Effects of Natural Land Cover, Anthropogenic Disturbance, Space, and Climate on Oribatid Mite Communities in Canada’s Oil Sands Region
Abstract
1. Introduction
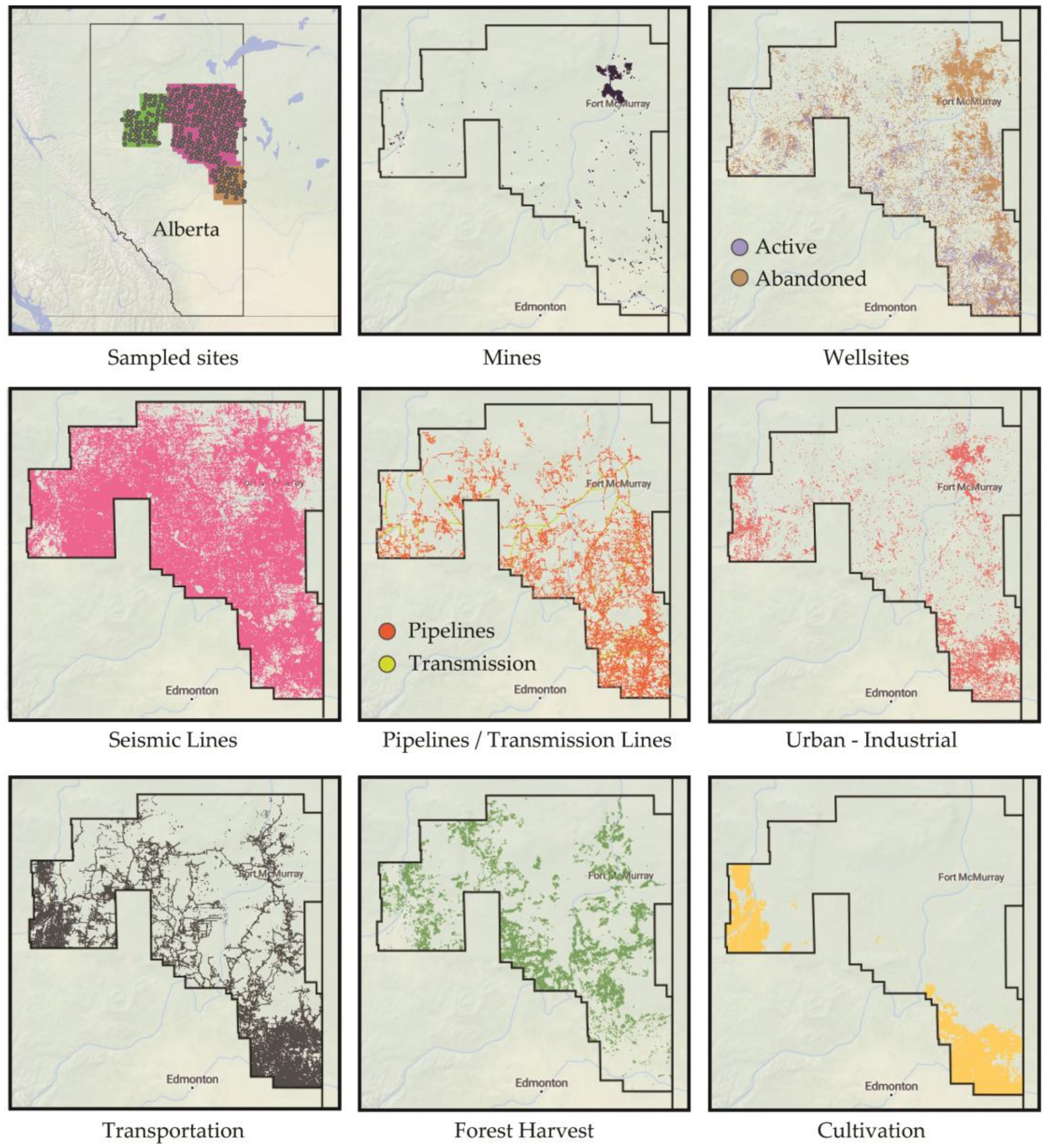
2. Materials and Methods
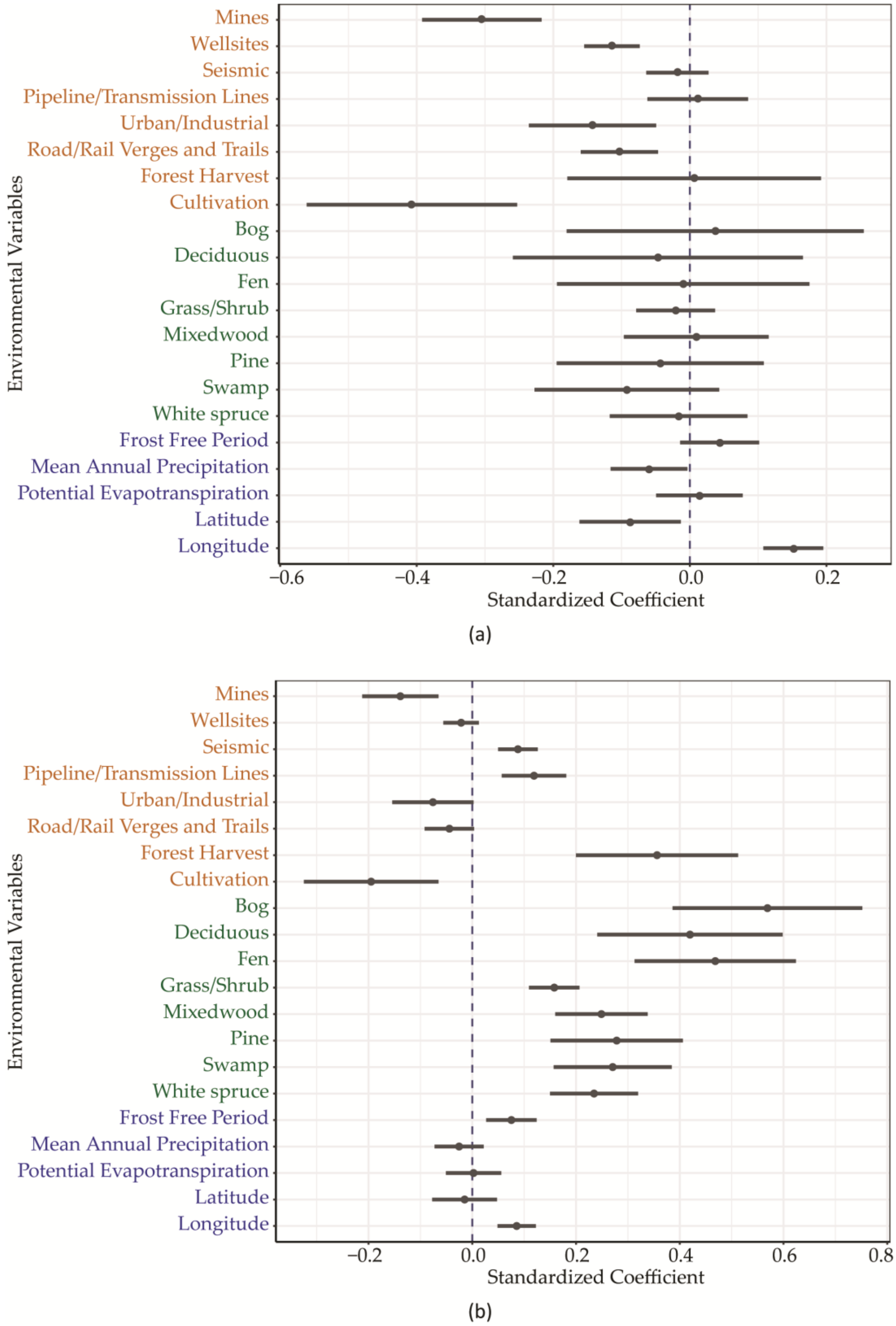
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Superfamily | Family | Species | Author |
|---|---|---|---|
| Parhypochthonioidea | Gehypochthoniidae | Gehypochthonius sp. 1 LML | |
| Brachychthonioidea | Brachychthoniidae | Eobrachychthonius latior | (Berlese, 1910) |
| Hypochthonioidea | Eniochthoniidae | Eniochthonius crosbyi | (Ewing, 1909) |
| Eniochthonius mahunkai | Norton and Behan-Pelletier, 2007 | ||
| Eniochthonius minutissimus | (Berlese, 1903) | ||
| Eniochthonius sp. 1 LML | |||
| Hypochthoniidae | Hypochthonius luteus | Oudemans, 1917 | |
| Hypochthonius rufulus | C.L. Koch, 1836 | ||
| Euphthiracaroidea | Euphthiracaridae | Euphthiracarus cf. flavus | (Ewing, 1908) |
| Euphthiracarus cf. fulvus | (Ewing, 1909) | ||
| Rhysotritia ardua | (C.L. Koch, 1841) | ||
| Oribotritiidae | Mesotritia nuda | (Berlese, 1887) | |
| Protoribotritia sp. 1 DEW | |||
| Phthiracaroidea | Phthiracaridae | Atropacarus striculus | (C. L. Koch, 1835) |
| Hoplophthiracarus illinoisensis | (Ewing, 1909) | ||
| Phthiracaridae sp. | |||
| Phthiracarus boresetosus | Jacot, 1930 | ||
| Phthiracarus cf. borealis | (Trägårdh, 1910) | ||
| Crotonioidea | Crotoniidae | Camisia biurus | (C.L. Koch, 1839) |
| Camisia biverrucata | (CL Koch, 1839) | ||
| Camisia horrida | (Hermann, 1804) | ||
| Camisia sp. 1 DEW | |||
| Camisia spinifer | (C.L. Koch, 1835) | ||
| Heminothrus longisetosus | Willmann, 1925 | ||
| Heminothrus thori | (Berlese, 1904) | ||
| Neonothrus humicola | Forsslund, 1955 | ||
| Platynothrus peltifer | (C.L. Koch, 1839) | ||
| Platynothrus sibiricus | Sitnikova, 1975 | ||
| Platynothrus sp. 1 DEW | |||
| Platynothrus yamasakii | Aoki, 1958 | ||
| Malaconothridae | Malaconothrus cf. mollisetosus | Hammer, 1952 | |
| Trimalaconothrus foveolatus | (Willmann, 1931) | ||
| Trimalaconothrus maior | (Berlese, 1910) | ||
| Trimalaconothrus sp. 3 DEW | |||
| Nanhermanniidae | Nanhermannia sp. 1 DEW | ||
| Nothridae | Nothrus anauniensis | Canestrini and Fanzago, 1876 | |
| Nothrus borussicus | Sellnick, 1928 | ||
| Nothrus cf. pratensis | Sellnick, 1928 | ||
| Nothrus sp. B DEW | |||
| Trhypochthoniidae | Mainothrus badius | (Berlese, 1905) | |
| Mucronothrus nasalis | (Willmann, 1929) | ||
| Trhypochthoniellus setosus canadensis | Hammer, 1952 | ||
| Trhypochthonius cf. cladonicola | (Willmann, 1919) | ||
| Trhypochthonius cf. nigricans | Willmann, 1928 | ||
| Trhypochthonius tectorum | (Berlese, 1896) | ||
| Achipterioidea | Achipteriidae | Achipteria coleoptrata | (Linnaeus, 1758) |
| Achipteria sp. 1 DEW | |||
| Anachipteria cf. howardi | (Berlese, 1908) | ||
| Anachipteria sp. 1 DEW | |||
| Parachipteria bella | (Sellnick, 1928) | ||
| Parachipteria sp. | |||
| Parachipteria sp. 1 DEW | |||
| Tegoribatidae | Tegoribates americanus | Hammer, 1958 | |
| Tegoribates subniger | Ewing, 1917 | ||
| Carabodoidea | Carabodidae | Carabodes granulatus | Banks, 1895 |
| Carabodes labyrinthicus | (Michael, 1879) | ||
| Carabodes polyporetes | Reeves, 1991 | ||
| Carabodes wonalancetanus | Reeves, 1990 | ||
| Cepheoidea | Cepheidae | Cepheus sp. 1 DEW | |
| Cepheus sp. 2 DEW | |||
| Cepheus sp. 2B DEW | |||
| Oribatodes mirabilis | Banks, 1895 | ||
| Ceratozetoidea | Ceratozetidae | Ceratozetes cuspidatus | Jacot, 1939 |
| Ceratozetes gracilis | (Michael, 1884) | ||
| Ceratozetes mediocris | Berlese, 1908 | ||
| Ceratozetes parvulus | Sellnick, 1922 | ||
| Ceratozetes sp. 1 LML | |||
| Ceratozetes sp. 2 LML | |||
| Ceratozetes thienemanni | Willmann, 1943 | ||
| Dentizetes ledensis | Behan-Pelletier, 2000 | ||
| Diapterobates humeralis | (Hermann, 1804) | ||
| Diapterobates sp. | |||
| Diapterobates variabilis | Hammer, 1955 | ||
| Fuscozetes fuscipes | (C.L. Koch, 1844) | ||
| Lepidozetes singularis | Berlese, 1910 | ||
| Lepidozetes sp. 1 DEW | |||
| Neogymnobates luteus | (Hammer, 1955) | ||
| Neogymnobates sp. 1 DEW | |||
| Scutozetes lanceolatus | Hammer, 1952 | ||
| Sphaerozetes arcticus | Hammer, 1952 | ||
| Sphaerozetes sp. 1 DEW | |||
| Trichoribates copperminensis | Hammer, 1952 | ||
| Trichoribates sp. | |||
| Trichoribates sp. 2 DEW | |||
| Trichoribates sp. 3 DEW | |||
| Trichoribates sp. 5 LML | |||
| Trichoribates striatus | Hammer, 1952 | ||
| Chamobatidae | Chamobates cf. cuspidatus | (Michael, 1884) | |
| Chamobates sp. 2 DEW | |||
| Punctoribatidae | Mycobates hylaeus | Behan-Pelletier, 1994 | |
| Mycobates incurvatus | Hammer, 1952 | ||
| Mycobates perates | Behan-Pelletier, 1994 | ||
| Pelopsis bifurcatus | (Ewing, 1909) | ||
| Punctoribates palustris | (Banks, 1895) | ||
| Zetomimidae | Heterozetes aquaticus | (Banks, 1895) | |
| Zetomimus francisi | (Habeeb, 1974) | ||
| Cymbaeremaeoidea | Cymbaeremaeidae | Scapheremaeus palustris | (Sellnick, 1924) |
| Damaeoidea | Damaeidae | Dyobelba sp. 1 DEW | |
| Epidamaeus arcticolus | (Hammer, 1952) | ||
| Epidamaeus canadensis | (Banks, 1909) | ||
| Epidamaeus cf. fortispinosus | Hammer, 1967 | ||
| Epidamaeus coxalis | (Hammer, 1952) | ||
| Epidamaeus floccosus | Behan-Pelletier and Norton, 1985 | ||
| Epidamaeus koyukon | Behan-Pelletier and Norton, 1985 | ||
| Epidamaeus sp. 1 DEW | |||
| Epidamaeus sp. 2 DEW | |||
| Epidamaeus sp. 3 DEW | |||
| Epidamaeus sp. 4 DEW | |||
| Epidamaeus sp. 5 DEW | |||
| Epidamaeus sp. 8 DEW | |||
| Epidamaeus tritylos | Behan-Pelletier and Norton, 1983 | ||
| Quatrobelba montana | Norton, 1980 | ||
| Galumnoidea | Galumnidae | Galumna sp. 1 DEW | |
| Pergalumna sp. 1 DEW | |||
| Pilogalumna sp. | |||
| Pilogalumna sp. 1 DEW | |||
| Pilogalumna sp. 2 DEW | |||
| Gustavioidea | Astegistidae | Astegistes sp. 1 DEW | |
| Gustaviidae | Gustavia sp. 1 DEW | ||
| Liacaridae | Dorycranosus cf. acutidens | (Aoki, 1965) | |
| Dorycranosus parallelus | (Hammer, 1967) | ||
| Dorycranosus sp. 4 DEW | |||
| Peloppiidae | Ceratoppia bipilis | (Hermann, 1804) | |
| Ceratoppia quadridentata arctica | Hammer, 1955 | ||
| Tenuialidae | Hafenferrefia sp. 1 DEW | ||
| Hermannielloidea | Hermanniellidae | Hermanniella robusta | Ewing, 1918 |
| Licneremaeoidea | Passalozetidae | Bipassalozetes cf. intermedius | (Mihelčič, 1954) |
| Limnozetoidea | Hydrozetidae | Hydrozetes octosetosus | Willmann, 1932 |
| Hydrozetes sp. | |||
| Hydrozetes sp. 1 DEW | |||
| Hydrozetes sp. 2 DEW | |||
| Hydrozetes sp. 3 DEW | |||
| Hydrozetes sp. E RAN | |||
| Limnozetidae | Limnozetes canadensis | Hammer, 1952 | |
| Oppioidea | Autognetidae | Autogneta sp. 2 DEW | |
| Oppiidae | Moritzoppia sp. 1 DEW | ||
| Multioppia sp. 1 DEW | |||
| Oppiella cf. washburni | (Hammer, 1952) | ||
| Oppiella sp. 2 DEW | |||
| Oppiella sp. 3 DEW | |||
| Oppiella sp. 4 LML | |||
| Ramusella sp. 2 DEW | |||
| Thyrisomidae | Banksinoma lanceolata canadensis | Fujikawa, 1979 | |
| Banksinoma spinifera | (Hammer, 1952) | ||
| Oribatelloidea | Oribatellidae | Oribatella banksi | Behan-Pelletier and Walter, 2012 |
| Oribatella ewingi | Behan-Pelletier and Walter, 2012 | ||
| Oribatella jacoti | Behan-Pelletier, 2011 | ||
| Oribatella reticulatoides | Hammer, 1955 | ||
| Oribatella yukonensis | Behan-Pelletier and Walter, 2012 | ||
| Oripodoidea | Haplozetidae | Peloribates canadensis | Hammer, 1952 |
| Peloribates pilosus | Hammer, 1952 | ||
| Peloribates sp. | |||
| Peloribates sp. 3 DEW | |||
| Peloribates sp. 4 DEW | |||
| Protoribates haughlandae | Walter and Latonas, 2013 | ||
| Protoribates robustior | (Jacot, 1937) | ||
| Protoribates sp. | |||
| Protoribates sp. 3 LML | |||
| Mochlozetidae | Podoribates longipes | (Berlese, 1887) | |
| Oribatulidae | Eporibatula sp. 1 DEW | ||
| Lucoppia burrowsii | (Michael, 1890) | ||
| Oribatula sp. 1 DEW | |||
| Oribatula sp. 2 LML | |||
| Phauloppia boletorum | (Ewing, 1913) | ||
| Zygoribatula bulanovae | Kulijew, 1961 | ||
| Zygoribatula sp. 1 DEW | |||
| Zygoribatula sp. 2 DEW | |||
| Parakalummidae | Neoribates sp. 1 DEW | ||
| Neoribates sp. 2 DEW | |||
| Scheloribatidae | Dometorina plantivaga | (Berlese, 1895) | |
| Hemileius haydeni | (Higgins and Woolley, 1975) | ||
| Paraleius leontonycha | (Berlese, 1910) | ||
| Scheloribates laevigatus | (C.L. Koch, 1835) | ||
| Scheloribates pallidulus | (C.L. Koch, 1841) | ||
| Scheloribates sp. | |||
| Scheloribates sp. 3 DEW | |||
| Phenopelopoidea | Phenopelopidae | Eupelops cf. septentrionalis | (Trägårdh, 1910) |
| Eupelops sp. 2 DEW | |||
| Eupelops sp. 3 DEW | |||
| Peloptulus sp. 1 DEW | |||
| Propelops alaskensis | (Hammer, 1955) | ||
| Propelops canadensis | (Hammer, 1952) | ||
| Unduloribatidae | Unduloribates dianae | Behan-Pelletier and Walter, 2009 | |
| Plateremaeoidea | Gymnodamaeidae | Gymnodamaeus cf. ornatus | Hammer, 1952 |
| Pleodamaeus sp. 1 DEW | |||
| Roynortonella gildersleeveae | (Hammer, 1952) | ||
| Roynortonella sp. 1 DEW | |||
| Tectocepheoidea | Tectocepheidae | Tectocepheus sarekensis | Trägårdh, 1910 |
| Tectocepheus velatus | (Michael, 1880) | ||
| Trizetoidea | Suctobelbidae | Allosuctobelba gigantea | (Hammer, 1955) |
| Allosuctobelba sp. 2 DEW | |||
| Suctobelbella punctata | (Hammer, 1955) | ||
| Suctobelbella sp. 2 DEW | |||
| Suctobelbella sp. 3 DEW | |||
| Zetorchestoidea | Eremaeidae | Eremaeus sp. | |
| Eremaeus translamellatus | Hammer, 1952 | ||
| Eueremaeus cf. quadrilamellatus | (Hammer, 1952) | ||
| Eueremaeus foveolatus | (Hammer, 1952) | ||
| Eueremaeus marshalli | Behan-Pelletier, 1993 | ||
| Eueremaeus masinasin | Behan-Pelletier, 1993 | ||
| Eueremaeus trionus | (Higgins, 1979) |
| Coefficient | Standard | Standardized | Standard | ||
|---|---|---|---|---|---|
| Error | Coefficient | Error | |||
| Energy Footprint | |||||
| Mines | −2.314 | 0.664 | −0.305 | 0.087 | *** |
| Well sites | −3.733 | 1.329 | −0.114 | 0.041 | ** |
| Seismic | −0.426 | 1.062 | −0.018 | 0.045 | |
| Pipeline/Transmission Lines | 0.108 | 0.696 | 0.011 | 0.074 | |
| Other Human Footprint | |||||
| Urban/Industrial | −0.982 | 0.641 | −0.143 | 0.093 | |
| Road/Rail Verges and Trails | −2.181 | 1.197 | −0.103 | 0.057 | |
| Forest Harvest | 0.021 | 0.619 | 0.006 | 0.186 | |
| Cultivation | −1.653 | 0.626 | −0.407 | 0.154 | ** |
| Natural Land Cover | |||||
| Bog | 0.104 | 0.610 | 0.037 | 0.218 | |
| Deciduous | −0.134 | 0.611 | −0.047 | 0.212 | |
| Fen | −0.033 | 0.618 | −0.010 | 0.185 | |
| Grass/Shrub | −0.279 | 0.773 | −0.021 | 0.058 | |
| Mixedwood | 0.056 | 0.640 | 0.009 | 0.106 | |
| Pine | −0.178 | 0.621 | −0.043 | 0.152 | |
| Swamp | −0.430 | 0.630 | −0.092 | 0.135 | |
| White spruce | −0.107 | 0.654 | −0.017 | 0.101 | |
| Climate | |||||
| Frost Free Period | 0.006 | 0.008 | 0.044 | 0.058 | |
| Mean Annual Precipitation | −0.002 | 0.002 | −0.060 | 0.056 | |
| Potential Evapotranspiration | 0.000 | 0.002 | 0.014 | 0.063 | |
| Space | |||||
| Latitude | −0.077 | 0.066 | −0.087 | 0.074 | |
| Longitude | 0.068 | 0.020 | 0.152 | 0.044 | *** |
| Coefficient | Standard | Standardized | Standard | ||
|---|---|---|---|---|---|
| Error | Coefficient | Error | |||
| Energy Footprint | |||||
| Mines | −1.291 | 0.381 | −0.257 | 0.076 | *** |
| Well sites | −1.058 | 0.761 | −0.049 | 0.035 | |
| Seismic | 0.757 | 0.609 | 0.049 | 0.039 | |
| Pipeline/Transmission Lines | 0.409 | 0.399 | 0.066 | 0.064 | |
| Other Human Footprint | |||||
| Urban/Industrial | −0.653 | 0.367 | −0.144 | 0.081 | |
| Road/Rail Verges and Trails | −0.961 | 0.686 | −0.069 | 0.049 | |
| Forest Harvest | 0.418 | 0.355 | 0.190 | 0.161 | |
| Cultivation | −0.937 | 0.359 | −0.349 | 0.134 | ** |
| Natural Land Cover | |||||
| Bog | 0.612 | 0.349 | 0.330 | 0.188 | |
| Deciduous | 0.365 | 0.350 | 0.192 | 0.184 | |
| Fen | 0.585 | 0.354 | 0.265 | 0.160 | |
| Grass/Shrub | 0.681 | 0.443 | 0.077 | 0.050 | |
| Mixedwood | 0.572 | 0.367 | 0.143 | 0.092 | |
| Pine | 0.268 | 0.356 | 0.099 | 0.131 | |
| Swamp | 0.301 | 0.361 | 0.098 | 0.117 | |
| White spruce | 0.469 | 0.375 | 0.109 | 0.087 | |
| Climate | |||||
| Frost Free Period | 0.005 | 0.004 | 0.055 | 0.050 | |
| Mean Annual Precipitation | −0.001 | 0.001 | −0.038 | 0.049 | |
| Potential Evapotranspiration | 0.001 | 0.001 | 0.030 | 0.055 | |
| Space | |||||
| Latitude | −0.017 | 0.038 | −0.030 | 0.064 | |
| Longitude | 0.036 | 0.011 | 0.121 | 0.038 | ** |
| Coefficient | Standard | Standardized | Standard | ||
|---|---|---|---|---|---|
| Error | Coefficient | Error | |||
| Energy Footprint | |||||
| Mines | −0.716 | 0.380 | −0.139 | 0.074 | |
| Well sites | −0.477 | 0.760 | −0.021 | 0.034 | |
| Seismic | 1.398 | 0.608 | 0.088 | 0.038 | * |
| Pipeline/Transmission Lines | 0.764 | 0.398 | 0.119 | 0.062 | |
| Other Human Footprint | |||||
| Urban/Industrial | −0.355 | 0.367 | −0.076 | 0.078 | |
| Road/Rail Verges and Trails | −0.634 | 0.685 | −0.044 | 0.048 | |
| Forest Harvest | 0.807 | 0.354 | 0.356 | 0.156 | * |
| Cultivation | −0.538 | 0.358 | −0.195 | 0.130 | |
| Natural Land Cover | |||||
| Bog | 1.085 | 0.349 | 0.569 | 0.183 | ** |
| Deciduous | 0.821 | 0.350 | 0.420 | 0.179 | * |
| Fen | 1.065 | 0.354 | 0.469 | 0.156 | ** |
| Grass/Shrub | 1.439 | 0.442 | 0.158 | 0.049 | ** |
| Mixedwood | 1.022 | 0.366 | 0.249 | 0.089 | ** |
| Pine | 0.775 | 0.355 | 0.278 | 0.128 | * |
| Swamp | 0.857 | 0.360 | 0.271 | 0.114 | * |
| White spruce | 1.035 | 0.374 | 0.235 | 0.085 | ** |
| Climate | |||||
| Frost Free Period | 0.007 | 0.004 | 0.075 | 0.049 | |
| Mean Annual Precipitation | −0.001 | 0.001 | −0.025 | 0.047 | |
| Potential Evapotranspiration | 0.000 | 0.001 | 0.003 | 0.053 | |
| Space | |||||
| Latitude | −0.009 | 0.038 | −0.015 | 0.062 | |
| Longitude | 0.026 | 0.011 | 0.086 | 0.037 | * |
| Df | Sum of Sqs | R2 | F | Pr (>F) | ||
|---|---|---|---|---|---|---|
| Energy Footprint | ||||||
| Mines | 1 | 1.604 | 0.008 | 5.717 | 0.001 | *** |
| Well sites | 1 | 0.410 | 0.002 | 1.462 | 0.080 | |
| Seismic | 1 | 0.380 | 0.002 | 1.354 | 0.116 | |
| Pipeline/Transmission Lines | 1 | 1.049 | 0.005 | 3.737 | 0.001 | *** |
| Other Human Footprint | ||||||
| Urban/Industrial | 1 | 1.292 | 0.006 | 4.603 | 0.001 | *** |
| Road/Rail Verges and Trails | 1 | 1.083 | 0.005 | 3.859 | 0.001 | *** |
| Forest Harvest | 1 | 6.713 | 0.032 | 23.920 | 0.001 | *** |
| Cultivation | 1 | 7.534 | 0.036 | 26.847 | 0.001 | *** |
| Natural Land Cover | ||||||
| Bog | 1 | 6.531 | 0.031 | 23.271 | 0.001 | *** |
| Deciduous | 1 | 9.256 | 0.044 | 32.982 | 0.001 | *** |
| Fen | 1 | 2.335 | 0.011 | 8.319 | 0.001 | *** |
| Grass/Shrub | 1 | 0.473 | 0.002 | 1.685 | 0.025 | * |
| Mixedwood | 1 | 0.400 | 0.002 | 1.426 | 0.094 | |
| Pine | 1 | 2.651 | 0.013 | 9.445 | 0.001 | *** |
| Swamp | 1 | 0.857 | 0.004 | 3.055 | 0.001 | *** |
| White spruce | 1 | 0.645 | 0.003 | 2.297 | 0.003 | ** |
| Climate | ||||||
| Frost Free Period | 1 | 3.147 | 0.015 | 11.213 | 0.001 | *** |
| Mean Annual Precipitation | 1 | 1.066 | 0.005 | 3.797 | 0.001 | *** |
| Potential Evapotranspiration | 1 | 1.362 | 0.007 | 4.852 | 0.001 | *** |
| Space | ||||||
| Latitude | 1 | 0.778 | 0.004 | 2.774 | 0.003 | ** |
| Longitude | 1 | 1.519 | 0.007 | 5.414 | 0.001 | *** |
| Df | R2 | Adj.R2 | |
|---|---|---|---|
| NLC | 8 | 0.131 | 0.118 |
| HF | 9 | 0.070 | 0.055 |
| CS | 5 | 0.064 | 0.056 |
| NLC + HF | 17 | 0.173 | 0.148 |
| NLC + CS | 13 | 0.165 | 0.146 |
| HF + CS | 14 | 0.126 | 0.105 |
| NLC + HF + CS | 22 | 0.209 | 0.178 |
References
- Lavelle, P.; Decaëns, T.; Aubert, M.; Barot, S.; Blouin, M.; Bureau, F.; Margerie, P.; Mora, P.; Rossi, J.-P. Soil invertebrates and ecosystem services. Eur. J. Soil Biol. 2006, 42, S3–S15. [Google Scholar] [CrossRef]
- Turbé, A.; De Toni, A.; Benito, P.; Lavelle, P.; Lavelle, P.; Ruiz, N.; Van der Putten, W.H.; Labouze, E.; Mudgal, S. Soil Biodiversity: Functions, Threats and Tools for Policy Makers. Bio Intelligence Service, IRD, and NIOO, Report for European Commission (DG Environment). 2010. Available online: https://ec.europa.eu/environment/archives/soil/pdf/biodiversity_report.pdf (accessed on 28 November 2022).
- Nielsen, U.N.; Wall, D.H.; Six, J. Soil biodiversity and the environment. Annu. Rev. Environ. Resour. 2015, 40, 63–90. [Google Scholar] [CrossRef]
- Wall, D.H.; Nielsen, U.N.; Six, J. Soil biodiversity and human health. Nature 2015, 528, 69–76. [Google Scholar] [CrossRef] [PubMed]
- FAO; ITPS; GSBI; SCBD; EC. State of Knowledge of Soil Biodiversity-Status, Challenges and Potentialities. Report 2020; FAO: Rome, Italy, 2020. [CrossRef]
- de Ruiter, P.C.; Neutel, A.-M.; Moore, J.C. Modelling food webs and nutrient cycling in agro-ecosystems. Trends Ecol. Evol. 1994, 9, 378–383. [Google Scholar] [CrossRef]
- De Deyn, G.B.; Van der Putten, W.H. Linking aboveground and belowground diversity. Trends Ecol. Evol. 2005, 20, 625–633. [Google Scholar] [CrossRef]
- Wolters, V.; Silver, W.L.; Bignell, D.E.; Coleman, D.C.; Lavelle, P.; Van der Putten, W.H.; De Ruiter, P.; Rusek, J.; Wall, D.H.; Wardle, D.A.; et al. Effects of global changes on above- and belowground biodiversity in terrestrial ecosystems: Implications for ecosystem functioning. Bioscience 2000, 50, 1089–1098. [Google Scholar] [CrossRef]
- Kulmatiski, A.; Anderson-Smith, A.; Beard, K.H.; Doucette-Riise, S.; Mazzacavallo, M.; Nolan, N.E.; Ramirez, R.A.; Stevens, J.R. Most soil trophic guilds increase plant growth: A meta-analytical review. Oikos 2014, 123, 1409–1419. [Google Scholar] [CrossRef]
- Geisen, S.; Wall, D.H.; van der Putten, W.H. Challenges and opportunities for soil biodiversity in the Anthropocene. Curr. Biol. 2019, 29, PR1036–PR1044. [Google Scholar] [CrossRef]
- Dabros, A.; Pyper, M.; Castilla, G. Seismic lines in the boreal and arctic ecosystems of North America: Environmental impacts, challenges, and opportunities. Environ. Rev. 2018, 26, 214–229. [Google Scholar] [CrossRef]
- Janz, A.; Whitson, I.R.; Lupardus, R. Soil quality and land capability of reclaimed oil and gas well pads in southern Alberta: Long-term legacy effects. Can. J. Soil Sci. 2019, 99, 262–276. [Google Scholar] [CrossRef]
- Azeria, E.T.; Santala, K.; McIntosh, A.C.S.; Aubin, I. Plant traits as indicators of recovery of reclaimed wellsites in forested areas: Slow but directional succession trajectory. For. Ecol. Manag. 2020, 468, 118180. [Google Scholar] [CrossRef]
- Mackenzie, D.D.; Naeth, M.A. Native seed, soil and atmosphere respond to boreal forest topsoil (LFH) storage. PLoS ONE 2019, 14, e0220367. [Google Scholar] [CrossRef]
- Gorzelak, M.; McAmmond, B.M.; Van Hamme, J.D.; Birnbaum, C.; Thomsen, C.; Hart, M. Soil microbial communities in long-term soil storage for sand mine reclamation. Ecol. Restor. 2020, 38, 13–23. [Google Scholar] [CrossRef]
- Rockett, L.C. Agricultural impact on the horizontal distribution of oribatid mites (Acari: Oribatida). Int. J. Acarol. 1986, 12, 175–180. [Google Scholar] [CrossRef]
- Behan-Pelletier, V.M. Oribatid mite biodiversity in agroecosystems: Role for bioindication. Agric. Ecosyst. Environ. 1999, 74, 411–423. [Google Scholar] [CrossRef]
- Nawaz, M.F.; Bourrié, G.; Trolard, F. Soil compaction impact and modelling. A review. Agron. Sustain. Dev. 2013, 33, 291–309. [Google Scholar] [CrossRef]
- Schaefer, V.; Hocking, M. Detecting the threshold between ornamental landscapes and functional ecological communities: Soil microarthropods as indicator species. Urban Ecosyst. 2015, 18, 1071–1080. [Google Scholar] [CrossRef]
- Mangová, B.; Hulejová Sládkovičová, V.; Krumpál, M.; Kozánek, M. The impact of different urban conditions on structural characteristics of oribatid mite communities. Biologia 2019, 74, 153–168. [Google Scholar] [CrossRef]
- Wang, X.; Chow, J.C.; Kohl, S.D.; Percy, K.E.; Legge, A.H.; Watson, J.G. Characterization of PM2.5 and PM10 fugitive dust source profiles in the Athabasca Oil Sands Region. J. Air Waste Manag. Assoc. 2015, 65, 1421–1433. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Liu, T.; Eisenhauer, N.; Zhang, W.; Wang, X.; Xiong, Y.; Liang, C.; Fu, S. Plants mitigate detrimental nitrogen deposition effects on soil biodiversity. Soil Biol. Biochem. 2018, 127, 178–186. [Google Scholar] [CrossRef]
- Hsu, Y.-M.; Bytnerowicz, A.; Fenn, M.E.; Percy, K.E. Atmospheric dry deposition of sulfur and nitrogen in the Athabasca Oil Sands Region, Alberta, Canada. Sci. Total Environ. 2016, 568, 285–295. [Google Scholar] [CrossRef]
- Tibbett, M.; Fraser, T.D.; Duddigan, S. Identifying potential threats to soil biodiversity. PeerJ 2020, 8, e9271. [Google Scholar] [CrossRef]
- Cameron, E.K.; Knysh, K.M.; Proctor, H.C.; Bayne, E.M. Influence of two exotic earthworm species with different foraging strategies on abundance and composition of boreal microarthropods. Soil Biol. Biochem. 2013, 57, 334–340. [Google Scholar] [CrossRef]
- McAdams, B.N. Oribatid Mite Communities after Ecosystem Disturbance in Alberta. Master’s Thesis, University of Alberta, Edmonton, AB, Canada, 2017. [Google Scholar]
- Steiner, W.A. Influence of air pollution on moss-dwelling animals: 3. Terrestrial fauna, with emphasis on Oribatida and Collembola. Acarologia 1995, 36, 149–173. [Google Scholar]
- St John, M.G.; Bagatto, G.; Behan-Pelletier, V.; Lindquist, E.E.; Shorthouse, J.D.; Smith, I.M. Mite (Acari) colonization of vegetated mine tailings near Sudbury, Ontario, Canada. Plant Soil 2002, 245, 295–305. [Google Scholar] [CrossRef]
- Battigelli, J.P.; Spence, J.R.; Langor, D.W.; Berch, S.M. Short-term impact of forest soil compaction and organic matter removal on soil mesofauna density and oribatid mite diversity. Can. J. For. Res. 2004, 34, 1136–1149. [Google Scholar] [CrossRef]
- Caruso, T.; Migliorini, M. Micro-arthropod communities under human disturbance: Is taxonomic aggregation a valuable tool for detecting multivariate change? Evidence from Mediterranean soil oribatid coenoses. Acta Oecol. 2006, 30, 46–53. [Google Scholar] [CrossRef]
- Gulvik, M.E. Mites (Acari) as indicators of soil biodiversity and land use monitoring: A review. Pol. J. Ecol. 2007, 55, 415–440. [Google Scholar]
- Skubała, P.; Zaleski, T. Heavy metal sensitivity and bioconcentration in oribatid mites (Acari, Oribatida): Gradient study in meadow ecosystems. Sci. Total Environ. 2012, 414, 364–372. [Google Scholar] [CrossRef]
- Feketeová, Z.; Sládkovičová, V.H.; Mangová, B.; Pogányová, A.; Šimkovic, I.; Krumpál, M. Biological properties of extremely acidic cyanide-laced mining waste. Ecotoxicology 2016, 25, 202–212. [Google Scholar] [CrossRef] [PubMed]
- McAdams, B.N.; Quideau, S.A.; Swallow, M.J.B.; Lumley, L.M. Oribatid mite recovery along a chronosequence of afforested boreal sites following oil sands mining. For. Ecol. Manag. 2018, 422, 281–293. [Google Scholar] [CrossRef]
- Manu, M.; Honciuc, V.; Neagoe, A.; Băncilă, R.I.; Iordache, V.; Onete, M. Soil mite communities (Acari: Mesostigmata, Oribatida) as bioindicators for environmental conditions from polluted soils. Sci. Rep. 2019, 9, 20250. [Google Scholar] [CrossRef] [PubMed]
- Meehan, M.L.; Song, Z.; Lumley, L.M.; Cobb, T.P.; Proctor, H. Soil mites as bioindicators of disturbance in the boreal forest in northern Alberta, Canada: Testing taxonomic sufficiency at multiple taxonomic levels. Ecol. Indic. 2019, 102, 349–365. [Google Scholar] [CrossRef]
- Meier, F.A.; Scherrer, S.; Honegger, R. Faecal pellets of lichenivorous mites contain viable cells of the lichen-forming ascomycete Xanthoria parietina and its green algal photobiont, Trebouxia arboricola. Biol. J. Linn. Soc. 2002, 76, 259–268. [Google Scholar] [CrossRef]
- Norton, R.A. Evolutionary aspects of oribatid mite life histories and consequences for the origin of the Astigmata. In Mites. Ecological and Evolutionary Analysis of Life-History Patterns; Houck, M., Ed.; Chapman & Hall: New York, NY, USA, 1994; pp. 99–135. [Google Scholar] [CrossRef]
- Gergócs, V.; Hufnagel, L. Application of oribatid mites as indicators (review). Appl. Ecol. Environ. Res. 2009, 7, 79–98. [Google Scholar] [CrossRef]
- André, H.M.; Noti, M.-I.; Lebrun, P. The soil fauna: The other last biotic frontier. Biodivers. Conserv. 1994, 3, 45–56. [Google Scholar] [CrossRef]
- Lehmitz, R.; Russell, D.; Hohberg, K.; Christian, A.; Xylander, W.E.R. Active dispersal of oribatid mites into young soils. Appl. Soil Ecol. 2012, 55, 10–19. [Google Scholar] [CrossRef]
- Lindo, Z.; Visser, S. Forest floor microarthropod abundance and oribatid mite (Acari: Oribatida) composition following partial and clear-cut harvesting in the mixedwood boreal forest. Can. J. For. Res. 2004, 34, 998–1006. [Google Scholar] [CrossRef]
- Minor, M.A.; Ermilov, S.G.; Philippov, D.A.; Prokin, A.A. Relative importance of local habitat complexity and regional factors for assemblages of oribatid mites (Acari: Oribatida) in Sphagnum peat bogs. Exp. Appl. Acarol. 2016, 70, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Wehner, K.; Heethoff, M.; Brückner, A. Seasonal fluctuation of oribatid mite communities in forest microhabitats. PeerJ 2018, 6, e4863. [Google Scholar] [CrossRef]
- Barreto, C.; Lindo, Z. Drivers of decomposition and the detrital invertebrate community differ across a hummock-hollow microtopology in Boreal peatlands. Écoscience 2018, 25, 39–48. [Google Scholar] [CrossRef]
- Walter, D.E.; Latonas, S.; Byers, K.; Lumley, L.M. Almanac of Alberta Oribatida Part I, Version 2.4. 2014. Available online: https://www.researchgate.net/publication/352842283_Almanac_of_Alberta_Oribatida_Part_I_Version_24 (accessed on 8 March 2023).
- Beaulieu, F.; Knee, W.; Nowell, V.; Schwarzfeld, M.; Lindo, Z.; Behan-Pelletier, V.M.; Lumley, L.; Young, M.R.; Smith, I.; Proctor, H.C.; et al. Acari of Canada. ZooKeys 2019, 819, 77–168. [Google Scholar] [CrossRef] [PubMed]
- Behan-Pelletier, V.M.; Lindo, Z. Checklist of oribatid mites (Acari: Oribatida) of Canada and Alaska. Zootaxa 2019, 4666, 1–180. [Google Scholar] [CrossRef] [PubMed]
- Barreto, C.; Lindo, Z. Checklist of oribatid mites (Acari: Oribatida) from two contrasting boreal fens: An update on oribatid mites of Canadian peatlands. Syst. Appl. Acarol. 2021, 26, 866–884. [Google Scholar] [CrossRef]
- Kevan, P.G.; Forbes, B.C.; Kevan, S.M.; Behan-Pelletier, V. Vehicle tracks on high Arctic tundra: Their effects on the soil, vegetation, and soil arthropods. J. Appl. Ecol. 1995, 32, 655–667. [Google Scholar] [CrossRef]
- Minor, M.A.; Cianciolo, J.M. Diversity of soil mites (Acari: Oribatida, Mesostigmata) along a gradient of land use types in New York. Appl. Soil Ecol. 2007, 35, 140–153. [Google Scholar] [CrossRef]
- Kim, J.W.; Jung, C. Abundance of soil microarthropods associated with forest fire severity in Samcheok, Korea. J. Asia Pac. Entomol. 2008, 11, 77–81. [Google Scholar] [CrossRef]
- Ivan, O.; Vasiliu, N.A. Oribatid mites (Acari, Oribatida)–bioindicators of forest soils pollution with heavy metals and fluorine. Ann. For. Res. 2009, 52, 11–18. [Google Scholar]
- Cao, Z.; Han, X.; Hu, C.; Chen, J.; Zhang, D.; Steinberger, Y. Changes in the abundance and structure of a soil mite (Acari) community under long-term organic and chemical fertilizer treatments. Appl. Soil Ecol. 2011, 49, 131–138. [Google Scholar] [CrossRef]
- Hugo-Coetzee, E.A.; Avenant, N.L. The effect of fire on soil oribatid mites (Acari: Oribatida) in a South African grassland. Zoosymposia 2011, 6, 210–220. [Google Scholar] [CrossRef]
- Magro, S.; Gutiérrez-López, M.; Casado, M.A.; Jiménez, M.D.; Trigo, D.; Mola, I.; Balaguer, L. Soil functionality at the roadside: Zooming in on a microarthropod community in an anthropogenic soil. Ecol. Eng. 2013, 60, 81–87. [Google Scholar] [CrossRef]
- Skubala, P.; Rola, K.; Osyczka, P.; Kafel, A. Oribatid mite communities on lichens in heavily contaminated post-smelting dumps. Arch. Environ. Contam. Toxicol. 2014, 67, 578–592. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, R.; Palacios-Vargas, J.G.; Castaño-Meneses, G. Comparison of oribatid mites from agricultural soils with contrasting irrigation types in Hidalgo State, Mexico: A case study. Rev. Mex. Biodivers. 2019, 90, e902780. [Google Scholar] [CrossRef]
- Buch, A.C.; Sautter, K.D.; Marques, E.D.; Silva-Filho, E.V. Ecotoxicological assessment after the world’s largest tailing dam collapse (Fundão dam, Mariana, Brazil): Effects on oribatid mites. Environ. Geochem. Health 2020, 42, 3575–3595. [Google Scholar] [CrossRef]
- Todria, N.; Murvanidze, M.; Mumladze, L. Oribatid (Acari: Oribatida) diversity in natural and altered open arid ecosystems of South-Eastern Caucasus. Pedobiologia 2021, 87-88, 150750. [Google Scholar] [CrossRef]
- Alberta Biodiversity Monitoring Institute. The Status of Human Footprint in Alberta. Available online: https://abmi.ca/home/reports/2022/human-footprint (accessed on 8 March 2023).
- Government of Alberta. About Oil Sands. Available online: https://www.alberta.ca/about-oil-sands.aspx (accessed on 8 March 2023).
- Alberta Biodiversity Monitoring Institute. Biodiversity Browser–Soil Mites. Available online: https://beta.abmi.ca/biobrowser/species-group/mites-intro.html (accessed on 8 March 2023).
- Alberta Biodiversity Monitoring Institute. The Alberta Biodiversity Monitoring Institutes Survey Locations, File No. 158, 2008-01-01. Available online: https://abmi.ca/home/publications/151-200/158.html (accessed on 8 March 2023).
- Alberta Biodiversity Monitoring Institute. Terrestrial Field Data Collection Protocols (Abridged Version), v. 2021-04-11. Available online: https://ftp-public.abmi.ca/home/publications/documents/601_ABMI_2021_TerrestrialFieldDataProtocols_ABMI.pdf (accessed on 8 March 2023).
- Alberta Biodiversity Monitoring Institute. Standard Operating Procedures for Oribatid Mites: Processing, Taxonomy and Curation, v.3, 2021-03-31. Available online: https://www.abmi.ca/home/publications/601-650/602 (accessed on 8 March 2023).
- Alberta Biodiversity Monitoring Institute. Wall-to-Wall Vegetation and Human Footprint Inventory. Available online: https://abmi.ca/home/data-analytics/da-top/da-product-overview/Human-Footprint-Products/HF-inventory.html (accessed on 8 March 2023).
- Daly, C.; Gibson, W.P.; Taylor, G.H.; Johnson, G.L.; Pasteris, P. A knowledge-based approach to the statistical mapping of climate. Clim. Res. 2002, 22, 99–113. [Google Scholar] [CrossRef]
- Graham, M.H. Confronting multicollinearity in ecological multiple regression. Ecology 2003, 84, 2809–2815. [Google Scholar] [CrossRef]
- Naimi, B.; Hamm, N.A.S.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where is positional uncertainty a problem for species distribution modelling? Ecography 2014, 37, 191–203. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 8 March 2023).
- Jost, L. Entropy and diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package, v.2.5-7. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 28 November 2022).
- Andrés, P.; Mateos, E. Soil mesofaunal responses to post-mining restoration treatments. Appl. Soil Ecol. 2006, 33, 67–78. [Google Scholar] [CrossRef]
- Todria, N.; Murvanidze, M.; Mumladze, L. Oribatid mite communities on former clay quarries under different reclamation strategy. Ann. Agrar. Sci. 2019, 17, 304–311. [Google Scholar]
- Ashwood, F.; Barreto, C.; Butt, K.R.; Lampert, M.; Doick, K.; Vanguelova, E.I. Earthworms and soil mesofauna as early bioindicators for landfill restoration. Soil Res. 2022. online early view. [Google Scholar] [CrossRef]
- Siepel, H.; de Ruiter-Dijkman, E.M. Feeding guilds of oribatid mites based on their carbohydrase activities. Soil Biol. Biochem. 1993, 25, 1491–1497. [Google Scholar] [CrossRef]
- Schneider, K.; Migge, S.; Norton, R.A.; Scheu, S.; Langel, R.; Reineking, A.; Maraun, M. Trophic niche differentiation in soil microarthropods (Oribatida, Acari): Evidence from stable isotope ratios (15N/14N). Soil Biol. Biochem. 2004, 36, 1769–1774. [Google Scholar] [CrossRef]
- Maraun, M.; Scheu, S. The structure of oribatid mite communities (Acari, Oribatida): Patterns, mechanisms and implications for future research. Ecography 2000, 23, 374–382. [Google Scholar] [CrossRef]
- De Deyn, G.B.; Kooistra, L. The role of soils in habitat creation, maintenance and restoration. Phil. Trans. R. Soc. B. 2021, 376, 20200170. [Google Scholar] [CrossRef]
- Hansen, R.A.; Coleman, D.C. Litter complexity and composition are determinants of the diversity and species composition of oribatid mites (Acari: Oribatida) in litterbags. Appl. Soil Ecol. 1998, 9, 7–23. [Google Scholar] [CrossRef]
- Migliorini, M.; Petrioli, A.; Bernini, F. Comparative analysis of two edaphic zoocoenoses (oribatid mites and carabid beetles) in five habitats of the ‘Pietraporciana’ and ‘Lucciolabella’ Nature Reserves (Orcia Valley, central Italy). Acta Oecol. 2002, 23, 361–374. [Google Scholar] [CrossRef]
- Erdmann, G.; Scheu, S.; Maraun, M. Regional factors rather than forest type drive the community structure of soil living oribatid mites (Acari, Oribatida). Exp. Appl. Acarol. 2012, 57, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Wissuwa, J.; Salamon, J.-A.; Frank, T. Oribatida (Acari) in grassy arable fallows are more affected by soil properties than habitat age and plant species. Eur. J. Soil Biol. 2013, 59, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Maraun, M.; Thomas, T.; Fast, E.; Treibert, N.; Caruso, T.; Schaefer, I.; Lu, J.-Z.; Scheu, S. New perspectives on soil animal trophic ecology through the lens of C and N stable isotope ratios of oribatid mites. Soil Biol. Biochem. 2023, 177, 108890. [Google Scholar] [CrossRef]
- Lupardus, R.C.; Battigelli, J.P.; Janz, A.; Lumley, L.M. Can soil invertebrates indicate soil biological quality on well pads reclaimed back to cultivated lands? Soil Tillage Res. 2021, 213, 105082. [Google Scholar] [CrossRef]
- Ballard, T.M. Impacts of forest management on northern forest soils. For. Ecol. Manag. 2000, 133, 37–42. [Google Scholar] [CrossRef]
- McCann, K.S. The diversity-stability debate. Nature 2000, 405, 228–233. [Google Scholar] [CrossRef]
- Yachi, S.; Loreau, M. Biodiversity and ecosystem productivity in a fluctuating environment: The insurance hypothesis. Proc. Natl. Acad. Sci. USA 1999, 96, 1463–1468. [Google Scholar] [CrossRef]
- Wagg, C.; Dudenhöffer, J.-H.; Widmer, F.; van der Heijden, M.G.A. Linking diversity, synchrony and stability in soil microbial communities. Funct. Ecol. 2018, 32, 1280–1292. [Google Scholar] [CrossRef]
- Macdonald, E.; Quideau, S.; Landhäusser, S. Rebuilding boreal forest ecosystems after industrial disturbance. In Restoration and Reclamation of Boreal Ecosystems: Attaining Sustainable Development; Vitt, D., Bhatti, J., Eds.; Cambridge University Press: New York, NY, USA, 2012; pp. 123–160. [Google Scholar]
- Alberta Energy Regulator. ST37: List of Wells in Alberta. Available online: http://www1.aer.ca/ProductCatalogue/10.html (accessed on 8 March 2023).
- Government of Alberta. Guidelines for Oil and Gas. Available online: https://www.alberta.ca/land-conservation-and-reclamation-guidelines-for-oil-and-gas.aspx (accessed on 8 March 2023).
- Environmental and Sustainable Resource Development. 2010 Reclamation Criteria for Wellsites and Associated Facilities for Cultivated Lands (Updated July 2013); Government of Alberta: Edmonton, AB, Canada, 2013; 92p. Available online: https://open.alberta.ca/dataset/ee82f0ab-fef2-4b78-805d-8c6d341aabd2/resource/54dd817c-225a-483a-a3f1-09cab3136743/download/2013-2010-reclamation-criteria-wellsites-cultivated-lands-2013-07.pdf (accessed on 8 March 2023).
- Pinno, B.D.; Hawkes, V.C. Temporal trends of ecosystem development on different site types in reclaimed boreal forests. Forests 2015, 6, 2109–2124. [Google Scholar] [CrossRef]
- Lupardus, R.C.; McIntosh, A.C.S.; Janz, A.; Farr, D. Succession after reclamation: Identifying and assessing ecological indicators of forest recovery on reclaimed oil and natural gas well pads. Ecol. Indic. 2019, 106, 105515. [Google Scholar] [CrossRef]
- Bayne, E.; Dennett, J.; Dooley, J.; Kohler, M.; Ball, J.; Bidwell, M.; Braid, A.; Chetelat, J.; Dillegeard, E.; Farr, D.; et al. A before after dose response (BADR): Terrestrial biological monitoring framework for the oil sands. OSM Tech. Rep. Ser. 2021, 7, 1–46. Available online: https://open.alberta.ca/publications/9781460151341 (accessed on 8 March 2023).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lumley, L.M.; Azeria, E.T.; Giacobbo, V.A.; Cobb, T.P. Effects of Natural Land Cover, Anthropogenic Disturbance, Space, and Climate on Oribatid Mite Communities in Canada’s Oil Sands Region. Diversity 2023, 15, 469. https://doi.org/10.3390/d15040469
Lumley LM, Azeria ET, Giacobbo VA, Cobb TP. Effects of Natural Land Cover, Anthropogenic Disturbance, Space, and Climate on Oribatid Mite Communities in Canada’s Oil Sands Region. Diversity. 2023; 15(4):469. https://doi.org/10.3390/d15040469
Chicago/Turabian StyleLumley, Lisa M., Ermias T. Azeria, Victoria A. Giacobbo, and Tyler P. Cobb. 2023. "Effects of Natural Land Cover, Anthropogenic Disturbance, Space, and Climate on Oribatid Mite Communities in Canada’s Oil Sands Region" Diversity 15, no. 4: 469. https://doi.org/10.3390/d15040469
APA StyleLumley, L. M., Azeria, E. T., Giacobbo, V. A., & Cobb, T. P. (2023). Effects of Natural Land Cover, Anthropogenic Disturbance, Space, and Climate on Oribatid Mite Communities in Canada’s Oil Sands Region. Diversity, 15(4), 469. https://doi.org/10.3390/d15040469







