Lichen-Associated Oribatid Mites in the Taiga Zone of Northeast European Russia: Taxonomical Composition and Geographical Distribution of Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Area
2.2. Material Collection and Processing Methods
3. Results and Discussion
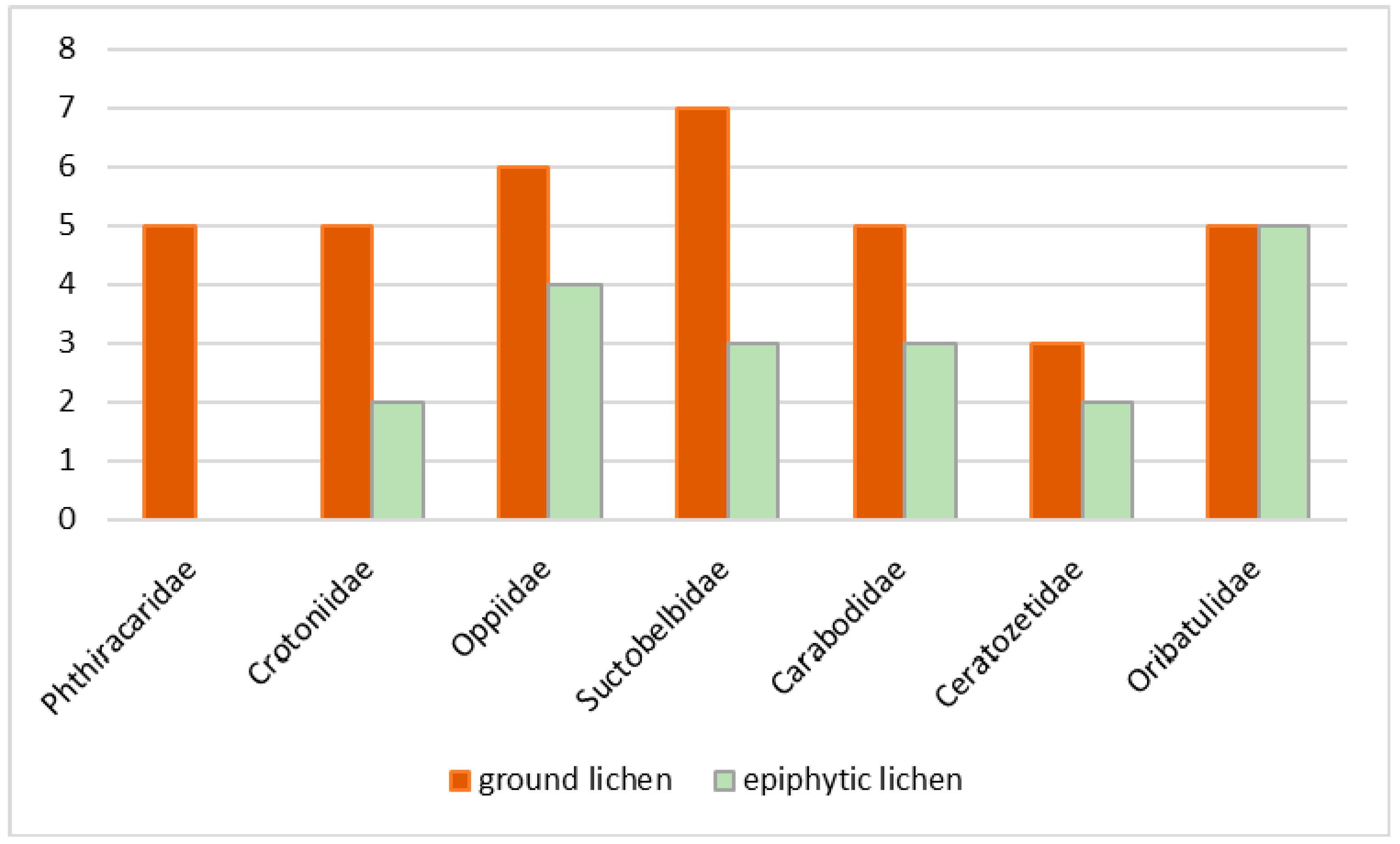
3.1. Taxonomic Diversity
3.2. Life Forms of Oribatid Mites
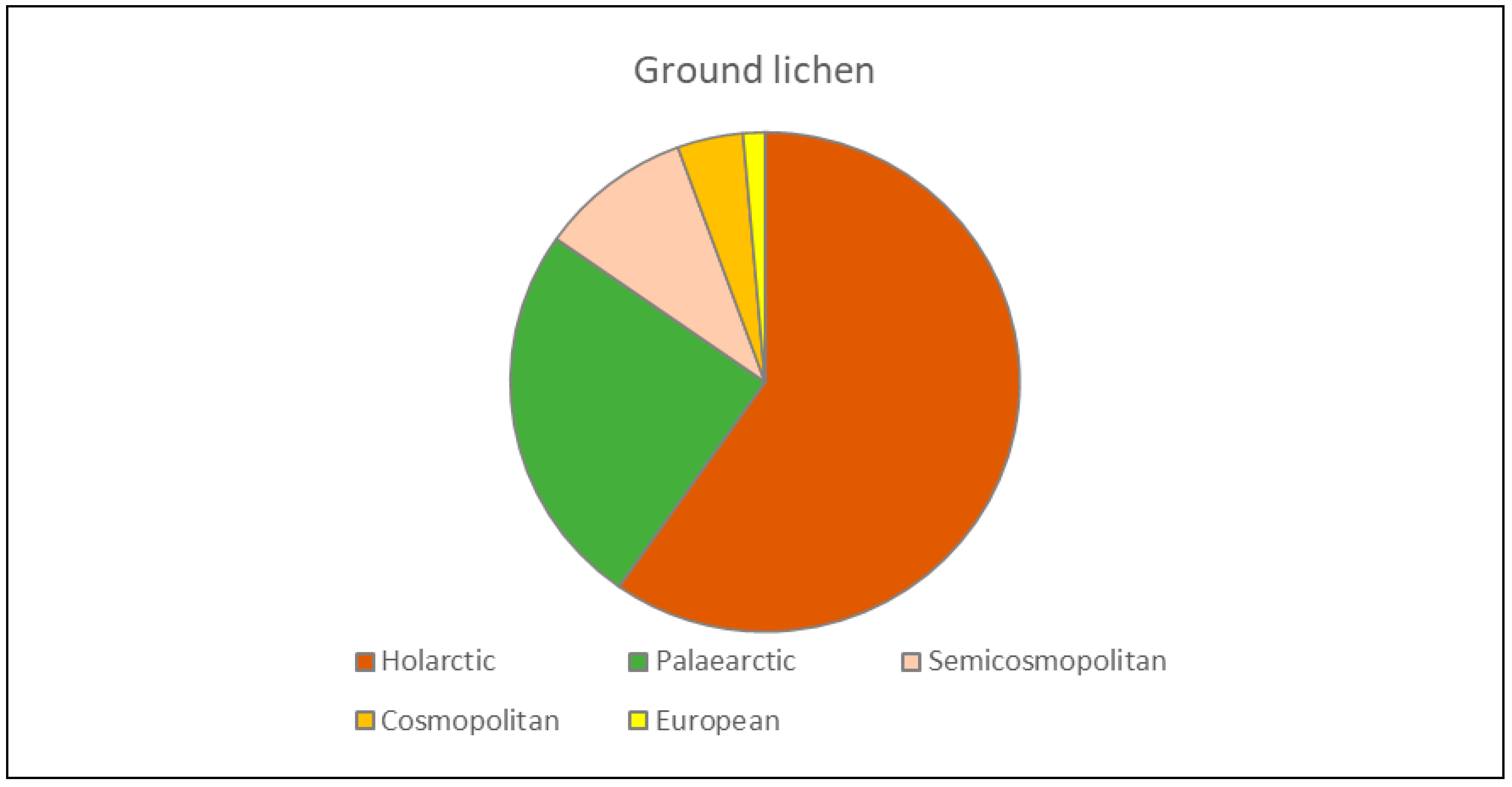
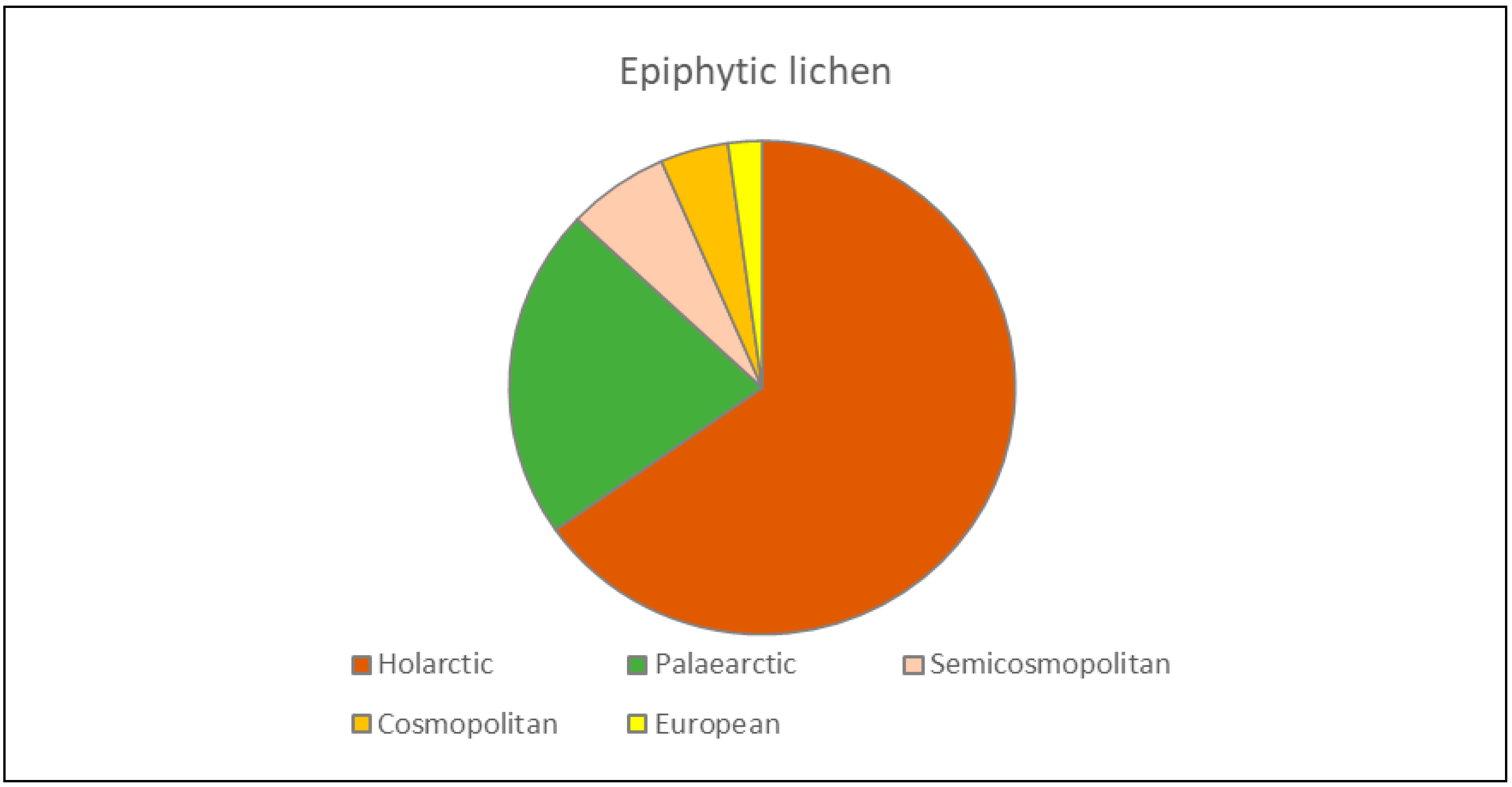
3.3. Zoogeographic Structure of the Fauna
3.3.1. Boreal–Alpine Species
3.3.2. Species Rare to the Taiga Zone
4. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Brachychthonioidea Thor, 1934 |
| Brachychthoniidae Thor, 1934 |
| 1. Liochthonius (Liochthonius) lapponicus (Trägårdh, 1910) Distribution. Holarctic Parmelia sulcata, Cetrariella delisei. |
| Euphthiracaroidea Jacot, 1930 |
| Oribotritiidae Balogh, 1943 |
| 2. Oribotritia (Oribotritia) berlesei (Michael, 1898) Distribution. Palaearctic Cetraria islandica, Cladonia stellaris, Cladonia digitata, Cladonia fimbriata. |
| Euphthiracaridae Jacot, 1930 |
| 3. Euphthiracarus (Euphthiracarus) cribrarius s. str. (Berlese, 1904) Distribution. Holarctic Cetraria islandica, Cladonia arbuscula, Cladonia mitis, Cladonia rangiferina, Cladonia stellaris. |
| Phthiracaroidea Perty, 1841 |
| Phthiracaridae Perty, 1841 |
| 4. Atropacarus (Atropacarus) striculus s. str. (Koch, 1835) Distribution. Semicosmopolitan Cetraria islandica, Cladonia stellaris, Cladonia uncialis, Stereocaulon sp., Peltigera canina. |
| 5. Hoplophthiracarus (Hoplophthiracarus) illinoisensis (Ewing, 1909) (=Hoploderma pavidum Berlese, 1913) Distribution. Semicosmopolitan Peltigera canina. |
| 6. Phthiracarus (Phthiracarus) laevigatus (Koch, 1844) (=Hoplophora nitens Nicolet, 1855) Distribution. Holarctic Cetraria islandica, Cladonia arbuscula, Cladonia rangiferina, Cladonia stellaris, Cladonia crispata, Cladonia gracilis, Peltigera canina. |
| 7. Phthiracarus (P.) longulus (Koch, 1841) (=Hoploderma boreale Trägårdh, 1910) Distribution. Holarctic Cetraria islandica, Cladonia arbuscula, Cladonia mitis, Cladonia rangiferina, Cladonia stellaris, Cladonia digitata, Peltigera aphthosa. |
| 8. Phthiracarus (Archiphthiracarus) piger (Scopoli, 1763) Distribution. Holarctic Cetraria islandica, Cladonia stellaris. |
| Crotonioidea Thorell, 1876 |
| Trhypochthoniidae Willmann, 1931 |
| 9. Trhypochthonius cladonicolus (Willmann, 1919) Distribution. Holarctic Cetraria islandica, Cladonia arbuscula, Cladonia mitis, Cladonia rangiferina, Cladonia stellaris, Cladonia digitata, Cladonia uncialis. |
| 10. Trhypochthonius tectorum s. str. (Berlese, 1896) Distribution. Semicosmopolitan Cetraria islandica, Cladonia stellaris. |
| Nothridae Berlese, 1896 |
| 11. Nothrus silvestris s. str. Nicolet, 1855 Distribution. Holarctic Hypogymnia physodes, Peltigera leucophlebia. |
| Crotoniidae Thorell, 1876 |
| 12. Camisia (Camisia) biurus s. str. (Koch, 1839) Distribution. Holarctic Hypogymnia physodes, Cetraria islandica, Cladonia arbuscula, Cladonia rangiferina, Cladonia stellaris, Cladonia uncialis, Peltigera aphthosa, Peltigera canina. |
| 13. Camisia (C.) borealis (Thorell, 1871) Distribution. Boreoalpine. Holarctic Cetraria islandica, Cladonia crispata, Cladonia fimbriata. |
| 14. Camisia (C.) segnis (Hermann, 1804) Distribution. Semicosmopolitan Leptogium saturninum, Melanohalea olivacea, Parmelia sulcata, Phaeophyscia ciliata, Physconia distorta, Cetrariella delisei, Peltigera leucophlebia. |
| 15. Camisia (Ensicamisia) lapponica (Trägårdh, 1910) Distribution. Boreoalpine. Holarctic Cladonia stellaris, Stereocaulon sp. |
| 16. Heminothrus (Heminothrus) longisetosus (Willmann, 1925) Distribution. Holarctic Cetraria islandica, Cladonia arbuscula, Cladonia rangiferina, Cladonia stellaris. |
| Nanhermannioidea Sellnick, 1928 |
| Nanhermanniidae Sellnick, 1928 |
| 17. Nanhermannia (Nanhermannia) dorsalis (Banks, 1896) (=Nanhermannia coronata Berlese, 1913) Distribution. Holarctic Cetraria islandica, Cladonia rangiferina, Cladonia stellaris. |
| Gymnodamaeoidea Grandjean, 1954 Gymnodamaeidae Grandjean, 1954 |
| 18. Gymnodamaeus bicostatus (Koch, 1835) Distribution. Holarctic Peltigera leucophlebia. |
| 19. Jacotella frondeus (Kulijev, 1979) (Plesiodamaeus) (=Plesiodamaeus ornatus Mahunka, 1979) Distribution. Palaearctic Hypogymnia physodes. |
| Damaeoidea Berlese, 1896 |
| Damaeidae Berlese, 1896 |
| 20. Damaeus (Epidamaeus) bituberculatus (Kulczynski, 1902) Distribution. Palaearctic Lobaria pulmonaria, Chaenotheca chrysocephala, Lepraria incana, Physconia distorta, Cetraria islandica, Cladonia arbuscula, Cladonia rangiferina, Cladonia stellaris, Peltigera leucophlebia. Hypogymnia physodes. |
| Cepheusoidea Berlese, 1896 |
| Cepheusidae Berlese, 1896 |
| 21. Cepheus cepheiformis (Nicolet, 1855) Distribution. Holarctic Parmelia sulcata, Peltigera aphthosa. |
| Gustavioidea Oudemans, 1900 |
| Astegistidae Balogh, 1961 |
| 22. Furcoppia (Mexicoppia) dentata (Willmann, 1950) (Cultroribula) Distribution. Holarctic Bryoria fuscescens, Usnea subfloridana, Hypogymnia physodes. |
| 23. Furcoribula furcillata (Nordenskiöld, 1901) Distribution. Holarctic Cladonia rangiferina. |
| Ceratoppiidae Grandjean, 1954 |
| 24. Ceratoppia quadridentata (Haller, 1882) Distribution. Holarctic Bryoria fuscescens, Evernia mesomorpha, Ramalina calicaris, Hypogymnia physodes, Leptogium saturninum, Lobaria pulmonaria, Melanohalea septentrionalis, Parmelia sulcata, Platismatia glauca, Tuckermannopsis chlorophilla, Chaenotheca chrysocephala, Lepraria incana, Lepra albescens, Physconia distorta, Cetraria islandica, Cetrariella delisei, Cladonia rangiferina, Cladonia stellaris, Peltigera canina, Peltigera leucophlebia. |
| Liacaridae Sellnick, 1928 |
| 25. Adoristes (Adoristes) ovatus s. str. (Koch, 1839) Distribution. Holarctic Cetraria islandica, Cladonia arbuscula, Cladonia rangiferina, Cladonia stellaris, Cladonia uncialis. |
| 26. Adoristes (A.) ovatus poppei (Oudemans, 1906) Distribution. Holarctic Hypogymnia physodes, Cetraria islandica, Cladonia arbuscula, Cladonia mitis, Cladonia rangiferina, Cladonia stellaris, Cladonia crispata, Cladonia uncialis, Stereocaulon sp., Peltigera leucophlebia. |
| Tenuialidae Jacot, 1929 |
| 27. Hafenrefferia gilvipes (Koch, 1839) Distribution. Palaearctic Cladonia stellaris. |
| Eremaeoidea Oudemans, 1900 |
| Eremaeidae Oudemans, 1900 |
| 28. Eueremaeus oblongus s. str. (Koch, 1835) Distribution. Holarctic Hypogymnia physodes, Parmelia sulcata, Cetraria islandica, Cladonia stellaris. |
| 29. Eueremaeus oblongus silvestris (Forsslund, 1956) Distribution. Palaearctic Leptogium saturninum, Lepraria incana, Phaeophyscia ciliata, Cetraria islandica, Cladonia arbuscula, Cladonia rangiferina, Cladonia stellaris. |
| Oppioidea Sellnick, 1937 |
| Oppiidae Sellnick, 1937 |
| 30. Graptoppia (Apograptoppia) foveolata (Paoli, 1908) Distribution. Holarctic Bryoria fuscescens, Usnea subfloridana Parmeliopsis ambigua, Hypogymnia physodes, Leptogium saturninum, Platismatia glauca, Phaeophyscia ciliata, Cetrariella delisei, Cladonia rangiferina, Cladonia stellaris, Cladonia uncialis, Stereocaulon sp., Peltigera aphthosa. |
| 31. Ramusella (Ramusella) clavipectinata (Michael, 1885) (=Oppia assimilis Mihelčič, 1956) Distribution. Semicosmopolitan Cladonia digitata. |
| 32. Rhinoppia (Rhinoppia) subpectinata (Oudemans, 1900) (=Oppia globosa Mihelčič, 1956) (=Oppia tuberculata Bulanova-Zachvatkina, 1964) Distribution. Holarctic Peltigera canina. |
| 33. Dissorhina ornata s. str. (Oudemans, 1900) Distribution. Holarctic Peltigera canina. |
| 34. Lauroppia maritima s. str. (Willmann, 1929) Distribution. Holarctic Hypogymnia physodes. |
| 35. Moritzoppia (M.) unicarinata s. str. (Paoli, 1908) Distribution. Holarctic Peltigera canina. |
| 36. Oppiella (Oppiella) nova s. str. (Oudemans, 1902) Distribution. Cosmopolitan Hypogymnia physodes, Cladonia arbuscula, Cladonia stellaris, Cladonia fimbriata. |
| 37. Oppiella (Moritzoppiella) neerlandica (Oudemans, 1900) (=Dameosoma translamellatum Willmann, 1923) Distribution. Holarctic Hypogymnia physodes. |
| Quadroppiidae Balogh, 1983 |
| 38. Quadroppia (Quadroppia) quadricarinata (Michael, 1885) Distribution. Semicosmopolitan Hypogymnia physodes. |
| Trizetoidea Ewing, 1917 |
| Suctobelbidae Jacot, 1938 |
| 39. Suctobelbella (Suctobelbella) acutidens s. str. (Forsslund, 1941) Distribution. Holarctic Hypogymnia physodes, Cladonia arbuscula. |
| 40. Suctobelbella (S.) acutidens duplex (Strenzke, 1950) (=Suctobelba hammerae Krivolutsky, 1965) Distribution. Holarctic Bryoria fuscescens, Hypogymnia physodes, Platismatia glauca, Lepraria incana, Cetraria islandica, Cladonia arbuscula, Cladonia rangiferina, Cladonia stellaris, Cladonia uncialis. |
| 41. Suctobelbella (S.) acutidens lobata (Strenzke, 1950) (=Suctobelba ornata Krivolutsky, 1966) Distribution. Palaearctic Cladonia arbuscula, Cladonia rangiferina, Cladonia stellaris, Cladonia uncialis, Stereocaulon sp. |
| 42. Suctobelbella (S.) palustris (Forsslund, 1950) Distribution. Holarctic Cladonia arbuscula, Cladonia stellaris, Cladonia digitata, Cladonia fimbriata, Cladonia gracilis. |
| 43. Suctobelbella (S.) singularis (Strenzke, 1950) Distribution. Palaearctic Cladonia arbuscula, Cladonia rangiferina, Cladonia stellaris, Cladonia crispata. |
| 44. Suctobelbella (Flagrosuctobelba) forsslundi s. str. (Strenzke, 1950) Distribution. Palaearctic Cladonia rangiferina, Peltigera aphthosa. |
| 45. Suctobelbella (Flagrosuctobelba) subtrigona (Oudemans, 1900) Distribution. Holarctic Hypogymnia physodes. |
| 46. Suctobelbella (Ussuribata) latirostris (Strenzke, 1950) Distribution. Palaearctic Cetraria islandica, Cladonia arbuscula, Cladonia mitis. |
| Carabodoidea Koch, 1843 |
| Carabodidae Koch, 1843 |
| 47. Carabodes (Carabodes) femoralis (Nicolet, 1855) Distribution. Palaearctic Cetraria islandica, Cladonia arbuscula. |
| 48. Carabodes (C.) labyrinthicus (Michael, 1879) Distribution. Holarctic Bryoria fuscescens, Evernia mesomorpha, Parmeliopsis ambigua, Hypogymnia physodes, Leptogium saturninum, Lobaria pulmonaria, Platismatia glauca, Tuckermannopsis chlorophilla, Vulpicida pinastri, Lepraria incana, Lepra albescens, Phaeophyscia ciliata, Cetraria islandica, Cetrariella delisei, Cladonia arbuscula, Cladonia rangiferina, Cladonia stellaris. |
| 49. Carabodes (C.) marginatus (Michael, 1884) Distribution. Palaearctic Hypogymnia physodes, Cetraria islandica, Cladonia arbuscula, Cladonia mitis, Cladonia rangiferina, Cladonia stellaris, Cladonia fimbriata, Cladonia uncialis, Stereocaulon sp., Peltigera canina, Peltigera leucophlebia. |
| 50. Carabodes (C.) ornatus Štorkán, 1925 (=Carabodes forsslundi Sellnick, 1953) Distribution. Palaearctic Peltigera leucophlebia. |
| 51. Carabodes (C.) subarcticus Trägårdh, 1902 Distribution. Palaearctic Bryoria fuscescens, Hypogymnia physodes, Parmelia sulcata, Cetraria islandica, Cladonia arbuscula, Cladonia mitis, Cladonia rangiferina, Cladonia stellaris, Cladonia crispata, Cladonia digitata, Cladonia fimbriata, Cladonia gracilis, Stereocaulon sp., Peltigera aphthosa, Peltigera canina, Peltigera leucophlebia. |
| Tectocepheoidea Grandjean, 1954 |
| Tectocepheidae Grandjean, 1954 |
| 52. Tectocepheus velatus s. str. (Michael, 1880) Distribution. Cosmopolitan Bryoria fuscescens, Parmeliopsis ambigua, Hypogymnia physodes, Vulpicida pinastri, Lepraria incana, Cetraria islandica, Cladonia arbuscula, Cladonia rangiferina, Cladonia stellaris, Cladonia crispata, Cladonia digitata, Cladonia fimbriata, Cladonia gracilis, Cladonia uncialis, Stereocaulon sp., Peltigera canina, Peltigera leucophlebia. |
| 53. Tectocepheus velatus sarekensis Trägårdh, 1910 Distribution. Cosmopolitan Cladonia rangiferina, Cladonia stellaris, Peltigera leucophlebia. |
| Ameronothroidea Vitzthum, 1943 |
| Ameronothridae Vitzthum, 1943 |
| 54. Ameronothrus oblongus Sitnikova, 1975 Distribution. Holarctic Hypogymnia physodes. |
| Cymbaeremaeoidea Sellnick, 1928 |
| Cymbaeremaeidae Sellnick, 1928 |
| 55. Cymbaeremaeus cymba (Nicolet, 1855) Distribution. Palaearctic Bryoria fuscescens, Evernia mesomorpha, Hypogymnia physodes, Melanohalea olivacea, Parmelia sulcata, Platismatia glauca, Lepraria incana, Lepra albescens. |
| 56. Scapheremaeus palustris (Sellnick, 1924) Distribution. Holarctic Bryoria fuscescens, Hypogymnia physodes, Melanohalea olivacea, Chaenotheca chrysocephala, Cetraria islandica, Cladonia mitis, Cladonia stellaris. Hipoorden PORONOTICAE Grandjean, 1954 |
| Licneremaeoidea Grandjean, 1954 |
| Micreremidae Grandjean, 1954 |
| 57. Micreremus brevipes (Michael, 1888) (=Micreremus gracilior Willmann, 1931) Distribution. Palaearctic Bryoria fuscescens. |
| Licneremaeidae Grandjean, 1954 |
| 58. Licneremaeus licnophorus (Michael, 1882) Distribution. Holarctic Hypogymnia physodes. |
| Achipterioidea Thor, 1929 |
| Achipteriidae Thor, 1929 |
| 59. Achipteria (Achipteria) coleoptrata s. str. (Linnaeus, 1758) Distribution. Holarctic Peltigera aphthosa. |
| 60. Achipteria (A.) acuta Berlese, 1908 (=Oribata nitens Nicolet, 1855) Distribution. Holarctic Cladonia rangiferina. |
| 61. Parachipteria punctata (Nicolet, 1855) Distribution. Holarctic Cetraria islandica, Cladonia arbuscula, Peltigera canina. |
| 62. Campachipteria (Triachipteria) fanzagoi (Jacot, 1929) (=Parachipteria willmanni Hammen, 1952) Distribution. Holarctic Cetraria islandica, Cladonia rangiferina, Cladonia stellaris. |
| Oribatelloidea Jacot, 1925 |
| Oribatellidae Jacot, 1925 |
| 63. Oribatella (Oribatella) calcarata (Koch, 1835) Distribution. Holarctic Hypogymnia physodes. |
| 64. Oribatella (O.) reticulata Berlese, 1916 Distribution. Holarctic Cladonia stellaris. |
| Ceratozetoidea Jacot, 1925 |
| Ceratozetidae Jacot, 1925 |
| 65. Melanozetes mollicomus (Koch, 1839) Distribution. Boreoalpine. Holarctic Cladonia arbuscula. |
| 66. Sphaerozetes piriformis (Nicolet, 1855) Distribution. Palaearctic Bryoria fuscescens, Ramalina calicaris, Lobaria pulmonaria, Parmelia sulcata, Chaenotheca chrysocephala, Cetraria islandica, Cetrariella delisei. |
| 67. Trichoribates (Trichoribates) berlesei (Jacot, 1929) (=Murcia trimaculata Koch, 1835) Distribution. Holarctic Bryoria fuscescens, Ramalina calicaris, Hypogymnia physodes, Lobaria pulmonaria, Melanohalea olivacea, Platismatia glauca, Tuckermannopsis chlorophilla, Chaenotheca chrysocephala, Mycoblastus sanguinarius, Lepra albescens, Cetraria islandica, Cladonia arbuscula, Cladonia rangiferina, Stereocaulon sp. |
| Chamobatidae Thor, 1937 |
| 68. Chamobates (Chamobates) pusillus (Berlese, 1895) (=Notaspis cuspidatus borealis Trägårdh, 1902) Distribution. Holarctic Hypogymnia physodes, Leptogium saturninum, Melanohalea septentrionalis, Parmelia sulcata, Lepraria incana, Cladonia arbuscula, Cladonia rangiferina, Cladonia stellaris, Cladonia gracilis, Stereocaulon sp., Peltigera aphthosa, Peltigera canina, Peltigera leucophlebia. |
| Humerobatidae Grandjean, 1971 |
| 69. Diapterobates dubinini Shaldybina, 1971 Distribution. Palaearctic Cetraria islandica. |
| 70. Diapterobates humeralis (Hermann, 1804) Distribution. Holarctic Bryoria fuscescens, Ramalina calicaris, Hypogymnia physodes, Melanohalea septentrionalis, Vulpicida pinastri, Physconia distorta, Cetraria islandica, Cladonia stellaris. |
| 71. Diapterobates oblongus (L. Koch, 1879) Distribution. Palaearctic Bryoria fuscescens, Usnea subfloridana, Hypogymnia physodes, Leptogium saturninum, Lobaria pulmonaria, Melanohalea septentrionalis, Parmelia sulcata, Platismatia glauca, Lepraria incana, Cetraria islandica, Cladonia rangiferina, Cladonia stellaris, Cladonia gracilis, Peltigera canina. |
| Punctoribatidae Thor, 1937 |
| 72. Mycobates (Calyptozetes) tridactylus Willmann, 1929 Distribution. Holarctic Bryoria fuscescens, Evernia mesomorpha |
| Oripodoidea Jacot, 1925 |
| Oribatulidae Thor, 1929 |
| 73. Oribatula (Oribatula) pannonica Willmann, 1949 Distribution. Palaearctic Cetraria islandica. |
| 74. Oribatula (O.) tibialis s. str. (Nicolet, 1855) Distribution. Holarctic Hypogymnia physodes, Lobaria pulmonaria, Melanohalea septentrionalis, Parmelia sulcata, Chaenotheca chrysocephala, Mycoblastus sanguinarius, Cetraria islandica, Cladonia arbuscula, Cladonia rangiferina, Cladonia stellaris, Cladonia fimbriata, Cladonia gracilis, Peltigera aphthosa, Peltigera canina, Peltigera leucophlebia. |
| 75. Oribatula (Zygoribatula) exilis s. str. (Nicolet, 1855) Distribution. Holarctic Bryoria fuscescens, Evernia mesomorpha, Usnea subfloridana, Parmeliopsis ambigua, Hypogymnia physodes, Leptogium saturninum, Lobaria pulmonaria, Parmelia sulcata, Vulpicida pinastri, Chaenotheca chrysocephala, Lepraria incana, Mycoblastus sanguinarius, Physconia distorta, Cetraria islandica, Cetrariella delisei, Cladonia arbuscula, Cladonia digitata, Peltigera canina, Peltigera leucophlebia, |
| 76. Oribatula (Z.) frisiae (Oudemans, 1900) (=Zygoribatula tenuelamellata Mihelčič, 1956) Distribution. Holarctic Ramalina calicaris, Usnea subfloridana, Platismatia glauca, Mycoblastus sanguinarius, Cetraria islandica. |
| 77. Oribatula (Z.) propinqua (Oudemans, 1902) Distribution. Palaearctic Bryoria fuscescens, Evernia mesomorpha, Usnea subfloridana, Hypogymnia physodes, Lobaria pulmonaria, Melanohalea olivacea, Chaenotheca chrysocephala, Lepra albescens. |
| 78. Phauloppia nemoralis (Berlese, 1916) Distribution. European Bryoria fuscescens, Evernia mesomorpha, Usnea subfloridana, Hypogymnia physodes, Lobaria pulmonaria, Melanohalea septentrionalis, Parmelia sulcata, Platismatia glauca, Vulpicida pinastri, Lepraria incana, Mycoblastus sanguinarius, Phaeophyscia ciliata, Physconia distorta, Cetraria islandica, Cladonia rangiferina, Cladonia stellaris, Cladonia fimbriata, Cladonia uncialis. |
| Hemileiidae Balogh et P. Balogh, 1984 |
| 79. Hemileius (Hemileius) initialis (Berlese, 1908) (=Scheloribates confundatus Sellnick, 1928) Distribution. Semicosmopolitan Cladonia rangiferina, Cladonia fimbriata, Stereocaulon sp. |
| Liebstadiidae Balogh et P. Balogh, 1984 |
| 80. Liebstadia (Liebstadia) humerata Sellnick, 1928 Distribution. Holarctic Hypogymnia physodes. |
| 81. Liebstadia (L.) pannonica s. str. (Willmann, 1951) (=Protoribates novus Willmann, 1953) Distribution. Holarctic Hypogymnia physodes, Parmelia sulcata. |
| Scheloribatidae Grandjean, 1933 |
| 82. Scheloribates (Scheloribates) laevigatus s. str. (Koch, 1835) Distribution. Semicosmopolitan Bryoria fuscescens, Parmeliopsis ambigua, Hypogymnia physodes, Parmelia sulcata, Lepraria incana, Cetraria islandica, Cladonia arbuscula, Cladonia mitis, Cladonia rangiferina, Cladonia stellaris, Cladonia crispata, Cladonia digitata, Cladonia fimbriata, Cladonia gracilis, Cladonia uncialis, Stereocaulon sp., Peltigera aphthosa, Peltigera canina, Peltigera leucophlebia. |
| 83. Scheloribates (S.) pallidulus latipes (Koch, 1844) Distribution. Semicosmopolitan Cetraria islandica. |
| Parakalummidae Grandjean, 1936 |
| 84. Neoribates (Neoribates) aurantiacus (Oudemans, 1914) Distribution. Holarctic Bryoria fuscescens, Hypogymnia physodes, Lobaria pulmonaria, Melanohalea septentrionalis, Tuckermannopsis chlorophilla, Cetraria islandica, Cetrariella delisei, Cladonia arbuscula, Cladonia rangiferina, Cladonia stellaris, Cladonia uncialis, Peltigera leucophlebia. |
| 85. Neoribates (N.) roubali (Berlese, 1910) Distribution. Palaearctic Cladonia stellaris. |
| Galumnoidea Jacot, 1925 |
| Galumnidae Jacot, 1925 |
| 86. Galumna (Galumna) lanceata (Oudemans, 1900) (=Zetes dorsalis Koch, 1835) Distribution. Palaearctic Cetraria islandica, Cladonia rangiferina |
| 87. Pergalumna (Pergalumna) nervosa s. str. (Berlese, 1914) Distribution. Holarctic Hypogymnia physodes, Parmelia sulcata, Vulpicida pinastri, Cetraria islandica, Cetrariella delisei, Cladonia arbuscula, Cladonia mitis, Cladonia stellaris, Cladonia crispata, Peltigera aphthosa, Peltigera canina. |
References
- Trave, J. Ecologie et biologie des Oribates (Acariens) saxicoles et arboricoles. Vie Milleu 1963, 14, 267. [Google Scholar]
- Biazrov, L.G.; Medvedev, L.N.; Chernova, N.M. Lichen consortia in broadleaf–coniferous forests of the Moscow oblast. In Bio-Geocenological Studies in Broad-Leaved–Spruce Forests; Nauka: Moscow, Russia, 1971; pp. 252–270. [Google Scholar]
- Gerson, U. Lichen-Arthropod Associations. Lichenologist 1973, 5, 434–443. [Google Scholar] [CrossRef]
- Gilbert, O.L. A Lichen-Arthropod Community. Lichenologist 1976, 8, 96. [Google Scholar] [CrossRef]
- Gerson, U.; Seaward, M.R.D. Lichen-invertebrate associations. In Lichen Ecology; Seaward, M.R.D., Ed.; Academic Press: London, UK, 1977; pp. 69–119. [Google Scholar]
- Søchting, U.; Gjelstrup, P. Lichen communities and the associated fauna on a rocky sea shore on Bornholm in the Baltic. Ecography 1985, 8, 66–75. [Google Scholar] [CrossRef]
- Stubbs, C.S. Patterns of Distribution and Abundance of Corticolous Lichens and Their Invertebrate Associates on Quercus rubra in Maine. Bryologist 1989, 92, 453. [Google Scholar] [CrossRef]
- Dalenius, J. Studies on the Oribatei (Acari) on the Tornetrásk Territory in Swedish Lapland. 1. A list of the habitats, and the composition of their Oribatid fauna. Oikos 1960, 11, 80–124. [Google Scholar] [CrossRef]
- MacLean, S.F.J. Introduction invertebrates. In International Biological Programme 25. Tundra ecosystems: A Comparative Analysis; Cambridge University Press: Cambridge, UK, 1981; pp. 509–516. [Google Scholar]
- MacLean, S.F.; Behan, V.; Fjellberg, A. Soil Acari and Collembola from Chaun Bay, Northern Chukotka. Arct. Alp. Res. 1978, 10, 559. [Google Scholar] [CrossRef]
- Ryan, J.K. Invertebrate faunas at IBP tundra sites. In International Biological Programme. 25. Tundra Ecosystems: A Comparative Analysis; Cambridge University Press: Cambridge, UK, 1981; pp. 517–539. [Google Scholar]
- Solhøy, T.; Koponen, S. Oribatei fauna (Acari) on alpine heath at Kevo, Finland. Repts. Kevo. Subarctic. Res. Stat. 1981, 17, 41–43. [Google Scholar]
- Pschorn-Walcher, H.; Gunhold, P. Zur kenntnis der tiergemeinschaft in moos- und flechtenrasen an park- und Waldbaumen. Zoomorphology 1957, 46, 342–354. [Google Scholar] [CrossRef]
- Niedbala, V.Y. The fauna of woody oribatid mites in the vicinity of Poznan. In Oribatids (Oribatei), Their Role in Soil-Forming Processes; Mosklas: Vilnius, Lithuania, 1970; pp. 103–112. [Google Scholar]
- Andre, H. Observations on Belgian corticolous mites. Found Univ. Luxemb. Ser. Notes Rech. 1975, 4, 1–31. [Google Scholar]
- Andre, H. Notes on the ecology of corticolous epiphytic dwellers. 1. The mite fauna of fruticose lichens. Recent Adv. Acarol. 1979, 1, 551–557. [Google Scholar]
- Andre, H. Notes on the ecology of corticolous epiphytic dwellers. 3. Oribatida. Acarologia 1984, 25, 385–395. [Google Scholar]
- Andre, H.M. Associations between corticolous microarthropod communities and epiphytic cover on bark. Ecography 1985, 8, 113–119. [Google Scholar] [CrossRef]
- Gjelstrup, P. Epiphytic Criptostigmatid mites on some beech- and birch-trees in Denmark. Pedobiologia 1979, 19, 1–8. [Google Scholar]
- Biazrov, L.G. Invertebrate animals in epiphytic lichens of different life forms in the forests of Moscow oblast. In Soil Biology of Northern Europe; Nauka: Moscow, Russia, 1988; pp. 149–154. (In Russian) [Google Scholar]
- Forsslund, K.-H.; Strenzke, K. Untersuchungen über die Tiergemeinschaften des Bodens: Die Oribatiden und ihre Synusien in den Böden Norddeutschlands. Oikos 1952, 4, 197. [Google Scholar] [CrossRef]
- Tarba, Z.M. Microarthropods of rock and epiphytic lichens of Abkhazia. Vestn. Zool. 1992, 2, 10–14. [Google Scholar]
- Gjelstrup, P.; Søchting, U. Criptostigmatid mites (Acarina) associated with Ramalina siliquosa (Lichens) on Bornholm in the Baltic. Pedobiologia 1979, 19, 237–245. [Google Scholar]
- Gjelstrup, P.; Søchting, U. Oribatid mites (Acarina) dominant on some lichen and moss species of maritime rocks on Bornholm in the Baltic. Acarology 1984, 6, 528–533. [Google Scholar]
- Coloff, M.J. Oribatid mites associated with marine and maritime lichens on the Islands of Great Cumbrae. Glasg. Nat. 1983, 20, 347–359. [Google Scholar]
- Niemi, R.; Vilkamaa, P. Microarthropods dwelling on two species of lichens of the coastal cliffs of the Finnish archipelago. In Biology of Soils of Northern Europe; Nauka: Moscow, Russia, 1988; pp. 145–148. (In Russian) [Google Scholar]
- Sowter, J.A. Mites (Acari) and lichens. Lichenologist 1971, 5, 176. [Google Scholar] [CrossRef]
- Seyd, E.L.; Seaward, M.R.D. The association of oribatid mites with lichens. Zoöl. J. Linn. Soc. 1984, 80, 369–420. [Google Scholar] [CrossRef]
- Seyd, E.L. The moss mites of the Cheviot (Acari: Oribatei). Biol. J. Linn. Soc. 1988, 34, 349–362. [Google Scholar] [CrossRef]
- Stary, J. Pancirnici (Acari: Oribatida) nekterych vrchovist na Sumave, jizni Cechy. Sbor. Jihoces. Muz. Ces. Budejovicich Prir. Vedy 1988, 28, 99–107. [Google Scholar]
- Melekhina, E.N.; Krivolutsky, D.A. Long-term dynamics of the population of microarthropods of epiphytic lichens in the area of the Chernobyl Nuclear Power Plant. In Radioecological Studies in the 30-km Zone of the Chernobyl Accident; Komi Science Center of Ural Branch of Russian Academy of Sciences Publisher: Syktyvkar, Russia, 1993; Volume 127, pp. 60–72. (In Russian) [Google Scholar]
- Barlow, S.L.; Ferry, B.W. Population dynamics of lichenicolous mites at Dungeness. Bot. J. Linn. Soc. 1989, 101, 111–124. [Google Scholar] [CrossRef]
- Biazrov, L.G.; Melekhina, E.N. Oribatid mites in lichen consortia of Northern Scandinavia (a case study of the Varangerfjord). In Bulletin of Moscow Society of Naturalists; Biological Series; Moscow University Press: Moscow, Russia, 1992; Volume 97, pp. 73–79. (In Russian) [Google Scholar]
- Biazrov, L.G.; Melekhina, E.N. Oribatid mites in lichen consortia of the forest–tundra of Northern Lapland (Finland). In Bulletin of Moscow Society of Naturalists; Biological Series; Moscow University Press: Moscow, Russia, 1994; Volume 99, pp. 40–45. (In Russian) [Google Scholar]
- Melekhina, E.N.; Biazrov, L.G. Long-term changes in the biodiversity of oribatid mites in epiphytic lichens of the Moscow region. In Dynamics of Biodiversity of the Animals: Proceedings of the Meeting; Institute of Problems of Ecology and Evolution im. A.N. Severtsov RAS; Institute of Problems of Ecology and Evolution: Moscow, Russia, 1997; pp. 120–123. (In Russian) [Google Scholar]
- Shtanchaeva, U.Y. Oribatids of consortions of lichens in pine forest. In Problems of Soil Zoology: Materials of the I All -Russian Meeting; Regional Institute for the Improvement of Teachers: Rostov-on-Don, Russia, 1996; pp. 195–196. (In Russian) [Google Scholar]
- Shtanchaeva, U.Y. Oribatid mites (Acariformes, Oribatida) of soil and epiphytic lichens of a pine forest. Acarina 1997, 5, 79–85. [Google Scholar]
- Root, H.T.; McGee, G.G.; Norton, R.A. Arboreal Mite Communities on Epiphytic Lichens of the Adirondack Mountains of New York. Northeast. Nat. 2007, 14, 425–438. [Google Scholar] [CrossRef]
- Melekhina, E.N. Diversity of oribatid mites of lichen groups of the taiga zone of the Komi Republic. In Study and Protection of the Diversity of the Fauna, Flora, and Major Ecosystems of Eurasia; Pavlov, D.S., Shatunovsky, M.I., Eds.; Institute of Problems of Ekology and Evolution named after A.N. Severtsov Russian Akademy of Sciences: Moscow, Russia, 2000; pp. 184–191. (In Russian) [Google Scholar]
- Melekhina, E.N. Biodiversity of oribatid mites–dwellers of epiphytic lichens in the taiga zone of the Komi Republic. In Fauna and Ecology of Invertebrate Animals of the European North-East; Komi Science Center of Ural Branch of Russian Academy of Sciences: Syktyvkar, Russia, 2001; Volume 166, pp. 111–120. (In Russian) [Google Scholar]
- Melekhina, E.N. Oribatid Mites as Inhabitants of Lichens in the Taiga Zone of Northeastern Europe: Biotopic Association and Ecological Groups of Species. Biol. Bull. 2020, 47, 522–534. [Google Scholar] [CrossRef]
- Atlas of the Komi Republic on Climate and Hydrology; Drofa: Moscow, Russia, 1997; p. 116. (In Russian)
- Vlasova, V.V.; Dronova, T.I.; Degteva, S.V.; Elsakov, V.V.; Zherebtsov, I.L.; Zainullin, V.G.; Zakharov, A.B.; Matsuk, M.A.; Sharapov, V.E.; Sazhina, S.A.; et al. Atlas of the Republic of Komi; Theoria: Moscow, Russia, 2011; p. 448. (In Russian) [Google Scholar]
- Yudin, Y.P. Productive Forces of the Komi ASSR; Akad. Nauk SSSR: Flora, Moscow, 1954; Volume 3, pp. 332–360. (In Russian) [Google Scholar]
- Forests of the Komi Republic; Kozubov, G.M.; Taskaev, A.I. (Eds.) Publishing Centre Design. Information. Cartography; Forests of the Komi Republic: Moscow, Russia, 1999; p. 332. (In Russian) [Google Scholar]
- Pystina, T.N. Lichens of Taiga Forests of the European Northeast (Subzones of the Southern and Middle Taiga); Ural Branch of the Russian Academy of Sciences: Yekaterinburg, Russia, 2003; p. 239. [Google Scholar]
- Soil Atlas of the Komi Republic; Dobrovolsky, G.V.; Taskaev, A.I.; Zaboeva, I.V. (Eds.) Soil Atlas of the Komi Republic; Komi Republican Printing House: Syktyvkar, Russia, 2001; p. 356. (In Russian) [Google Scholar]
- Westberg, M.; Moberg, R.; Myrdal, M.; Nordin, A.; Ekman, S. Santesson’s Checklist of Fennoscandian Lichen-Forming and Lichenicolous Fungi; Uppsala University: Museum of Evolution 2021; Uppsala University: Uppsala, Sweden, 2021; p. 933. [Google Scholar]
- Golubkova, N.S.; Biazrov, L.G. Life forms of lichens and lichenosinusia. Bot. Zh. 1989, 74, 794–805. (In Russian) [Google Scholar]
- Krivolutsky, D.A.; Lebren, F.; Kunst, M.; Akimov, I.A.; Bayartogtokh, B.; Vasiliu, N.; Golosova, L.D.; Grishina, L.G.; Karppinen, E.; Kramnoy, V.J.; et al. Oribatid Mites: Morphology, Development, Phylogeny, Ecology, Research Methods, and Characteristics of the Model Species Nothrus Palustris C.L. Koch, 1839; Nauka: Moscow, Russia, 1995; p. 223. (In Russian) [Google Scholar]
- Gilyarov, M.S. The Key to Identify Soil Mites. Sarcoptiformes; Nauka: Moscow, Russia, 1975; p. 488. (In Russian) [Google Scholar]
- Subías, L.S. Listado sistemático, sinonímico y biogeográfico de los ácaros oribátidos (Acariformes, Oribatida) del mundo (ex-cepto fósiles). Monografías Electrónicas S.E.A. 2022, 12, 1–538. [Google Scholar]
- Karppinen, E.; Krivolutsky, D.A. List of oribatid mites (Acarina, Oribatei) of northern palaearctic region. 1. Europe. Acta Entomol. Fenn. 1982, 41, 1–18. [Google Scholar]
- Golosova, L.; Karppinen, E.; Krivolutsky, D.A. List of oribatid mites (Acarina, Oribatei) of northern palaearctic region. II. Siberia and the Far East. Acta Entomol. Fenn. 1983, 43, 1–14. [Google Scholar]
- Karppinen, E.; Krivolutsky, D.A.; Poltavskaja, M.P. List of oribatid mites (Acarina, Oribatei) of northern palaearctic region. III. Arid lands. Ann. Entomol. Fenn. 1986, 52, 81–94. [Google Scholar]
- Grishina, L.G. Oribatid mites of the North of Siberia. In Arthropods of Siberia and the Far East; Nauka: Novosibirsk, Russia, 1985; pp. 14–24. (In Russian) [Google Scholar]
- Grishina, L.G.; Andrievsky, V.S. Oribatid mites of the steppes of Western Siberia and Kazakhstan. In Arthropods of Siberia and the Far East; Nauka: Novosibirsk, Russia, 1985; pp. 28–39. (In Russian) [Google Scholar]
- Grishina, L.G.; Dobrotvorsky, A.K. Peculiarities of the population of oribatid mites in recreational pine forests of the Upper Ob region. In Arthropods of Siberia and the Far East; Nauka: Novosibirsk, Russia, 1985; pp. 23–28. (In Russian) [Google Scholar]
- Pankov, A.N.; Ryabinin, N.A.; Golosova, L.D. Catalogue of Oribatid Mites of the Far East of Russia. Part I. Catalogue of Oribatid Mites of Kamchatka, Sakhalin and Kuril Islands; Far Eastern Branch of the Russian Academy of Sciences: Vladivostok-Khabarovsk, Russia, 1997; p. 87. [Google Scholar]
- Ryabinin, N.A.; Pankov, A.N. Catalogue of Oribatid Mites of the Far East of Russia. Part II. Continental Part of the Far East; Far Eastern Branch of the Russian Academy of Sciences: Vladivostok-Khabarovsk, Russia, 2002; p. 92. [Google Scholar]
- Behan-Pelletier, V. Oribatid mites (Acari: Oribatida) of the Yukon. In Insects of the Yukon; Danks, H.V., Downes, J.A., Eds.; Biological survey of Canada: Ottawa, ON, Canada, 1997; pp. 115–149. [Google Scholar]
- Behan, V.M.; Hill, S.B. Distribution and diversity of North American Arctic soil Acari. In Soil Biology as Related to Land Use Practices; Dindal, D.L., Ed.; McGill University: Montréal, QC, Canada, 1980; pp. 717–740. [Google Scholar]
- Shtanchaeva, U.Y. Catalog of oribatid mites of the Caucasus (Acari, Oribatida). Acarina 2001, 9, 177–221. [Google Scholar]
- Sidorchuk, E.A. New Data on the fauna of oribatid mites (Acari, Oribatida) from the polar Urals. EÈntomol. Rev. 2009, 89, 554–563. [Google Scholar] [CrossRef]
- Bayartogtokh, B.; Schatz, H.; Ekrem, T. Distribution and diversity of the soil mites of Svalbard, with redescription of three known species (Acari: Oribatida). Int. J. Acarol. 2011, 37, 467–484. [Google Scholar] [CrossRef]
- Coulson, S.J.; Convey, P.; Aakra, K.; Aarvik, L.; Ávila-Jiménez, M.L.; Babenko, A.; Biersma, E.M.; Boström, S.; Brittain, J.E.; Carlsson, A.M.; et al. The terrestrial and freshwater invertebrate biodiversity of the archipelagoes of the Barents Sea; Svalbard, Franz Josef Land and Novaya Zemlya. Soil Biol. Biochem. 2014, 68, 440–470. [Google Scholar] [CrossRef]
- Coulson, S.J.; Fjellberg, A.; Melekhina, E.N.; Taskaeva, A.A.; Lebedeva, N.V.; Belkina, O.A.; Seniczak, S.; Seniczak, A.; Gwiazdowicz, D.J. Microarthropod communities of industrially disturbed or imported soils in the High Arctic; the abandoned coal mining town of Pyramiden, Svalbard. Biodivers. Conserv. 2015, 24, 1671–1690. [Google Scholar] [CrossRef]
- Melekhina, E.N. Diversity of fauna and geographical distribution of the oribatid mites (Oribatida) of the taiga zone of the Eu-ropean North-East. In Regularities in the Zonal Organization of Complexes of the Animal Population in the European North-East; Komi Science Center of Ural Branch of Russian Academy of Sciences: Syktyvkar, Russia, 2005; Volume 177, pp. 258–274. (In Russian) [Google Scholar]
- Melekhina, E.N. Taxonomic diversity and areology of oribatid mites (Oribatei) of the European North of Russia. Proc. Komi Sci. Cent. Ural Div. Russ. Acad. Sci. 2011, 2, 30–37. (In Russian) [Google Scholar]
- Melekhina, E.N. Analysis of oribatid fauna of the eastern European tundra with first reported data from Subpolar Urals. Diversity 2020, 12, 235. [Google Scholar] [CrossRef]
- Melekhina, E.N.; Zinovyeva, A.N. First data on oribatid mites (Acari: Oribatida) of Pay-Khoy ridge (Yugor peninsula). Proc. Komi Sci. Cent. Ural. Branch Russ. Acad. Sci. 2012, 2, 42–50. (In Russian) [Google Scholar]
- Melekhina, E.N.; Matyukhin, A.V.; Glazov, P.M. Oribatid mites in nests of the Lapland Bunting (Calcarius lapponicus) on the arctic island of Vaygach (with analysis of the islands fauna). Proc. Karelian Res. Cent. Russ. Acad. Sci. 2019, 8, 108–122. [Google Scholar] [CrossRef]
- Melekhina, E.N.; Kanev, V.A.; Deneva, S.V. Karst Ecosystems of Middle Timan, Russia: Soils, Plant Communities, and Soil Oribatid Mites. Diversity 2022, 14, 718. [Google Scholar] [CrossRef]
- Danks, H.V. Arctic Artropods. A Reviev of Systematics and Ecology with Particular Reference to the North American Fauna; Entomological Society of Canada: Ottawa, ON, Canada, 1981; p. 608. [Google Scholar]
- Thunes, K.H.; Søli, G.E.E.; Thuróczy, C.; Fjellberg, A.; Olberg, S.; Roth, S.; Coulianos, C.-C.; Disney, R.H.L.; Starý, J.; Vierbergen, G.; et al. The Arthropod Fauna of Oak (Quercus spp., Fagaceae) Canopies in Norway. Diversity 2021, 13, 332. [Google Scholar] [CrossRef]
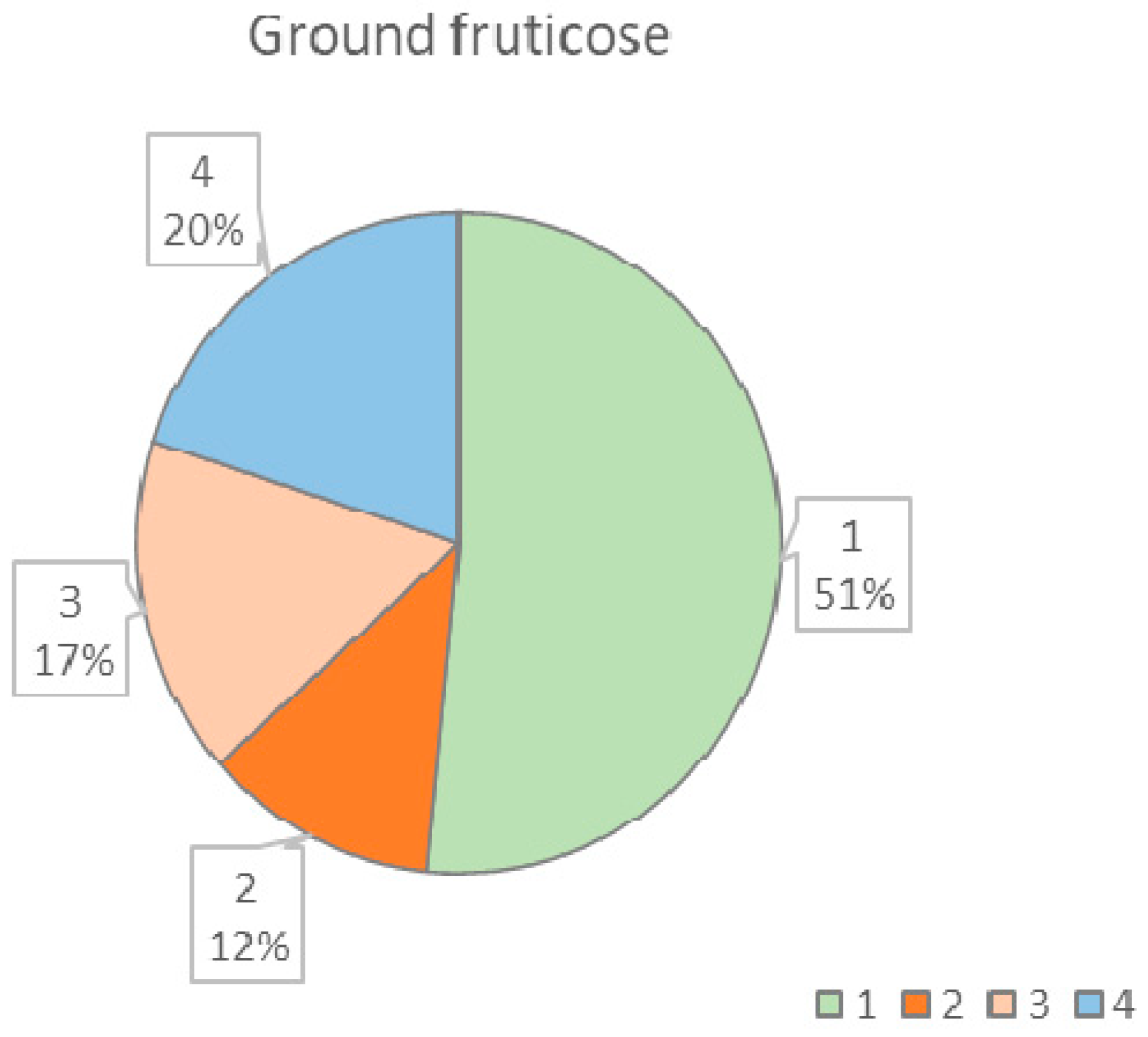
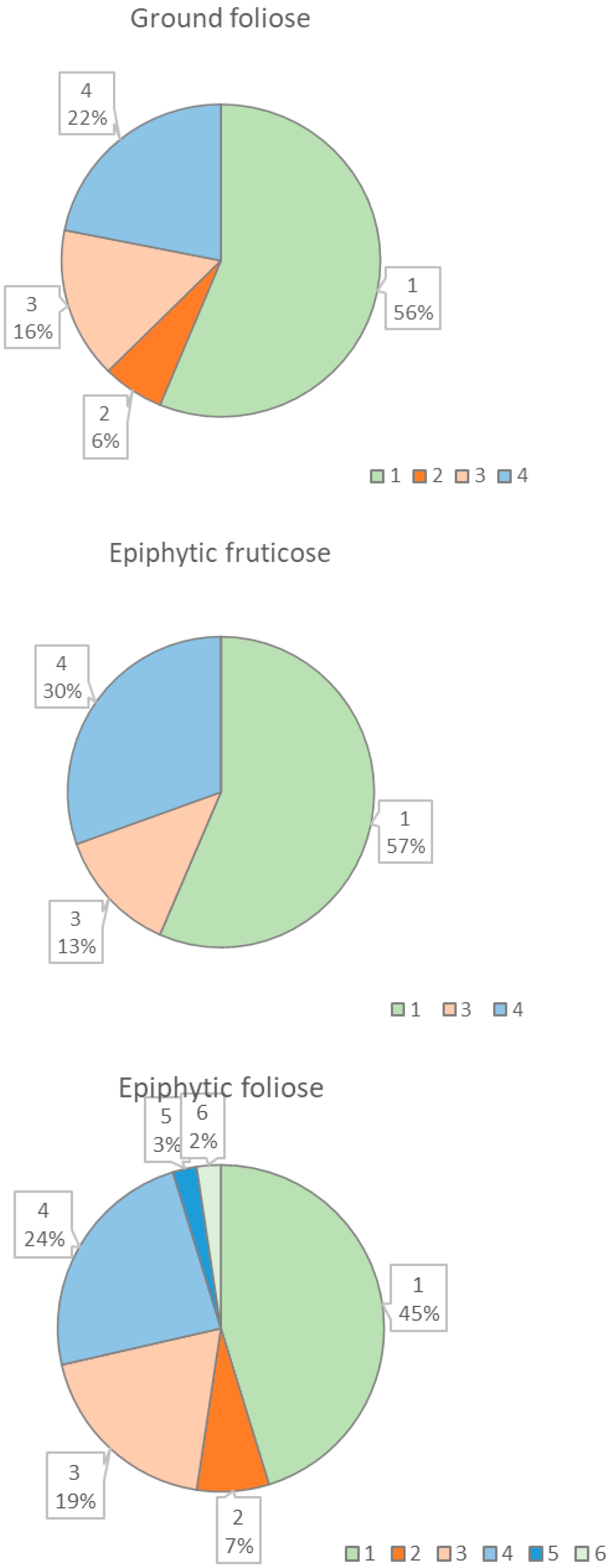

| Life Forms of Lichens | Lichen Species |
|---|---|
| Ground fruticose lichen | Cetraria islandica (L.) Ach. (1, 2, 3, 4, 5, 6), Cetrariella delisei (Bory ex Schaer.) Kärnefelt & A.Thell (1, 2, 3, 4), Cladonia arbuscula (Wallr.) Flot. (1, 2, 3, 4, 5), Cladonia mitis Sandst. (1, 2, 3), Cladonia rangiferina (L.) F.H.Wigg. (1, 2, 3, 4, 5, 6), Cladonia stellaris (Opiz) Pouzar & Vězda (1, 2, 3, 4, 5, 6), Cladonia crispata (Ach.) Flot. var. crispata (1, 2, 3, 5, 6), Cladonia digitata (L.) Hoffm. (1, 5, 6), Cladonia fimbriata (L.) Fr. (1, 2, 5), Cladonia gracilis (L.) Willd. subsp. gracilis (1, 2, 3), Cladonia uncialis (L.) Weber ex F.H. Wigg. (1, 2, 3), Stereocaulon sp. (1, 2, 3) |
| Ground foliose lichen | Peltigera aphthosa (L.) Willd. (1, 4, 5), Peltigera canina (L.) Willd. (5, 6, 7), Peltigera leucophlebia (Nyl.) Gyeln. (1, 5, 6) |
| Epiphytic fruticose lichen | Bryoria fuscescens (Gyeln.) Brodo & D.Hawksw. (1, 2, 3, 4, 5, 6), Evernia mesomorpha Nyl. (3, 4, 5), Ramalina calicaris (L.) Fr. (3, 4, 5, 6), Usnea subfloridana Stirt. (3, 4, 5, 6) |
| Epiphytic foliose lichen | Parmeliopsis ambigua (Wulfen) Nyl. (3, 4, 5, 6, 8, 9), Hypogymnia physodes (L.) Nyl. (1, 2, 3, 4, 5, 6, 11), Leptogium saturninum (Dicks.) Nyl. (6, 9), Lobaria pulmonaria (L.) Hoffm. (4, 5, 9), Melanohalea olivacea (L.) O.Blanco et al. (8, 9), Melanohalea septentrionalis (Lynge) O.Blanco et al. (5, 8, 9), Parmelia sulcata Taylor (5, 8, 9, 10), Platismatia glauca (L.) W.L. Culb. & C.F. Culb. (5, 6), Tuckermannopsis chlorophilla (Willd.) Hale (5, 6, 8, 9), Vulpicida pinastri (Scop.) J.E. Mattsson & M.J. Lai (1, 3, 5) |
| Epiphytic crustose lichen | Chaenotheca chrysocephala (Turner ex Ach.) Th. Fr. (5, 6), Lepraria incana (L.) Ach. (5, 6), Mycoblastus sanguinarius (L.) Norman (3, 4), Lepra albescens (Huds.) Hafellner (5, 6), Phaeophyscia ciliata (Hoffm.) Moberg (8), Physconia distorta (With.) J.R. Laundon (4, 5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melekhina, E.N. Lichen-Associated Oribatid Mites in the Taiga Zone of Northeast European Russia: Taxonomical Composition and Geographical Distribution of Species. Diversity 2023, 15, 599. https://doi.org/10.3390/d15050599
Melekhina EN. Lichen-Associated Oribatid Mites in the Taiga Zone of Northeast European Russia: Taxonomical Composition and Geographical Distribution of Species. Diversity. 2023; 15(5):599. https://doi.org/10.3390/d15050599
Chicago/Turabian StyleMelekhina, Elena N. 2023. "Lichen-Associated Oribatid Mites in the Taiga Zone of Northeast European Russia: Taxonomical Composition and Geographical Distribution of Species" Diversity 15, no. 5: 599. https://doi.org/10.3390/d15050599
APA StyleMelekhina, E. N. (2023). Lichen-Associated Oribatid Mites in the Taiga Zone of Northeast European Russia: Taxonomical Composition and Geographical Distribution of Species. Diversity, 15(5), 599. https://doi.org/10.3390/d15050599







