7, e45121. [Google Scholar] [CrossRef]
- Touhami, F.; Idrissi, H.R.; Benhoussa, A. Foraging behaviour of wintering shorebirds at Merja Zerga lagoon (Atlantic coast, Morocco). Ostrich 2020, 91, 244–251. [Google Scholar] [CrossRef]
- Aarif, K.M. Some Aspects of Feeding Ecology of Lesser Sand Plover in Three Different Zones in the Kadalundi Estuary, Kerala and South India. Podoces 2009, 4, 100–107. [Google Scholar]
- Aarif, K.M.; Muzaffar, S.B.; Babu, S.; Prasadan, P.K. Shorebird assemblages respond to anthropogenic stress by altering habitat use in a wetland in India. Biodivers. Conserv. 2014, 23, 727–740. [Google Scholar] [CrossRef]
- Aarif, K.; Nefla, A.; Nasser, M.; Prasadan, P.; Athira, T.; Bin Muzaffar, S. Multiple environmental factors and prey depletion determine declines in abundance and timing of departure in migratory shorebirds in the west coast of India. Glob. Ecol. Conserv. 2021, 26, e01518. [Google Scholar] [CrossRef]
- Steibl, S.; Laforsch, C. The importance of Maldives as a wintering ground for migratory birds of the Central Asian Flyway. Forktail 2021, 37, 80–87. [Google Scholar]
- Szabo, J.; Mundkur, T. Conserving Wetlands for Migratory Waterbirds in South Asia. In Wetland Science Perspectives from South Asia; Prusty, B., Chandra, R., Azeez, P., Eds.; Springer: New Delhi, India, 2017; pp. 105–127. [Google Scholar] [CrossRef]
- Piersma, T.; Lok, T.; Chen, Y.; Hassell, C.J.; Yang, H.-Y.; Boyle, A.; Slaymaker, M.; Chan, Y.-C.; Melville, D.S.; Zhang, Z.-W.; et al. Simultaneous declines in summer survival of three shorebird species signals a flyway at risk. J. Appl. Ecol. 2016, 53, 479–490. [Google Scholar] [CrossRef]
- Naser, H.A. Marine Ecosystem Diversity in the Arabian Gulf: Threats and Conservation. In Biodiversity. The Dynamic Balance of the Planet; Grillo, O., Ed.; Intechopen: London, UK, 2014; pp. 297–328. Available online: https://www.intechopen.com/books/3821 (accessed on 9 February 2023).
- Al-Sayed, H.; Naser, H.; Al-Wedaei, K. Observations on macrobenthic invertebrates and wader bird assemblages in a protected marine mudflat in Bahrain. Aquat. Ecosyst. Health Manag. 2008, 11, 450–456. [Google Scholar] [CrossRef]
- Al-Osaimi, A.; Ali, T.S.; Al-Zubari, W.; Nasser, H. Effect of brine discharge from Al-Dur RO desalination plant on the infauna species composition in the East Coast of Bahrain. Desalination Water Treat. 2020, 176, 29–37. [Google Scholar] [CrossRef]
- Marzooq, H.; Naser, H.A.; Elkanzi, E.M. Quantifying exposure levels of coastal facilities to oil spills in Bahrain, Arabian Gulf. Environ. Monit. Assess. 2019, 191, 160. [Google Scholar] [CrossRef]
- Feary, D.A.; Burt, J.; Bauman, A.G.; Al Hazeem, S.; Abdel-Moati, M.A.; Al-Khalifa, K.A.; Anderson, D.M.; Amos, C.; Baker, A.; Bartholomew, A.; et al. Critical research needs for identifying future changes in Gulf coral reef ecosystems. Mar. Pollut. Bull. 2013, 72, 406–416. [Google Scholar] [CrossRef]
- Evans, M.I. Important Bird Areas in the Middle East; Birdlife International: Cambridge, UK, 1994. [Google Scholar]
- Hirschfeld, E. Migration patterns of some regularly occurring waders in Bahrain 1990–1992. Wader Study Group Bull. 1994, 74, 36–49. [Google Scholar]
- Hirschfeld, E.; Mohamed, S.A.; Stawarczyk, T. Migration pattern, weight, measurements and moult of waders ringing in August-September 1992 in Bahrain. Wader Study Group Bull. 1996, 80, 69–77. [Google Scholar]
- Linden, O. Report of Marine Pollution and Fisheries in Bahrain; FAO: Rome, Italy, 1982. [Google Scholar]
- Naser, H.A.; Hoad, G. An investigation of salinity tolerance and salt secretion in protected mangroves, Bahrain. In Proceedings of the Gulf II: An International Conference. The State of the Gulf Ecosystem: Functioning and Services, Kuwait City, Kuwait, 7–9 February 2011. [Google Scholar]
- Naser, H.A. Community Structures of Benthic Macrofauna in Reclaimed and Natural Intertidal Areas in Bahrain, Arabian Gulf. J. Mar. Sci. Eng. 2022, 10, 945. [Google Scholar] [CrossRef]
- Howes, J.G.; Bakewell, D. Shorebird Studies Manual; AWB Publication: Kuala Lumpur, Malaysia, 1989; p. 362. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 25 August 2022).
- Ahmed, S.; Khan, S.; Shah, J.N.; Al Hammadi, A.A.; Al Hammadi, E.A.; Javed, S. Eurasian Stone Curlew breeding in Abu Dhabi, UAE. Phoenix 2013, 29, 2–4. [Google Scholar]
- Almalki, M.; AlRashidi, M.; Shobrak, M.; Székely, T. Breeding distribution and conservation of the Crab Plover (Dromas ardeola) in Saudi Arabia (Aves: Charadriiformes). Zool. Middle East 2014, 60, 6–12. [Google Scholar] [CrossRef]
- Bom, R.A.; Al-Nasrallah, K. Counts and breeding biology of Crab Plovers Dromas ardeola on Bubiyan Islands, Kuwait, in 2012–2014. Wader Study 2015, 122, 212–220. [Google Scholar] [CrossRef]
- Ayala-Perez, V.O.; Carmona, R.; Arce, N.; Albores-Barajas, Y.V. Over-summering shorebirds in Guerrero Negro, Baja California Sur, Mexico and the particular case of the Marbled Godwit. Wader Study 2021, 128, 109–116. [Google Scholar] [CrossRef]
- Klaassen, M.; Hoye, B.J.; Nolet, B.A.; Buttemer, W.A. Ecophysiology of avian migration in the face of current global hazards. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1719–1732. [Google Scholar] [CrossRef] [PubMed]
- Kuwae, T.; Miyoshi, E.; Hosokawa, S.; Ichimi, K.; Hosoya, J.; Amano, T.; Moriya, T.; Kondoh, M.; Ydenberg, R.C.; Elner, R.W. Variable and complex food web structures revealed by exploring missing trophic links between birds and biofilm. Ecol. Lett. 2012, 15, 347–356. [Google Scholar] [CrossRef]
- Schnurr, P.J.; Drever, M.C.; Elner, R.W.; Harper, J.; Arts, M.T. Peak Abundance of Fatty Acids From Intertidal Biofilm in Relation to the Breeding Migration of Shorebirds. Front. Mar. Sci. 2020, 7, 63. [Google Scholar] [CrossRef]
- Canham, R.; Flemming, S.A.; Hope, D.D.; Drever, M.C. Sandpipers go with the flow: Correlations between estuarine conditions and shorebird abundance at an important stopover on the Pacific Flyway. Ecol. Evol. 2021, 11, 2828–2841. [Google Scholar] [CrossRef]
- Mishra, H.; Kumar, V.; Kumar, A. Resource Partitioning between Two Species of Migratory Waders, Common Redshank Tringa totanus (Linnaeus, 1758) and Little Stint Calidris minuta (Leisler, 1812) (Scolopacidae): A Behavioural Comparison in a Wetland Ecosystem in Bakhira Tal, Uttar Pradesh, India. Acta Zool. Bulg. 2019, 71, 103–111. [Google Scholar]
- Estrella, S.M.; Masero, J.A.; Pérez-Hurtado, A. Small-Prey Profitability: Field Analysis of Shorebirds’ use of Surface Tension of Water to Transport Prey. Ornithology 2007, 124, 1244–1253. [Google Scholar] [CrossRef]
- Rubeena, K.; Nefla, A.; Aarif, K.; AlMaarofi, S.S.; Gijjappu, D.R.; Reshi, O.R. Alterations in hydrological variables and substrate qualities and its impacts on a critical conservation reserve in the southwest coast of India. Mar. Pollut. Bull. 2023, 186, 114463. [Google Scholar] [CrossRef]
- Athira, T.R.; Nefla, A.; Shifa, C.T.; Shamna, H.; Aarif, K.M.; AlMaarofi, S.S.; Rashiba, A.P.; Reshi, O.R.; Jobiraj, T.; Thejass, P.; et al. The impact of long-term environmental change on zooplankton along the southwestern coast of India. Environ. Monit. Assess. 2022, 194, 316. [Google Scholar] [CrossRef]
- A Hobson, K.; Kuwae, T.; Drever, M.C.; E Easton, W.; Elner, R.W. Biofilm and invertebrate consumption by western sandpipers (Calidris mauri) and dunlin (Calidris alpina) during spring migratory stopover: Insights from tissue and breath CO2 isotopic (δ13C, δ15N) analyses. Conserv. Physiol. 2022, 10, coac006. [Google Scholar] [CrossRef]
- Huang, P.; Poon, E.S.K.; Chan, L.Y.; Chan, D.T.C.; Huynh, S.; So, I.W.Y.; Sung, Y.; Sin, S.Y.W. Dietary diversity of multiple shorebird species in an Asian subtropical wetland unveiled by DNA metabarcoding. Environ. DNA 2022, 4, 1381–1396. [Google Scholar] [CrossRef]
- Lu, X.; Yang, H.; Piersma, T.; Sun, L.; Chen, Q.; Jia, Y.; Lei, G.; Cheng, L.; Rao, X. Food resources for Spoon-billed Sandpipers (Calidris pygmaea) in the mudflats of Leizhou Bay, southern China. Front. Mar. Sci. 2022, 9, 1733. [Google Scholar] [CrossRef]
- BirdLife International. Country Profile: Bahrain. 2022. Available online: http://datazone.birdlife.org/country/bahrain/resources (accessed on 28 October 2022).
- Naser, H. Human Impacts on Marine Biodiversity: Macrobenthos in Bahrain, Arabian Gulf. In The Importance of Biological Interactions in the Study of Biodiversity; Lopez-Pujol, J., Ed.; InTech Publishing: London, UK, 2011; pp. 109–126. [Google Scholar] [CrossRef]
- Navedo, J.G.; Ruiz, J. Oversummering in the southern hemisphere by long-distance migratory shorebirds calls for reappraisal of wetland conservation policies. Glob. Ecol. Conserv. 2020, 23, e01189. [Google Scholar] [CrossRef]
- Martín, B.; Delgado, S.; de la Cruz, A.; Tirado, S.; Ferrer, M. Effects of human presence on the long-term trends of migrant and resident shorebirds: Evidence of local population declines. Anim. Conserv. 2014, 18, 73–81. [Google Scholar] [CrossRef]
- Goss-Custard, J.D.; Burton, N.H.K.; Clark, N.A.; Ferns, P.N.; McGrorty, S.; Reading, C.J.; Rehfisch, M.M.; Stillman, R.A.; Townend, I.; West, A.D.; et al. Test of a Behavior-Based Individual-Based Model: Response of Shorebird Mortality to Habitat Loss. Ecol. Appl. 2006, 16, 2215–2222. [Google Scholar] [CrossRef]
- Naser, H.A. Using Macrobenthos as a Tool in Ecological Impact Assessment: Applications in Environmental Impact Assessment (EIA); Lambert Academic Publishing: Saarbrucken, Germany, 2010. [Google Scholar]
- Maggini, I.; Kennedy, L.V.; Elliott, K.H.; Dean, K.M.; MacCurdy, R.; Macmillan, A.; Pritsos, C.A.; Guglielmo, C.G. Trouble on takeoff: Crude oil on feathers reduces escape performance of shorebirds. Ecotoxicol. Environ. Saf. 2017, 141, 171–177. [Google Scholar] [CrossRef]
- Takeshita, R.; Bursian, S.J.; Colegrove, K.M.; Collier, T.K.; Deak, K.; Dean, K.M.; De Guise, S.; DiPinto, L.M.; Elferink, C.J.; Esbaugh, A.J.; et al. A review of the toxicology of oil in vertebrates: What we have learned following the Deepwater Horizon oil spill. J. Toxicol. Environ. Health Part B 2021, 24, 355–394. [Google Scholar] [CrossRef] [PubMed]
- Lagassé, B.J.; Lanctot, R.B.; Barter, M.; Brown, S.; Chiang, C.-Y.; Choi, C.-Y.; Gerasimov, Y.N.; Kendall, S.; Liebezeit, J.R.; Maslovsky, K.S.; et al. Dunlin subspecies exhibit regional segregation and high site fidelity along the East Asian–Australasian Flyway. Condor 2020, 122, duaa054. [Google Scholar] [CrossRef]
- Watts, B.D.; Smith, F.M.; Hines, C.; Duval, L.; Hamilton, D.J.; Keyes, T.; Paquet, J.; Pirie-Dominix, L.; Rausch, J.; Truitt, B.; et al. The annual cycle for whimbrel populations using the Western Atlantic Flyway. PLoS ONE 2021, 16, e0260339. [Google Scholar] [CrossRef]
- Henkel, J.R.; Sigel, B.J.; Taylor, C.M. Large-Scale Impacts of the Deepwater Horizon Oil Spill: Can Local Disturbance Affect Distant Ecosystems through Migratory Shorebirds? Bioscience 2012, 62, 676–685. [Google Scholar] [CrossRef]
- Burt, J.A. The environmental costs of coastal urbanization in the Arabian Gulf. City 2014, 18, 760–770. [Google Scholar] [CrossRef]
- Duan, H.; Zhang, H.; Huang, Q.; Zhang, Y.; Hu, M.; Niu, Y.; Zhu, J. Characterization and environmental impact analysis of sea land reclamation activities in China. Ocean Coast. Manag. 2016, 130, 128–137. [Google Scholar] [CrossRef]
- Wu, W.; Yang, Z.; Tian, B.; Huang, Y.; Zhou, Y.; Zhang, T. Impacts of coastal reclamation on wetlands: Loss, resilience, and sustainable management. Estuarine Coast. Shelf Sci. 2018, 210, 153–161. [Google Scholar] [CrossRef]
- Burt, J.A.; Bartholomew, A. Towards more sustainable coastal development in the Arabian Gulf: Opportunities for ecological engineering in an urbanized seascape. Mar. Pollut. Bull. 2019, 142, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Aarif, K.; Kaiser, S.A.; Nefla, A.; Almaroofi, S. Over-summering abundance, species composition, and habitat use patterns at a globally important site for migratory shorebirds. Wilson J. Ornithol. 2020, 132, 165–172. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).